Abstract
Summary
Unawareness of postprandial hypoglycemia for 5 years was identified in a 66-year-old man at a local clinic. The patient was referred to our hospital because of this first awareness of hypoglycemia (i.e. lightheadedness and impaired consciousness) developing after lunch. In a 75 g oral glucose tolerance test, the plasma glucose concentration was decreased to 32 mg/dL (1.8 mmol/L) at 150 min with relatively high concentrations of insulin (8.1 μU/mL), proinsulin (70.3 pmol/L), and C-peptide (4.63 ng/mL). In a prolonged fasting test, the plasma glucose concentration was decreased to 43 mg/dL (2.4 mmol/L) at 66 h with an insulin concentration of 1.4 μU/mL and a C-peptide concentration of 0.49 ng/mL. Computed tomography showed an 18 mm hyperenhancing tumor in the uncinate process of the pancreas. A selective arterial calcium stimulation test showed an elevated serum insulin concentration in the superior mesenteric artery. The patient was then diagnosed with insulinoma and received pancreaticoduodenectomy. Continuous glucose monitoring (CGM) using the Dexcom G6 system showed unawareness of hypoglycemia mainly during the daytime before surgery. When the sensor glucose value was reduced to 55 mg/dL (3.1 mmol/L), the Dexcom G6 system emitted an urgent low glucose alarm to the patient four times for 10 days. Two months after surgery, an overall increase in daily blood glucose concentrations and resolution of hypoglycemia were shown by CGM. We report a case of insulinoma with unawareness of postprandial hypoglycemia in the patient. The Dexcom G6 system was helpful for assessing preoperative hypoglycemia and for evaluating outcomes of treatment by surgery.
Learning points
Insulinoma occasionally leads to postprandial hypoglycemia.
The CGM system is useful for revealing the presence of unnoticed hypoglycemia and for evaluating treatment outcomes after surgical resection.
The Dexcom G6 system has an urgent low glucose alarm, making it particularly suitable for patients who are unaware of hypoglycemia.
Patient Demographics: Adult, Male, Asian-Japanese, Japan
Clinical Overview: Pancreas, Diabetes, Endocrine-related cancer, Tumours and neoplasia
Publication Details: Insight into disease pathogenesis or mechanism of therapy, September, 2023
Background
Insulinoma is a rare neuroendocrine tumor of the pancreas that produces excess endogenous insulin leading to hypoglycemia (1). Insulinoma accounts for 20.9% of all pancreatic endocrine tumors in Japan (2). More than 80% of patients with insulinoma develop symptoms of hypoglycemia in the fasting state (3). However, distinguishing insulinoma from other conditions presenting with reactive hypoglycemia (i.e. early-onset diabetes mellitus) is difficult, especially if a patient develops postprandial hypoglycemia. Furthermore, patients with recurrent hypoglycemia may be unaware of this condition (4). This unawareness causes difficulty in diagnosing insulinoma. There have been some reports using continuous glucose monitoring (CGM) for the assessment of hypoglycemia induced by insulinoma and subsequent resolution of hypoglycemia by surgical resection of insulinoma (5, 6, 7). The Dexcom G6 (Dexcom, Inc., San Diego, CA, USA) is a CGM system, which was released in Japan in July 2021. Unlike other CGM systems, the Dexcom G6 system does not require calibration by self-monitoring of capillary blood glucose. Additionally, the Dexcom G6 system has the characteristic feature of providing an urgent low glucose alarm when sensor glucose concentrations are reduced to 55 mg/dL (3.1 mmol/L).
We report a case of insulinoma in a patient who presented with unawareness of postprandial hypoglycemia in whom the Dexcom G6 system was helpful for diagnosing insulinoma and evaluating the outcome after surgical resection.
