Abstract
Aims
Left bundle branch area pacing (LBBAP) is most often delivered using lumenless leads (LLLs), but may also be performed using stylet-driven leads (SDLs). There are limited reports on the comparison of these tools, mainly limited to reports describing initial operator experience or without detailed procedural data. Our aim was to perform an in-depth comparison of SDLs and LLLs for LBBAP at implantation and follow-up in a larger cohort of patients with experience that extends beyond that of the initial learning curve.
Methods and results
A total of 306 consecutive patients (age 77 ± 11 years, 183 males) undergoing LBBAP implantation at a single centre were prospectively included. The population was split into two groups of 153 patients based on the initial use of an SDL (from 4 manufacturers) or an LLL. After having discounted the initial learning curve of 50 patients, there was no difference in the success rate between the initial use of lead type (96.0% with SDL vs. 94.3% with LLL, P = 0.56). There were no significant differences in success between lead models. Electrocardiogram and electrical parameters were comparable between the groups. Post-operative macro-dislodgement occurred in 4.3% of patients (essentially within the first day following implantation) and presumed micro-dislodgement with loss of conduction system capture or rise in threshold (occurring mostly during the first month) was observed in 4.7% of patients, without differences between groups.
Conclusion
Left bundle branch area pacing may be safely and effectively performed using either LLLs or SDLs, which provides implanters with alternatives for delivering this therapy.
Keywords: Conduction system pacing, Left bundle branch area pacing, Lumenless lead, Stylet-driven lead, Success, Failure, Complications
Graphical Abstract
Graphical Abstract.
What’s new?
Left bundle branch area pacing (LBBAP) with stylet-driven leads (SDLs) are comparable with lumenless leads in terms of implantation success rate, electrical parameters, and complications (except for a higher rate of helix damage with SDLs).
Stylet-driven leads from four manufacturers were successfully implanted.
The initial learning curve for LBBAP is ∼50 patients with a gradual increase in success rate thereafter.
Lead macro-dislodgements and presumed micro-dislodgements (with loss of conduction system capture) were relatively frequent in our series (∼3–6%) and warrants further evaluation.
Introduction
Conduction system pacing (CSP) has gained interest for avoiding ventricular dysfunction encountered with right ventricular pacing and has been introduced in the 2021 European pacing guidelines.1 Left bundle branch area pacing (LBBAP) is associated with favourable electrical parameters, high success rates, and low complication rates.2 This entity includes left bundle branch pacing (LBBP), left fascicular pacing, and left ventricular septal pacing (LVSP).3
Left bundle branch area pacing is most frequently performed using lumenless leads (LLLs).2,4,5 Stylet-driven leads (SDLs) are also used and have the advantage of greater stiffness to facilitate septal penetration, as well as being able to continuously pace via the stylet to assess lead depth while screwing the lead.3 However, SDLs have a larger diameter than LLL and may be less robust due to the retractable helix design.
The safety and feasibility of SDL compared with LLL has been described in a few series.2,6–8 However, these reports either had a limited number of patients in the groups (<35), implying that this was a description of their initial experience,6–8 or did not compare the procedural observations and findings at follow-up of the leads.2
Our aim was to perform an in-depth comparison of SDLs and LLLs for LBBAP at implantation and follow-up in a larger cohort of patients with experience that extends beyond that of the initial learning curve.
Methods
Study population
We included consecutive patients undergoing LBBAP implantation recruited from the University Hospital of Geneva from February 2020 to March 2023. The patients were assigned to either SDL or LLL groups based on the initial choice of lead type, which was left to the operator’s discretion. All patients were part of the Geneva Conduction System Pacing Registry, for which institutional ethics committee approval had been granted and informed patient consent obtained.
Data collection
A prospective database of each LBBAP procedure (successful or not) is recorded at our institution to capture implantation data including numbers and duration of lead positioning, tools used, reason for failure and complications (perforation, dislodgement, chest pain, and lead damage), electrical parameters (capture threshold, sensing, and impedance), and per-operative electrocardiogram (ECG) measurements for confirming conduction system capture. Demographic variables and follow-up data (including 12-lead ECGs during device controls) were retrieved via the hospital electronic medical records.
Data analysis
Implant success rate (defined as achieving LBBAP) with the initial lead type (SDL vs. LLL) and procedural findings (number of lead positions before achieving successful implantation, duration of lead positioning, electrical and ECG parameters, per-operative complications, etc.) were recorded. As LBBAP lead implantation was sometimes performed with additional procedures (e.g. coronary sinus lead implantation, lead extraction, etc.), total procedure and fluoroscopy durations were not analysed.
Left bundle branch area pacing implantation
The procedures were mainly performed by H.B. as the first operator, who also trained two additional operators (E.B. and N.K.). The SDLs used in this study were Solia S 60 (Biotronik, Berlin, Germany), Tendril STS (Abbott, Sylmar, CA, USA), Ingevity (Boston Scientific, Marlborough, MA, USA), and Vega (Microport, Shanghai, China) delivered using the Selectra 3D 55 or 65 curve (39 or 42 cm catheter length) delivery system. The LLL was the 3830-69 SelectSecure lead (Medtronic, Minnesota, MN, USA) delivered using the C-315 His sheath (or a Selectra 3D sheath if previously used unsuccessfully with an SDL). The C304-His sheath was also initially used but abandoned after a few cases because of issues with introducing the lead past the distal curve. The Solia S 60 was used as a bailout in selected cases if implantation with other SDLs or with an LLL was unsuccessful. Likewise, the LLL was used as a bailout in selected cases of failed Solia S 60 implantation. In cases where right ventricular septal pacing was deemed to be acceptable (e.g. in case of infrequent ventricular pacing), we chose not to cross over to another lead in order to limit costs. Lead characteristics are given in Table 1.
