Abstract
Background
Obesity and arterial stiffness are strongly associated with cardiovascular disease; however, their relationship remains controversial.
Methods
Body mass index was measured using anthropometric evaluation, and visceral fat area was calculated using an absorptiometry scan.
Results
The data of 5309 participants were collected from NHANES (National Health and Nutrition Examination Survey) (2011–2018). Based on the normal-weight normal visceral fat group that was considered as a reference, ePWV increased in all other groups, with the obese grade 2 visceral obesity group increasing the most by 26.35 cm/s (95% CI: 13.52, 39.18, P < 0.001), followed by normal-weight visceral obesity group 24.43 cm/s (95% CI: 1.88, 46.98, P = 0.035), which was even higher than obese grade 1 visceral obesity (β: 21.16, 95% CI: 9.24, 33.07, P = 0.001), obese grade 2 normal visceral fat group (β: 13.8; 95% CI: 0.10, 27.5, P = 0.048) and overweight visceral obesity group (β: 10.23; 95% CI: 1.89, 18.57, P = 0.018). For the 10-year cardiovascular risk, the obese grade 2 visceral obesity group had a 9.56-fold increase in compared with the control (OR: 10.56, 95% CI: 4.06, 27.51, P < 0.0001). Normal-weight visceral obesity, obese grade 1 visceral obesity, and overweight visceral obesity groups increased by 8.03-fold (OR: 9.03, 95% CI: 2.66, 30.69; P < 0.001), 7.91-fold (OR: 8.91, 95% CI: 3.82, 20.79, P < 0.001), and 7.28-fold (OR: 8.28, 95% CI: 3.19, 21.46, P < 0.001). The risk was lower in the normal visceral fat group. Except for the obese grade 2 normal visceral fat group, there was no significant difference in other groups.
Conclusions
Normal-weight visceral obesity was associated with higher arterial stiffness and 10-year cardiovascular risk.
Keywords: estimated pulse wave velocity, body mass index, visceral fat area, arterial stiffness, obesity
Introduction
Estimated pulse wave velocity with arterial stiffness
Radial arterial pulsation is a vital indicator in ancient Chinese that allowed experienced traditional practitioners to examine their patients. The ancient Chinese discovered thousands of years ago that many diseases are closely related to arterial pulsation and arterial stiffness. Arterial stiffness is widely considered to be closely associated with hypertension and is an independent predictor of cardiovascular diseases (1). Contemporary science has replaced the palpation practice of experienced traditional Chinese practitioners with machines to determine arterial stiffness. Carotid–femoral pulse wave velocity (cf-PWV) is the gold standard for assessing arterial stiffness. Higher cf-PWV and faster conduction velocity indicate less vascular elasticity and more severe sclerosis. However, cf-PWV is not widely used due to the limitations of personnel and equipment (2). Estimated pulse wave velocity (ePWV) can effectively predict cf-PWV in response to arterial stiffness (3). ePWV is calculated using mean blood pressure (MBP) and age and is more easily applied clinically. Notably, reducing arterial stiffness can decrease global cardiovascular mortality (4).
Body mass index and visceral fat mass with obesity
The proportion of people with obesity has increased globally over the past 50 years (5). Obesity is an adiposity-based chronic disease. From ancient times to the present, obesity has been defined in various ways, ranging from simple physical observations to various body weight index calculations. However, the body mass index (BMI) is the most simple and effective and has been used for 200 years (6, 7, 8). Clinically, BMI is calculated as weight (kg)/height2 (m2). According to BMI, the mainstream standards classify the population into underweight (BMI < 35 kg/m2), obese grade 2 (35 kg/m2 ≤ BMI < 40 kg/m2), and obese grade 3 (BMI ≥ 40 kg/m2) (9). Obesity, defined using BMI, is associated with an increased risk of death from cardiovascular diseases (CVD), type 2 diabetes mellitus, metabolic syndrome, and several diseases, while underweight is associated with chronic wasting diseases, smoking, and other death-causing diseases (10). Few obesity-related guidelines have explored the risk of disease associated with normal BMI groups (11), and it is thought that a higher BMI (overweight or obesity) is potentially associated with higher morbidity and mortality, especially CVD (12). However, some studies have reported reduced mortality among newly diagnosed patients with diabetes in obese populations than those with normal BMI (10, 13). Scholars have increasingly found limitations in assessing obesity using BMI alone, such as its ineffectiveness in estimating fat distribution and differentiating between muscle/fat mass (6, 14). BMI is more suitable for general-obesity assessment (15). A central fat-related biomarker, visceral adipose tissue (VAT), has emerged as a good remedy for the shortcomings of BMI in central obesity assessment. VAT is considered an independent risk factor for CVD and even predicts cardiovascular event risk better than BMI. It enhances the validity of BMI for cardiovascular risk prediction in patients with CVD (8, 13). Available dual-energy x-ray absorptiometry techniques can accurately measure visceral fat mass by visceral fat area (VFA) (8).
Obesity with arterial stiffness
The relationship between obesity and arterial stiffness remains controversial. A study of 2354 people from Spain showed that adiposity measures were negatively associated with arterial stiffness. The study also assessed adiposity using BMI, waist-to-height ratio, and body roundness index, among others. The arterial stiffness was determined by measuring the cardio–ankle vascular index (CAVI) and brachial-ankle pulse wave velocity (16). However, 3512 human experiments from Tokyo demonstrated that obesity indices such as VAT, BMI, and body roundness index were positively correlated with brachial–ankle pulse wave velocity (17). Clinically, their findings indicated the existence of a specific subgroup of patients, who are individuals with normal weight with visceral obesity and are more common in Asian populations (10, 15, 18). The diagnosis of arterial stiffness using a single general obesity index (including BMI) or central obesity index (including waist-to-height ratio) is somewhat limited.
Aim of this study
The aims of this study were as follows: (1) to explore the relationship between obesity and vascular stiffness; (2) to redefine obesity by combining the general obesity index (BMI) and central obesity index (VAT) to identify the primary prevention population of CVD; (3) to explore risk factors related to vascular stiffness and identify targets for vascular stiffness treatment; (4) to assess early diagnosis, early prevention, and personalized treatment for CVD populations to reduce the global burden of CVD worldwide.
Materials and methods
Data source
National Health and Nutrition Examination Survey (NHANES) is a continuous population-based cross-sectional survey conducted by the Centers for Disease Control and Prevention (CDC) to collect information on the health and nutrition status of the US household population. The project began in 1999, is conducted on a two-year cycle, and includes interviews and physical examinations. The National Center for Health Statistics Research Ethics Review Board approved the survey, and all participants signed the written informed consent. Our study collected some of the relevant data from the NHANES database.