Case presentation
A 66-year-old man was treated for hypertension for 5 years in a local clinic and received a blood examination every 6 months that was performed approximately 4 h after lunch. The plasma glucose concentration, which was measured on his first visit to a local clinic 5 years previously, was 44 mg/dL (2.4 mmol/L), and it remained between 32 mg/dL (1.8 mmol/L) and 60 mg/dL (3.3 mmol/L) thereafter. However, the patient was unaware of postprandial hypoglycemia. He was referred to our hospital owing to his first awareness of hypoglycemia (i.e. lightheadedness and impaired consciousness occurring after lunch) while driving. These symptoms were relieved by the ingestion of bread. There were no remarkable findings on a physical examination. His blood pressure, height, weight, and BMI were 146/81 mmHg, 166 cm, 65.8 kg, and 23.9 kg/m2, respectively. Although weight was not measured regularly, it had increased by 7 kg in the last 3 years according to the patient’s recollection. Therefore, we suspected that he unconsciously ate an excess amount of food owing to the potential fear of hypoglycemia even though he was unaware of hypoglycemia. He was not obese and did not have a history of type 2 diabetes mellitus or surgery. He was taking a calcium antagonist and renin–angiotensin system inhibitor for hypertension. He had a family history of type 2 diabetes mellitus in his mother and an older brother, but no history of multiple endocrine neoplasia type 1.
Investigation
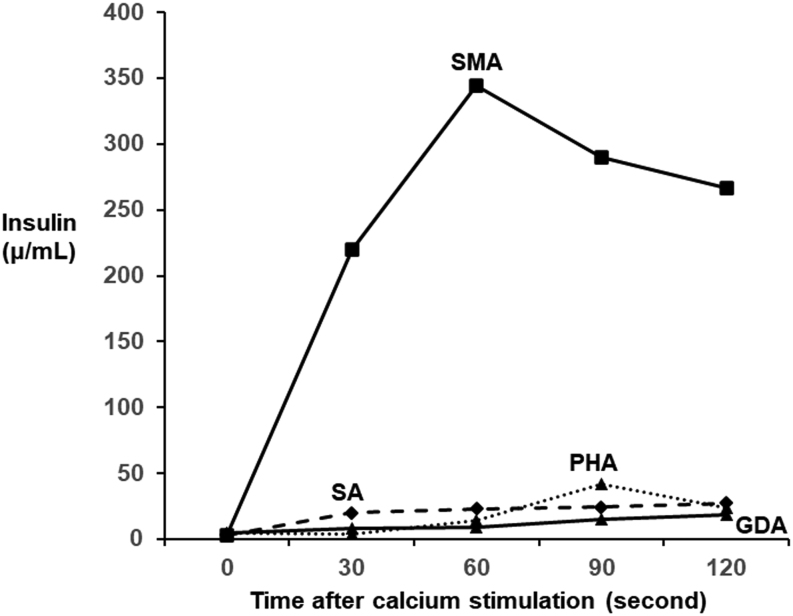
A blood examination performed at 8 a.m. in the fasting state during hospitalization (Table 1) showed that the plasma glucose concentration was 70 mg/dL (3.9 mmol/L), which was at the lower limit of the normal value. Anti-insulin antibody was negative, and none of the laboratory data suggested pituitary or thyroid diseases. In a 75 g oral glucose tolerance test (OGTT), baseline fasting plasma glucose, serum insulin, and serum C-peptide concentrations were 77 mg/dL (4.3 mmol/L), 6.0 μU/mL, and 1.26 ng/mL, respectively (Table 2). His plasma glucose concentration was reduced to 32 mg/dL (1.8 mmol/L) at 150 min after glucose loading, and hypoglycemia persisted thereafter (Table 2). Serum insulin, proinsulin, and C-peptide concentrations were relatively high at 8.1 μU/mL, 70.3 pmol/L, and 4.63 ng/mL, respectively, despite a low plasma glucose concentration (32 mg/dL (1.78 mmol/L)), suggesting the presence of endogenous hyperinsulinemia. In a prolonged fasting test, the plasma glucose concentration was reduced to 43 mg/dL (2.4 mmol/L) at 66 h after the last meal (Table 3). However, insulin (1.4 μU/mL) and C-peptide (0.49 ng/mL) concentrations did not reach 3.0 μU/mL and 0.6 ng/mL, respectively. In contrast to insulin and C-peptide concentrations, the 3-β-hydroxybutyrate concentration was suppressed to 1.4 mmol/L, which suggested hyperinsulinemic hypoglycemia. Subsequent intravenous administration of 1 mg of glucagon insufficiently increased the plasma glucose concentration to 58 mg/dL (3.2 mmol/L). Notably, the patient was unaware of hypoglycemia during the 75 g OGTT and the prolonged fasting test. Computed tomography showed an 18 mm hyperenhancing tumor in the uncinate process of the pancreas (Fig. 1). A selective arterial calcium stimulation test confirmed elevated insulin concentrations from 3.0 μU/mL to 344.3 μU/mL in the superior mesenteric artery (Fig. 2). A clinical diagnosis of insulinoma was made on the basis of these findings.
