Abstract
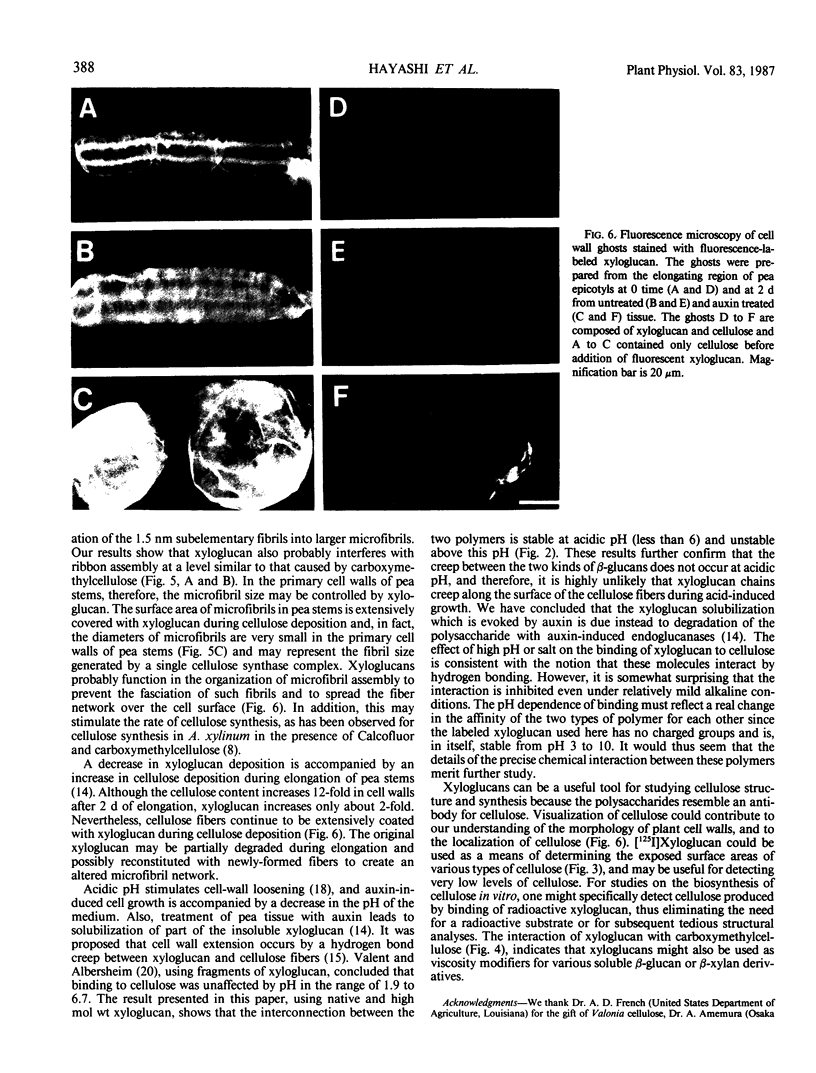
Since xyloglucan is believed to bind to cellulose microfibrils in the primary cell walls of higher plants and, when isolated from the walls, can also bind to cellulose in vitro, the binding mechanism of xyloglucan to cellulose was further investigated using radioiodinated pea xyloglucan. A time course for the binding showed that the radioiodinated xyloglucan continued to be bound for at least 4 hours at 40°C. Binding was inhibited above pH 6. Binding capacity was shown to vary for celluloses of different origin and was directly related to the relative surface area of the microfibrils. The binding of xyloglucan to cellulose was very specific and was not affected by the presence of a 10-fold excess of (1→2)-β-glucan, (1→3)-β-glucan, (1→6)-β-glucan, (1→3, 1→4)-β-glucan, arabinogalactan, or pectin. When xyloglucan (0.1%) was added to a cellulose-forming culture of Acetobacter xylinum, cellulose ribbon structure was partially disrupted indicating an association of xyloglucan with cellulose at the time of synthesis. Such a result suggests that the small size of primary wall microfibrils in higher plants may well be due to the binding of xyloglucan to cellulose during synthesis which prevents fasciation of small fibrils into larger bundles. Fluorescent xyloglucan was used to stain pea cell wall ghosts prepared to contain only the native xyloglucan:cellulose network or only cellulose. Ghosts containing only cellulose showed strong fluorescence when prepared before or after elongation; as predicted, the presence of native xyloglucan in the ghosts repressed binding of added fluorescent xyloglucan. Such ghosts, prepared after elongation when the ratio of native xyloglucan:cellulose is substantially reduced, still showed only faint fluorescence, indicating that microfibrils continue to be coated with xyloglucan throughout the growth period.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer W. D., Talmadge K. W., Keegstra K., Albersheim P. The Structure of Plant Cell Walls: II. The Hemicellulose of the Walls of Suspension-cultured Sycamore Cells. Plant Physiol. 1973 Jan;51(1):174–187. doi: 10.1104/pp.51.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. M., Jr, Montezinos D. Cellulose microfibrils: visualization of biosynthetic and orienting complexes in association with the plasma membrane. Proc Natl Acad Sci U S A. 1976 Jan;73(1):143–147. doi: 10.1073/pnas.73.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. M., Jr, Willison J. H., Richardson C. L. Cellulose biosynthesis in Acetobacter xylinum: visualization of the site of synthesis and direct measurement of the in vivo process. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4565–4569. doi: 10.1073/pnas.73.12.