Study population and design
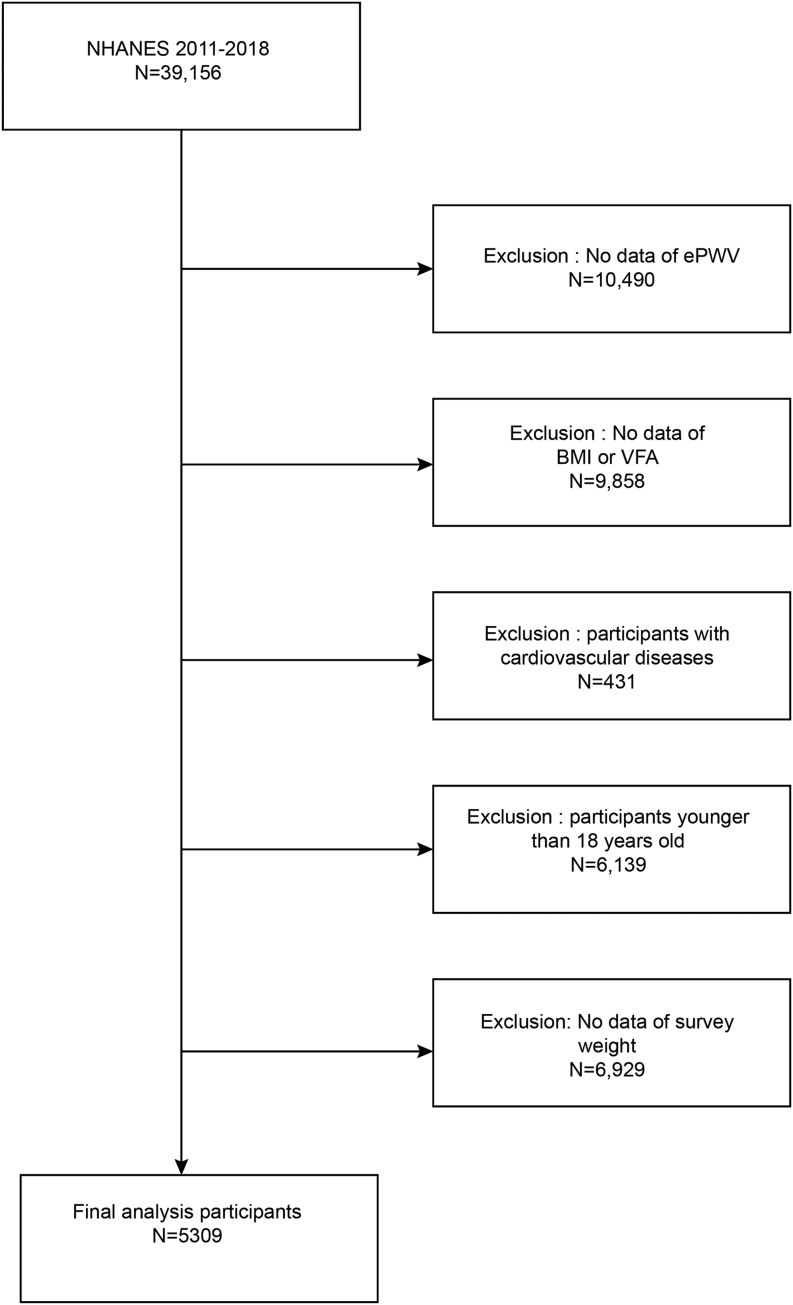
Because VFA has only been measured since 2011, we used data from four NHANES cycles of 2011–2012, 2013–2014, 2015–2016, and 2017–2018 (N = 39,156 originally). The exclusion criteria were as follows: (i) lack of ePWV data; (ii) lack of BMI data; (iii) lack of VFA data; (iv) self-reported a previous diagnosis of CVD (including coronary heart disease, angina, myocardial infarction, and stroke); (v) less than 18 years old; (vi) pregnancy (VFA was not measured in pregnant women); (vii) lack of complete survey weight. Ultimately, our study included 5309 people aged 18–59 years, representing a CVD-free US population of 144,907,159 (Fig. 1). The Research Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University, approved this study as exempt.
Figure 1.
Flowchart.
Assessment of the body mass index and visceral fat
BMI was calculated as weight (kg)/height (m2), and weight and height were measured by trained professionals. The population was grouped according to BMI: 18.5 kg/m2 ≤ BMI < 25kg/m2, 25 kg/m2 ≤ BMI < 30 kg/m2, 30 kg/m2 ≤ BMI < 35 kg/m2, BMI ≥ 35kg/m2, representing normal weight, overweight, obese grade 1, and obese grade 2 and above, respectively (9, 19). Visceral fat tissues were assessed using VFA. The VFA (cm2) was obtained by measuring the VAT area at the approximate interspace location of the L4 and L5 vertebra using dual-energy x-ray absorptiometry. Moreover, the VFA was divided into two groups: VFA < 100 cm2 and VFA ≥100 cm2, indicating normal visceral fat and visceral obesity (15).
Assessment of arterial stiffness
The outcome variable for arterial stiffness was evaluated using ePWV, which was calculated using the following equation:
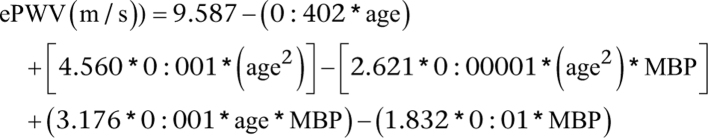 |
Age was in years. MBP was calculated using the formula MBP = DBP + (0:4 × (SBP − DBP)) (2, 20, 21). Blood pressure was measured by a trained health technician, and participants obtained three consecutive sphygmomanometric counts after 5 min of sitting still, and SDP and DBP were determined by calculating the average of all the blood pressure readings obtained.
Assessment of 10-year cardiovascular risk
We also assessed the association of BMI and VFA combined status with 10-year cardiovascular risk. The Framingham Heart Study risk score was used (22, 23). Because this calculation was applied to people aged 30–74 years without CVDs history, we excluded 672 people without complete 10-year cardiovascular risk data and 1415 people who were less than 30 years old. The detailed calculation step of the cardiovascular risk score is available in previous literature (22). Ten-year cardiovascular risk score was treated as a dichotomous variable, and a high-risk group was identified as individuals with a 10-year cardiovascular risk score was ≥ 20%.
Assessment of covariates
Covariates selection for this study was based on previous studies (2, 15, 23). The selected covariates were as follows: age, sex (male/female), race (Mexican American/Other Hispanic/Non-Hispanic White/Non-Hispanic Black/Other Race including Multi-Racial), education (less than ninth grade/9–11th grade/high school graduate/some college or associate of arts degree/college graduate or above), marital status (married/widowed/divorced/separated/never married/living with a partner), family income ratio, diabetes mellitus (yes/no), hypertension (yes/no), hyperlipidemia (yes/no), leisure time physical activity (poor/intermediate/optimal), smoking (none/past smoker/smoker now), drinking (none/moderate/heavy), and body surface area. The demographic variables (age, gender, race, education, marital status, and family income ratio) were acquired during the household interview. Laboratory tests (triglycerides, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, fasting plasma glucose, and glycated hemoglobin) were obtained by taking a blood sample. We calculated the body surface area using the following formula: body surface area = 0.007184 × (height (cm)0.725) × (weight (kg)0.425) (24). Fasting indicators required a minimum fast of 9 h. Medical history information was obtained through a questionnaire. Diabetes mellitus was defined as glycohemoglobin ≥ 6.5%, fasting glucose ≥ 126 mg/dL, self-reported diabetes mellitus, or current use of antihyperglycemic medication (25). Hypertension was defined as SBP ≥ 140 mmHg, DBP ≥ 90 mmHg, self-reported hypertension, or current use of antihypertensive medication (26). Hyperlipidemia was defined as fasting serum total cholesterol ≥ 200 mg/dL, fasting serum triglyceride ≥ 160 mg/dL, or self-reported high cholesterol level and current use of cholesterol-lowering drugs. Optimal leisure time physical activity was defined as ≥ 75 min/week of vigorous leisure time physical activity or ≥ 150 min/week of moderate leisure time physical activity. The absence of any leisure time physical activity was defined as poor. Moreover, in-between poor and optimal leisure time physical activity was defined as intermediate. Drinking ≥2 bottles of alcoholic drinks/day for women or ≥3 bottles of alcoholic drinks/day for men was defined as heavy drinking, 1 bottle of alcoholic drink/day for women or one to two bottles of alcoholic drinks/day for men was defined as moderate, and no alcohol consumption was defined as none.
Statistical analysis
Statistical analyses were conducted using EmpowerStats (http://www.empowerstats.com) and R (http://www.R-project.org) software packages. The statistical significance was set at P < 0.05. Sampling weights were used that were calculated according to National Health and Nutrition Examination Survey (NHANES) recommendations. In the baseline characteristics distribution, continuous variables were presented as survey-weighted means (95% CI), and the P-value was calculated by using survey-weighted linear regression; categorical variables were presented as survey-weighted percentages (95% CI), and the P-value was determined by using a survey-weighted chi-square test (Table 1). Subsequently, multivariate analysis was performed using a generalized linear model to analyze the relationship between BMI/VFA status and ePWV, with three adjusting models. The crude model was adjusted for no variable. The minimally adjusted model was adjusted for age, sex, race, education, marital status, and family income ratio. The fully adjusted model was adjusted for diabetes mellitus, hypertension, hyperlipidemia, leisure time physical activity, smoking, drinking, and body surface area in addition to the factors included in the minimally adjusted model. BMI (VFA) was treated as a continuous variable to analyze the change in ePWV owing to a one s.d. increase BMI (VFA) and was then used as a categorical variable to observe the effect values. Moreover, the population was divided into eight groups (Tables 2 and 3, Fig. 2) according to various combinations of BMI and VFA values, with the normal BMI normal VFA group being used as a control group. Subgroup analysis was performed and the P-value of interaction was calculated (Table 4). The generalized additive model and the smooth curve fitting function of EmpowerStats were used to show relationships between the BMI/VFA status and ePWV (Figs. 3 and 4). Logistic regression models were used to calculate the 10-year cardiovascular risk for different groups defined by BMI combined with VFA values (Table 5). Finally, the relationship between the various patient groups defined by BMI/VFA and all-cause mortality was calculated using COX regression (Supplementary Table 3, see section on supplementary materials given at the end of this article).