Table 1.
Baseline data based on blood was collected at 8 a.m. in the fasting state.
| Parameters | Values |
|---|---|
| Complete blood count | |
| White blood cells (/μL) | 5600 |
| Hemoglobin (g/dL) | 13.5 |
| Platelets (×104/μL) | 20.8 |
| Biochemistry | |
| Total protein (g/dL) | 6.5 |
| Albumin (g/dL) | 4.1 |
| Blood urea nitrogen (mg/dL) | 12 |
| Creatinine (mg/dL) | 0.72 |
| Sodium (mEq/L) | 139 |
| Chloride (mEq/L) | 106 |
| Potassium (mEq/L) | 4.2 |
| Aspartate transaminase (IU/L) | 22 |
| Alanine transaminase (IU/L) | 29 |
| Lactate dehydrogenase (IU/L) | 193 |
| Alkaline phosphatase (IU/L) | 176 |
| γ-Glutamyl transpeptidase (IU/L) | 24 |
| Creatinine phosphokinase (IU/L) | 155 |
| Glucose (mg/dL) | 70 |
| Glucose (mmol/L) | 3.9 |
| Hemoglobin A1c (%) | 4.4 |
| Glycated albumin (%) | 9.6 |
| Hormones and others | |
| Insulin (μU/mL) | 5.2 |
| C-peptide (ng/dL) | 1.4 |
| Adrenocorticotropic hormone (pg/mL) | 13.7 |
| Cortisol (μg/dL) | 6.32 |
| Thyroid-stimulating hormone (μU/mL) | 1.0 |
| Free triiodothyronine (pg/mL) | 2.63 |
| Free thyroxine (ng/dL) | 0.97 |
| Growth hormone (ng/mL) | 0.04 |
| Insulin-like growth factor-1 (ng/mL) | 118 |
| Luteinizing hormone (mIU/mL) | 6.94 |
| Follicle-stimulating hormone (mIU/mL) | 10.93 |
| Testosterone (ng/mL) | 4.78 |
| Prolactin (ng/mL) | 11.7 |
| Intact parathyroid hormone (pg/mL) | 50 |
| Glucagon (pg/mL) | 7.2 |
| Anti-insulin antibody (%) | <0.4 |
Table 2.
Seventy-five gram oral glucose tolerance test results.
| Time (min) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 30 | 60 | 90 | 120 | 150 | 180 | 210 | 240 | 270 | 300 | |
| Plasma glucose (mg/dL) | 77 | 158 | 114 | 97 | 83 | 32 | 52 | 54 | 58 | 61 | 65 |
| Plasma glucose (mmol/L) | 4.3 | 8.8 | 6.3 | 5.4 | 4.6 | 1.8 | 2.9 | 3.0 | 3.2 | 3.4 | 3.6 |
| Insulin (μU/mL) | 6.0 | 135.8 | 170.4 | 102.4 | 65.1 | 8.1 | 3.0 | 2.6 | 1.8 | 1.6 | 1.9 |
| C-peptide (ng/mL) | 1.26 | 12.42 | 16.18 | 14.84 | 12.82 | 4.63 | 2.22 | 1.42 | 1.15 | 1.03 | 0.88 |
Table 3.