Table 1.
Technical characteristics of leads used in the study
| Lead model | 3830 | Solia S | Tendril STS | Vega | Ingevity |
|---|---|---|---|---|---|
| Medtronic (n = 157) | Biotronik (n = 124) | Abbott (n = 27) | Microport (n = 8) | Boston Scientific (n = 5) | |
| Stylet | No | Yes | Yes | Yes | Yes |
| Diameter (F) | 4.1 | 5.6 | 5.6 | 6 | 5.7 |
| Length (cm) | 69 | 60 | 58 | 58 | 59 |
| Helix type | Fixed | Retractable | Retractable | Retractable | Retractable |
| Helix length (mm) | 1.8 | 1.8 | 2.0 | 1.5 | 1.8 |
| Body material | Polyurethane | Polyurethane outer (silicone inner) | Optim | Silicone (with silglide treatment) | Polyurethane |
The implantation technique evolved during the course of the study to conform to the 2023 European Heart Rhythm Association (EHRA) consensus document on CSP implantation.3,9 In brief, the His bundle was located in the right anterior oblique 20–30° view (during maximum of 1 min) using unipolar mapping of the pacing lead inserted in a three-dimensional delivery catheter and served as a reference point (if not found, the atrio-ventricular ring was located using endocavitary signals, or occasionally, by contrast injection). The lead insertion site was located 15–35 mm from the His bundle or the summit of the tricuspid annulus. Pace mapping was performed to locate the insertion site, aiming initially for discordance in Lead II (positive or isoelectric) and Lead III (isoelectric or negative), with a W pattern in Lead V1 (not mandatory). For SDLs, initial mapping of the His and pace mapping were performed with the helix retracted to avoid snagging of tissue. The screw was then extended and additionally pre-tensioned and locked using the funnel tool (Solia lead) or pinch tool maintained in place (other SDLs) to avoid screw retraction. The stylet was fully inserted and connected to a cathodal crocodile clip to deliver unipolar pacing continuously while screwing, whereas intermittent pacing was performed during interrupted screwing with LLLs. The lead was rapidly rotated with forward pressure in the left anterior oblique 30–40° view and at an angle of 10–40° with respect to the horizontal plane while screening by fluoroscopy to maintain lead/sheath alignment and to observe progression in the septum.
Lead depth was assessed by fixation/template beat morphology, paced QRS morphology, amplitude of sensed current of injury (COI) in the unipolar unfiltered (0.5–500 Hz) channel or presence of a fascicular potential (best visualized in the filtered 30–500 Hz channel), by monitoring unipolar pacing impedance, and in some instances by contrast injection via the sheath to delineate the septum. In case of perforation, the lead was repositioned at a new site. Conduction system capture was aimed for in all cases, but LVSP (without CSP) was accepted in case where this was not obtained. During the last months of patient inclusion, lead stability was tested prior to catheter slitting by pushing the lead to form an α-shaped loop and in some instances by gently pulling on the lead and observing a forward motion of the catheter and feeling resistance on the lead.
Micro-dislodgments were diagnosed intra-operatively by changes in paced QRS morphology or loss of visualization of a fascicular potential without any visible fluoroscopic changes in lead position, whereas macro-dislodgments were visible by fluoroscopy.
Perforation was diagnosed when the lead could be freely pushed into the left ventricular cavity or when there was a marked fall in COI amplitude (usually <4–5 mV or with a ‘QS’ morphology) along with a drop in pacing impedance to <450 Ω,10 usually with a rise in capture threshold.
Electrocardiogram analysis
Confirmed or likely conduction system capture, LVSP (without conduction system capture), and deep septal pacing (DSP) without criteria for LBBAP were based on the EHRA expert consensus.3Confirmed conduction system capture was diagnosed by the presence of a clear QRS transition with decrementing output during unipolar pacing (considered to be the gold standard if observed), V6 R-wave peak time (V6RWPT) <75 ms (or <80 ms in case of left bundle or bifascicular block, non-specific intra-ventricular conduction delay, wide escape rhythm or paced rhythm), or V6-V1 interpeak interval >44 ms.11Likely conduction system capture was diagnosed by V6RWPT <85 ms (or <100 ms and the presence of the aforementioned conduction disturbances). If these criteria were not fulfilled but the patient had a paced terminal r/R wave in V1, LVSP was diagnosed (which is also considered as being LBBAP). If all criteria were absent, DSP was diagnosed and LBBAP implantation was considered a failure. Programmed stimulation is not routinely performed at our institution and was not used to define capture. Electrocardiograms at implantation and follow-up were performed during asynchronous pacing from the LBBAP lead in order to avoid the confounding effect of fusion. Measurements at implantation were performed by the same investigator (H.B.), using a Boston Scientific Labsystem Pro electrophysiology recording system with digital calipers at 100 mm/s sweep speed. Standard 12-lead ECGs at device follow-up were analysed using digital calipers with measurements of V6RWPT and V6V1 interpeak interval. QRS duration was measured from the onset of the pacing spike (and not the onset of the rapid QRS upslope). All measurements were performed by a single observer (A.S.), and confirmation of conduction system capture was validated by a second investigator (H.B.).