Table 1.
Characteristic distribution of participants in NHANES 2011-2018, weighted (population size represented N = 144,907,159).
| Variables | All | Normal weight | Overweight | Obese grade 1 | Obese grade 2 | P | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Normal visceral fat | Visceral obesity | Normal visceral fat | Visceral obesity | Normal visceral fat | Visceral obesity | Normal visceral fat | Visceral obesity | |||
| Participants’ number | 5199 | 1528 | 128 | 898 | 733 | 327 | 715 | 145 | 725 | |
| Age (years) | 38.30 (37.82, 38.78) | 34.13 (33.12, 35.13) | 50.96 (49.08, 52.84) | 34.14 (33.11, 35.18) | 46.14 (45.32, 46.95) | 32.75 (31.20, 34.31) | 43.25 (42.31, 44.20) | 31.12 (29.26, 32.98) | 40.73 (39.39, 42.08) | <0.0001 |
| Body surface area (m2) | 1.93 (1.92, 1.94) | 1.73 (1.72, 1.74) | 1.85 (1.81, 1.88) | 1.91 (1.90, 1.93) | 1.94 (1.92, 1.96) | 2.03 (2.01, 2.06) | 2.05 (2.02, 2.07) | 2.19 (2.15, 2.22) | 2.23 (2.21, 2.26) | <0.0001 |
| Body mass index (kg/m2) | 28.80 (28.47, 29.13) | 22.19 (22.07, 22.30) | 23.59 (23.36, 23.83) | 27.12 (27.01, 27.22) | 27.80 (27.64, 27.96) | 32.04 (31.87, 32.21) | 32.30 (32.16, 32.44) | 38.95 (38.03, 39.87) | 41.02 (40.49, 41.56) | <0.0001 |
| Visceral fat area (cm2) | 102.65 (99.74, 105.57) | 50.43 (49.07, 51.80) | 126.53 (121.12, 131.94) | 70.59 (69.09, 72.08) | 133.66 (130.86, 136.45) | 75.64 (73.21, 78.06) | 152.34 (148.58, 156.09) | 78.52 (75.20, 81.84) | 182.35 (177.39, 187.32) | <0.0001 |
| ePWV (cm/s) | 709.39 (704.88, 713.89) | 664.52 (658.57, 670.46) | 824.72 (792.19, 857.26) | 670.38 (662.01, 678.74) | 763.83 (754.27, 773.39) | 669.48 (657.67, 681.29) | 756.57 (744.88, 768.26) | 674.82 (658.84, 690.79) | 751.44 (738.36, 764.52) | <0.0001 |
| Male (%) | 51.94 (50.36, 53.51) | 45.27 (41.98, 48.60) | 73.04 (60.52, 82.73) | 58.26 (54.27, 62.15) | 63.40 (59.01, 67.57) | 55.55 (47.16, 63.64) | 56.13 (50.70, 61.42) | 36.12 (25.77, 47.94) | 40.46 (36.23, 44.84) | <0.0001 |
| Non-Hispanic white (%) | 61.49 (57.48, 65.35) | 62.83 (58.30, 67.15) | 68.07 (57.35, 77.16) | 59.23 (53.84, 64.41) | 64.31 (59.08, 69.22) | 47.77 (38.75, 56.93) | 65.11 (58.71, 71.00) | 29.10 (19.43, 41.12) | 64.90 (59.66, 69.79) | <0.0001 |
| Hypertension (%) | 25.91 (24.20, 27.70) | 9.91 (8.08, 12.11) | 41.51 (30.15, 53.85) | 15.31 (12.78, 18.24) | 34.98 (30.10, 40.20) | 24.82 (18.48, 32.48) | 38.96 (34.44, 43.68) | 27.51 (18.98, 38.08) | 47.09 (42.50, 51.73) | <0.0001 |
| Diabetes (%) | 8.26 (7.29, 9.34) | 1.49 (1.01, 2.20) | 16.65 (9.46, 27.62) | 3.06 (2.03, 4.57) | 10.82 (8.39, 13.84) | 3.79 (2.21, 6.43) | 12.64 (9.86, 16.07) | 7.27 (4.31, 12.01) | 22.39 (19.11, 26.06) | <0.0001 |
| Hyperlipidemia | 51.02 (49.09, 52.94) | 34.12 (30.89, 37.50) | 73.70 (61.80, 82.92) | 41.23 (36.96, 45.63) | 75.02 (70.34, 79.18) | 44.02 (38.79, 49.39) | 66.48 (61.21, 71.36) | 36.97 (27.32, 47.79) | 61.80 (56.14, 67.15) | <0.0001 |
| Active leisure time physical activity (%) | 40.67 (38.54, 42.83) | 49.41 (44.97, 53.87) | 29.44 (20.01, 41.02) | 52.09 (48.29, 55.86) | 33.04 (28.36, 38.09) | 48.75 (41.67, 55.88) | 29.60 (25.29, 34.30) | 47.29 (37.48, 57.32) | 27.36 (23.12, 32.04) | <0.0001 |
| Current smoker (%) | 19.90 (17.91, 22.06) | 20.49 (16.99, 24.50) | 34.88 (24.28, 47.21) | 17.21 (14.00, 20.97) | 21.78 (18.11, 25.96) | 20.28 (15.38, 26.25) | 18.65 (15.41, 22.38) | 17.88 (11.89, 26.01) | 18.37 (15.38, 21.79) | <0.0001 |
| Heavy drinking (%) | 49.20 (46.81, 51.59) | 48.49 (44.55, 52.45) | 45.89 (33.99, 58.27) | 50.99 (46.10, 55.86) | 45.67 (40.56, 50.88) | 47.54 (40.49, 54.68) | 52.81 (48.77, 56.82) | 49.39 (39.79, 59.02) | 49.92 (45.39, 54.46) | 0.0183 |
For continuous variables: survey-weighted mean (95% CI), P-value was by survey-weighted linear regression (svyglm).
For categorical variables: survey-weighted percentage (95% CI), P-value was by survey-weighted chi-square test (svytable).
ePWV, estimated pulse wave velocity.
Table 2.
Multiple linear regression analysis of the association between BMI/visceral fat status with ePWV (cm/s), weighted.
| BMI/VFA status | Crude model | Minimally adjusted model | Fully adjusted model |
|---|---|---|---|
| β (95% CI), P | β (95% CI), P | β (95% CI), P | |
| BMI (per s.d.: 6.99 kg/m2) | 25.70 (20.78, 30.62), <0.001 | 14.81 (12.30, 17.32), <0.001 | 7.97 (3.69, 12.26), <0.001 |
| Normal weight 18.5 kg/m2 ≤ BMI < 25 kg/m2 | Reference | Reference | Reference |
| Overweight 25 kg/m2 ≤ BMI <30 kg/m2 | 36.53 (27.01, 46.05), <0.001 | 8.36 (1.73, 14.98), 0.018 | 2.50 (−3.76, 8.76), 0.418 |
| Obese grade 1 30 kg/m2 ≤ BMI < 35 kg/m2 | 56.06 (44.69, 67.43), <0.001 | 26.52 (18.00, 35.05), <0.001 | 12.55 (2.82, 22.29), 0.013 |
| Obese grade 2 BMI ≥ 35 kg/m2 | 65.13 (51.52, 78.73), <0.001 | 41.22 (33.15, 49.27), <0.001 | 20.08 (9.02, 31.13), <0.001 |
| VFA (per s.d.: 56.97 m2) | 50.72 (46.53, 54.91), <0.001 | 16.94 (13.18, 20.69), <0.001 | 8.40 (3.68, 13.12), 0.002 |
| Normal visceral fat VFA < 100 cm2 | Reference | Reference | Reference |
| Visceral obesity VFA ≥ 100 cm2 | 93.85 (85.24, 102.45), <0.001 | 30.45 (24.09, 36.82), <0.001 | 14.76 (7.71, 21.82), <0.001 |
R 2 for crude model, minimally adjusted model, and fully adjusted model is 0.05 ,0.62, and 0.68, respectively (BMI as continuous variables per s.d.).
R 2 for crude model, minimally adjusted model, and fully adjusted model is 0.05, 0.62, and 0.68, respectively (BMI as categorical variables).
R 2 for crude model, minimally adjusted model, and fully adjusted model is 0.22, 0.63, and 0.67, respectively (VFA as continuous variables per s.d.).
R 2 for crude model, minimally adjusted model, and fully adjusted model is 0.17, 0.62, and 0.68, respectively (VFA as categorical variables).