Prolonged fasting test results.
| Fasting time | After a 1 mg glucagon administration | |||
|---|---|---|---|---|
| 66 h | 10 min | 20 min | 30 min | |
| Plasma glucose (mg/dL) | 43 | 58 | 58 | 58 |
| Plasma glucose (mmol/L) | 2.4 | 3.2 | 3.2 | 3.2 |
| Insulin (μU/mL) | 1.4 | 9.7 | 5.8 | 5 |
| C-peptide (ng/mL) | 0.49 | 1.82 | 1.61 | 1.28 |
| 3-β-hydroxybutyrate (mmol/L) | 1.4 | |||
Figure 1.
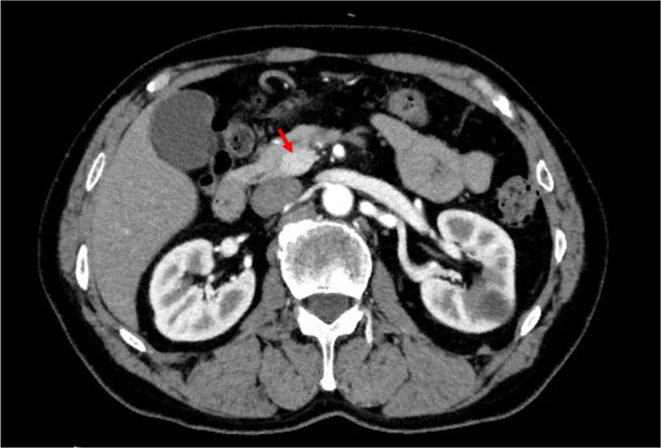
Contrast-enhanced computed tomography image of the abdomen. The arrowhead indicates an 18 mm hyperenhancing tumor in the uncinate process of the pancreas (red arrow).
Figure 2.
Selective arterial calcium stimulation test. Insulin concentrations were elevated in the SMA. GDA, gastroduodenal artery; PHA, proper hepatic artery; SMA, superior mesenteric artery; SA, splenic artery.
Treatment
The patient underwent pancreaticoduodenectomy. A pathological examination showed that the tumor size was 17 × 12 × 17 mm. Immunohistochemical studies showed that the tumor was a neuroendocrine tumor (NET) graded G1 and was positive for chromogranin A (focal), synaptophysin, and insulin (Fig. 3). The Ki67 proliferative index was 0.2–0.5%. His pathological staging was T1cN0M0 (Stage IA) according to the Union for International Cancer Control and European Neuroendocrine Tumor Society tumor-node-metastasis staging system.
Figure 3.
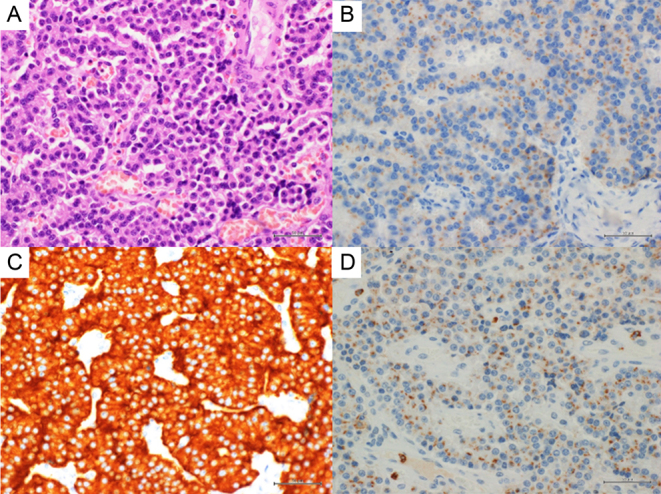
Pathological examination of the pancreatic tumor. (A) Hematoxylin and eosin staining (×400). Immunostaining for (B) insulin (×400), (C) synaptophysin (× 400), and (D) chromogranin A (× 400).
