Abstract
Drug resistance to antibacterial and anticancer drugs is one of the most important global problems in the treatment field that is constantly expanding and hinders the recovery and survival of patients. Therefore, it is necessary to identify compounds that have antibacterial and anticancer properties or increase the effectiveness of existing drugs. One of these approaches is using natural compounds that have few side effects and are effective. Gallic acid (GA) has been identified as one of the most important plant polyphenols that health‐promoting effects in various aspects such as bacterial and viral infections, cancer, inflammatory, neuropsychological, gastrointestinal, and metabolic disease. Various studies have shown that GA inhibits bacterial growth by altering membrane structure, and bacterial metabolism, and inhibits biofilm formation. Also, GA inhibits cancer cell growth by targeting different signaling pathways in apoptosis, increasing reactive oxygen species (ROS) production, targeting the cell cycle, and inhibiting oncogenes and matrix metalloproteinases (MMPs) expression. Due to the powerful function of GA against bacteria and cancer cells. In this review, we describe the latest findings in the field of the sources and chemical properties of GA, its pharmacological properties and bioavailability, the antibacterial and anticancer activities of GA, and its derivatives alone, in combination with other drugs and in the form of nanoformulation. This review can be a comprehensive perspective for scientists to use medicinal compounds containing GA in future research and expand its clinical applications.
Keywords: antibacterial, anticancer, gallic acid, health benefits, polyphenol
Gallic acid (GA) has been identified as one of the most important plant polyphenols that have health‐promoting effects in various aspects. Various studies have shown that GA inhibits bacterial growth by altering membrane structure, and bacterial metabolism, and inhibits biofilm formation and growth. GA inhibits cancer cell growth by targeting different signaling pathways in apoptosis, increasing reactive oxygen species (ROS) production, targeting the cell cycle, and inhibiting oncogenes and matrix metalloproteinases (MMPs) expression.
1. INTRODUCTION
Nowadays, the growing resistance to antibiotic and anticancer drugs and treatment failure leads to increased economic and treatment costs besides long‐term hospitalization, which requires finding new approaches. Given the importance of antibiotic resistance, the World Health Organization (WHO) predicts that if this problem is not addressed, deaths from resistant bacterial infections will be the leading cause of death in the world by 2050 (Manso et al., 2021) Increased resistance of pathogenic microorganisms has often been attributed to inappropriate use of antibiotics and transmission of resistance within and between individuals. Given that the production of new antibiotics in the industry has not attracted the attention of investors and is not cost‐effective, new strategies are needed to prevent the emergence and spread of drug resistance, inhibit bacterial growth, and prolong the life of conventional antibiotics (Minarini et al., 2020; Prestinaci et al., 2015).
On the other hand, resistance to anticancer drugs has limited the effectiveness of existing treatments for cancer. Drug resistance can develop as inherent resistance of cancer cells to the drug or as acquired during treatment which the growth of resistant cells leads to treatment failure (Holohan et al., 2013). Therefore, overcoming resistance can increase the efficiency of treatment.
Recently, the use of natural compounds to control and overcome drug resistance in cancer cells as well as against bacteria has been considered by researchers (Álvarez‐Martínez et al., 2020; Keyvani‐Ghamsari et al., 2020). Among the natural compounds, phenolic compounds in plants are considered secondary plant metabolites and have one or more aromatic rings with hydroxyl groups. So far, about 8000 phenolic compounds are known, including tannins, flavonoids, ligands, coumarins, and xanthines. Many of them have anticancer and antibacterial effects (Curti et al., 2017; Khorsandi et al., 2020). Gallic acid (GA) is one of the most important natural polyphenols. Carl Wilhelm Scheele, a Swedish chemist, first isolated GA from plants in 1786, after which studies began on the function of GA and its derivatives (Yang, Zhang, et al., 2020). It is a natural secondary plant metabolite with extensive biological activities such as anti‐inflammatory, antibacterial, antifungal, antiulcerogenic, and anticancer (Fernandes & Salgado, 2016) (Figure 2). In addition, due to its ability to inhibit free radicals, it is a powerful antioxidant used as a preservative in food packaging ingredients, prepared and processed foods, beverages, drugs, and cosmetics to prevent the effects of peroxidation and fat breakdown (Yen et al., 2002).
FIGURE 2.
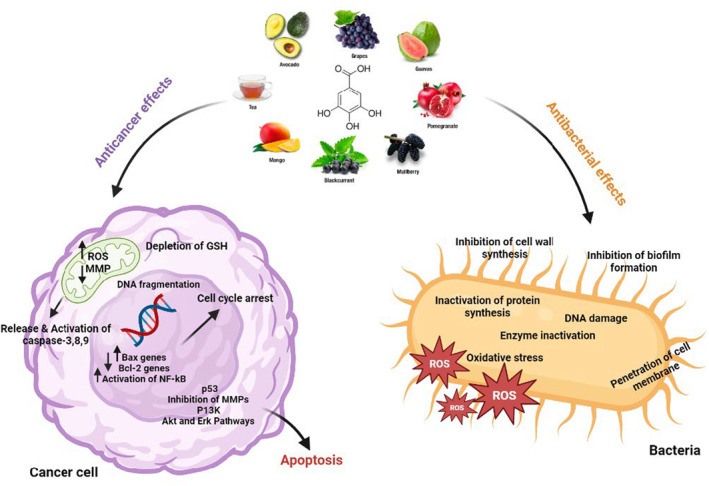
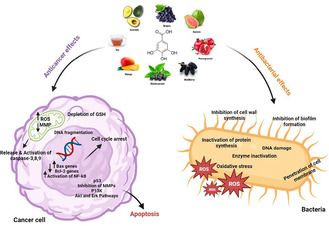
Schematic description of anticancer and antibacterial mechanisms of gallic acid.
GA and its derivatives alone and in combination with other drugs can prevent the growth of planktonic and biofilm of different types of bacteria by various mechanisms (Subramanian et al., 2016). Also, GA in cancer cells can induce apoptosis by affecting the expression of oncogenes, apoptotic proteins, antiapoptosis, matrix metalloproteinases (MMPs), ROS production, and targeting the cell cycle (Moghtaderi et al., 2018; Subramanian et al., 2016; Yang et al., 2022).
In this review, we describe the antibacterial and anticancer function of GA and its derivatives alone, in combination with other drugs, and in the nanoparticle formulation against various cancer cells and bacteria.
2. THE SOURCES AND CHEMICAL PROPERTIES OF GA AND ITS DERIVATIONS
GA (3, 4, 5‐trihydroxybenzoic acid) with the chemical formula of C6H2(OH)3COOH is an organic acid that is known as a powerful antioxidant in plants, vegetables, and fruits. It is one of the most abundant phenolic acids in herbs and foods (Moghtaderi et al., 2018). GA is present in the roots, skin, stems, leaves, flowers, and seeds of plants mainly in the form of ester derivatives combined with sugars, phenols, and polyols (Badhani et al., 2015). It is present in various components of medicinal plants such as Phyllanthus amarus (Euphorbiaceae), Mentha spicata (Lamiaceae), Abutilon pictum (Malvaceae), Achillea millefolium (Asteraceae), and Momordica cabrae (Cucurbitaceae) (Bai et al., 2021). The amount of GA extracted from plants is very different and ranges from 0.001 to 135.08 mg/g. The highest levels of it were found in Phyllanthus A., followed by Momordica C. and Achillea S. (Zhang, Liu, et al., 2020). It is also naturally present in dietary substances such as sundew, blackberry, bearberry, gallnut, vinegar, tea leaves, cloves, sumac, hot chocolate, and beverages such as red wine, and green tea (Subramanian et al., 2015).
It is a yellowish‐white crystalline compound with a molecular mass of 170.12 g/mol, a melting point of 210°C, pKa of 4.40, a density of 1.69 kg/L (20°C), and a solubility of 1.1% in water at 20°C and soluble in alcohol, ether, acetone, and glycerol (Choubey et al., 2015). GA is widely used in pharmacy, cosmetics, the food industry, and medical and chemical research (Brewer, 2011).
GA esters are also present in many plants and are identified as gallates. GA derivatives exist in two forms: ester and catechins (Figure 1). Its ester derivatives are in the form of alkyl esters, such as methyl gallate, propyl gallate, and octyl gallate, and the most important catechin derivatives are epicatechin gallate, epicatechin, and gallocatechin gallate (Badhani et al., 2015; Yang, Zhang, et al., 2020). They have antioxidant capacity against active species of oxygen and nitrogen such as hydrogen peroxide (H2O2), hydroxyl (HO·), peroxyl (ROO·), and azide radicals. These ester derivatives, like propyl gallate, octyl gallate, and lauryl gallate, act as scavengers of ROS and are used in the packaging of food and processed foods to prevent oxidative spoilage (Badhani et al., 2015). In addition, GA esters can increase the bioavailability of drug compounds by acting on cellular transporters such as uridine diphosphate‐glucuronosyl transferase 2B (UGT2B) (Choubey et al., 2015).
FIGURE 1.
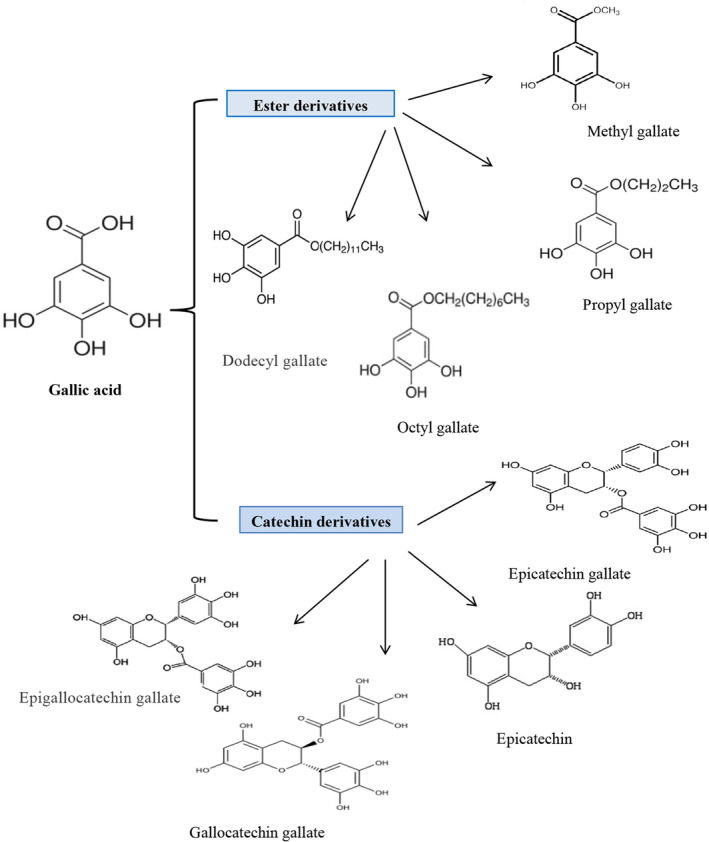
Chemical structure of GA and its derivatives.
Fuiza et al. investigated the effect of GA esters on the human cervical adenocarcinoma cells (HeLa line). They reported the size, substituted groups of hydroxyl rings, the number of carbons in the alkyl chain, and lipophilicity of the structure have a decisive effect on the anticancer activity of the GA. The results showed compounds containing propyl esters have a more inhibitory effect on Hela cells compared to derivatives containing methyl and octyl groups. These results probably depend on the balance between the drug's hydrophobicity and hydrophilicity. Because for a drug to exert its effect in the cell, it must pass through two environments: the membrane (hydrophobic environment) and the cytoplasm (hydrophilic environment). Considering that the degree of hydrophobicity of ester derivatives depends on the number of carbons in the alkyl chain, therefore, compounds with moderate hydrophobicity and hydrophilicity (moderate carbon chain) can exert more toxic effects on the cells (Fiuza et al., 2004). Based on quantitative structure–activity relationship (QSAR) models, Bouarab‐Chibane et al. (2019) also reported the antibacterial activity of polyphenols depends on their lipophilicity, electronic properties, and electrical charge which leads to the interaction of the compound with the bacterial surface. Another group of researchers has shown that most of the time, GA derivatives have more antibacterial activity compared to GA itself. This can be due to the more lipophilicity of derivatives, which increases their ability to pass through the cell membrane (Choińska et al., 2021).