Crude model was adjusted for none.
Minimally adjusted model was adjusted for age, gender, race, education, marital status, and family income ratio.
Fully adjusted model was adjusted for age, gender, race, education, marital status, family income ratio, diabetes mellitus, hypertension, hyperlipidemia, leisure time physical activity, smoking, drinking, and body surface area.
BMI, body mass index; ePWV, estimated pulse wave velocity; VFA, visceral fat area.
Table 3.
Multiple linear regression analysis of the association between BMI with visceral fat area status with ePWV (cm/s), weighted.
| BMI + VFA status | Crude model | Minimally adjusted model | Fully adjusted model |
|---|---|---|---|
| β (95% CI), P | β (95% CI), P | β (95% CI), P | |
| Normal weight, normal visceral fat | Reference | Reference | Reference |
| Normal weight, visceral obesity | 160.21 (127.27, 193.15), <0.001 | 31.60 (7.74, 55.47), 0.013 | 24.43 (1.88, 46.98), 0.035 |
| Overweight, normal visceral fat | 5.86 (−4.34, 16.06), 0.265 | 5.47 (−2.67, 13.61), 0.196 | 2.29 (−5.16, 9.75), 0.529 |
| Overweight, visceral obesity | 99.32 (88.03, 110.60), <0.001 | 19.43 (11.34, 27.52), <0.0001 | 10.23 (1.89, 18.57), 0.018 |
| Obese grade 1, normal visceral fat | 4.96 (−7.97, 17.89), 0.455 | 15.15 (6.48, 23.82), 0.001 | 4.85 (−4.82, 14.52), 0.308 |
| Obese grade 1, visceral obesity | 92.06 (80.23, 103.88), <0.001 | 35.80 (25.65, 45.94), <0.0001 | 21.16 (9.24, 33.07), 0.001 |
| Obese grade 2, normal visceral fat | 10.30 (-6.96, 27.56), 0.247 | 24.48 (11.57, 37.39), <0.0001 | 13.80 (0.10, 27.50), 0.048 |
| Obese grade 2, visceral obesity | 86.92 (71.76, 102.08), <0.001 | 46.91 (38.10, 55.73), <0.0001 | 26.35 (13.52, 39.18), <0.001 |
Bold indicates statistical significance.
R 2 for crude model, minimally adjusted model, and fully adjusted model is 0.18 ,0.63, and 0.68, respectively.
Crude model was adjusted for none.
Minimally adjusted model was adjusted for age, gender, race, education, marital status, and family income ratio.
Fully adjusted model was adjusted for age, gender, race, education, marital status, family income ratio, diabetes mellitus, hypertension, hyperlipidemia, leisure time physical activity, smoking, drinking, and body surface area.
BMI, body mass index; ePWV, estimated pulse wave velocity; VFA, visceral fat area.
Figure 2.
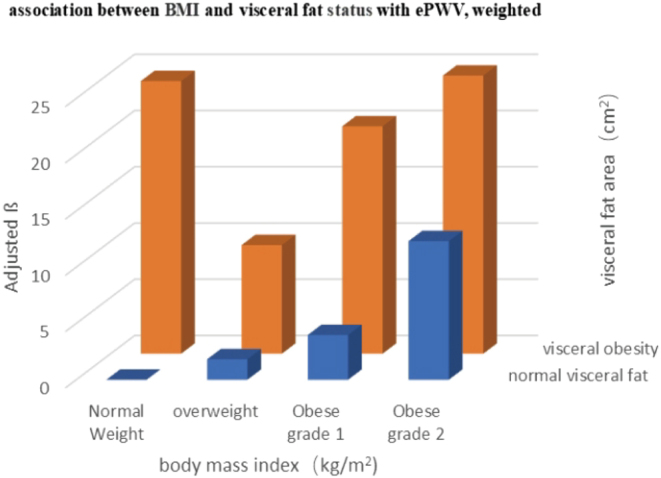
The relationship between BMI with visceral fat area status with ePWV (cm/s), after adjusting for age, gender, race, education, marital status, family income ratio, diabetes mellitus, hypertension, hyperlipidemia, leisure time physical activity, smoking, drinking, and body surface area. ePWV, estimated pulse wave velocity.
Table 4.
Effect size of BMI with visceral fat area status on ePWV (cm/s) in prespecified and exploratory subgroups, weighted.
| Stratification | BMI/VFA status | P | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Normal weight normal visceral fat β (95% CI), P | Normal Weight visceral obesity β (95% CI), P | Overweight normal visceral fat β (95% CI), P | Overweight visceral obesity β (95% CI), P | Obese grade 1 normal visceral fat β (95% CI), P | Obese grade 1 visceral obesity β (95% CI), P | Obese grade 2 normal visceral fat β (95% CI), P | Obese grade 2 visceral obesity β (95% CI), P | ||
| Gender | 0.575 | ||||||||
| Male | Reference | 56.3 (23.9, 88.7), 0.0026 | −3.9 (−13.4, 5.6), 0.4283 | 16.3 (4.4, 28.3), 0.0134 | −4.5 (−16.7, 7.7), 0.4744 | 28.2 (12.5, 43.9), 0.0020 | 8.6 (−15.2, 32.4), 0.4863 | 29.9 (11.4, 48.3), 0.0044 | |
| Female | Reference | 51.6 (15.8, 87.3), 0.0098 | 2.7 (−10.5, 15.9), 0.6939 | 32.5 (18.1, 47.0), 0.0002 | −0.1 (−16.6, 16.4), 0.9877 | 33.7 (14.5, 52.9), 0.0023 | 10.7 (−8.1, 29.5), 0.2750 | 26.2 (8.9, 43.4), 0.0071 | |
| Age | 0.243 | ||||||||
| <40 years | Reference | 31.4 (8.5, 54.3), 0.0133 | 1.2 (−5.8, 8.3), 0.7372 | 16.5 (5.9, 27.1), 0.0058 | 2.5 (−6.8, 11.9), 0.5977 | 29.4 (18.7, 40.1), <0.0001 | 14.5 (0.0, 29.0), 0.0624 | 30.7 (16.5, 44.9) 0.0003 | |
| ≥40 years | Reference | 59.4 (32.8, 86.0), 0.0002 | −3.8 (−21.4, 13.8), 0.6774 | 24.3 (9.5, 39.1), 0.0040 | −13.3 (−39.2, 12.6), 0.3261 | 31.9 (12.2, 51.7), 0.0044 | −3.9 (−44.9, 37.1), 0.8539 | 25.4 (2.3, 48.5) 0.0422 | |
| Diabetes | 0.151 | ||||||||
| No | Reference | 54.6 (26.9, 82.3), 0.0008 | 0.7 (−7.4, 8.7), 0.8754 | 21.6 (11.7, 31.5), 0.0003 | −2.1 (−13.3, 9.0), 0.7099 | 29.8 (15.3, 44.3), 0.0006 | 11.3 (−4.8, 27.4), 0.1826 | 30.2 (14.8, 45.6), 0.0009 | |
| Yes | Reference | 53.4 (−0.8, 107.6) 0.0664 | −46.5 (−92.8, −0.3), 0.0615 | 23.7 (−25.8, 73.3), 0.3579 | −7.7 (−67.1, 51.6), 0.8015 | 29.0 (−21.4, 79.5), 0.2716 | −13.9 (−67.4, 39.5), 0.6146 | 9.1 (−34.0, 52.2), 0.6824 | |
| Hypertension | 0.086 | ||||||||
| No | Reference | 57.5 (34.2, 80.8), 0.0001 | −0.5 (−8.2, 7.3), 0.9073 | 26.1 (14.6, 37.7), 0.0002 | 4.2 (−7.3, 15.8), 0.4773 | 33.5 (20.7, 46.4), <0.0001 | 15.8 (0.6, 31.0), 0.0541 | 36.7 (23.6, 49.7), <0.0001 | |
| Yes | Reference | 37.2 (−20.1, 94.4), 0.2168 | -9.8 (-40.9, 21.2), 0.5408 | −0.3 (−26.4, 25.9), 0.9843 | −36.2 (−68.4, −4.0), 0.0382 | 8.7 (−24.9, 42.4), 0.6163 | −23.5 (−62.6, 15.5), 0.2502 | −2.7 (−35.2, 29.7), 0.8715 | |