Outcome and follow-up
CGM using the Dexcom G6 system was performed for 10 days before and after surgery (Figs. 4 and 5). Postprandial hypoglycemia was suspected on the basis of the patient’s interview and letter of referral from another hospital. Hypoglycemia (glucose concentrations <70 mg/dL (3.9 mmol/L)) was frequently observed in the postprandial state (Fig. 4). However, fasting hypoglycemia was also observed at midnight and before breakfast. Moreover, when the sensor glucose value was reduced to 55 mg/dL (3.1 mmol/L), the Dexcom G6 system emitted an urgent low glucose alarm four times for 10 days (235 h) (Fig. 4). Notably, the patient was unaware of hypoglycemia during CGM. After surgery, the Dexcom G6 system showed an overall increase in daily blood glucose concentrations with the resolution of hypoglycemia (Fig. 5). Mean sensor glucose concentrations were elevated from 90 ± 25 mg/dL (5.3 ± 1.4 mmol/L) before surgery to 155 ± 33 mg/dL (8.6 ± 1.8 mmol/L) after surgery. The percentages of sensor glucose concentrations <54 mg/dL (3.0 mmol/L) and <70mg/dL (3.9 mmol/L) were 1% and 11%, respectively, before surgery. However, none of the sensor glucose concentrations under these values were observed after surgery.
Figure 4.
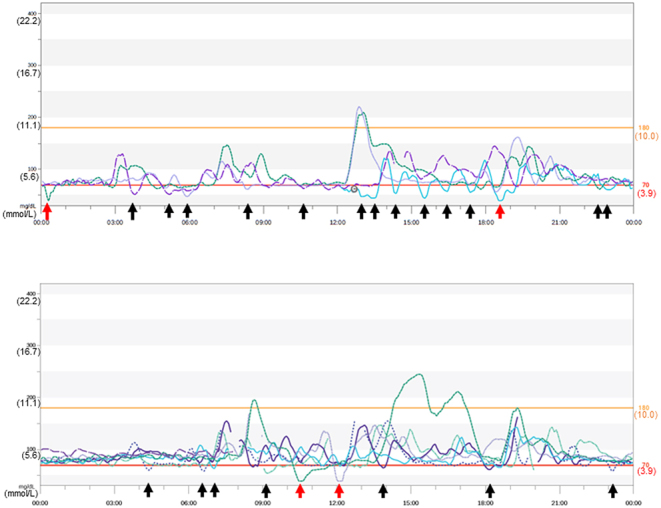
Continuous glucose monitoring using the Dexcom G6 system before surgery. Continuous glucose monitoring (CGM) was performed for 10 days before surgery. CGM shows hypoglycemia (black arrows). CGM emitted an urgent low glucose alarm when the sensor glucose concentration decreased to 55 mg/dL (3.1 mmol/L) (red arrows).
Figure 5.
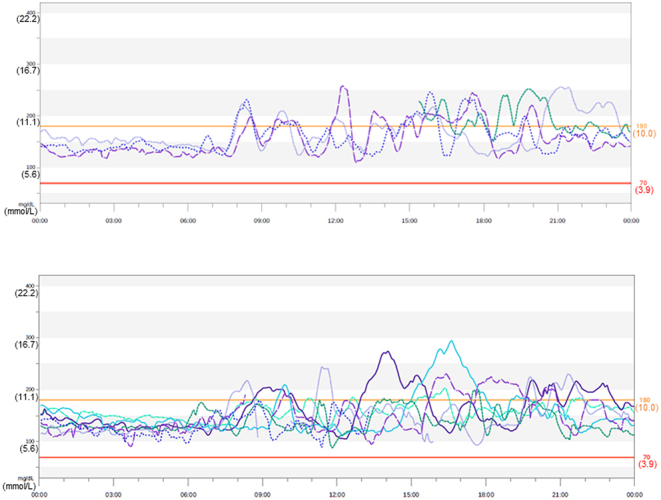
Continuous glucose monitoring using the Dexcom G6 system after surgery. Continuous glucose monitoring (CGM) was performed for 10 days 2 months after surgery. An overall increase in daily blood glucose concentrations and resolution of hypoglycemia after surgery were observed.