Follow-up
Data from patients who were followed up at the institutional pacemaker clinic were recorded, with follow-up defined as the last visit with paced ECG data uploaded to the electronic health records.
Statistical analysis
The analyses were performed by using SPSS v28. Continuous variables were analysed by using an unpaired Student’s t-test after testing for Gaussian distribution by Kolmogorov–Smirnov and Shapiro–Wilk tests, otherwise non-parametrical tests (Mann–Whitney U test) were used. Categorical variables were analysed with Pearson’s χ2 test (or Fisher’s exact test). The level of significance was P < 0.05, and hypothesis testing was done two-sided.
Results
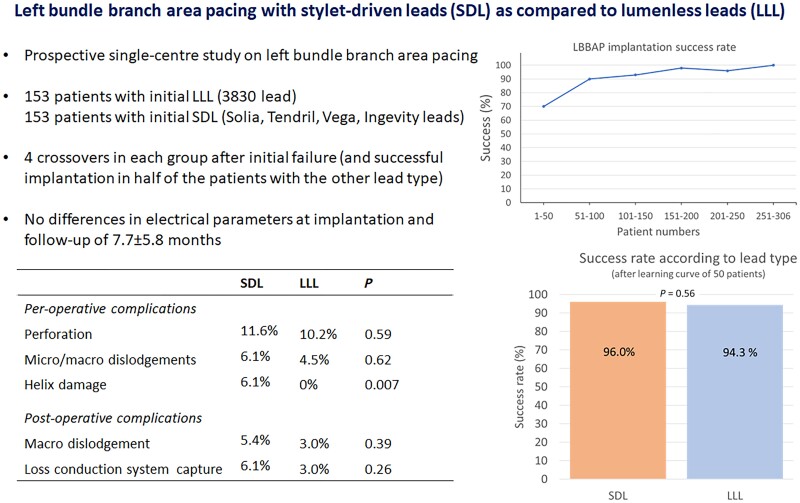
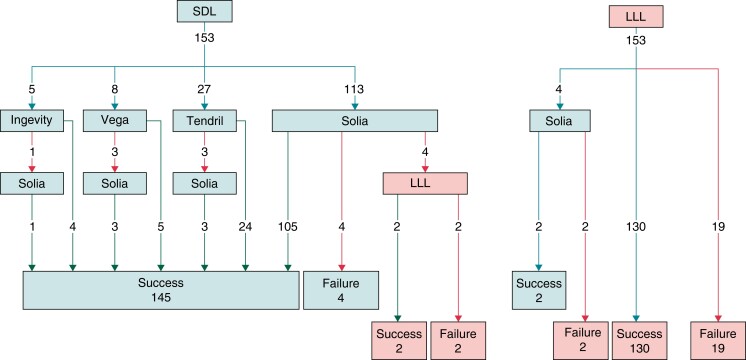
A total of 306 patients were included, of whom 153 were in the SDL group and 153 were in the LLL group (see Figure 1). Patient demographics are given in Table 2. The first 48 patients were all implanted using an LLL over the initial 13 months (His bundle pacing was being performed in the majority of cases during that period—data not provided), following which there was a marked rise in LBBP implantation, along with an increasing adoption of SDLs (see Figure 2).
Figure 1.
Patient groups based on the initial strategy of SDL or LLL. LLL, lumenless lead; SDL, stylet-driven lead.
Table 2.
Patient demographics
| SDL (n = 153) | LLL (n = 153) | P-value | |
|---|---|---|---|
| Age (years) | 80 ± 11 | 74 ± 13 | <0.001 |
| Male/female | 91/62 | 92/61 | 0.85 |
| Indication | |||
| Pace and ablate | 19 (12%) | 24 (16%) | 0.94 |
| AVB-I | 5 (3%) | 4 (3%) | |
| AVB-II Wenckebach | 13 (8%) | 11 (8%) | |
| AVB-II Mobitz 2 | 11 (7%) | 9 (6%) | |
| AVB-III | 54 (35%) | 55 (36%) | |
| LOT-CRT | 3 (2%) | 2 (1%) | |
| In lieu of BiV-CRT | 7 (5%) | 10 (7%) | |
| Syncope and BBB | 22 (14%) | 16 (10%) | |
| Slow AF | 19 (12%) | 22 (14%) | |
| Baseline rhythm | |||
| SR | 101 (66%) | 100 (65%) | 0.95 |
| AF | 46 (30%) | 46 (30%) | |
| Flutter | 6 (4%) | 7 (5%) | |
| Comorbidities | |||
| Ischaemic heart disease | 63 (41%) | 43 (28%) | 0.016 |
| Dilated cardiomyopathy | 11 (7%) | 12 (8%) | 0.83 |
| PTCA | 34 (22%) | 21 (14%) | 0.48 |
| CABG | 12 (8%) | 4 (3%) | 0.151 |
| Valve surgery | 12 (8%) | 17 (11%) | 0.44 |
| TAVI | 20 (13%) | 6 (4%) | 0.004 |
| Diabetes | 49 (32%) | 47 (31%) | 0.81 |
| Hypertension | 124 (81%) | 112 (73%) | 0.11 |
| Kidney failure | 50 (33%) | 44 (29%) | 0.46 |
| LVEF | 54.8 ± 2.1 | 53 ± 2.3 | 0.25 |
| Left-sided approach | 147 (96%) | 146 (95%) | 1.00 |
| Right-sided approach | 6 (4%) | 7 (5%) | |
Numbers in bold are statistically significant.