| Hyperlipidemia | 0.390 | ||||||||
| No | Reference | 21.6 (−38.3, 81.6), 0.4868 | −4.8 (−14.3, 4.7), 0.3344 | 14.6 (−3.5, 32.8), 0.1279 | 0.6 (−11.9, 13.1), 0.9259 | 23.1 (6.3, 40.0), 0.0135 | 9.1 (−8.6, 26.8), 0.3228 | 26.4 (9.5, 43.2), 0.0056 | |
| Yes | Reference | 71.2 (48.8, 93.6), <0.0001 | 6.2 (−6.2, 18.6), 0.3395 | 29.1 (15.2, 42.9), 0.0005 | −4.1 (−21.9, 13.7), 0.6571 | 38.0 (21.5, 54.6), 0.0002 | 10.9 (−14.9, 36.7), 0.4153 | 31.5 (13.4, 49.6), 0.0025 | |
| Leisure time physical activity | 0.162 | ||||||||
| Poor | Reference | 49.2 (0.5, 97.9), 0.0662 | −7.6 (−21.9, 6.8), 0.3165 | 17.5 (2.5, 32.4), 0.0367 | −1.7 (−17.7, 14.3), 0.8367 | 30.6 (10.0, 51.2), 0.0106 | 10.7 (−20.9, 42.2), 0.5174 | 31.9 (13.9, 49.9), 0.0034 | |
| Intermediate | Reference | 49.1 (14.9, 83.2), 0.0130 | −1.8 (−19.9, 16.3), 0.8479 | 26.0 (8.0, 43.9), 0.0125 | -39.1 (−67.7, −10.4), 0.0174 | 18.2 (−2.5, 38.8), 0.1049 | −4.7 (−29.5, 20.2), 0.7172 | 20.7 (−5.1, 46.4), 0.1373 | |
| Optimal | Reference | 68.2 (31.9, 104.6), 0.0022 | 4.0 (−6.8, 14.9), 0.4755 | 26.5 (7.1, 46.0), 0.0175 | 7.6 (−5.1, 20.4), 0.2597 | 37.4 (19.1, 55.7), 0.0012 | 12.0 (−5.0, 28.9), 0.1867 | 20.8 (1.0, 40.7), 0.0574 | |
| Body surface area | 0.163 | ||||||||
| <1.88 m2 | Reference | 63.7 (32.6, 94.8), 0.0006 | 0.1 (−11.3, 11.6), 0.9827 | 36.5 (24.1, 48.9), <0.0001 | −4.5 (−22.1, 13.0), 0.6175 | 28.0 (4.7, 51.3), 0.0278 | −15.8 (-59.2, 27.6), 0.4840 | 6.7 (−19.5, 32.9), 0.6215 | |
| ≥1.88 m2 | Reference | 49.7 (3.8, 95.5), 0.0454 | 1.6 (−11.6, 14.9), 0.8115 | 17.7 (3.6, 31.7), 0.0222 | 2.0 (−13.0, 17.1), 0.7952 | 35.2 (17.7, 52.8), 0.0007 | 14.9 (−3.8, 33.5), 0.1324 | 32.2 (16.2, 48.3), 0.0007 | |
Results are shown as (for ePWV) survey-weighted coefficients (95% CI), P-value.
P-values are calculated by global chi-square test.
aThe relationship between BMI/visceral fat status on ePWV (cm/s) stratified by gender, age, hypertension, hyperlipidemia, diabetes, leisure time physical activity, and body surface area after adjusting gender, age, race, education, marital status, family poverty income ratio, diabetes, hypertension, hyperlipidemia, smoking, alcohol drinking, leisure time physical activity, and body surface area. The model is not adjusted for the stratification variable.
BMI, body mass index; ePWV, estimated pulse wave velocity; VFA, visceral fat area.
Figure 3.
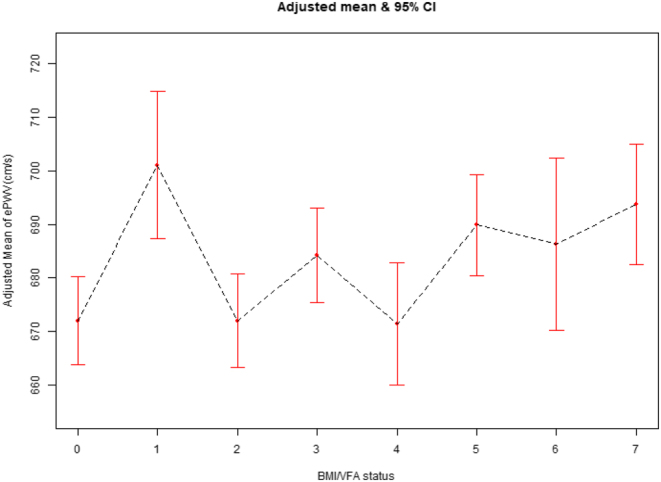
The relationship between BMI and visceral fat status with ePWV (cm/s) using the curve fitting of EmpowerStats, after adjusting for age, gender, race, education, marital status, family income ratio, diabetes mellitus, hypertension, hyperlipidemia, leisure time physical activity, smoking, drinking, and body surface area. Numbers 0–7 on the x-axis represent different BMI/VFA status: 0 = normal Weight and normal visceral fat, 1 = normal weight and visceral obesity, 2 = overweight and normal visceral fat, 3 = overweight and visceral obesity), 4 = obese grade 1 and normal visceral fat, 5 = obese grade 1 and visceral obesity, 6 = obese grade 2 and normal visceral fat, 7 = obese grade 2 and visceral obesity. BMI, body mass index; ePWV, estimated pulse wave velocity; VFA, visceral fat area.
Figure 4.
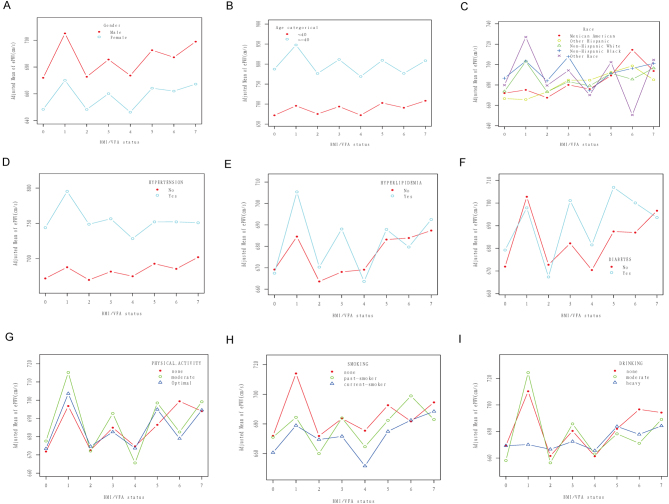
The relationship between BMI and visceral fat status on ePWV (cm/s) stratified by gender (A), age (B), race (C), hypertension (D), hyperlipidemia (E), diabetes (F), leisure time physical activity (G), smoking status (H), and drinking status (I) after adjusting for gender, age, race, education, marital status, family poverty income ratio, diabetes, hypertension, hyperlipidemia, smoking, alcohol drinking, leisure time physical activity, and body surface area. The model is not adjusted for the stratification variable. Numbers 0–7 on the x-axis represent different BMI/VFA status: 0 = normal weight and normal visceral fat, 1 = normal weight and visceral obesity, 2 = overweight and normal visceral fat, 3 = overweight and visceral obesity), 4 = obese grade 1 and normal visceral fat, 5 = obese grade 1 and visceral obesity, 6 = obese grade 2 and normal visceral fat, 7 = obese grade 2 and visceral obesity. BMI, body mass index; ePWV, estimated pulse wave velocity; VFA, visceral fat area.
Table 5.
association of BMI and visceral fat area status with 10-year cardiovascular risk, weighted.
| Crude model | Adjusted model 1 | |
|---|---|---|
| OR (95% CI), P | OR (95% CI), P | |
| Normal weight, normal visceral fat | Reference | Reference |
| Normal weight, visceral obesity | 33.98 (12.32,93.70), <0.0001 | 9.03 (2.66,30.69), <0.001 |
| Overweight, normal visceral fat | 1.49 (0.47, 4.73), 0.501 | 1.97 (0.59, 6.65), 0.278 |
| Overweight, visceral obesity | 14.58 (6.10, 34.83), <0.0001 | 8.28 (3.19,21.46),<0.001 |
| Obese grade 1, normal visceral fat | 0.88 (0.18,4.43), 0.881 | 1.48 (0.31, 7.15), 0.627 |
| Obese grade 1, visceral obesity | 11.65 (5.27, 25.78), <0.0001 | 8.91 (3.82,20.79), <0.001 |
| Obese grade 2, normal visceral fat | 0.00001 (0, 0.00002), <0.0001 | 0.00001 (0, 0.00003), <0.0001 |
| Obese grade 2, visceral obesity | 11.67 (4.79, 28.41), <0.0001 | 10.56 (4.06,27.51), <0.0001 |
Bold indicates statistical significance.