Consumption of GA worldwide is about several thousand tons per year, while its natural production is limited and does not meet this need. At present, GA can be produced industrially by the oxidation of natural gallotanins such as tannic acids (Aguilar‐Zárate et al., 2015). Acids and alkalines can hydrolyze tannic acid to produce GA. One of the common methods of precipitating GA from aqueous solutions is the use of concentrated sulfuric acid. But because of its disadvantages such as high cost, low efficiency of acid hydrolysis, and the production of large toxic effluents, the use of new methods based on biocompatible compounds is essential. Nowadays, microorganisms are considered a suitable factor for the production of GA due to their ability to hydrolyze tannic acid by producing the enzyme tannase that catalyzes the hydrolysis of ester bonds present in gallotannins (Brewer, 2011; Moghtaderi et al., 2018). Fungi such as Fusaria, Aspergilii, Trichoderma, and Penicilii, and bacteria such as Lactobacillus sp., Bacillus, Corynebacterium sp., Serratia sp., Streptococcus, Pseudomonas, Enterococcus, have been reported as tannase producing microorganisms (Aguilar‐Zárate et al., 2015).
3. THE PHARMACOKINETICS AND BIOAVAILABILITY OF GA
A group of researchers has found that GA after entering the body spreads rapidly to all tissues, with the highest distribution being in the kidney, heart, spleen, liver, and lungs. While another group believes that GA is distributed only in the liver and kidneys (Chen et al., 2018). Researchers have shown that GA is extensively metabolized to various compounds after entering the gastrointestinal tract; for example, Barnes et al. examined the uptake and metabolism of mango (as a source of GA) in healthy volunteers for 10 days. They identified seven metabolites of GA in their urine, including pyrogallol‐1‐O‐glucuronide, 4‐OMeGA, 4‐ OMeGA‐3‐O‐sulfate, pyrogallol‐O‐sulfate, deoxy pyrogallol‐Osulfate, and O‐methylpyrogallol‐O‐sulfate (Barnes et al., 2016). In fact, various GA metabolites have been identified in the body, such as ethyl gallate, 3‐dihydrochemical acid, and different type's galloyl‐glucose derivatives. Also, GA can also be converted to syringic acid via the methylation of oxygen (Bai et al., 2021). Methylated, sulfated, and glucoside metabolites are major constituents of GA. Konishi et al. examined the intestinal absorption of GA in rats after oral administration. The rats received a single 100 μmol/L of GA dose. The drug was absorbed at a slow rate and GA and 4‐O‐methyl GA (4OMGA) were observed in the serum (Konishi et al., 2004). 4OMGA was identified as the major metabolite of GA, tannic acid, propyl gallate, and lauryl gallate in the urine of rats and rabbits (Moghtaderi et al., 2018). In humans, 4OMGA is also the first metabolite of GA produced in plasma and urine. Studies in humans have shown that GA is broken down and absorbed in the gastrointestinal tract. It was metabolized in the liver and eventually excreted by the kidneys. After oral administration, about 70% of it was absorbed and then excreted as 4OMGA in the urine. GA has a high absorption compared to hundreds of food polyphenols and as a source of natural product, its health effects have been confirmed in humans (Gulcin et al., 2019; Manach et al., 2005).
Studies on the bioavailability of GA have been performed in animals and humans. It has been reported that rapid metabolism and excretion of GA reduce its effectiveness. Polyphenol compounds are generally metabolized rapidly in the gut, they are rapidly absorbed and excreted in the body, resulting in low bioavailability (Crozier et al., 2009; Laddomada et al., 2015; Zhang, McClements, et al., 2020). To solve this problem, the biopolymer‐based delivery system is designed using proteins, polysaccharides, and phospholipids to increase physicochemical properties, stability, and the absorption of polyphenols through the intestine and reducing their excretion rate, thereby increasing their bioavailability in the body. This method has been able to greatly increase the effectiveness of polyphenols in the food industry and biomedical applications (Bhattacharyya et al., 2013; Zhang, McClements, et al., 2020). Nanotechnology is also an ideal transport system for polyphenolic compounds such as GA. Nanoformulation drug delivery systems such as micelles, emulsions, niosomes, polymers, and metal nanoparticles can overcome the limitations of using polyphenols, protect active substances, and control their release. However, if nanoparticles are used, their safety should be thoroughly evaluated after entering the body (Yang, Dong, et al., 2020).
In vivo and in vitro studies have shown that the toxicity of GA is relatively weak, so it is effective and safe in low concentrations in most cells, while in high concentrations it is slightly toxic (Bai et al., 2021). For example, acute GA toxicity was observed in albino mice when the LD50 of GA was above 2000 mg/kg. Hematological examinations, gross necropsy, and histopathological studies at dose sub‐acute toxicity of GA (≤ 900 mg/kg/day) for 28 days did not cause any significant changes in blood homeostasis, tissue histology, and morphological and behavioral parameters of the male and female albino mice, and confirmed the safety of GA in mice (Variya et al., 2019). Another group of researchers investigated the effect of GA on neuronal cells. They reported that concentrations of 5–50 μM GA did not affect BV‐2 and Nerve‐2A cell survival, while concentrations above 100 μM were toxic to the cells (Kim et al., 2011). Ghafor et al. investigated different concentrations (0–500 μg/mL) of GA‐loaded graphene oxide (GO) (GAGO) on zebrafish embryogenesis for 24‐96 h. The results showed that low concentrations of GAGO (0–150 μg/mL) had no toxic effect on cells at all times and are completely safe but at higher concentrations, mortality significantly increased, hatching rate delayed, and heart rate decreased (Abdul Ghafor, 2020). Other researchers reported the concentration of 75 μM GA caused apoptosis in the A549 cell line via the PI3K/Akt Pathway, while this concentration of GA did not have a destructive effect on the normal lung fibroblast cells (W‐26 cell line) (Ko et al., 2022). Devl et al. compared the concentration of 5–100 μg/mL of GA on HCT150 cells and normal human lymphocyte cells. Their results showed that GA caused severe DNA damage in the colon cancer cells and had potent anticancer effects on these cells, while no toxic effect was observed on lymphocytes (Devi et al., 2015). Therefore, the conducted studies show that GA is a safe agent for normal cells, although its dosage should be considered.
4. APPLICATIONS OF GA AND ITS DERIVATIVES
The therapeutic effects of GA are widespread. It can act as an antibacterial, anticancer, antiviral, antioxidant, antiallergy, and antiinflammatory agent (Figure 2). It is also effective in cardiovascular diseases, and metabolic diseases such as diabetes, obesity, and degenerative diseases (Gao et al., 2019). In the following sections, we describe the latest findings about the antibacterial and anticancer activity of GA and its derivatives.
4.1. Antibacterial activity of GA
GA as an active phenolic compound in plants has a wide range of antimicrobial activities against humans and plant pathogens as well as pathogenic yeasts in humans (Table 1) (Choubey et al., 2018). Researchers have shown that GA not only has antimicrobial effects against various bacteria but also increases the effectiveness of antibacterial compounds such as ciprofloxacin, erythromycin, norfloxacin, oxacillin, ampicillin, gentamicin, and penicillin via synergistic function (Rajamanickam et al., 2019).
TABLE 1.
The antimicrobial activity of GA.
| Compounds | Strains | Functions | References |
|---|---|---|---|
| GA | Escherichia coli | Blocked the catalase activity, inhibition of biofilm formation by the effect on the pgaABCD genes expression | Kang, Li, et al. (2018), Wang et al. (2018) |
| GA | Klebsiella pneumoniae | Disrupted membrane integrity, inhibited the growth and production of capsular polysaccharide | Khorsandi et al. (2021) |
| GA | Staphylococcus aureus | Inhibited the biofilm formation by the effect on the expression of the ica operon | Liu et al. (2017) |
| GA | Shigella flexneri | In the destroyed cell, the bacterial morphology inhibited biofilm formation by the effect on the expression of the mdoH gene and the OpgH protein | Kang, Liu, et al. (2018) |
| Octyl gallate | E. coli and S. aureus | Destroying the cell wall, Penetration the cell, interacting with DNA, damaging the activity of the respiratory chain, increasing the production of ROS | Shi et al. (2021) |
| GA‐grafted‐chitosans + ampicillin penicillin oxacillin | MRSA | Had synergic effects on bacteria, induced loss of membrane integrity, and released intracellular components | Lee et al. (2014) |
| GA+ hydroxytyrosol | E. coli S. pyogenes, K. Pneumoniae, S. aureus | Inhibited the growth of bacteria and had a synergistic effect against all four strains | Tafesh et al. (2011) |
| GA+ ceftiofur | S. Typhimurium | This compound had an additive effect on bacteria, inhibited the growth of plankton and bacterial biofilm, changed the morphology of bacteria, and inhibited the swimming and swarming motilities of bacterial | Hossain, Park, Lee, et al. (2020) |
| GA+ thiamphenicol | E. coli | Altered bacterial morphology, synergistically inhibited the growth of plankton and bacterial biofilms | Hossain, Park, Park, et al. (2020) |
| Au‐NP‐ GA | P. shigelloides S. flexneri | Destruction of membrane surface macromolecules | Daduang et al. (2016) |
| GA‐ loaded – ZnO NPs | MRSA | Had strong antioxidant and antibiotic effects | Lee et al. (2017) |
| GAGO | MRSA | The nanoformulation significantly increased the antibacterial activity of GA | Shamsi et al. (2018) |
| GA‐g‐chitin‐glucan complex | E. coli B. subtilis | Had stronger antibacterial properties compared to the unmodified chitin‐glucan complex | Singh et al. (2019) |
Abbreviations: GA‐g‐chitin‐glucan, GA grafted chitin‐glucan complex; GAGO, GA Loaded Graphene Oxide; P. shigelloides, Plesiomonas shigelloides; S. flexneri, Shigella Flexner.
Borges et al. have shown the antibacterial activity of GA against E. coli, Pseudomonas aeruginosa (P. aeruginosa), S. aureus, and Listeria monocytogenes (L. monocytogenes). GA caused membrane pores in the bacteria and led to irreversible changes in bacteria by altering membrane permeability, hydrophobicity, and physicochemical properties of bacteria (Borges et al., 2013). Rangel et al. have proposed that ester derivatives of GA reduce drug resistance in Saccharomyces cerevisiae (S. cerevisiae) through inhibition of ABC transporter Pdr5p. They proved that ester derivatives with 8–16 carbon in the side chain have a strong inhibitory effect on transporter Pdr5p (Pereira Rangel et al., 2010). GA‐grafted‐Chitosan inhibited the growth of gram‐positive and gram‐negative bacteria by destroying cell membranes and increasing their permeability (Choubey et al., 2018). A comparative study of the antibacterial effects of chlorogenic acid and its metabolites showed that GA has a strong antibacterial activity among other metabolites, especially against Meticillin‐Sensitive S. aureus (MSSA) and Methicillin‐resistant S. aureus (MRSA). GA was found to destroy membrane adhesions and increase membrane permeability, leading to increased bacterial fluid conductivity (Lu et al., 2021). Another study showed GA effectively can inhibit Streptococcus mutans (S. mutans) biofilm formation (de Lima Pimenta et al., 2013). Passos et al. have studied GA and ethyl gallate isolated from the fruit and seeds of Libidibia ferrea against Streptococcus mutans. The results showed the compounds reduced the expression of gtfB, gtfC, and gtfD genes in biofilm. In addition, they inhibited adhesion, and reduced exopolysaccharide, biomass, and bacterial microclines in the biofilms (Passos et al., 2021). Kang et al. have shown that GA inhibits the growth of planktonic S. flexneri by reducing cell viability, and membrane adhesion, and causing morphological changes. It also effectively inhibited the formation of biofilms in bacteria by reducing the expression of the mdoH gene and the OpgH protein that stopped the synthesis of polysaccharides within the biofilm, thus preventing the formation and stability of biofilms (Kang, Liu, et al., 2018) (Pereira Rangel et al., 2010). GA inhibited biofilm growth in E. coli by regulating pgaABCD gene expression and reducing the synthesis of polysaccharides, proteins, and DNA in the biofilm. Thus, GA has been proposed as a new natural and safe approach to control biofilm‐related infections (Kang, Li, et al., 2018). Bisignano et al. have studied the effect of pistachio polyphenols such as GA on 44 S. aureus clinical isolates (9 MRSA) derived from skin infections and surgery. The results showed the significant bactericidal effects of GA on S. aureus (Bisignano et al., 2013). Another group of researchers studied phenolic compounds (caffeic, ellagic, and GA, kaempferol, quercetin, and rutin) derived from plants on skin infections including Staphylococcus epidermidis (S. epidermidis), S. aureus, and K. pneumonia. The results showed that the antibacterial effects of GA against bacteria were greater than other compounds. The minimum bactericidal concentration (MBC) of GA against the investigated bacteria was finally 0.04 mg/mL, at this concentration, the survival of animal fibroblast cells was more than 70%. Therefore, GA can be used as a safe compound against these bacteria in infectious wounds without harming fibroblast cells (Pinho et al., 2014).