Groups are split according to the lead type used initially.
AF, atrial fibrillation; AVB, atrio-ventricular block; BBB, bundle branch block; BiV-CRT, biventricular cardiac resynchronization therapy; CABG, coronary artery bypass surgery; LLL, lumenless lead; LOT-CRT, left bundle branch optimized cardiac resynchronization therapy; LVEF, left ventricular ejection fraction; PTCA, percutaneous coronary angioplasty; SDL, stylet-driven lead; SR, sinus rhythm; TAVI, transcatheter aortic valve implantation.
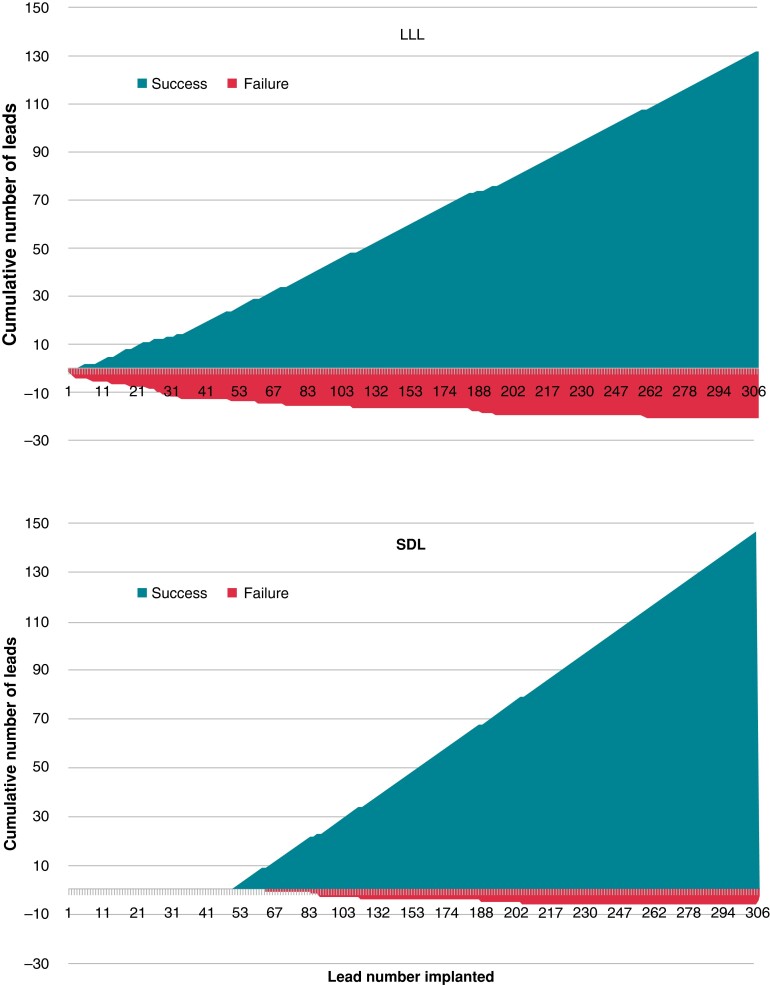
Figure 2.
Cumulative success and failure for left bundle area lead implantation for LLL and SDL groups. LLL, lumenless lead; SDL, stylet-driven lead.
Procedural findings
Success rate
The overall success rate for LBBAP implantation was 91.2%. The success rate for LBBAP was higher with an initial strategy of SDL: 147/153 (96.1%) compared with the initial LLL 132/153 (86.3%, P = 0.004; see Figures 1 and 2). This included four patients in each group (eight patients in total) who crossed over to the other lead type because of the inability to penetrate the septum, of whom half were implanted successfully in each group using the other lead type. The reasons for the failed implantation in the six patients in the initial SDL group were difficulty in penetrating the septum in four patients and a wide-paced QRS in two patients. For the 21 patients with failed implantation with the initial strategy of LLL, the main reasons were difficulty in penetrating the septum in 12 patients, lead instability in 5 patients, and a wide-paced QRS in 4 patients.
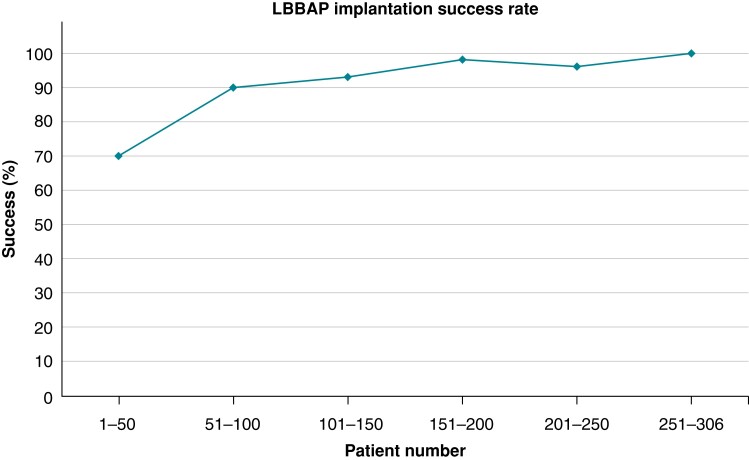
The steepest part of the learning curve was during the first 50 patients with a success rate of 70% (implantation in the first 4 consecutive patients failed), following which the success rate flattened out (with 100% success in patients 250–306; see Figure 3). After discounting the first 50 cases, the success rate with an initial strategy of SDL was 145/151 (96.0%) and with LLL, it was 99/105 (94.3%; P = 0.56).