Crude model was adjusted for none.
Adjusted model was adjusted for age, gender, and race.
BMI, body mass index; ePWV, estimated pulse wave velocity; VFA, visceral fat area.
Results
Population characteristics
This study included 5309 participants (18–59 years) representing 144,907,159 Americans currently free of CVD. This population included 51.94% males, with a weighted mean age of 38.3 years and a weighted mean ePWV of 709.39 cm/s. Table 1 and Supplementary Table 1 shows the basic characteristics of the participant population classified according to different combined group of BMI and VFA (110 individuals in the low BMI group are not shown in the table). The eight groups differed significantly in age, family income ratio, BMI, VFA, SBP, DBP, ePWV, sex proportion, race, education level, marital status, hypertension, diabetes, hyperlipidemia, leisure time physical activity, smoking status, alcohol intake, and body surface area (all P < 0.05).
Association of body mass index, visceral fat area, and body mass index and visceral fat area combination status with arterial stiffness
The relationship between BMI, VFA, BMI/VFA combination and ePWV was observed in the crude, minimally adjusted, and fully adjusted models individually.
BMI was positively correlated with ePWV. When BMI was treated as a categorical variable, ePWV increased more markedly in the higher BMI group. The results show consistency in all three models considered. In the fully adjusted model, ePWV increased by 7.97 cm/s (95% CI: 3.69, 12.26, P < 0.001) for each SD unit increase in BMI. In the obese grade 1 group, ePWV increased by 12.55 cm/s (95% CI: 2.82, 22.29, P = 0.013), while the obese grade 2 group showed an ePWV increase of 20.08 cm/s (95% CI: 9.02, 31.13, P < 0.001) (Table 2). Moreover, VFA also showed a positive correlation with ePWV: ePWV increased by 8.40 cm/s (95% CI: 3.68, 13.12, P= 0.002) for each SD unit increase in VFA in the fully adjusted model. ePWV increased by 14.76 cm/s (95% CI: 7.71, 21.82, P < 0.001) in the higher VFA group (VFA ≥ 100 m2) as compared to the lower VFA group (VFA < 100 m2). The increase in ePWV was more marked in the higher VFA group (Table 2). The same trend was observed in the crude and minimally adjusted models. To detect an association with arterial stiffness (ePWV), we divided the cohort into eight groups as follows: normal-weight normal visceral fat; normal weight visceral obesity; overweight normal visceral fat; overweight visceral obesity; obese grade 1 normal visceral fat; obese grade 1 visceral obesity; obese grade 2 normal visceral fat; and obese grade 2 visceral obesity groups. We used the normal-weight normal visceral fat group as a reference. All groups showed a positive correlation with ePWV. Surprisingly, ePWV increased by 24.43 cm/s (95% CI: 1.88, 46.98, P = 0.035) in the normal-weight visceral obesity population, and this increase was higher than the 10.23 cm/s (95% CI: 1.89, 18.57, P = 0. 018) observed in the overweight visceral obesity population and 21.16 cm/s seen in the obesity grade 1 visceral obesity population (95% CI: 9.24, 33.07, P = 0.001), and was close to 26.35 cm/s (95% CI: 13.52, 39.18, P < 0.001) in the obese class 2 visceral obesity population after adjusting for age, sex, race, education, marital status, family income ratio, diabetes mellitus, hypertension, hyperlipidemia, leisure time physical activity, smoking, drinking, and body surface area (Table 3, Fig. 2).
Sensitivity and subgroup analyses
We conducted further stratified analyses to assess the correlation between BMI/VFA combination status and ePWV in different subgroups. As shown in Table 4, none of the variables, including sex (male with female, P = 0.575), age (<40 with > =40, P = 0.243), diabetes (yes with no, P = 0.151), hypertension (yes with no, P = 0.086), hyperlipidemia (yes with no, P = 0.39), leisure time physical activity (poor, intermediate with optimal, P = 0.162), body surface area (<1.88 m2 with ≥1.88 m2, P = 0.163), significantly changes the relationship between BMI/VFA combination status and ePWV. However, we observed a significant interaction between race and BMI/VFA combination status on ePWV (P < 0.0001). A similar interaction was observed with smoking (P = 0.007), and alcohol consumption (P <0.0001). The statistically significant positive correlation between BMI/VFA combination status and ePWV seen in other group comparisons disappeared among Mexican Americans, non-Hispanic black and other races, previous smokers, current smokers, and all drinking status groups (Supplementary Table 2).
The above results are shown as curve fitting plots using Empower Stats software (Figs. 3, 4A, B, C, D, E, F, G, H, and I).
Association of body mass index and visceral fat area combination status with 10-year cardiovascular risk
The 10-year cardiovascular risk in different BMI and VFA combination groups was evaluated. After adjusting for age, sex, and race, the 10-year cardiovascular risk was 8.03 times higher in the normal-weight visceral obesity group than in the control group (OR: 9.03, 95% CI: 2.66,30.69, P < 0.001), and this was higher than the risk observed in the overweight visceral obesity (OR: 8.28, 95% CI: 3.19, 21.46, P < 0.001) and obese grade 1 visceral obesity groups (OR: 8.91, 95% CI: 3.82, 20.00, P < 0.001; CI: 3.82, 20.79, P < 0.001) and was close to that seen in the obese grade 2 visceral obesity group (OR: 10.56, 95% CI: 4.06, 27.51, P < 0.0001). In the normal visceral fat group, the risk of being obese grade 2 was reduced (OR: 0.00001, 95% CI: 0, 0.00003, P < 0.0001) and was significantly different. The rest of the groups were not significantly different (Table 5).
Association of body mass index and visceral fat area combination status with all-cause mortality
The correlation of BMI and VFA combination status with all-cause mortality is shown in Supplementary Table 3. Normal-weight visceral obesity is associated with a higher all-cause mortality value (HR: 5.47, 95% CI: 0.47, 3.25, P = 0.006).
Discussion
This study, based on data collected from the NHANES 2011–2018 surveys comprises a final population of 5,309 people aged 18 to 59 years after considering the exclusion criteria and represents a US population of 144,907,159 people without a history of CVD. It is the first report that examines the association of normal-weight visceral obesity with arterial stiffness and cardiovascular risk in CVD-free people. Using the normal-weight normal visceral fat group as a reference, the other seven groups showed an increase in ePWV, specifically, an increase in vascular stiffness, with the obese grade 2 visceral obesity group showing the greatest increase of 26.35 cm/s (95% CI: 13.52, 39.18, P < 0.001), followed by 24.43 cm/s in the normal-weight visceral obesity group (95% CI: 1.88, 46.98, P = 0.035). Effect values in the normal-weight visceral obesity group were even higher than those in the obese grade 1 visceral obesity (β: 21.16, 95% CI: 9.24, 33.07, P = 0.001) and overweight visceral obesity groups (β: 10.23; 95% CI: 1.89, 18.57, P = 0.018). Moreover, ePWV values increased in the overweight normal visceral fat, obese grade 1 normal visceral fat, and obese grade 2 normal visceral fat groups. However, only the ePWV increase in the obese grade 2 normal visceral fat group (β: 13.8; 95% CI: 0.10, 27.5, P = 0.048) showed statistical significance (Table 3). Additionally, the 10-year cardiovascular risk assessment showed the two groups with the highest risk, obese grade 2 visceral obesity group had a 9.56-fold increase in risk compared with the control (OR: 10.56, 95% CI: 4.06, 27.51, P < 0.0001), normal weight visceral obesity group increased by 8.03 times (OR: 9.03, 95% CI: 2.66, 30.69; P < 0.001), followed by obese grade 1 visceral obesity group by 7.91 times (OR: 8.91, 95% CI: 3.82, 20.79; P < 0.001), and overweight visceral obesity group (OR: 8.28, 95% CI: 3.19, 27.51, P < 0.001). The 10-year cardiovascular risk was significantly lower in the normal visceral fat group than in the visceral obesity group, with no significant difference in any of the other groups except for the obese grade 2 normal visceral fat group (OR: 0.00001, 95% CI: 0, 0.00003, P < 0.0001) (Table 5).