Birhanu et al. reported methyl gallate, a GA derivative inhibited invasion, adhesion, and intracellular survival of Salmonella Typhimurium (S. Typhimurium) (Birhanu et al., 2018). Hydroxyl groups in GA derivatives can inactivate bacterial proteins such as enzymes, receptors, ion channels, and carriers by binding with them and forming ionic and protonic bonds (Buchmann et al., 2022; Hossain, Park, Park, et al., 2020). Another group investigated the antibacterial action of alkyl gallates on E. coli and S. aureus. Studies have shown that alkyl chain length plays a major role in the activity of GA derivatives, so octyl gallate showed strong antibacterial activity. Octyl gallate had a bactericidal function in both bacteria by destroying the cell wall, damaging the respiratory chain's activity, and increasing ROS production (Shi et al., 2021).
According to the results obtained from the action of GA on bacteria, it can be used to promote antimicrobial technologies in the field of treatment and the food industry.
4.1.1. Antibacterial activity of GA in combination with other antibacterial agents
Due to the widespread resistance of bacteria to existing antibiotics, combination therapy is one of the most effective ways to increase the effectiveness of existing antibiotics. In addition, combination therapy reduces the spread of drug resistance due to reduced antibiotic doses (Buchmann et al., 2022). Hossain et al. investigated the combined effect of GA and its derivatives with traditional antibiotics on E. coli. The results showed the combination of GA with Thiamphenicol had a synergistic effect on E. coli. GA with cefotaxime, marbofloxacin, and amphetamine had an additive effect. Subsequent analysis showed GA‐ampicillin inhibited the growth and viability of biofilm in E. coli in humans and animals (Hossain, Park, Park, et al., 2020).
Another group of researchers studied the combined effect of GA ethyl ester with conventional antibiotics, such as fusidic acid, minocycline, and rifampicin, against S. aureus strains. The results showed that the combination of GA ethyl ester with all antibiotics had an additive effect against the studied strains (Kyaw et al., 2012). Another study revealed that phenols isolated from the alcoholic extract of Thai mango kernel including GA and methyl gallate had a synergistic effect with penicillin G against MRSA clinical isolates and increased the antibacterial function of this antibiotic in the isolates by bacteriostatic effect (Jiamboonsri et al., 2011). Gutiérrez‐Fernández et al. studied the antibacterial effects of three natural phenols (thymol, carvacrol, and GA), and two synthetic phenols (octyl gallate, and butylated hydroxyanisole) alone and in combination against resistant Enterococcus faecalis (E. faecalis) dairy isolates. The results noted that GA in combination with octyl gallate is effective in controlling bacteria in dairy products in such a way that their combined effect significantly reduced MIC of compounds compared to their use alone (Gutiérrez‐Fernández et al., 2013). Lima et al. demonstrated the synergistic effect of GA with norfloxacin and gentamicin against S. aureus. The MIC of norfloxacin alone against bacteria was 156.3 μg/mL, which was reduced to 49.21 μg/mL in combination therapy. Therefore, the combined treatment led to a reduction of MIC up to 31.48%. The MIC of gentamicin alone also decreased from 49.21 μg/mL to 2.44 μg/mL (4.95% reduction compared to control) (Lima et al., 2016). Wang et al. investigated the effect of GA in the presence of UV‐A light against E. coli. GA alone had a mild antibacterial effect. UV‐A light with a wavelength of 360 nm and average intensity of 3425 μW/cm2 for 30 min enhanced the effectiveness of GA. Initially, UV‐A light increased the uptake of GA into the bacteria. The interaction of GA and UV increased the production of ROS, inhibited the activity of superoxide dismutase, and caused oxidative stress in the bacteria by unbalancing the redox state of the cells. Oxidative stress caused the death of bacteria by affecting macromolecules, metabolism, and bacterial structure (Wang et al., 2017).
4.1.2. Antimicrobial activity of GA in nanoparticle formulation
Nanoparticles are a suitable drug transmitter for the treatment of infections caused by resistant bacteria because the possibility of bacterial resistance to nanoparticles is very low. In addition, they increase the stability and biocompatibility of drug compounds and targeted drug delivery (Mazzotta et al., 2021; Shamsi et al., 2018). Sun et al. designed a nanocomposite containing chitosan‐copper‐GA and then used it as an antibacterial dressing on wounds infected with S. aureus. In addition to the positive effect on the removal of bacteria, the designed composition had no side effects on the normal cells. Its effective application makes its use effective in the field of biomedicine (Sun et al., 2021). Hoyo et al. designed nano‐hybrid‐coated contact lenses containing ZnO NPs‐chitosan‐GA. The synthesized nanocomposite had antioxidant effects and increased the wettability of lenses. It also had a high antibacterial effect against S. aureus, which is directly related to problems with contact lenses such as microbial‐associated keratitis, peripheral ulcer, and acute red eye (Hoyo et al., 2019). Another study showed that the conjugation of GA with Au‐NPs increased the antibacterial effects of GA against foodborne pathogens. FTIR studies have shown that Au‐NP‐GA leads to changes in lipids, nucleic acids, and proteins in bacterial membranes (Daduang et al., 2016). Shamsi et al. evaluated the antibacterial effects of GAGO as a popular graphene‐based compound on MRSA and MSSA. The results showed the preparation of this nanoformulation significantly increased the antibacterial efficacy of GA against MRSA compared to GA alone. It is suggested that this compound can be a suitable antibacterial agent against multi‐drug‐resistant bacteria (Shamsi et al., 2022). Another study showed that the effectiveness of GAGO against MSSA and MRSA was comparable to the first 2 hours of exposure of bacteria to GA and GO alone. They reported that its efficacy on bacteria at concentrations of ≥150 μg/mL is comparable to cefoxitin. Moreover, this nanoformulation increased the biocompatibility of GA in physiological environments and GAGO had little toxicity in 3T3 murine fibroblast cells and zebrafish embryos cells at all investigated times (24, 48, 72 h) compared to GA alone (Shamsi et al., 2022). Another group of researchers designed liposomes loaded with GA (GA‐LIP) and GA‐LIP decorated with Lactoferrin (LF‐GA‐LIP). LF‐ GA‐ LIP showed stronger antibacterial activity than GA‐LIP against E. coli and S. aureus and it had a slower release rate in a simulated digestive system. Therefore LF‐GA‐LIP was introduced as a suitable transport system in the food industry (Zhang, Pu, et al., 2019). Li et al. synthesized Ag‐NPs coated with GA and studied their antibacterial effects on E. coli, and S. aureus. The results showed high activity of GA‐AgNPs which had very little toxicity on normal cells. It was suggested that the GA‐AgNPs could be used in medical devices, pharmaceutical applications, and silver‐based wound care products to eliminate bacterial infections, although their safety in animals should be examined more closely (Li et al., 2015). The other group synthesized modified Ag‐NPs using GA and chitosan (GC‐AgNps) by ultrasonication and investigated their antibacterial effects on E. coli. In addition to being a quick, easy, and cost‐effective method, the synthesized combination showed strong antibacterial results. GC‐AgNPs bind to the peptidoglycan membrane destroyed the lipopolysaccharide barrier, and penetrated the membrane protoplasm, also increasing ROS production due to contact with intracellular components and scavengers' depletion (Guzmán et al., 2019).
Overall, the results show that the use of GA as nanoparticles increases its efficacy, although the performance and safety of GA‐carrying nanoparticles in vitro and in vivo need to be evaluated. Because polyphenols such as GA as a natural substance should be consumed for a long time and considering that different types of nanoparticles can have a negative effect on the lungs, liver, kidneys, brain, and sexual organs, their use as GA carriers should be considered and its side effects should be minimized (Carvalho et al., 2018; Lin et al., 2022).
The antibacterial effects of GA alone, in combination with other drugs, and as a nanoparticle formulation against different types of bacteria are summarized in Table 1.
4.1.3. Anticancer activity of gallic acid
GA and its derivatives can be used as anticancer agents. Several studies have demonstrated that GA and its derivatives have an anticancer effect in cancers such as prostate cancer, melanoma, leukemia, oral cancer, colon cancer, lymphoma, and breast cancer cells. The following sections described the molecular actions initiated by GA and its derivatives on various cancer cells (Subramanian et al., 2015).
Anticancer activity on prostate cancer cells
In DU145 prostate cancer cells, GA was the primary anticancer compound that suppressed the cells' growth. GA reduces the cell survival of DU145 cells by generating ROS and mitochondria‐mediated apoptosis. Also, GA leads to cell cycle arrest at the G2/M phases by activating Chk1 and Chk2 and inactivating Cdc25C and Cdc2 (Chen et al., 2009). The autoxidation of GA is effective on malignant prostate cells and increases ROS levels. Loss of mitochondrial potential and release of cytochrome c from the mitochondria to the cytosol led to the activation of caspases 3, 8, and 9. In prostate cancer cells, GA leads to dose‐dependent apoptosis (Russell Jr et al., 2012).
A study investigated the antitumor effect of GA on PC3 prostate cancer cells. GA can induce DNA damage and recruits several DNA repair genes (Liu et al., 2015). Also, it was investigated that GA can eliminate the migration and invasion of PC3 human prostate cancer cells (Meng, 2011). There was inhibition of JNK, PKC, p38, and P13K/AKT signaling pathways in PC3 cells treated with GA and it finally blocked MMP‐2 and ‐9 in these cells (Subramanian et al., 2015).
Anticancer activity on melanoma cells
The GA showed significant cell proliferation inhibition and apoptosis induction on A375S2 human melanoma cells (Kondo et al., 2009). After GA treatment, the proliferation of cells decreased in a dose and timely manner. The apoptosis molecular mechanism is done by the downregulation of antiapoptotic Bcl‐2 and upregulation of the proapoptotic B‐cell lymphoma 2 (Bcl‐Fp132) associated X protein (Bax). By decreasing the mitochondrial membrane potential in a time‐dependent manner, GA induced the release of cytochrome c, promoting the activation of caspase 9 and 3, and finally apoptosis. Also, GA induced the expression of endonuclease G (Endo G) and apoptosis‐inducing factors (AIF) (Lo et al., 2011). A study showed the influence of GA on the gene expression and protein levels of MMPs and in vitro migration of melanoma cells; as a result, the treatment of the GA leads to a reduction in the MMPs signal pathway and mRNA levels in A375S2 cells. Thus, GA acted as an antimetastatic agent. In addition, this was included in the Ras, and p‐ERK signaling pathways, causing the suppression of MMP‐2 in A375S2 melanoma cells (Subramanian et al., 2015).
Anticancer activity on colon cancer cells
A study investigated the anticancer property of GA against COLO 205 cells of colon cancer. GA treatment leads to the fragmentation of DNA. Morphological changes, especially observing apoptotic bodies, indicated that GA caused apoptosis (Yoshioka et al., 2000). Another study mentioned an antitumor effect of GA on HCT‐15 colon cancer cells. GA decreased the survival of colon cancer cells in a dose‐dependent manner. Cell contraction, cell rounding, and separation from the substrate were notable changes in GA‐treated cells compared to the control (Devi et al., 2014). Generally, GA derivatives treatment reduces cell viability; also, the antioxidant influence and structure of GA derivates showed a similar manner (Khaledi et al., 2011).
Anticancer activity on leukemia
Several studies showed the anticancer property of GA against HL 60 and HL‐60RG promyelocytic leukemia and also K562 human leukemia cells through cell cycle arrest at the G0/G1 phase. Inhibition of ribonucleotide reductase, DNA damage, and fragmentation, the release of cytochrome c, upregulation Bcl‐2 protein, AIF & Endo G, activation of caspase 4, 9, and 3, inhibition of BCR/ABL tyrosine kinase, activation of NF‐κB, increasing levels of Bax & Fas ligand and p53 have been reported as another anticancer effect of GA. (Chandramohan Reddy et al., 2012; Htay et al., 2002; Locatelli et al., 2008; Madlener et al., 2007; Moriwaki et al., 1991; Yeh et al., 2011) (Table 2).
TABLE 2.