Figure 3.
Implantation success rates according to the number of patients implanted, including all types of leads and the four patients who had successful implantation after crossing over the lead type. The success rate increases markedly after the first 50 cases (from 70 to 90%) and then gradually reaches 100% for the last 56 cases. LBBAP, left bundle branch area pacing.
The 3830 lead was successful in 132/157 (84.1%) patients in whom it was used (including the 4 crossovers in whom an SDL was implanted first). After discounting the initial learning curve of 50 cases, the lead was successfully implanted in 99/105 (94.3%) patients. The Solia lead was successful in 114/124 (91.9%) patients in whom it was used (including the 4 crossovers in whom an LLL was implanted first, P = 0.61 compared with LLL used after the learning curve). The Tendril STS lead was successful in 24/27 (88.9%) patients. The Ingevity lead was successful in 4/5 (80.0%) patients and the Vega lead in 5/8 (62.5%) patients (P = 0.056 for differences between SDLs).
Lead positioning
The number of lead positions required before the final successful site was found was similar in both groups (SDL 2.8 ± 0.3, LLL 3.0 ± 0.4, P = 0.44). The duration of lead implantation (which included testing and ECG measurements) was, however, shorter for SDL than for LLL (15.9 ± 1.9 vs. 19.2 ± 2.6 min, respectively, P = 0.044). A fascicular potential was visible in 29 (19%) patients in each group.
Electrocardiogram and electrical parameters
Electrocardiogram findings are presented in Table 3. Unipolar capture threshold at implantation (0.5 ms pulsewidth) was the same for both lead types (SDL 0.74 ± 0.34 V, LLL 0.70 ± 0.24 V, P = 0.28). A capture threshold <1.0 V was encountered in 123/147 (83.7%) patients with SDL and 113/132 (85.6%) patients with LLL (P = 0.62). Bipolar sensing amplitudes were also similar (SDL 9.3 ± 3.5 mV, LLL 10.0 ± 4.6 mV, P = 0.12). Bipolar impedance at implantation was lower with SDL (617 ± 117 Ω) than with LLL (702 ± 136 Ω, P < 0.001).
Table 3.
Electrocardiogram parameters at implantation and follow-up (35 patients were lost to follow-up after initial discharge, and 12 patients with macro-dislodgement were removed)
| ECG parameters at implantation | Successful final SDL (n = 147) | Successful final LLL (n = 132) | P-value |
|---|---|---|---|
| V6RWPT (ms) | 78 ± 14 | 76 ± 13 | 0.55 |
| QRS duration (ms) | 149 ± 24 | 140 ± 21 | 0.21 |
| QRS axis | 0.18 | ||
| Positive in II and III | 19 (12.9%) | 27 (20.5%) | |
| Positive/iso II and iso/negative III | 62 (42.2%) | 56 (42.4%) | |
| Negative II and III | 66 (44.9%) | 49 (37.1%) | |
| Conduction system capture | 0.55 | ||
| Confirmed | 98 (66.7%) | 95 (72.0%) | |
| Likely | 37 (25.1%) | 26 (19.7%) | |
| LVSP | 12 (8.2%) | 11 (8.3%) |
| ECG parameters at last follow-up | Successful final SDL (n = 118) | Successful final LLL (n = 114) | P-value |
|---|---|---|---|
| V6RWPT (ms) | 75 ± 17 | 71 ± 13 | 0.06 |
| QRS duration (ms) | 140 ± 20 | 136 ± 16 | 0.65 |
| Conduction system capture | |||
| Confirmed | 76 (64.4%) | 80 (70.2%) | 0.70 |
| Likely | 25 (21.1%) | 21 (18.4%) | |
| LVSP | 11 (9.3%) | 10 (8.8%) | |
| DSPa | 6 (5.1%) | 3 (2.6%) | |
DSP, deep septal pacing; ECG, electrocardiogram; iso, isoelectric; LLL, lumenless leads; LVSP, left ventricular septal pacing; SDL, stylet-driven leads; V6RWPT, V6 R-wave peak time.
DSP was newly diagnosed at follow-up (patients with DSP at implantation were considered to be failed procedures).
Per-operative complications
Complications are listed in Table 4. All per-operative micro- and macro-dislodgments were repositioned during the procedure. Helix damage caused by entanglement with tissue was observed in 10/164 (6.1%) patients in whom an SDL had been used (eight Solia leads and two Tendril leads) and in 0/157 with an LLL (P = 0.007). One patient with an SDL experienced intense chest pain without ECG modifications, which disappeared after the lead was repositioned. Complete heart block (all in patients with underlying left bundle branch block) was observed in two patients with each lead type.
Table 4.