Related studies
An obesity paradox exists in many diseases. Although obesity is an independent risk factor for heart disease, overweight and obese people seem to have a better prognosis than normal-weight people in heart failure (27), atrial fibrillation (28), aortic stenosis (29), and cardiovascular disease (30). Yanagisawa et al. found that a lower body weight was associated with a poorer outcome in elderly patients with atrial fibrillation (28). Follow-up data on 148 patients with severe aortic stenosis after TAVI showed a reduction in all-cause mortality in overweight and obese patients (29). In recent years, more and more studies have focused on BMI along with fat distribution. Normal-weight visceral obesity has been studied more often in cardiovascular secondary prevention populations. Coutinho et al. found that long-term survival was lowest in the normal-weight centrally obese population with CVD, and the normal-weight centrally obese population had a 10% increase in mortality compared with normal weight without central obese population ((HR): 1.10; 95% CI: 1.05–1.17) (13). Consistent results were obtained in another study of participants over 65 years of age with CVD (31). Additionally, an 83% increase in all-cause mortality (adjusted HR: 1.83; 95% CI: 1.04–3.31) and a 62% increase in major adverse cardiovascular events (HR: 1.62; 95% CI: 1.18–2.27) were found among Chinese men with premature acute coronary syndrome in the normal weight centrally obese population compared with the non-normal-weight centrally obese population (32). A multicenter study of patients with diabetes from China showed that normal-weight visceral obesity had a higher 10-year cardiovascular risk than any other combination of BMI and VFA conditions and was twice higher than the overweight or obese population without visceral obesity group (15). Additionally, the researchers found that among postmenopausal women, all-cause mortality in the central obesity group increased by 31% in normal-weight (HR, 1.31 (95% CI, 1.20–1.42)),16% in overweight (HR, 1.16 (95% CI, 1.13–1.20)), and 30% in obesity (HR, 1.30 (95% CI, 1.27–1.34)). No central obesity groups all decreased (33). Another large study of the general population included a population stratified by sex. Normal-weight central obesity men had an increased mortality rate compared with any other group of men, with an 87% increase compared with normal-weight but not in central obesity men (HR, 1.87 (95% CI, 1.53–2.29)). More than two times higher with the overweight no centrally obese and obese no centrally obese groups. Mortality was 48% higher in the normal weight central obesity group than in the normal weight no central obesity group in women (HR, 1.48 (95% CI, 1.35 to 1.62)), and overweight no centrally obese (HR, 1.40 (95% CI, 1.27 to 1.54)) and obese no centrally obese groups (HR, 1.32 (95% CI, 1.15–1.51)) (18). Our study fills the research gap on the cardiovascular risk of normal-weight visceral obesity in the CVD-free population.
Possible mechanism
We believe that normal weight visceral obesity is associated with higher vascular stiffness and cardiovascular risk, which may be caused by possible factors as follows: First, the bias caused by the deficiencies of the BMI values, which use weight and height but do not take into account the differences in the proportions of muscle, bone, and fat. Notably, bone density is greater than muscle, and muscle density is greater than fat; hence, all three have the smallest bone volume and the largest fat volume for the same weight. This makes people with large bones, strong muscles, and low-fat content to be classified as ‘overweight’ or ‘obese,’ and this population makes the study results biased. Second, visceral fat versus subcutaneous fat causes bias. In a previous study by Samuel et al., 15 obese women who underwent abdominal liposuction (subcutaneous fat) lost significant weight but did not improve corresponding cardiovascular-related risk factors such as blood pressure, blood glucose, insulin, and adiposity, nor did they improve insulin sensitivity in muscle, liver, and fat (34). Adiposity includes subcutaneous and visceral fat. Excessive visceral fat accumulation is more associated with metabolic syndrome, including insulin resistance, diabetes, hyperlipidemia, and CVD. The ‘normal BMI population’ segment with low subcutaneous fat and a lean appearance, but high visceral fat, is another possible cause (35). This phenomenon leads to the masking of many health problems, which is why Asian populations are often at high risk for normal weight with visceral obesity (15). Finally, genes that make people fatter but healthier exist (10). Loos et al. found through a genome-wide association study that the IRS1 allele is associated with higher adiposity and lower risk of cardiovascular metabolisms, such as type 2 diabetes and coronary heart disease. This allele leads to more subcutaneous fat accumulation rather than visceral fat accumulation (36). However, more related mechanisms need to be further investigated.
Strengths and limitations
This study has many strengths. First, it is the first study on the correlation between obesity and arterial stiffness evaluated using the general-obesity index combined with the central-obesity index in a population without CVD. For clinical work, a simple, effective, and comprehensive assessment of cardiovascular primary prevention populations or even class 0 prevention populations is crucial to reducing the global cardiovascular risk burden. Second, since observational studies are inevitably influenced by multiple factors, we used validated statistical methods such as performing three adjustment models and stratified analyses to reduce bias. Additionally, we performed a multidimensional assessment of the independent and dependent variables, assessed the relationship between the general-obesity index BMI/central obesity index VFA and vascular stiffness (both continuous and categorical variables were included), the general-obesity index combined with the central-obesity index and vascular stiffness, and validated the association of the joint index with cardiovascular risk with 10-year cardiovascular event risk. Finally, we used the NHANES database with survey weights, and based on the weights, this experiment represents a large population base of 144,907,159 Americans.
This study has some drawbacks. First, the cross-sectional study can only determine the correlation between obesity and arterial stiffness rather than the cause and effect. Second, the study applied to no existing cardiovascular population and cannot be generalized. Finally, we could not make model comparisons because NHANES does not have more data on other central obesity indicators.
Conclusively, this study demonstrated that the normal-weight visceral obesity group was associated with higher arterial stiffness and 10-year cardiovascular risk In the US population without existing CVD, indicating that this group needs to begin management for primary cardiovascular prevention clinically.
Supplementary Materials
Declaration of interest
The author reports no conflicts of interest in this work.
Funding
The study was funded by grant 81800231 from the National Natural Science Foundation of China.
Author contribution statement
Yuan Huang designed the study, analyzed the data, and wrote the manuscript. Yunyun Hu and Bingshu Bao collected the data.
Ethics statement
The Research Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University, approved this study as exempt.
Acknowledgements
The authors thank Haoan Jin, Chijia Jin, Yajin Mei, and Chengguo Huang for their support.