The anticancer activity of GA.
| Compounds | Cancer | Function | References |
|---|---|---|---|
| GA | Colon cancer (Male albino Wistar rats) | Increased superoxide dismutase, catalase, glutathione reductase, and glutathione peroxidase activity | Giftson et al. (2010) |
| GA | Nonsmall‐cell lung cancer (A549 and NCI‐H1299 cell lines) | Inhibited proliferation and elevated apoptosis in cells by suppression of EGFR and reduced CARM1‐PELP1 complex formation | Wang and Bao (2020) |
| GA | Gastric adenocarcinoma cells | Induced apoptosis by up‐regulation of Fas, FasL, and DR5 expression | Tsai (2018) |
| AGS cells (ATCC CRL 1739). | |||
| GA | Bladder cancer (T24 cell line) | Inhibited the cell proliferation by disrupting PI3K/Akt/NF‐kB signaling pathways | Zeng et al. (2020) |
| GA |
Leukemia sensitive and its resistant sublines (HL60 cell HL60/VINC HL60/MX2) |
Altered cell cycle distribution and increased cell population in sub‐G1 | Maruszewska and Tarasiuk (2019) |
| Modulated ROS production in cells in a time‐ and dose‐dependent manner | |||
| GA + paclitaxel carboplatin | Breast cancer (MCF‐7 cell line) | Cell cycle arrest at the G2/M phase, the minimum concentration of GA increased the induction of apoptosis by drugs, and triplet combination upregulated the expression of P53, Bax, and CASP‐3. | Aborehab et al. (2021) |
| GA+ Paclitaxel | Cervical (HeLa cells) | Increased apoptosis by upregulation of p53 and caspase 3 | Aborehab and Osama (2019) |
| GA+ Caffeic acid | Breast cancer (MCF‐7 cells) | Activation of apoptosis signaling pathways by regulating the expression of P53, Mcl‐1, and P21 | Rezaei‐Seresht et al. (2019) |
| GA+ low‐level laser | Melanoma and breast cancer (A375, and MDA‐MB‐231 cells) | Generated ROS and inducted apoptosis and ferroptosis | Khorsandi et al. (2020) |
| GA+ Cisplatin | Small cell lung cancer (H446 cells) | Increased cisplatin effect by producing ROS, increased Bax, Apaf‐1, DIABLO, and p53 expression, decreased XIAP expression, and MMP degradation | Wang et al. (2016) |
| GA+ Temozolomide | Human glioma cell (U87MG cells) | Increased the performance of the drug by reducing of Bcl‐2 expression and Akt activation, and activation of the p38‐MAPK pathway | Yang et al. (2022) |
| GA‐Gold NPs + radiotherapy | Human glioma cells (U251 cells) | Inhibited cell viability, increased radiotherapy function, induced apoptosis by increasing BAX expression, downregulated Bcl‐2 expression, and stopped cell cycle in S and G2/M phases. | Jing et al. (2021) |
| Graphene Oxide‐GA | Liver cancer cells (HepG2 cells) | Inhibited the growth of cancer cells without damaging normal cells | Dorniani et al. (2016) |
| Conjugated of the GA with PAMAM dendrimers | Human colorectal carcinoma (HCT 116 cells) | Synthesized particles increased GA uptake, inhibited cell proliferation and their colonogenic ability, decreased cancer cell migration by reducing MMP expression | Priyadarshi et al. (2021) |
| Promoted apoptosis in cancer cells by inhibiting NF‐κB activation | |||
| GA nanoparticles coated with alginate‐chitosan | Breast cancer cells (T47D cells) | Had strong cytotoxicity in cancer cells | Arsianti et al. (2020) |
Anticancer activity on cervical cancer cells
HeLa cervical cancer cells treated by GA showed depletion in GSH, decrease in mitochondrial membrane potential, downregulation of the EGFR, Erk/p‐Erk, and Akt/p‐Akt signaling pathways, activation of caspases and poly (ADP) ribose polymerase cleavage and cell cycle arrest in G1 phase (You et al., 2010; You & Park, 2011; Zhao & Hu, 2013).
Another group of scientists synthesized nine chimeric gallate‐cinnamate derivatives using QSAR (Figure 3). Then their cytotoxicity activity was investigated on HeLa cells and nontumorigenic HaCaT cells. QSAR model showed the importance of structure, attachment of specific groups to phenolic rings, lipophilicity, size, and electronic charge of gallate‐cinnamate derivatives to inhibit the growth of HeLa cells. Among the designed models, 3,4,5‐trimethoxybenzyl 3,4‐dimethoxy benzoate (N6), 3,4,5‐trimethoxybenzyl 3,4,5‐trimethoxybenzoate (N5), cinnamyl 3,4,5‐trimethoxybenzoate (N1), 3,4,5‐trimethoxybenzyl 3,4,5‐trimethoxycinnamate(N9) had the more effect on inhibiting Hela cells (with IC50ExpHeLa 7.26–11.95 μM), while they had no toxic effect on HaCaT cells. Finally, molecular docking studies showed that the binding of compounds to tubulins inhibits the growth of cancer cells (Nolasco‐Quintana et al., 2023).
FIGURE 3.
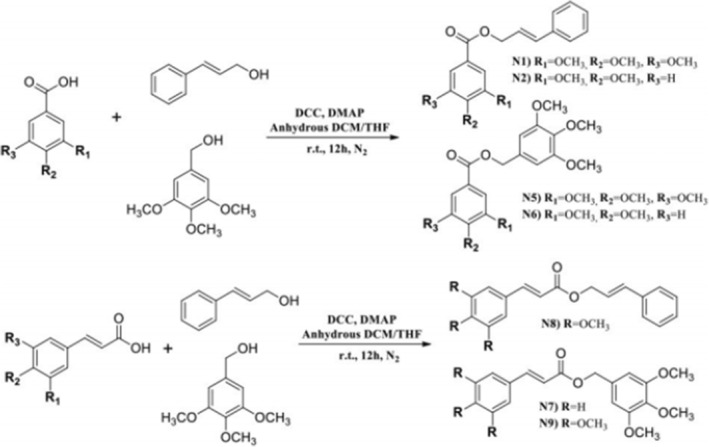
Gallate analogs.
4.1.4. Anticancer activity of GA in combination with anticancer agents
The anticancer mechanism of GA alone or in combination with cisplatin was found in nonsmall cell‐lung cancer (NSCLC). The studies show that GA prompted the apoptosis of NSCLC A549 cells and restrained the proliferation in the dose‐ and time‐dependent manners with downregulating Bcl 2 and upregulating B‐cell lymphoma 2 (Bcl‐Fp132)‐associated X protein (Bax). Also, the findings showed that GA increases Bax expression and inhibits Bcl 2 expression which finally increases the anticancer property of cisplatin and induces cell apoptosis. Furthermore, these studies indicated that GA alone has anticancer effects on NSCLC A549 cells and increases cisplatin's anticancer effects by inducing the JAK/STAT3 signaling pathway and downstream apoptotic molecules (Zhang, Ma, et al., 2019).
Khorsandi et al. found that pretreatment with low‐level lasers and then the GA treatment on breast cancer and melanoma cells induce more cell death in comparison to GA alone. They indicated that ROS production in cells treated with low‐level lasers and then GA was more than in cells treated only with GA. It is noted that the combination of low‐level laser irradiation and then GA may increase cell death through cell death apoptosis and ferroptosis mechanisms compared to GA alone (Khorsandi et al., 2020).
Paclitaxel/carboplatin is one of the most common drugs in treating cervical cancer. Furthermore, chemotherapeutic drugs are very beneficial, but intensive side effects and the development of drug resistance limit these drugs' use. Using natural products with anticancer activity may help to overcome these problems. These findings indicated that GA increases the paclitaxel property. A combination of paclitaxel and GA could display a promising replacement with lower side effects for paclitaxel/carboplatin combination in cervical cancer treatment (Aborehab & Osama, 2019).
GA and curcumin (Cur) are two natural phenolic compounds and demonstrated their anticancer properties on several types of cancer. A study investigated combining these factors on MDA‐MB‐231 breast cancer cells. The results showed that the combination of GA and Cur significantly reduced MDA‐MB‐231 cell growth. Furthermore, in MDA‐MB‐231 cells, this compound elevated cytotoxic activity, ROS level, and glutathione depletion. Flow cytometry analysis indicated that the compound of GA and Cur elevated the sub‐G1 cell population. Moreover, fluorescent staining and Annexin V/PI assay showed that apoptotic cells increased notably in the existence of GA and Cur. Finally, in MDA‐MB‐231 cells, protein expression found that the combination of GA and Cur notably decreased Bcl‐2 levels but increased PARP, Bax, and cleaved‐caspase 3 levels (Moghtaderi et al., 2018).
Pirarubicin (Pira) is an antitumor drug used to treat leukemia. The studies showed that GA/Pira compounds in K562 and K562/Dox cancer cells decreased cell survival, ATP levels, and mitochondrial activity in a GA concentration‐dependent manner. In GA‐treated K562/Dox cancer cells, GA suppressed P‐glycoprotein‐mediated efflux of Pira. Also, GA increased the antitumor effect of Pira on K562 and K562/Dox cancer cells by disrupting cellular energy status and reversing drug resistance in live K562/Dox cancer cells by blocking P‐glycoprotein function (Aye et al., 2021).
4.1.5. Anticancer activity of GA in nanoparticle formulations
The bioactive copper‐GA nanoscale metal‐organic framework was synthesized and studied for the codelivery of GA as an anticancer factor and methylene blue as a photosensitizer for cancer cells. Synth copper‐bioactive frameworks (bio‐MOFs) were employed as carriers of two antitumor factors. GA was considered part of the framework structure (building block). Methylene blue (MB) is loaded as a guest molecule in the context of the amphiphilic pores. In vitro and in vivo assays have shown increased cytotoxicity of two nano pharmaceutical frameworks compared to equivalent doses of free drugs in the presence of light (Sharma et al., 2019).
One study redesigned the antitumor nanocomposite formulation using polyethylene glycol‐coated iron oxide nanoparticles and GA as the anticancer drug (Fe3O4‐PEG‐GA). In vitro, research indicated that characteristics and drug loading percentage were better than the previously reported formulation. The anticancer effect of GA without a nanocarrier (Fe3O4‐PEG) and compare with Fe3O4‐PEG‐GA in human breast cancer cells (MCF‐7), human lung cancer cells (A549), and human colon cancer cells (HT‐29) after incubation for 24, 48, and 72 h using MTT assay. The formulated (Fe3O4‐PEG‐GA) showed an improved anticancer effect compared to GA alone (Rosman et al., 2018).
The antitumor effect of encapsulated polyherbal nanoparticles (GA and quercetin nanocomposite) and polyherbal extract (amla and pomegranate fruit peels) were investigated in DMH‐induced colorectal cancer in rats. In normal and DMH‐induced rats, a pharmacokinetic study showed that polyunsaturated nanoparticles had a typical sustained release profile (Sustained release profile form is defined as well characterized and reproducible profile form, which is designed to control drug release profile at a specified rate to achieve desired drug concentration either in blood plasma or at the target site) with a fourfold higher bioavailability than polyunsaturated extracts. After oral application, pharmacokinetic parameters for multi‐plant nanoparticles and multi‐plant extracts were determined using a single‐compartment approach based on the serum concentrations profile of multi‐plant nanoparticles and multi‐plant extracts. This study showed that encapsulation of GA and quercetin in polymer nanoparticles enhance oral bioavailability and anticolon cancer activity (Patil & Killedar, 2021a).
One study synthesized environmentally, and low‐cost silver nanoparticles (AgNPs) using GA in bentonite/starch biocomposites (BNCs) for oral use and evaluated its antibacterial and anticancer effects. The composition of AgNPs was confirmed by the UV‐vis absorption peak at 412 nm. The results showed that AgNPs‐GA synthesized in BNCs could be a potential candidate for suppressing the growth of bacteria, and they have shown significant cytotoxicity against MCF‐7 cancer cells, too (Thapliyal & Chandra, 2018). Another study used single‐dispersed high‐performance (Ag‐Se) single‐particle nanoparticles by quercetin and GA. Bimetallic nanoparticles were synthesized at room temperature. Various reaction factors such as quercetin GA and Ag/Se salt concentration, temperature, pH, and reaction time are optimized for controlling nanoparticle properties. Different analytical techniques characterized the nanoparticles, and their size was 30–35 nm. Results suggest that flavonoids and phenolics caused nanoparticle reduction and stabilization. This research explained the efficacy of quercetin and GA‐mediated synthesis of bimetallic (Ag–Se) nanoparticles, which are antioxidant, anticancer, and antimicrobial in vitro. Synthesized Ag‐Se nanoparticles were used as antitumor factors for Dalton lymphoma cells, and results show that 80% of their viability was decreased (Mittal et al., 2014).
A study on the synergistic impact of GA from amla fruit and quercetin from pomegranate peel in chitosan for targeted delivery to colorectal cancer showed significant changes in crypt misplaced foci in CS nanoparticles compared to polyunsaturated extracts, with a significant reduction in levels of catalase, glutathione, and colonic superoxide dismutase (Patil & Killedar, 2021b).