Per-operative and post-operative complications
| SDL | LLL | P-value | |
|---|---|---|---|
| Per-operative | n = 164 | n = 157 | |
| Perforation | 19 (11.6%) | 16 (10.2%) | 0.59 |
| Micro/macro-dislodgements | 10 (6.1%) | 7 (4.5%) | 0.62 |
| Helix damage | 10 (6.1%) | 0 (0%) | 0.007 |
| Chest pain | 1 (0.6%) | 0 (0%) | 1.00 |
| RBBB | 4 (2.4%) | 3 (1.9%) | 1.00 |
| AVB-III | 2 (1.2%) | 2 (1.3%) | 1.00 |
| Post-operative | n = 147 | n = 132 | |
| Macro-dislodgement | 8 (5.4%) | 4 (3.0%) | 0.39 |
| Loss CS capture | 9 (6.1%) | 4 (3.0%) | 0.26 |
AVB, atrio-ventricular block; CS, conduction system; LLL, lumenless lead; RBBB, right bundle branch block; SDL, stylet-driven lead.
Findings at follow-up
A total of 35 patients (21 patients with SDL and 14 patients with LLL) were lost to follow-up after initial hospital discharge. At the last follow-up of 7.7 ± 5.8 months, bipolar capture thresholds at 0.4 ms pulsewidth were comparable between lead types (SDL 0.88 ± 0.33 V and LLL 0.76 ± 0.29 V, P = 0.12). A threshold of <1.0 V was found in 78/126 (61.9%) patients with SDL and 86/118 (72.9%) patients with LLL (P = 0.08). The change in capture threshold amplitude at the last follow-up was not different between capture types at implantation: confirmed conduction system capture 0.13 ± 0.41 V, likely conduction system capture 0.11 ± 0.31 V and LVSP 0.04 ± 0.27 V (P = 0.45).
Macro-dislodgement was observed in a total of 8/147 patients (5.4%) implanted with an SDL and in 4/132 (3.0%) implanted with an LLL (P = 0.39; see Table 4 and Figure 4B). All macro-dislodgements were diagnosed on the same day, or the day following implantation, except for one patient with an SDL who was diagnosed at first post-discharge follow-up at 37 days (it was unclear from the trends of the automatic electrical measurements at what time point the dislodgement had actually occurred). Electrical parameters were satisfactory despite macro-dislodgement in three patients with SDL and in one patient with LLL. The leads were not repositioned in these four patients, and electrical parameters as well as clinical follow-up remained satisfactory.
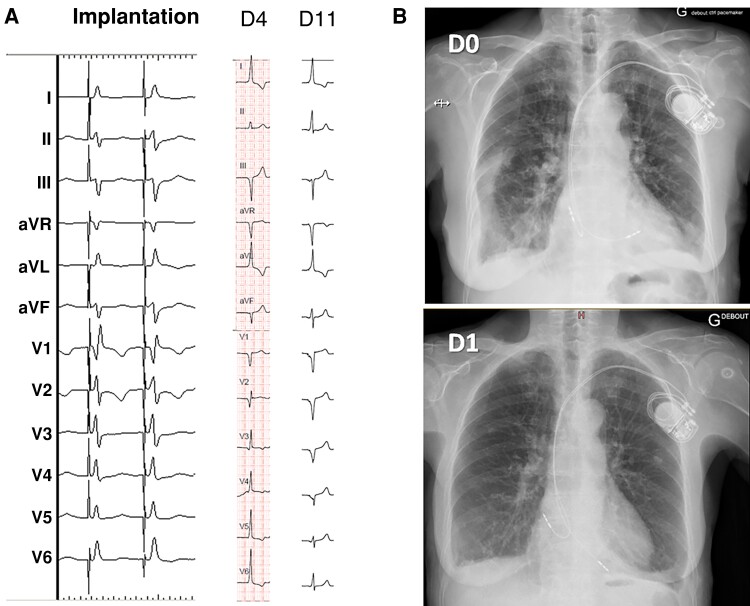
Figure 4.
Micro- and macro-dislodgement of LBBAP leads in two different patients. (A) Micro-dislodgement of a Solia lead. At implantation, the transition in QRS from non-selective left septal fascicular pacing to myocardial capture is visible (note the increase in V6 R-wave peak time). At Day 4 (D4), there is a loss of terminal R-wave in V1 with an increase in R-wave peak time in V6, with a further broadening of the QRS at Day 11 (D11). (B) Left bundle branch area pacing with a Solia lead on the day of implantation (D0) with lead macro-dislodgement on Day 1 (D1). Electrical parameters were good, and no repositioning was deemed necessary, with stable electrical parameters at 1 year and uneventful follow-up. LBBAP, left bundle branch area pacing.
Loss of conduction system capture without fluoroscopic evidence of lead displacement (i.e. likely micro-dislodgement) was observed in 9/147 (6.1%) patients with an SDL and 4/132 (3.0%) patients with an LLL (P = 0.26 compared with SDL; see Table 4 and Figure 4A). All cases were identified within 1 month of implantation except for a patient with a Solia lead who had a rise in thresholds with an intermittent complete loss of capture after 340 days. In this last case, it was unclear what the mechanism of loss of capture was. Another patient with a Solia lead experienced a rise in thresholds identified 5 days after implantation, and both of these patients underwent lead revision. One patient with a 3830 lead with loss of conduction system capture also underwent lead revision. There were no differences in the rates of combined macro- and likely micro-dislodgements between lead types (P = 0.14).