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambat G., Barnoud F., Joseleau J. P. Structure of the Primary Cell Walls of Suspension-Cultured Rosa glauca Cells: I. Polysaccharides Associated with Cellulose. Plant Physiol. 1984 Mar;74(3):687–693. doi: 10.1104/pp.74.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmer D. P. Biosynthesis of cellulose. Adv Carbohydr Chem Biochem. 1983;41:105–153. doi: 10.1016/s0065-2318(08)60057-8. [DOI] [PubMed] [Google Scholar]
- Glabe C. G., Harty P. K., Rosen S. D. Preparation and properties of fluorescent polysaccharides. Anal Biochem. 1983 Apr 15;130(2):287–294. doi: 10.1016/0003-2697(83)90590-0. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Maclachlan G. Pea Xyloglucan and Cellulose : III. Metabolism during Lateral Expansion of Pea Epicotyl Cells. Plant Physiol. 1984 Nov;76(3):739–742. doi: 10.1104/pp.76.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Maclachlan G. Pea xyloglucan and cellulose : I. Macromolecular organization. Plant Physiol. 1984 Jul;75(3):596–604. doi: 10.1104/pp.75.3.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Polonenko D. R., Camirand A., Maclachlan G. Pea Xyloglucan and Cellulose : IV. Assembly of beta-Glucans by Pea Protoplasts. Plant Physiol. 1986 Sep;82(1):301–306. doi: 10.1104/pp.82.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Wong Y. S., Maclachlan G. Pea Xyloglucan and Cellulose : II. Hydrolysis by Pea Endo-1,4-beta-Glucanases. Plant Physiol. 1984 Jul;75(3):605–610. doi: 10.1104/pp.75.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A. A new solid-state reagent to iodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal Biochem. 1982 Sep 15;125(2):427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
- McNeil M., Darvill A. G., Fry S. C., Albersheim P. Structure and function of the primary cell walls of plants. Annu Rev Biochem. 1984;53:625–663. doi: 10.1146/annurev.bi.53.070184.003205. [DOI] [PubMed] [Google Scholar]
- SCHRAMM M., HESTRIN S. Factors affecting production of cellulose at the air/liquid interface of a culture of Acetobacter xylinum. J Gen Microbiol. 1954 Aug;11(1):123–129. doi: 10.1099/00221287-11-1-123. [DOI] [PubMed] [Google Scholar]
- Valent B. S., Albersheim P. The structure of plant cell walls: v. On the binding of xyloglucan to cellulose fibers. Plant Physiol. 1974 Jul;54(1):105–108. doi: 10.1104/pp.54.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valent B. S., Darvill A. G., McNeil M., Robertsen B. K., Albersheim P. A general and sensitive chemical method for sequencing the glycosyl residues of complex carbohydrates. Carbohydr Res. 1980 Mar;79(2):165–192. doi: 10.1016/s0008-6215(00)83830-6. [DOI] [PubMed] [Google Scholar]