The anticancer effects of GA alone, in combination with other drugs, and in the form of nanoformulations are summarized in Table 2.
5. CONCLUSION AND PERSPECTIVE
Given that in recent years, anticancer and antibacterial therapies have faced widespread drug resistance that has led to treatment failure, the use of new drugs and their combinations with low toxicity and side effects is essential (Patel et al., 2021). Currently, the use of plant compounds as antibacterial and anticancer compounds has been popular (Bhandari et al., 2017; Khameneh et al., 2019; Shrihastini et al., 2021). In this regard, phenolic compounds such as GA have shown favorable results. Hence, in this study, we described the chemical and biological properties of GA and its derivatives, their pharmacokinetics and bioavailability, and the importance of using GA and its mechanism as an antibacterial and anticancer agent. As mentioned earlier, GA is one of the most important natural phenolic compounds found in many dietary substances including various plants, fruits, and vegetables. It has antioxidant and antiinflammatory properties and has pharmacological effects on various diseases. After administration, it is absorbed by the gastrointestinal tract and after being metabolized in the liver to various compounds, is excreted by the kidneys. The toxic effects of GA have rarely been seen in animal studies and clinical trials and its safety has been confirmed, but due to the rapid absorption and metabolism of GA, its bioavailability is low which can limit its therapeutic applications. To overcome this problem, the use of biopolymers or nanoformulations to increase their stability and absorption has been proposed as a suitable method. In addition, the combination of GA with many antibacterial or anticancer drugs showed synergistic effects against multidrug‐resistant pathogens or cancer cells and improved the efficacies of both antibacterial and anticancer drugs, which can be an effective way to reduce and prevent drug resistance. Because the combination of common antibacterial and anticancer drugs with GA can reduce their effective dosage and increase the sensitivity of cells to the existing drugs (Buchmann et al., 2022). In addition, GA and its derivation alone or in combination with other drugs can reverse drug resistance in bacteria and cancer cells by inhibiting efflux pumps such as ABC transporters, MrsA, NorA, TetK pumps, and P‐glycoproteins (Dashtbani‐Roozbehani & Brown, 2021; Pereira Rangel et al., 2010). Inhibition of pumps leads to an increase in the accumulation of drugs in the resistant cells and enhances their cytotoxicity. According to what was mentioned; GA and its derivatives have potent antibacterial and anticancer action. They inhibit the growth of plankton in gram‐positive and gram‐negative bacteria by degradation of the cell morphology, increasing membrane permeability, the release of intracellular components, and enhancing the production of ROS. Moreover, they inhibit biofilm formation by inhibiting the synthesis of proteins and polysaccharides in biofilm structure.
In different cancer cells, GA induces cell death and inhibits cell growth and migration by different mechanisms, including targeting intrinsic and extrinsic signaling pathways related to apoptosis, DNA damage through DNA fragmentation, and recruit's DNA repair genes, effect on the expression of apoptotic and nonapoptotic proteins, ROS production, activation of NF‐κB, depletion of GSH, down‐regulation of MMP expression, and cell cycle targeting. Therefore, according to studies, GA as a plant phenolic compound has the potential to treat and/ or manage bacterial infections and various cancers. Hence, GA and its derivatives have been considered by researchers in various fields of drug development worldwide. Further research in this area may lead to more insights, and identification of newer functions and effects of these compounds. Given that extensive clinical studies have not been performed so far and the exact mechanism of action is still unknown, further studies in humans are necessary to confirm the results obtained at the level of in vitro and place GA as a potential drug in the commercial market.
AUTHOR CONTRIBUTIONS
Saeedeh Keyvani‐Ghamsari: Conceptualization (equal); investigation (equal); methodology (equal); writing – original draft (equal); writing – review and editing (equal). Maryam Rahimi: Investigation (equal); methodology (equal); writing – original draft (equal). Khatereh Khorsandi: Conceptualization (equal); data curation (equal); methodology (equal); resources (equal); software (equal); supervision (equal); visualization (equal); writing – review and editing (equal).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
All figures were created in BioRender.com.
Keyvani‐Ghamsari, S. , Rahimi, M. , & Khorsandi, K. (2023). An update on the potential mechanism of gallic acid as an antibacterial and anticancer agent. Food Science & Nutrition, 11, 5856–5872. 10.1002/fsn3.3615
Contributor Information
Saeedeh Keyvani‐Ghamsari, Email: khorsandi.kh@ut.ac.ir.
Khatereh Khorsandi, Email: keyvani@ut.ac.ir.
DATA AVAILABILITY STATEMENT
The data presented in this study are available in this manuscript.
REFERENCES
- Abdul Ghafor, A. A. H. (2020). Toxicity assessment of gallic acid loaded graphene oxide (GAGO) nano‐formulation in zebrafish (Danio rerio) embryos. Pertanika Journal of Science & Technology, 28, 311–326. [Google Scholar]
- Aborehab, N. M. , Elnagar, M. R. , & Waly, N. E. (2021). Gallic acid potentiates the apoptotic effect of paclitaxel and carboplatin via overexpression of Bax and P53 on the MCF‐7 human breast cancer cell line. Journal of Biochemical and Molecular Toxicology, 35, e22638. 10.1002/jbt.22638 [DOI] [PubMed] [Google Scholar]
- Aborehab, N. M. , & Osama, N. (2019). Effect of gallic acid in potentiating chemotherapeutic effect of paclitaxel in HeLa cervical cancer cells. Cancer Cell International, 19, 154. 10.1186/s12935-019-0868-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar‐Zárate, P. , Cruz, M. A. , Montañez, J. , Rodríguez‐Herrera, R. , Wong‐Paz, J. E. , Belmares, R. E. , & Aguilar, C. N. (2015). Gallic acid production under anaerobic submerged fermentation by two bacilli strains. Microbial Cell Factories, 14, 209. 10.1186/s12934-015-0386-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Álvarez‐Martínez, F. J. , Barrajón‐Catalán, E. , & Micol, V. (2020). Tackling antibiotic resistance with compounds of natural origin: A comprehensive review. Biomedicine, 8, 405. 10.3390/biomedicines8100405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsianti, A. , Bahtiar, A. , Fadilah, F. , Wangsaputra, V. K. , Paramita, R. I. , Azizah, N. N. , Nadapdap, L. D. , Fajrin, A. M. , Tanimoto, H. , & Kakiuchi, K. (2020). Synthesis, characterization, and cytotoxicity evaluation of gallic acid nanoparticles towards breast T47D cancer cells. Pharmacognosy Journal, 12, 321–327. 10.5530/pj.2020.12.51 [DOI] [Google Scholar]
- Aye, K. , Wattanapongpitak, S. , Supawat, B. , Kothan, S. , Udomtanakunchai, C. , Tima, S. , Pan, J. , & Tungjai, M. (2021). Gallic acid enhances pirarubicin‐induced anticancer in living K562 and K562/Dox leukemia cancer cells through cellular energetic state impairment and P‐glycoprotein inhibition. Oncology Reports, 46, 227. 10.3892/or.2021.8178 [DOI] [PubMed] [Google Scholar]
- Badhani, B. , Sharma, N. , & Kakkar, R. (2015). Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Advances, 5, 27540–27557. 10.1039/C5RA01911G [DOI] [Google Scholar]
- Bai, J. , Zhang, Y. , Tang, C. , Hou, Y. , Ai, X. , Chen, X. , Zhang, Y. , Wang, X. , & Meng, X. (2021). Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation‐related diseases. Biomedicine & Pharmacotherapy, 133, 110985. 10.1016/j.biopha.2020.110985 [DOI] [PubMed] [Google Scholar]
- Barnes, R. C. , Krenek, K. A. , Meibohm, B. , Mertens‐Talcott, S. U. , & Talcott, S. T. (2016). Urinary metabolites from mango (Mangifera indica L. cv. Keitt) galloyl derivatives and in vitro hydrolysis of gallotannins in physiological conditions. Molecular Nutrition & Food Research, 60, 542–550. 10.1002/mnfr.201500706 [DOI] [PubMed] [Google Scholar]
- Bhandari, J. , Muhammad, B. , Thapa, P. , & Shrestha, B. G. (2017). Study of phytochemical, anti‐microbial, anti‐oxidant, and anti‐cancer properties of Allium wallichii. BMC Complementary and Alternative Medicine, 17, 102. 10.1186/s12906-017-1622-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya, S. , Ahammed, S. M. , Saha, B. P. , & Mukherjee, P. K. (2013). The gallic acid–phospholipid complex improved the antioxidant potential of gallic acid by enhancing its bioavailability. AAPS PharmSciTech, 14, 1025–1033. 10.1208/s12249-013-9991-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birhanu, B. T. , Park, N.‐H. , Lee, S.‐J. , Hossain, M. A. , & Park, S.‐C. (2018). Inhibition of Salmonella Typhimurium adhesion, invasion, and intracellular survival via treatment with methyl gallate alone and in combination with marbofloxacin. Veterinary Research, 49, 101. 10.1186/s13567-018-0597-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisignano, C. , Filocamo, A. , Faulks, R. M. , & Mandalari, G. (2013). In vitro antimicrobial activity of pistachio (Pistacia vera L.) polyphenols. FEMS Microbiology Letters, 341, 62–67. 10.1111/1574-6968.12091 [DOI] [PubMed] [Google Scholar]
- Borges, A. , Ferreira, C. , Saavedra, M. J. , & Simões, M. (2013). Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microbial Drug Resistance, 19, 256–265. 10.1089/mdr.2012.0244 [DOI] [PubMed] [Google Scholar]
- Bouarab‐Chibane, L. , Forquet, V. , Lantéri, P. , Clément, Y. , Léonard‐Akkari, L. , Oulahal, N. , Degraeve, P. , & Bordes, C. (2019). Antibacterial properties of polyphenols: Characterization and QSAR (quantitative structure–activity relationship) models. Frontiers in Microbiology, 10, 829. 10.3389/fmicb.2019.00829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer, M. S. (2011). Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Comprehensive Reviews in Food Science and Food Safety, 10, 221–247. 10.1111/j.1541-4337.2011.00156.x [DOI] [Google Scholar]
- Buchmann, D. , Schultze, N. , Borchardt, J. , Böttcher, I. , Schaufler, K. , & Guenther, S. (2022). Synergistic antimicrobial activities of epigallocatechin gallate, myricetin, daidzein, gallic acid, epicatechin, 3‐hydroxy‐6‐methoxyflavone and genistein combined with antibiotics against ESKAPE pathogens. Journal of Applied Microbiology, 132, 949–963. 10.1111/jam.15253 [DOI] [PubMed] [Google Scholar]
- Carvalho, R. S. , Carollo, C. A. , de Magalhães, J. C. , Palumbo, J. M. C. , Boaretto, A. G. , Nunes e Sá, I. C. , Ferraz, A. C. , Lima, W. G. , de Siqueira, J. M. , & Ferreira, J. M. S. (2018). Antibacterial and antifungal activities of phenolic compound‐enriched ethyl acetate fraction from Cochlospermum regium (mart. Et. Schr.) Pilger roots: Mechanisms of action and synergism with tannin and gallic acid. South African Journal of Botany, 114, 181–187. 10.1016/j.sajb.2017.11.010 [DOI] [Google Scholar]