A total of 27 patients died during the course of the study, 8 of unknown cause, 13 due to non-cardiovascular pathologies, 4 patients due to heart failure, 1 patient due to ventricular arrhythmia, and 1 patient due to tamponade. This last patient was a frail 88-year-old male with renal failure, prior stroke, ischaemic heart disease, and 30% left ventricular ejection fraction who underwent uneventful dual-chamber LBBAP implantation under oral anticoagulation due to Mobitz 2 atrio-ventricular block and was admitted 3 days after the procedure with tamponade attributed to perforation of the atrial Tendril STS lead. Despite pericardiocentesis, the patient’s condition remained precarious, and the patient died after 11 days.
Discussion
The main findings of our study are as follows: (i) SDLs (from four manufacturers) and LLL (the 3830 lead) are both effective for delivering LBBAP with comparable electrical parameters; (ii) Per-operative complications are comparable between lead types, although there is a higher risk of helix damage with SDLs; and (iii) Lead dislodgment was an issue with both lead types in this series.
Although the success rate of initial SDL was greater than that for LLL, it is difficult to compare these strategies as the initial learning curve (found to be 50 patients in our series) was acquired almost exclusively using LLL. However, although the number of lead positioning attempts was comparable between groups (around three on average), the total duration for lead implantation and testing was shorter with SDLs. This may be explained by the fact that one has the convenience of continuously pacing via the stylet while rotating the body of the SDL lead, without having to do this in an interrupted manner as with LLLs. There was a trend in the lower anatomical lead implantation site with SDLs (as indicated by the trend in a greater proportion of superior-paced QRS axis; see Table 3), possibly due to the difference in the delivery catheter curve and the added stiffness by the stylet, resulting in a straightening of the delivery catheter. There was also a trend of greater efficacy using the Solia lead compared with the other SDLs. This may be attributed to the low lead profile, polyurethane outer coating (contributing to stiffness and low friction) as well as the funnel-shaped tool, which may be used to lock the stylet and avoid its retraction during lead body rotations.
To the best of our knowledge, this is the first report of LBBAP using Vega leads. Although the results should be interpreted with caution because of the small number of these leads, they appeared to be more difficult to implant, probably due to the larger diameter and silicone body (both factors contributing to friction within the guiding catheter and loss of torque transfer to the lead tip) and shorter helix length. The choice of lead may be impacted by patient characteristics and generator manufacturer (e.g. to maintain magnetic resonance imaging conditionality). We favoured the 3830 lead in young patients (which explains the difference in age between the groups) because of its excellent longevity.12 Finally, apart from the consideration of not being constrained to working with a single manufacturer, it is useful to be able to implant both SDLs and LLLs, as failed implantation with one lead type may prove to be successful in half of the patients using the other lead type.
Despite the greater stiffness of SDLs as well as more support offered by the stiffer delivery catheter, the number of perforations was similar compared with LLL. Perforation is more related to striving to achieve the best possible paced ECG, sometimes resulting in ‘overshooting’ the target. Helix damage was only observed with SDLs and attributed to ‘entanglement’ with the cardiac tissue.3,13 The rate of helix damage with SDLs of 6.1% is much lower than the 25% reported by Tan et al.,7 as we repositioned leads at other sites if excessive torque build-up was felt and did not insist on trying to overcome the endocardial barrier.
Lead macro-dislodgement at follow-up was encountered with both lead types in 4.3% of patients overall and was most often diagnosed within the first post-operative day. It is most probably due to a ‘drill’ effect13 and may more rarely be attributed to perforation. This rate is higher than in our experience with standard right ventricular pacing, and also higher than the 1.5% rate reported in the MELOS study.2 It is unclear why this is so, but a greater proportion of SDLs (which tended to have more dislodgments) and exclusively prospective data in our series may account for some of the differences. Striving to obtain the best possible ECG in our series may also have resulted in a drill effect in some patients. With SDLs, release of the tension of the inner coil may result in backspin of the lead body with subsequent destabilization. This phenomenon was not appreciated during the study, and currently we release the tension on the inner coil before testing the lead and slitting the sheath. Testing of lead stability was performed only during the last months of the study, and the impact of these manoeuvres on avoiding dislodgements needs to be further assessed. Loss of conduction system capture and a rise in capture thresholds may be attributed to micro-dislodgement which occurred in 4.7% of patients overall, and is comparable with the 4.0% loss of terminal R/r in V1 and 0.7% incidence of a rise in threshold reported in MELOS.2 It is also comparable with the 4.6% incidence of loss of capture reported by Ponnusamy et al.,14 although contrary to our findings, they reported late conduction capture (after >6 weeks) in 60% of these cases. Tan et al.15 reported a higher rate of loss of conduction system capture of 13.5%, but which also occurred within 30 days as in our patients. Hopefully, future modifications in lead design will facilitate penetration and anchoring of the lead to avoid dislodgements.
Study limitations
This is a single-centre study, with implantations mainly performed by a single operator who had previous experience with >200 His bundle pacing implantations. The results may, therefore, not be generalized, but nevertheless provide a head-to-head comparison between SDLs and LLLs by the same operator, thereby avoiding confounding factors. Only Selectra 3D delivery catheters were used for SDL implantation, and other catheters available on the market were not evaluated. The number of Ingevity and Vega leads was limited, and results regarding these models are to be interpreted with caution. Long-term follow-up will be necessary to evaluate lead survival, which was not properly assessed by our report.
Conclusions
Left bundle branch area pacing may be safely and effectively performed using either LLLs or SDLs, which provides implanters with alternatives for delivering therapy, and in some cases may provide bailout to improve the success rate. Modifications in lead design (e.g. SDL with a fixed helix) and related accessories are likely to facilitate implantation and address some of the issues that we encountered during the procedure and at follow-up.