References
- 1.Vlachopoulos C, Terentes-Printzios D, Laurent S, Nilsson PM, Protogerou AD, Aznaouridis K, Xaplanteris P, Koutagiar I, Tomiyama H, Yamashina A, et al. Association of estimated pulse wave velocity with survival: a secondary analysis of Sprint. JAMA Network Open 20192e1912831. ( 10.1001/jamanetworkopen.2019.12831) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu LD Chu P Kong CH Shi Y Zhu MH Xia YY Li Z Zhang JX & Chen SL. Estimated pulse wave velocity is associated with all-cause mortality and cardiovascular mortality among adults with diabetes. Frontiers in Cardiovascular Medicine 2023101157163. ( 10.3389/fcvm.2023.1157163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greve SV Blicher MK Kruger R Sehestedt T Gram-Kampmann E Rasmussen S Vishram JKK Boutouyrie P Laurent S & Olsen MH. Estimated carotid-femoral pulse wave velocity has similar predictive value as measured carotid-femoral pulse wave velocity. Journal of Hypertension 2016341279–1289. ( 10.1097/HJH.0000000000000935) [DOI] [PubMed] [Google Scholar]
- 4.Wilkinson IB Mäki-Petäjä KM & Mitchell GF. Uses of arterial stiffness in clinical practice. Arteriosclerosis, Thrombosis, and Vascular Biology 2020401063–1067. ( 10.1161/ATVBAHA.120.313130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin X & Li H. Obesity: epidemiology, pathophysiology, and therapeutics. Frontiers in Endocrinology (Lausanne) 202112706978. ( 10.3389/fendo.2021.706978) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bray GA. Beyond BMI. Nutrients 2023152254. ( 10.3390/nu15102254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keys A Fidanza F Karvonen MJ Kimura N & Taylor HL. Indices of relative weight and obesity. International Journal of Epidemiology 201443655–665. ( 10.1093/ije/dyu058) [DOI] [PubMed] [Google Scholar]
- 8.Rao G, Powell-Wiley TM, Ancheta I, Hairston K, Kirley K, Lear SA, North KE, Palaniappan L, Rosal MC. & American Heart Association Obesity Committee of the Council on Lifestyle and Cardiometabolic Health. Identification of obesity and cardiovascular risk in ethnically and racially diverse populations: a scientific statement from the American Heart Association. Circulation 2015132457–472. ( 10.1161/CIR.0000000000000223) [DOI] [PubMed] [Google Scholar]
- 9.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH, American Heart Association & Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006113898–918. ( 10.1161/CIRCULATIONAHA.106.171016) [DOI] [PubMed] [Google Scholar]
- 10.Ahima RS & Lazar MA. Physiology. The health risk of obesity--better metrics imperative. Science 2013341856–858. ( 10.1126/science.1241244) [DOI] [PubMed] [Google Scholar]
- 11.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. Circulation 2014129(Supplement 2) S102–S138. ( 10.1161/01.cir.0000437739.71477.ee) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prospective Studies Collaboration, Whitlock G Lewington S Sherliker P Clarke R Emberson J Halsey J Qizilbash N Collins R & Peto R. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 20093731083–1096. ( 10.1016/S0140-6736(0960318-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coutinho T, Goel K, Corrêa de Sá D, Carter RE, Hodge DO, Kragelund C, Kanaya AM, Zeller M, Park JS, Kober L, et al. Combining body mass index with measures of central obesity in the assessment of mortality in subjects with coronary disease: role of “normal weight central obesity”. Journal of the American College of Cardiology 201361553–560. ( 10.1016/j.jacc.2012.10.035) [DOI] [PubMed] [Google Scholar]
- 14.Ishida A Taira H Shinzato T & Ohya Y. Association between visceral fat mass and arterial stiffness among community-based screening participants. Hypertension Research 2023. ( 10.1038/s41440-023-01350-7) [DOI] [PubMed] [Google Scholar]
- 15.Zheng J, Hu Y, Xu H, Lei Y, Zhang J, Zheng Q, Li L, Tu W, Chen R, Guo Q, et al. Normal-weight visceral obesity promotes a higher 10-year atherosclerotic cardiovascular disease risk in patients with type 2 diabetes mellitus-a multicenter study in China. Cardiovascular Diabetology 202322137. ( 10.1186/s12933-023-01876-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomez-Sanchez L, Garcia-Ortiz L, Patino-Alonso MC, Recio-Rodriguez JI, Rigo F, Martí R, Agudo-Conde C, Rodriguez-Sanchez E, Maderuelo-Fernandez JA, Ramos R, et al. Adiposity measures and arterial stiffness in primary care: the MARK prospective observational study. BMJ Open 20177e016422. ( 10.1136/bmjopen-2017-016422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haraguchi N Koyama T Kuriyama N Ozaki E Matsui D Watanabe I Uehara R & Watanabe Y. Assessment of anthropometric indices other than BMI to evaluate arterial stiffness. Hypertension Research 2019421599–1605. ( 10.1038/s41440-019-0264-0) [DOI] [PubMed] [Google Scholar]
- 18.Sahakyan KR, Somers VK, Rodriguez-Escudero JP, Hodge DO, Carter RE, Sochor O, Coutinho T, Jensen MD, Roger VL, Singh P, et al. Normal-weight central obesity: implications for total and cardiovascular mortality. Annals of Internal Medicine 2015163827–835. ( 10.7326/M14-2525) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jayanama K Theou O Godin J Mayo A Cahill L & Rockwood K. Relationship of body mass index with frailty and all-cause mortality among middle-aged and older adults. BMC Medicine 202220404. ( 10.1186/s12916-022-02596-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reference Values for Arterial Stiffness ’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: 'establishing normal and reference values'. European Heart Journal 2010312338–2350. ( 10.1093/eurheartj/ehq165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heffernan KS Stoner L Meyer ML & Loprinzi PD. Association between estimated pulse wave velocity and cognitive performance in older black and white adults in NHANES. Journal of Alzheimer’s Disease 202288985–993. ( 10.3233/JAD-220042) [DOI] [PubMed] [Google Scholar]
- 22.D'Agostino RB Vasan RS Pencina MJ Wolf PA Cobain M Massaro JM & Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008117743–753. ( 10.1161/CIRCULATIONAHA.107.699579) [DOI] [PubMed] [Google Scholar]
- 23.Wu Z, Zhang H, Li Z, Li H, Miao X, Pan H, Wang J, Liu X, Kang X, Li X, et al. Mutual effect of homocysteine and uric acid on arterial stiffness and cardiovascular risk in the context of predictive, preventive, and personalized medicine. EPMA Journal 202213581–595. ( 10.1007/s13167-022-00298-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor HCM Chaturvedi N Davey Smith G Ferreira DLS Fraser A Howe LD Hughes AD Lawlor DA Timpson NJ & Park CM. Is Height2.7 appropriate for indexation of left ventricular mass in healthy adolescents? The importance of sex differences. Hypertension 2023. ( 10.1161/HYPERTENSIONAHA.121.17109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American Diabetes Association Professional Practice Committee. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care 202245(Supplement 1) S17–S38. ( 10.2337/dc22-S002) [DOI] [PubMed] [Google Scholar]
- 26.Chaturvedi S. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7): is it really practical? National Medical Journal of India 200417227. [PubMed] [Google Scholar]
- 27.Casas-Vara A Santolaria F Fernández-Bereciartúa A González-Reimers E García-Ochoa A & Martínez-Riera A. The obesity paradox in elderly patients with heart failure: analysis of nutritional status. Nutrition 201228616–622. ( 10.1016/j.nut.2011.10.006) [DOI] [PubMed] [Google Scholar]
- 28.Yanagisawa S, Inden Y, Yoshida N, Kato H, Miyoshi-Fujii A, Mizutani Y, Ito T, Kamikubo Y, Kanzaki Y, Hirai M, et al. Body mass index is associated with prognosis in Japanese elderly patients with atrial fibrillation: an observational study from the outpatient clinic. Heart and Vessels 2016311553–1561. ( 10.1007/s00380-015-0765-y) [DOI] [PubMed] [Google Scholar]
- 29.Tokarek TA Dziewierz A Sorysz D Bagienski M Rzeszutko Ł Krawczyk-Ożóg A Dudek D & Kleczyński P. The obesity paradox in patients undergoing transcatheter aortic valve implantation: is there any effect of body mass index on survival? Kardiologia Polska 201977190–197. ( 10.5603/KP.a2018.0243) [DOI] [PubMed] [Google Scholar]
- 30.Gruberg L, Weissman NJ, Waksman R, Fuchs S, Deible R, Pinnow EE, Ahmed LM, Kent KM, Pichard AD, Suddath WO, et al. The impact of obesity on the short-term and long-term outcomes after percutaneous coronary intervention: the obesity paradox? Journal of the American College of Cardiology 200239578–584. ( 10.1016/s0735-1097(0101802-2) [DOI] [PubMed] [Google Scholar]
- 31.Sharma S, Batsis JA, Coutinho T, Somers VK, Hodge DO, Carter RE, Sochor O, Kragelund C, Kanaya AM, Zeller M, et al. Normal-weight central obesity and mortality risk in older adults with coronary artery disease. Mayo Clinic Proceedings 201691343–351. ( 10.1016/j.mayocp.2015.12.007) [DOI] [PubMed] [Google Scholar]
- 32.Wan J Zhou P Wang D Liu S Yang Y Hou J Li W & Wang P. Impact of normal weight central obesity on clinical outcomes in male patients with premature acute coronary syndrome. Angiology 201970960–968. ( 10.1177/0003319719835637) [DOI] [PubMed] [Google Scholar]
- 33.Sun Y, Liu B, Snetselaar LG, Wallace RB, Caan BJ, Rohan TE, Neuhouser ML, Shadyab AH, Chlebowski RT, Manson JE, et al. Association of normal-weight central obesity with all-cause and cause-specific mortality among postmenopausal women. JAMA Network Open 20192e197337. ( 10.1001/jamanetworkopen.2019.7337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klein S Fontana L Young VL Coggan AR Kilo C Patterson BW & Mohammed BS. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. New England Journal of Medicine 20043502549–2557. ( 10.1056/NEJMoa033179) [DOI] [PubMed] [Google Scholar]
- 35.Valenzuela PL Carrera-Bastos P Castillo-García A Lieberman DE Santos-Lozano A & Lucia A. Obesity and the risk of cardiometabolic diseases. Nature Reviews. Cardiology 202320475–494. ( 10.1038/s41569-023-00847-5) [DOI] [PubMed] [Google Scholar]
- 36.Antonopoulos AS & Tousoulis D. The molecular mechanisms of obesity paradox. Cardiovascular Research 20171131074–1086. ( 10.1093/cvr/cvx106) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a