- Chandramohan Reddy, T. , Bharat Reddy, D. , Aparna, A. , Arunasree, K. M. , Gupta, G. , Achari, C. , Reddy, G. V. , Lakshmipathi, V. , Subramanyam, A. , & Reddanna, P. (2012). Anti‐leukemic effects of gallic acid on human leukemia K562 cells: Downregulation of COX‐2, inhibition of BCR/ABL kinase and NF‐κB inactivation. Toxicology in Vitro, 26, 396–405. 10.1016/j.tiv.2011.12.018 [DOI] [PubMed] [Google Scholar]
- Chen, H.‐M. , Wu, Y.‐C. , Chia, Y.‐C. , Chang, F.‐R. , Hsu, H.‐K. , Hsieh, Y.‐C. , Chen, C.‐C. , & Yuan, S.‐S. (2009). Gallic acid, a major component of Toona sinensis leaf extracts, contains a ROS‐mediated anti‐cancer activity in human prostate cancer cells. Cancer Letters, 286, 161–171. 10.1016/j.canlet.2009.05.040 [DOI] [PubMed] [Google Scholar]
- Chen, X. , Zhu, P. , Liu, B. , Wei, L. , & Xu, Y. (2018). Simultaneous determination of fourteen compounds of Hedyotis diffusa Willd extract in rats by UHPLC–MS/MS method: Application to pharmacokinetics and tissue distribution study. Journal of Pharmaceutical and Biomedical Analysis, 159, 490–512. 10.1016/j.jpba.2018.07.023 [DOI] [PubMed] [Google Scholar]
- Choińska, R. , Dąbrowska, K. , Świsłocka, R. , Lewandowski, W. , & Świergiel, A. H. (2021). Antimicrobial properties of Mandelic acid, Gallic acid and their derivatives, mini‐reviews in medicinal. Chemistry, 21, 2544–2550. 10.2174/1389557521666210105123834 [DOI] [PubMed] [Google Scholar]
- Choubey, S. , Goyal, S. , Varughese, L. R. , Kumar, V. , Sharma, A. K. , & Beniwal, V. (2018). Probing Gallic acid for its broad Spectrum applications. Mini‐Reviews in Medicinal Chemistry, 18, 1283–1293. 10.2174/1389557518666180330114010 [DOI] [PubMed] [Google Scholar]
- Choubey, S. , Varughese, L. R. , Kumar, V. , & Beniwal, V. (2015). Medicinal importance of gallic acid and its ester derivatives: A patent review. Pharmaceutical Patent Analyst, 4, 305–315. 10.4155/ppa.15.14 [DOI] [PubMed] [Google Scholar]
- Crozier, A. , Jaganath, I. B. , & Clifford, M. N. (2009). Dietary phenolics: Chemistry, bioavailability and effects on health. Natural Product Reports, 26, 1001. 10.1039/b802662a [DOI] [PubMed] [Google Scholar]
- Curti, V. , Di Lorenzo, A. , Dacrema, M. , Xiao, J. , Nabavi, S. M. , & Daglia, M. (2017). In vitro polyphenol effects on apoptosis: An update of literature data. Seminars in Cancer Biology, 46, 119–131. 10.1016/j.semcancer.2017.08.005 [DOI] [PubMed] [Google Scholar]
- Daduang, J. , Klaynongsruang, S. , Leelayuwat, C. , Limpaiboon, T. , Lulitanond, A. , Boonsiri, P. , Srichan, S. , Soontaranon, S. , Rugmai, S. , & Rattanata, N. (2016). Gallic acid conjugated with gold nanoparticles: Antibacterial activity and mechanism of action on foodborne pathogens. International Journal of Nanomedicine, 11, 3347–3356. 10.2147/IJN.S109795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashtbani‐Roozbehani, A. , & Brown, M. H. (2021). Efflux pump mediated antimicrobial resistance by staphylococci in health‐related environments: Challenges and the quest for inhibition. Antibiotics, 10, 1502. 10.3390/antibiotics10121502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lima Pimenta, A. , Chiaradia‐Delatorre, L. D. , Mascarello, A. , de Oliveira, K. A. , Leal, P. C. , Yunes, R. A. , de Aguiar, C. B. N. M. , Tasca, C. I. , Nunes, R. J. , & Smânia, A. (2013). Synthetic organic compounds with potential for bacterial biofilm inhibition, a path for the identification of compounds interfering with quorum sensing. International Journal of Antimicrobial Agents, 42, 519–523. 10.1016/j.ijantimicag.2013.07.006 [DOI] [PubMed] [Google Scholar]
- Devi, Y. P. , Uma, A. , Narasu, M. L. , & Kalyani, C. (2014). Anticancer activity of gallic acid on cancer cell lines, HCT15 and MDA MB 231. International Journal of Research in Applied Natural and Social Sciences, 2, 269–272. [Google Scholar]
- Devi, Y. P. , Uma, A. , Narasu, M. L. , & Kalyani, C. (2015). A comparative study of the effect of gallic acid on cancer cells and normal cells. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 6, 460–464. [Google Scholar]
- Dorniani, D. , Saifullah, B. , Barahuie, F. , Arulselvan, P. , Bin Hussein, M. Z. , Fakurazi, S. , & Twyman, L. J. (2016). Graphene oxide‐Gallic acid nanodelivery system for cancer therapy. Nanoscale Research Letters, 11, 491. 10.1186/s11671-016-1712-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes, F. H. A. , & Salgado, H. R. N. (2016). Gallic acid: Review of the methods of determination and quantification. Critical Reviews in Analytical Chemistry, 46, 257–265. 10.1080/10408347.2015.1095064 [DOI] [PubMed] [Google Scholar]
- Fiuza, S. M. , Gomes, C. , Teixeira, L. J. , Girão da Cruz, M. T. , Cordeiro, M. N. D. S. , Milhazes, N. , Borges, F. , & Marques, M. P. M. (2004). Phenolic acid derivatives with potential anticancer properties – A structure–activity relationship study. Part 1: Methyl, propyl and octyl esters of caffeic and gallic acids. Bioorganic & Medicinal Chemistry, 12, 3581–3589. 10.1016/j.bmc.2004.04.026 [DOI] [PubMed] [Google Scholar]
- Gao, J. , Hu, J. , Hu, D. , & Yang, X. (2019). A role of gallic acid in oxidative damage diseases: A comprehensive review. Natural Product Communications, 14, 1934578X1987417. 10.1177/1934578X19874174 [DOI] [Google Scholar]
- Giftson, J. S. , Jayanthi, S. , & Nalini, N. (2010). Chemopreventive efficacy of gallic acid, an antioxidant and anticarcinogenic polyphenol, against 1,2‐dimethyl hydrazine induced rat colon carcinogenesis. Investigational New Drugs, 28, 251–259. 10.1007/s10637-009-9241-9 [DOI] [PubMed] [Google Scholar]
- Gulcin, I. , Kaya, R. , Goren, A. C. , Akincioglu, H. , Topal, M. , Bingol, Z. , Cetin Çakmak, K. , Ozturk Sarikaya, S. B. , Durmaz, L. , & Alwasel, S. (2019). Anticholinergic, antidiabetic and antioxidant activities of cinnamon (Cinnamomum verum) bark extracts: Polyphenol contents analysis by LC‐MS/MS. International Journal of Food Properties, 22, 1511–1526. 10.1080/10942912.2019.1656232 [DOI] [Google Scholar]
- Gutiérrez‐Fernández, J. , García‐Armesto, M. R. , Álvarez‐Alonso, R. , del Valle, P. , de Arriaga, D. , & Rúa, J. (2013). Antimicrobial activity of binary combinations of natural and synthetic phenolic antioxidants against Enterococcus faecalis . Journal of Dairy Science, 96, 4912–4920. 10.3168/jds.2013-6643 [DOI] [PubMed] [Google Scholar]
- Guzmán, K. , Kumar, B. , Vallejo, M. J. , Grijalva, M. , Debut, A. , & Cumbal, L. (2019). Ultrasound‐assisted synthesis and antibacterial activity of gallic acid‐chitosan modified silver nanoparticles. Progress in Organic Coating, 129, 229–235. 10.1016/j.porgcoat.2019.01.009 [DOI] [Google Scholar]
- Holohan, C. , Van Schaeybroeck, S. , Longley, D. B. , & Johnston, P. G. (2013). Cancer drug resistance: An evolving paradigm. Nature Reviews. Cancer, 13, 714–726. 10.1038/nrc3599 [DOI] [PubMed] [Google Scholar]
- Hossain, M. A. , Park, H.‐C. , Park, S.‐W. , Park, S.‐C. , Seo, M.‐G. , Her, M. , & Kang, J. (2020). Synergism of the combination of traditional antibiotics and novel phenolic compounds against Escherichia coli . Pathogens, 9, 811. 10.3390/pathogens9100811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain, M. A. , Park, H.‐C. , Lee, K.‐J. , Park, S.‐W. , Park, S.‐C. , & Kang, J. (2020). In vitro synergistic potentials of novel antibacterial combination therapies against Salmonella enterica serovar Typhimurium. BMC Microbiology, 20, 118. 10.1186/s12866-020-01810-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyo, J. , Ivanova, K. , Guaus, E. , & Tzanov, T. (2019). Multifunctional ZnO NPs‐chitosan‐gallic acid hybrid nanocoating to overcome contact lenses associated conditions and discomfort. Journal of Colloid and Interface Science, 543, 114–121. 10.1016/j.jcis.2019.02.043 [DOI] [PubMed] [Google Scholar]
- Htay, H. H. , Tsubouchi, R. , Haneda, M. , Murakami, K. , & Yoshino, M. (2002). Induction of apoptosis of HL60 cells by gallic acid derivatives. Biomedical Research, 23, 127–134. 10.2220/biomedres.23.127 [DOI] [Google Scholar]
- Jiamboonsri, P. , Pithayanukul, P. , Bavovada, R. , & Chomnawang, M. T. (2011). The inhibitory potential of Thai mango seed kernel extract against methicillin‐resistant Staphylococcus aureus . Molecules, 16, 6255–6270. 10.3390/molecules16086255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing, Z. , Li, M. , Wang, H. , Yang, Z. , Zhou, S. , Ma, J. , Meng, E. , Zhang, H. , Liang, W. , Hu, W. , Wang, X. , & Fu, X. (2021). Gallic acid‐gold nanoparticles enhance radiation‐induced cell death of human glioma U251 cells. IUBMB Life, 73, 398–407. 10.1002/iub.2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, J. , Li, Q. , Liu, L. , Jin, W. , Wang, J. , & Sun, Y. (2018). The specific effect of gallic acid on Escherichia coli biofilm formation by regulating pgaABCD genes expression. Applied Microbiology and Biotechnology, 102, 1837–1846. 10.1007/s00253-017-8709-3 [DOI] [PubMed] [Google Scholar]
- Kang, J. , Liu, L. , Liu, M. , Wu, X. , & Li, J. (2018). Antibacterial activity of gallic acid against Shigella flexneri and its effect on biofilm formation by repressing mdoH gene expression. Food Control, 94, 147–154. 10.1016/j.foodcont.2018.07.011 [DOI] [Google Scholar]
- Keyvani‐Ghamsari, S. , Khorsandi, K. , & Gul, A. (2020). Curcumin effect on cancer cells' multidrug resistance: An update. Phytotherapy Research, 34, ptr.6703. 10.1002/ptr.6703 [DOI] [PubMed] [Google Scholar]
- Khaledi, H. , Alhadi, A. A. , Yehye, W. A. , Ali, H. M. , Abdulla, M. A. , & Hassandarvish, P. (2011). Antioxidant, cytotoxic activities, and structure‐activity relationship of Gallic acid‐based indole derivatives. Archiv der Pharmazie, 344, 703–709. 10.1002/ardp.201000223 [DOI] [PubMed] [Google Scholar]
- Khameneh, B. , Iranshahy, M. , Soheili, V. , & Fazly Bazzaz, B. S. (2019). Review on plant antimicrobials: A mechanistic viewpoint. Antimicrobial Resistance and Infection Control, 8, 118. 10.1186/s13756-019-0559-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorsandi, K. , Keyvani‐Ghamsari, S. , Khatibi Shahidi, F. , Hosseinzadeh, R. , & Kanwal, S. (2021). A mechanistic perspective on targeting bacterial drug resistance with nanoparticles. Journal of Drug Targeting, 29, 941–959. 10.1080/1061186X.2021.1895818 [DOI] [PubMed] [Google Scholar]
- Khorsandi, K. , Kianmehr, Z. , Hosseinmardi, Z. , & Hosseinzadeh, R. (2020). Anti‐cancer effect of gallic acid in presence of low level laser irradiation: ROS production and induction of apoptosis and ferroptosis. Cancer Cell International, 20, 18. 10.1186/s12935-020-1100-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, M. J. , Seong, A. R. , Yoo, J. Y. , Jin, C. H. , Lee, Y. H. , Kim, Y. J. , Lee, J. , Jun, W. J. , & Yoon, H. G. (2011). Gallic acid, a histone acetyltransferase inhibitor, suppresses β‐amyloid neurotoxicity by inhibiting microglial‐mediated neuroinflammation. Molecular Nutrition & Food Research, 55, 1798–1808. 10.1002/mnfr.201100262 [DOI] [PubMed] [Google Scholar]