Discussion
We describe a case of insulinoma in a patient presenting with unawareness of hypoglycemia in whom CGM was helpful for assessing hypoglycemia and treatment outcomes after surgical resection of insulinoma.
According to retrospective data from the Mayo Clinic, hypoglycemia in insulinoma typically develops in the fasting state (73%), but it also occurs in the fasting and postprandial states (27%) and in the postprandial state exclusively (6%) (3). Our patient showed unawareness of postprandial hypoglycemia for 5 years. He was first aware of the symptoms of lightheadedness and impaired consciousness postprandially and was referred to our hospital. We found that his plasma glucose concentration was reduced to 32 mg/dL (1.8 mmol/L) at 150 min after glucose loading in the 75 g OGTT, and relatively high concentrations of insulin, proinsulin, and C-peptide were observed. These concentrations satisfied the criteria of spontaneous hyperinsulinemia proposed by the United States Endocrine Society (8). This society’s consensus report recommends insulin concentrations ≥3.0 μU/mL, proinsulin concentrations ≥ 5.0 pmol/L, and C-peptide concentrations of 0.6 ng/mL when glucose concentrations are <55 mg/dL (3.1 mmol/L). In the fasting state in our patient, plasma glucose concentrations were measured at 8 a.m. (Table 1) and at the start of the 75 g OGTT (Table 2) did not show hypoglycemia. In the prolonged fasting test, the plasma glucose concentration was reduced to 43 mg/dL (2.4 mmol/L) at 66 h after the last meal. Although insulin (1.4 µU/mL) and C-peptide (0.49 ng/mL) concentrations did not reach the values mentioned above, suppression of 3-β-hydroxybutyrate concentrations, which indicate increased insulin secretion, was observed (<2.7 mmol/L). This finding indicated the presence of hyperinsulinemic hypoglycemia. Therefore, these data suggested that spontaneous hyperinsulinemia was present in the fasting and postprandial states.
There have been some reports of patients with insulinoma in whom the CGM system or flash glucose monitoring (FGM) system was helpful for the corroboration of hypoglycemia induced by insulinoma and treatment outcomes after surgical resection (5, 6, 7). The FGM system is convenient because it does not require frequent calibration by self-monitoring of capillary blood glucose, but its accuracy estimated by the mean absolute relative difference is higher than that in the CGM system (9). The Dexcom G6 system, which is a CGM system that was released in Japan in July 2021, shows a preferable mean absolute relative difference of 9.0% without calibration by self-monitoring of capillary blood glucose (10). In our patient, fasting hypoglycemia was equivocal based on plasma glucose concentrations measured at 8 a.m. and at the start of the 75 g OGTT. However, the Dexcom G6 system clearly demonstrated fasting hypoglycemia at midnight and before breakfast, although fasting hypoglycemia was less frequent than postprandial hypoglycemia (Fig. 4). Our patient was unaware of hypoglycemia during the 75 g OGTT, the prolonged fasting test, and glucose monitoring by the Dexcom G6 system, despite glucose concentrations indicating hypoglycemia. When the sensor glucose concentration was reduced to 55 mg/dL (3.1mmol/L), the Dexcom G6 system produced an urgent low glucose alarm. Our patient received such an alarm four times for 10 days (235 h). Therefore, the Dexcom G6 is particularly suitable for preventing serious accidents caused by severe hypoglycemia, especially in case of being unaware of hypoglycemia. After surgical resection of insulinoma, an overall increase in daily glucose concentrations and resolution of hypoglycemia were confirmed by the Dexcom G6 system (Fig. 5).