Contributor Information
Aarthiga Sritharan, Cardiac Pacing Unit, Department of Cardiology, University Hospital of Geneva, Rue Gabrielle-Perret-Gentil 4, CH-1211 Genève, Switzerland.
Nikola Kozhuharov, Cardiac Pacing Unit, Department of Cardiology, University Hospital of Geneva, Rue Gabrielle-Perret-Gentil 4, CH-1211 Genève, Switzerland.
Nicolas Masson, Cardiac Pacing Unit, Department of Cardiology, University Hospital of Geneva, Rue Gabrielle-Perret-Gentil 4, CH-1211 Genève, Switzerland.
Elise Bakelants, Cardiac Pacing Unit, Department of Cardiology, University Hospital of Geneva, Rue Gabrielle-Perret-Gentil 4, CH-1211 Genève, Switzerland.
Valérian Valiton, Cardiac Pacing Unit, Department of Cardiology, University Hospital of Geneva, Rue Gabrielle-Perret-Gentil 4, CH-1211 Genève, Switzerland.
Haran Burri, Cardiac Pacing Unit, Department of Cardiology, University Hospital of Geneva, Rue Gabrielle-Perret-Gentil 4, CH-1211 Genève, Switzerland.
Funding
N.M. was funded by the institutional research foundation (GeCOR), which also covered open-access fees.
Data availability
The authors will provide data upon reasonable request.
References
- 1. Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IMet al. 2021 ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Europace 2022;24:71–164. [DOI] [PubMed] [Google Scholar]
- 2. Jastrzebski M, Kielbasa G, Cano O, Curila K, Heckman L, De Pooter Jet al. Left bundle branch area pacing outcomes: the multicentre European MELOS study. Eur Heart J 2022;43:4161–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burri H, Jastrzebski M, Cano Ó, Čurila K, de Pooter J, Huang Wet al. EHRA clinical consensus statement on conduction system pacing implantation: endorsed by the Asia Pacific Heart Rhythm Society (APHRS), Canadian Heart Rhythm Society (CHRS), and Latin American Heart Rhythm Society (LAHRS). Europace 2023;25:1208–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kircanski B, Boveda S, Prinzen F, Sorgente A, Anic A, Conte Get al. Conduction system pacing in everyday clinical practice: EHRA physician survey. Europace 2023;25:682–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Keene D, Anselme F, Burri H, Pérez ÓC, Čurila K, Derndorfer Met al. Conduction system pacing, a European survey: insights from clinical practice. Europace 2023;25:euad019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Pooter J, Calle S, Timmermans F, Van Heuverswyn F. Left bundle branch area pacing using stylet-driven pacing leads with a new delivery sheath: a comparison with lumen-less leads. J Cardiovasc Electrophysiol 2021;32:439–48. [DOI] [PubMed] [Google Scholar]
- 7. Tan ESJ, Lee JY, Boey E, Soh R, Sim MG, Yeo WTet al. Use of extendable helix leads for conduction system pacing: differences in lead handling and performance lead design impacts conduction system pacing. J Cardiovasc Electrophysiol 2022;33:1550–7. [DOI] [PubMed] [Google Scholar]
- 8. Braunstein ED, Kagan RD, Olshan DS, Gabriels JK, Thomas G, Ip JEet al. Initial experience with stylet-driven versus lumenless lead delivery systems for left bundle branch area pacing. J Cardiovasc Electrophysiol 2023;34:710–7. [DOI] [PubMed] [Google Scholar]
- 9. Burri H, Jastrzebski M, Cano Ó, Čurila K, de Pooter J, Huang Wet al. EHRA clinical consensus statement on conduction system pacing implantation: executive summary. Endorsed by the Asia-Pacific Heart Rhythm Society (APHRS), Canadian Heart Rhythm Society (CHRS) and Latin-American Heart Rhythm Society (LAHRS). Europace 2023;25:1237–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ponnusamy SS, Basil W, Vijayaraman P. Electrophysiological characteristics of septal perforation during left bundle branch pacing. Heart Rhythm 2022;19:728–34. [DOI] [PubMed] [Google Scholar]
- 11. Jastrzębski M, Burri H, Kiełbasa G, Curila K, Moskal P, Bednarek Aet al. The V6-V1 interpeak interval: a novel criterion for the diagnosis of left bundle branch capture. Europace 2022;24:40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Medtronic . CRHP Product Performance eSource. https://wwwp.medtronic.com/productperformance/model/3830-selectsecure.html (14 May 2023, date last accessed).
- 13. Jastrzebski M, Moskal P, Holda MK, Strona M, Bednarek A, Kielbasa Get al. Deep septal deployment of a thin, lumenless pacing lead: a translational cadaver simulation study. Europace 2020;22:156–61. [DOI] [PubMed] [Google Scholar]
- 14. Ponnusamy SS, Ganesan V, Vijayaraman P. Loss of capture during long term follow-up after left-bundle-branch-pacing. JACC Clin Electrophysiol 2023;9:418–20. [DOI] [PubMed] [Google Scholar]
- 15. Tan ESJ, Soh R, Boey E, Lee J-Y, Leon JD, Chan S-Pet al. Comparison of pacing performance and clinical outcomes between left bundle branch and His bundle pacing. JACC Clin Electrophysiol 2023;9:1393–403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors will provide data upon reasonable request.