- Ko, E.‐B. , Jang, Y.‐G. , Kim, C.‐W. , Go, R.‐E. , Lee, H. K. , & Choi, K.‐C. (2022). Gallic acid hindered lung cancer progression by inducing cell cycle arrest and apoptosis in A549 lung cancer cells via PI3K/Akt pathway. Biomolecules & Therapeutics, 30, 151–161. 10.4062/biomolther.2021.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo, T. , Oka, T. , Sato, H. , Shinnou, Y. , Washio, K. , Takano, M. , Morito, T. , Takata, K. , Ohara, N. , Ouchida, M. , Shimizu, K. , & Yoshino, T. (2009). Accumulation of aberrant CpG hypermethylation by Helicobacter pylori infection promotes development and progression of gastric MALT lymphoma. International Journal of Oncology, 35, 547–557. 10.3892/ijo_00000366 [DOI] [PubMed] [Google Scholar]
- Konishi, Y. , Hitomi, Y. , & Yoshioka, E. (2004). Intestinal absorption of p‐Coumaric and gallic acids in rats after oral administration. Journal of Agricultural and Food Chemistry, 52, 2527–2532. 10.1021/jf035366k [DOI] [PubMed] [Google Scholar]
- Kyaw, B. M. , Arora, S. , & Lim, C. S. (2012). Bactericidal antibiotic‐phytochemical combinations against methicillin resistant Staphylococcus aureus . Brazilian Journal of Microbiology, 43, 938–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laddomada, B. , Caretto, S. , & Mita, G. (2015). Wheat bran phenolic acids: Bioavailability and stability in whole wheat‐based foods. Molecules, 20, 15666–15685. 10.3390/molecules200915666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, D.‐S. , Eom, S.‐H. , Kim, Y.‐M. , Kim, H. S. , Yim, M.‐J. , Lee, S.‐H. , Kim, D.‐H. , & Je, J.‐Y. (2014). Antibacterial and synergic effects of gallic acid‐ grafted ‐chitosan with β‐lactams against methicillin‐resistant Staphylococcus aureus (MRSA). Canadian Journal of Microbiology, 60, 629–638. 10.1139/cjm-2014-0286 [DOI] [PubMed] [Google Scholar]
- Lee, J. , Choi, K.‐H. , Min, J. , Kim, H.‐J. , Jee, J.‐P. , & Park, B. J. (2017). Functionalized ZnO nanoparticles with gallic acid for antioxidant and antibacterial activity against methicillin‐resistant S. aureus . Nanomaterials, 7, 365. 10.3390/nano7110365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, D. , Liu, Z. , Yuan, Y. , Liu, Y. , & Niu, F. (2015). Green synthesis of gallic acid‐coated silver nanoparticles with high antimicrobial activity and low cytotoxicity to normal cells. Process Biochemistry, 50, 357–366. 10.1016/j.procbio.2015.01.002 [DOI] [Google Scholar]
- Lima, V. N. , Oliveira‐Tintino, C. D. M. , Santos, E. S. , Morais, L. P. , Tintino, S. R. , Freitas, T. S. , Geraldo, Y. S. , Pereira, R. L. S. , Cruz, R. P. , Menezes, I. R. A. , & Coutinho, H. D. M. (2016). Antimicrobial and enhancement of the antibiotic activity by phenolic compounds: Gallic acid, caffeic acid and pyrogallol. Microbial Pathogenesis, 99, 56–61. 10.1016/j.micpath.2016.08.004 [DOI] [PubMed] [Google Scholar]
- Lin, T.‐H. , Wu, C.‐C. , Tseng, C.‐Y. , Fang, J.‐H. , & Lin, C.‐T. (2022). Effects of gallic acid on capsular polysaccharide biosynthesis in Klebsiella pneumoniae . Journal of Microbiology, Immunology and Infection., 55, 1255–1262. 10.1016/j.jmii.2021.07.002 [DOI] [PubMed] [Google Scholar]
- Liu, C. , Ho, P. C.‐L. , Wong, F. C. , Sethi, G. , Wang, L. Z. , & Goh, B. C. (2015). Garcinol: Current status of its anti‐oxidative, anti‐inflammatory and anti‐cancer effects. Cancer Letters, 362, 8–14. 10.1016/j.canlet.2015.03.019 [DOI] [PubMed] [Google Scholar]
- Liu, M. , Wu, X. , Li, J. , Liu, L. , Zhang, R. , Shao, D. , & Du, X. (2017). The specific anti‐biofilm effect of gallic acid on Staphylococcus aureus by regulating the expression of the ica operon. Food Control, 73, 613–618. 10.1016/j.foodcont.2016.09.015 [DOI] [Google Scholar]
- Lo, C. , Lai, T.‐Y. , Yang, J.‐S. , Yang, J.‐H. , Ma, Y.‐S. , Weng, S.‐W. , Lin, H.‐Y. , Chen, H.‐Y. , Lin, J.‐G. , & Chung, J.‐G. (2011). Gallic acid inhibits the migration and invasion of A375.S2 human melanoma cells through the inhibition of matrix metalloproteinase‐2 and Ras. Melanoma Research, 21, 267–273. 10.1097/CMR.0b013e3283414444 [DOI] [PubMed] [Google Scholar]
- Locatelli, C. , Rosso, R. , Santos‐Silva, M. C. , de Souza, C. A. , Licínio, M. A. , Leal, P. , Bazzo, M. L. , Yunes, R. A. , & Creczynski‐Pasa, T. B. (2008). Ester derivatives of gallic acid with potential toxicity toward L1210 leukemia cells. Bioorganic & Medicinal Chemistry, 16, 3791–3799. 10.1016/j.bmc.2008.01.049 [DOI] [PubMed] [Google Scholar]
- Lu, L. , Zhao, Y. , Yi, G. , Li, M. , Liao, L. , Yang, C. , Cho, C. , Zhang, B. , Zhu, J. , Zou, K. , & Cheng, Q. (2021). Quinic acid: A potential antibiofilm agent against clinical resistant Pseudomonas aeruginosa . Chinese Medicine, 16, 72. 10.1186/s13020-021-00481-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madlener, S. , Illmer, C. , Horvath, Z. , Saiko, P. , Losert, A. , Herbacek, I. , Grusch, M. , Elford, H. L. , Krupitza, G. , Bernhaus, A. , Fritzer‐Szekeres, M. , & Szekeres, T. (2007). Gallic acid inhibits ribonucleotide reductase and cyclooxygenases in human HL‐60 promyelocytic leukemia cells. Cancer Letters, 245, 156–162. 10.1016/j.canlet.2006.01.001 [DOI] [PubMed] [Google Scholar]
- Manach, C. , Williamson, G. , Morand, C. , Scalbert, A. , & Rémésy, C. (2005). Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. The American Journal of Clinical Nutrition, 81, 230S–242S. 10.1093/ajcn/81.1.230S [DOI] [PubMed] [Google Scholar]
- Manso, T. , Lores, M. , & de Miguel, T. (2021). Antimicrobial activity of polyphenols and natural polyphenolic extracts on clinical isolates. Antibiotics, 11, 46. 10.3390/antibiotics11010046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruszewska, A. , & Tarasiuk, J. (2019). Antitumour effects of selected plant polyphenols, gallic acid and ellagic acid, on sensitive and multidrug‐resistant leukaemia HL60 cells. Phytotherapy Research, 33, 1208–1221. 10.1002/ptr.6317 [DOI] [PubMed] [Google Scholar]
- Mazzotta, E. , Orlando, C. , & Muzzalupo, R. (2021). New nanomaterials with intrinsic antioxidant activity by surface functionalization of niosomes with natural phenolic acids. Pharmaceutics, 13, 766. 10.3390/pharmaceutics13060766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, M. (2011). Gallic acid suppresses the migration and invasion of PC‐3 human prostate cancer cells via inhibition of matrix metalloproteinase‐2 and ‐9 signaling pathways. Oncology Reports, 26, 177–184. 10.3892/or.2011.1264 [DOI] [PubMed] [Google Scholar]
- Minarini, L. A. D. R. , de Andrade, L. N. , De Gregorio, E. , Grosso, F. , Naas, T. , Zarrilli, R. , & Camargo, I. L. B. C. (2020). Editorial: Antimicrobial resistance as a global public health problem: How can we address it? Frontiers in Public Health, 8, 612844. 10.3389/fpubh.2020.612844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal, A. K. , Kumar, S. , & Banerjee, U. C. (2014). Quercetin and gallic acid mediated synthesis of bimetallic (silver and selenium) nanoparticles and their antitumor and antimicrobial potential. Journal of Colloid and Interface Science, 431, 194–199. 10.1016/j.jcis.2014.06.030 [DOI] [PubMed] [Google Scholar]
- Moghtaderi, H. , Sepehri, H. , Delphi, L. , & Attari, F. (2018). Gallic acid and curcumin induce cytotoxicity and apoptosis in human breast cancer cell MDA‐MB‐231. BioImpacts: BI, 8, 185–194. 10.15171/bi.2018.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriwaki, T. , Zhao, C. , & Nishiuchi, M. (1991). Thermal deformation of machining center due to temperature change in the environment. Transactions of the Japan Society of Mechanical Engineers Series C, 57, 2447–2452. 10.1299/kikaic.57.2447 [DOI] [Google Scholar]
- Nolasco‐Quintana, N. Y. , González‐Maya, L. , Razo‐Hernández, R. S. , & Alvarez, L. (2023). Exploring the gallic and cinnamic acids chimeric derivatives as anticancer agents over HeLa cell line: An in silico and in vitro study. Molecular Informatics, 42, 2200016. 10.1002/minf.202200016 [DOI] [PubMed] [Google Scholar]
- Passos, M. R. , Almeida, R. S. , Lima, B. O. , Rodrigues, J. Z. S. , de Macêdo Neres, N. S. , Pita, L. S. , Marinho, P. D. F. , Santos, I. A. , da Silva, J. P. , Oliveira, M. C. , Oliveira, M. A. , Pessoa, S. M. B. , Silva, M. M. L. , Silveira, P. H. S. , Reis, M. M. , Santos, I. P. , Ricardo, L. O. N. , Andrade, L. O. S. B. , Soares, A. B. , … Yatsuda, R. (2021). Anticariogenic activities of Libidibia ferrea, gallic acid and ethyl gallate against Streptococcus mutans in biofilm model. Journal of Ethnopharmacology, 274, 114059. 10.1016/j.jep.2021.114059 [DOI] [PubMed] [Google Scholar]
- Patel, H. , Wu, Z.‐X. , Chen, Y. , Bo, L. , & Chen, Z.‐S. (2021). Drug resistance: From bacteria to cancer. Molecular Biomedicine, 2, 27. 10.1186/s43556-021-00041-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil, P. , & Killedar, S. (2021a). Improving gallic acid and quercetin bioavailability by polymeric nanoparticle formulation. Drug Development and Industrial Pharmacy, 47, 1656–1663. 10.1080/03639045.2022.2043353 [DOI] [PubMed] [Google Scholar]
- Patil, P. , & Killedar, S. (2021b). Formulation and characterization of gallic acid and quercetin chitosan nanoparticles for sustained release in treating colorectal cancer. Journal of Drug Delivery Science and Technology, 63, 102523. 10.1016/j.jddst.2021.102523 [DOI] [Google Scholar]
- Pereira Rangel, L. , Fritzen, M. , Yunes, R. A. , Leal, P. C. , Creczynski‐Pasa, T. B. , & Ferreira‐Pereira, A. (2010). Inhibitory effects of gallic acid ester derivatives on Saccharomyces cerevisiae multidrug resistance protein Pdr5p. FEMS Yeast Research, 10, 244–251. 10.1111/j.1567-1364.2010.00603.x [DOI] [PubMed] [Google Scholar]
- Pinho, E. , Ferreira, I. C. F. R. , Barros, L. , Carvalho, A. M. , Soares, G. , & Henriques, M. (2014). Antibacterial potential of northeastern Portugal wild plant extracts and respective phenolic compounds. BioMed Research International, 2014, 1–8. 10.1155/2014/814590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestinaci, F. , Pezzotti, P. , & Pantosti, A. (2015). Antimicrobial resistance: A global multifaceted phenomenon. Pathogens and Global Health, 109, 309–318. 10.1179/2047773215Y.0000000030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priyadarshi, K. , Shirsath, K. , Waghela, N. B. , Sharma, A. , Kumar, A. , & Pathak, C. (2021). Surface modified PAMAM dendrimers with gallic acid inhibit, cell proliferation, cell migration and inflammatory response to augment apoptotic cell death in human colon carcinoma cells. Journal of Biomolecular Structure & Dynamics, 39, 6853–6869. 10.1080/07391102.2020.1802344 [DOI] [PubMed] [Google Scholar]