In conclusion, we describe a case of insulinoma in a patient presenting with unawareness of hypoglycemia postprandially in whom the Dexcom G6 system facilitated the diagnosis and treatment outcomes.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Patient consent
Written informed consent for publication of the case report was obtained from the patient.
Author contribution statement
RN, HI, DS, JI, KI, MS, and HY were involved in the clinical care of the patient. MK performed surgery in this patient. NT assessed the pathological findings. RN and HY wrote the manuscript.
Acknowledgements
We thank Ellen Knapp, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
References
- 1.Service FJ McMahon MM O'Brien PC & Ballard DJ. Functioning insulinoma–incidence, recurrence, and long-term survival of patients: a 60-year study. Mayo Clinic Proceedings 199166711–719. ( 10.1016/s0025-6196(1262083-7) [DOI] [PubMed] [Google Scholar]
- 2.Ito T, Igarashi H, Nakamura K, Sasano H, Okusaka T, Takano K, Komoto I,Tanaka M, Imamura M, Jensen RT, et al. Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: a nationwide survey analysis. Journal of Gastroenterology 20155058–64. ( 10.1007/s00535-014-0934-2) [DOI] [PubMed] [Google Scholar]
- 3.Placzkowski KA Vella A Thompson GB Grant CS Reading CC Charbineau JW Andrews JC Lioyd RV & Service FJ. Secular trends in the presentation and management of functioning insulinoma at the Mayo Clinic, 1987–2007. Journal of Clinical Endocrinology and Metabolism 2009941069–1073. ( 10.1210/jc.2008-2031) [DOI] [PubMed] [Google Scholar]
- 4.Mitrakou A, Fanelli C, Veneman T, Perriello G, Calderone S, Platanisiotis D, Rambotti A, Raptis S, Bruntetti P, Cryer P, et al. Reversibility of unawareness of hypoglycemia in patients with insulinomas. New England Journal of Medicine 1993329834–839. ( 10.1056/NEJM199309163291203) [DOI] [PubMed] [Google Scholar]
- 5.Kikuchi T Chujo D Takahashi K Takahashi N Tanno Y Tonoike M Ihana N Tsujimoto T Tanabe A & Kajio H. Insulinoma presenting with reactive hypoglycemia: evaluating the effect of tumor resection via continuous glucose monitoring. Internal Medicine 2017563067–3071. ( 10.2169/internalmedicine.8766-16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugawa T, Murakami T, Yabe D, Kashima R, Tatsumi M, Ooshima S, Joo E, Wada K, Yoshizawa A, Masui T, et al. Hypoglycemia unawareness in insulinoma revealed with flash glucose monitoring systems. Internal Medicine 2018573407–3412. ( 10.2169/internalmedicine.1173-18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maher MD Desai DN & Bajaj M. Metastatic insulinoma presenting with postprandial hypoglycaemia. AACE Clinical Case Reports 20228154–157. ( 10.1016/j.aace.2022.03.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cryer PE, Axelrod L, Grossman AB, Heller SR, Montori VM, Seaquist ER, Service FJ. & Endocrine Society. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. Journal of Clinical Endocrinology and Metabolism 200994709–728. ( 10.1210/jc.2008-1410) [DOI] [PubMed] [Google Scholar]
- 9.Bailey T Bode BW Christiansen MP Klaff LJ & Alva S. The performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes Technology and Therapeutics 201517787–794. ( 10.1089/dia.2014.0378) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah VN Laffel LM Wadwa RP & Garg SK. Performance of a factory-calibrated real-time continuous glucose monitoring system utilizing an automated sensor applicator. Diabetes Technology and Therapeutics 201820428–433. ( 10.1089/dia.2018.0143) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a