- Rajamanickam, K. , Yang, J. , & Sakharkar, M. K. (2019). Gallic acid potentiates the antimicrobial activity of tulathromycin against two key bovine respiratory disease (BRD) causing‐pathogens. Frontiers in Pharmacology, 9, 1486. 10.3389/fphar.2018.01486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaei‐Seresht, H. , Cheshomi, H. , Falanji, F. , Movahedi‐Motlagh, F. , Hashemian, M. , & Mireskandari, E. (2019). Cytotoxic activity of caffeic acid and gallic acid against MCF‐7 human breast cancer cells: An in silico and in vitro study. Avicenna Journal of Phytomedicine, 9, 574–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosman, R. , Saifullah, B. , Maniam, S. , Dorniani, D. , Hussein, M. , & Fakurazi, S. (2018). Improved anticancer effect of magnetite nanocomposite formulation of GALLIC acid (Fe3O4‐PEG‐GA) against lung, breast and colon cancer cells. Nanomaterials, 8, 83. 10.3390/nano8020083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell, L. H., Jr. , Mazzio, E. , Badisa, R. B. , Zhi‐Ping, Z. H. U. , Agharahimi, M. , Oriaku, E. T. , & Goodman, C. B. (2012). Autoxidation of gallic acid induces ROS‐dependent death in human prostate cancer LNCaP cells. Anticancer Research, 32, 1595. [PMC free article] [PubMed] [Google Scholar]
- Shamsi, S. , Abdul Ghafor, A. A. H. , Norjoshukrudin, N. H. , Ng, I. M. J. , Abdullah, S. N. S. , Sarchio, S. N. E. , Md Yasin, F. , Abd Gani, S. , & Mohd Desa, M. N. (2022). Stability, toxicity, and antibacterial potential of gallic acid‐loaded graphene oxide (GAGO) against methicillin‐resistant Staphylococcus aureus (MRSA) strains. International Journal of Nanomedicine, 17, 5781–5807. 10.2147/IJN.S369373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamsi, S. , Elias, N. , Narti Edayu Sarchio, S. , & Md Yasin, F. (2018). Gallic acid loaded graphene oxide based Nanoformulation (GAGO) as potential anti‐bacterial agent against Staphylococcus aureus. Materials Today: Proceedings, 5, S160–S165. 10.1016/j.matpr.2018.08.059 [DOI] [Google Scholar]
- Sharma, S. , Mittal, D. , Verma, A. K. , & Roy, I. (2019). Copper‐gallic acid nanoscale metal‐organic framework for combined drug delivery and photodynamic therapy. ACS Applied Bio Materials, 2, 2092–2101. 10.1021/acsabm.9b00116 [DOI] [PubMed] [Google Scholar]
- Shi, Y. , Zhang, R. , Zhu, C. , Xu, M. , Gu, Q. , Ettelaie, R. , Lin, S. , Wang, Y. , & Leng, X. (2021). Antimicrobial mechanism of alkyl gallates against Escherichia coli and Staphylococcus aureus and its combined effect with electrospun nanofibers on Chinese Taihu icefish preservation. Food Chemistry, 346, 128949. 10.1016/j.foodchem.2020.128949 [DOI] [PubMed] [Google Scholar]
- Shrihastini, V. , Muthuramalingam, P. , Adarshan, S. , Sujitha, M. , Chen, J.‐T. , Shin, H. , & Ramesh, M. (2021). Plant derived bioactive compounds, their anti‐cancer effects and in silico approaches as an alternative target treatment strategy for breast cancer: An updated overview. Cancers, 13, 6222. 10.3390/cancers13246222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, A. , Dutta, P. K. , Kumar, H. , Kureel, A. K. , & Rai, A. K. (2019). Improved antibacterial and antioxidant activities of gallic acid grafted chitin‐glucan complex. Journal of Polymer Research, 26, 234. 10.1007/s10965-019-1893-3 [DOI] [Google Scholar]
- Subramanian, A. P. , Jaganathan, S. K. , Mandal, M. , Supriyanto, E. , & Muhamad, I. I. (2016). Gallic acid induced apoptotic events in HCT‐15 colon cancer cells. World Journal of Gastroenterology, 22, 3952–3961. 10.3748/wjg.v22.i15.3952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian, A. P. , John, A. A. , Vellayappan, M. V. , Balaji, A. , Jaganathan, S. K. , Supriyanto, E. , & Yusof, M. (2015). Gallic acid: Prospects and molecular mechanisms of its anticancer activity. RSC Advances, 5, 35608–35621. 10.1039/C5RA02727F [DOI] [Google Scholar]
- Sun, X. , Dong, M. , Guo, Z. , Zhang, H. , Wang, J. , Jia, P. , Bu, T. , Liu, Y. , Li, L. , & Wang, L. (2021). Multifunctional chitosan‐copper‐gallic acid based antibacterial nanocomposite wound dressing. International Journal of Biological Macromolecules, 167, 10–22. 10.1016/j.ijbiomac.2020.11.153 [DOI] [PubMed] [Google Scholar]
- Tafesh, A. , Najami, N. , Jadoun, J. , Halahlih, F. , Riepl, H. , & Azaizeh, H. (2011). Synergistic antibacterial effects of polyphenolic compounds from olive mill wastewater. Evidence‐Based Complementary and Alternative Medicine, 2011, 1–9. 10.1155/2011/431021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapliyal, A. , & Chandra, A. (2018). Antibacterial and anticancer potential of silver nanoparticles synthesized using gallic acid in bentonite/starch bio‐nanocomposites. International Journal of Applied Pharmaceutics, 10, 178. 10.22159/ijap.2018v10i5.27728 [DOI] [Google Scholar]
- Tsai, C. L. , Chiu, Y. M. , Ho, T. Y. , Hsieh, C. T. , Shieh, D. C. , Lee, Y. J. , Tsay, G. J. , & Wu, Y. Y. (2018). Gallic acid induces apoptosis in human gastric adenocarcinoma cells. Anticancer Research, 38, 2057–2067. 10.21873/anticanres.12445 [DOI] [PubMed] [Google Scholar]
- Variya, B. C. , Bakrania, A. K. , Madan, P. , & Patel, S. S. (2019). Acute and 28‐days repeated dose sub‐acute toxicity study of gallic acid in albino mice. Regulatory Toxicology and Pharmacology, 101, 71–78. 10.1016/j.yrtph.2018.11.010 [DOI] [PubMed] [Google Scholar]
- Wang, D. , & Bao, B. (2020). Gallic acid impedes non‐small cell lung cancer progression via suppression of EGFR‐dependent CARM1‐PELP1 complex. Drug Design, Development and Therapy, 14, 1583–1592. 10.2147/DDDT.S228123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q. , de Oliveira, E. F. , Alborzi, S. , Bastarrachea, L. J. , & Tikekar, R. V. (2017). On mechanism behind UV‐A light enhanced antibacterial activity of gallic acid and propyl gallate against Escherichia coli O157:H7. Scientific Reports, 7, 8325. 10.1038/s41598-017-08449-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, R. , Ma, L. , Weng, D. , Yao, J. , Liu, X. , & Jin, F. (2016). Gallic acid induces apoptosis and enhances the anticancer effects of cisplatin in human small cell lung cancer H446 cell line via the ROS‐dependent mitochondrial apoptotic pathway. Oncology Reports, 35, 3075–3083. 10.3892/or.2016.4690 [DOI] [PubMed] [Google Scholar]
- Wang, R. , Song, B. , Wu, J. , Zhang, Y. , Chen, A. , & Shao, L. (2018). Potential adverse effects of nanoparticles on the reproductive system. International Journal of Nanomedicine, 13, 8487–8506. 10.2147/IJN.S170723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, B. , Dong, Y. , Wang, F. , & Zhang, Y. (2020). Nanoformulations to enhance the bioavailability and physiological functions of polyphenols. Molecules, 25, 4613. 10.3390/molecules25204613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J.‐T. , Lee, I.‐N. , Chen, C.‐H. , Lu, F.‐J. , Chung, C.‐Y. , Lee, M.‐H. , Cheng, Y.‐C. , Chen, K.‐T. , Peng, J.‐Y. , & Chen, C.‐H. (2022). Gallic acid enhances the anti‐cancer effect of temozolomide in human glioma cell line via inhibition of Akt and p38‐MAPK pathway. Processes, 10, 448. 10.3390/pr10030448 [DOI] [Google Scholar]
- Yang, K. , Zhang, L. , Liao, P. , Xiao, Z. , Zhang, F. , Sindaye, D. , Xin, Z. , Tan, C. , Deng, J. , Yin, Y. , & Deng, B. (2020). Impact of gallic acid on gut health: Focus on the gut microbiome, immune response, and mechanisms of action. Frontiers in Immunology, 11, 580208. 10.3389/fimmu.2020.580208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh, R.‐D. , Chen, J.‐C. , Lai, T.‐Y. , Yang, J.‐S. , Yu, C.‐S. , Chiang, J.‐H. , Lu, C.‐C. , Yang, S.‐T. , Yu, C.‐C. , Chang, S.‐J. , Lin, H.‐Y. , & Chung, J.‐G. (2011). Gallic acid induces G0/G1 phase arrest and apoptosis in human leukemia HL‐60 cells through inhibiting cyclin D and E, and activating mitochondria‐dependent pathway. Anticancer Research, 31, 2821–2832. [PubMed] [Google Scholar]
- Yen, G.‐C. , Duh, P.‐D. , & Tsai, H.‐L. (2002). Antioxidant and pro‐oxidant properties of ascorbic acid and gallic acid. Food Chemistry, 79, 307–313. 10.1016/S0308-8146(02)00145-0 [DOI] [Google Scholar]
- Yoshioka, K. , Kataoka, T. , Hayashi, T. , Hasegawa, M. , Ishi, Y. , & Hibasami, H. (2000). Induction of apoptosis by gallic acid in human stomach cancer KATO III and colon adenocarcinoma COLO 205 cell lines. Oncology Reports, 7, 1221–1223. 10.3892/or.7.6.1221 [DOI] [PubMed] [Google Scholar]
- You, B. R. , Moon, H. J. , Han, Y. H. , & Park, W. H. (2010). Gallic acid inhibits the growth of HeLa cervical cancer cells via apoptosis and/or necrosis. Food and Chemical Toxicology, 48, 1334–1340. 10.1016/j.fct.2010.02.034 [DOI] [PubMed] [Google Scholar]
- You, B. R. , & Park, W. H. (2011). The effects of mitogen‐activated protein kinase inhibitors or small interfering RNAs on Gallic acid‐induced HeLa cell death in relation to reactive oxygen species and glutathione. Journal of Agricultural and Food Chemistry, 59, 763–771. 10.1021/jf103379d [DOI] [PubMed] [Google Scholar]
- Zeng, M. , Su, Y. , Li, K. , Jin, D. , Li, Q. , Li, Y. , & Zhou, B. (2020). Gallic acid inhibits bladder cancer T24 cell progression through mitochondrial dysfunction and PI3K/Akt/NF‐κB signaling suppression. Frontiers in Pharmacology, 11, 1222. 10.3389/fphar.2020.01222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, K. , Liu, C. , Yang, T. , Li, X. , Wei, L. , Chen, D. , Zhou, J. , Yin, Y. , Yu, X. , & Li, F. (2020). Systematically explore the potential hepatotoxic material basis and molecular mechanism of radix Aconiti Lateralis based on the concept of toxicological evidence chain (TEC). Ecotoxicology and Environmental Safety, 205, 111342. 10.1016/j.ecoenv.2020.111342 [DOI] [PubMed] [Google Scholar]
- Zhang, L. , McClements, D. J. , Wei, Z. , Wang, G. , Liu, X. , & Liu, F. (2020). Delivery of synergistic polyphenol combinations using biopolymer‐based systems: Advances in physicochemical properties, stability and bioavailability. Critical Reviews in Food Science and Nutrition, 60, 2083–2097. 10.1080/10408398.2019.1630358 [DOI] [PubMed] [Google Scholar]
- Zhang, T. , Ma, L. , Wu, P. , Li, W. , Li, T. , Gu, R. , Dan, X. , Li, Z. , Fan, X. , & Xiao, Z. (2019). Gallic acid has anticancer activity and enhances the anticancer effects of cisplatin in non‐small cell lung cancer A549 cells via the JAK/STAT3 signaling pathway. Oncology Reports, 41, 1779–1788. 10.3892/or.2019.6976 [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Pu, C. , Tang, W. , Wang, S. , & Sun, Q. (2019). Gallic acid liposomes decorated with lactoferrin: Characterization, in vitro digestion and antibacterial activity. Food Chemistry, 293, 315–322. 10.1016/j.foodchem.2019.04.116 [DOI] [PubMed] [Google Scholar]
- Zhao, B. , & Hu, M. (2013). Gallic acid reduces cell viability, proliferation, invasion and angiogenesis in human cervical cancer cells. Oncology Letters, 6, 1749–1755. 10.3892/ol.2013.1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in this manuscript.


