Abstract
Several vaccines have been developed against highly pathogenic avian influenza (HPAI), mostly inactivated whole‐virus vaccines for chickens. In the EU, one vaccine is authorised in chickens but is not fully efficacious to stop transmission, highlighting the need for vaccines tailored to diverse poultry species and production types. Off‐label use of vaccines is possible, but effectiveness varies. Vaccines are usually injectable, a time‐consuming process. Mass‐application vaccines outside hatcheries remain rare. First vaccination varies from in‐ovo to 6 weeks of age. Data about immunity onset and duration in the target species are often unavailable, despite being key for effective planning. Minimising antigenic distance between vaccines and field strains is essential, requiring rapid updates of vaccines to match circulating strains. Generating harmonised vaccine efficacy data showing vaccine ability to reduce transmission is crucial and this ability should be also assessed in field trials. Planning vaccination requires selecting the most adequate vaccine type and vaccination scheme. Emergency protective vaccination is limited to vaccines that are not restricted by species, age or pre‐existing vector‐immunity, while preventive vaccination should prioritise achieving the highest protection, especially for the most susceptible species in high‐risk transmission areas. Model simulations in France, Italy and The Netherlands revealed that (i) duck and turkey farms are more infectious than chickens, (ii) depopulating infected farms only showed limitations in controlling disease spread, while 1‐km ring‐culling performed better than or similar to emergency preventive ring‐vaccination scenarios, although with the highest number of depopulated farms, (iii) preventive vaccination of the most susceptible species in high‐risk transmission areas was the best option to minimise the outbreaks' number and duration, (iv) during outbreaks in such areas, emergency protective vaccination in a 3‐km radius was more effective than 1‐ and 10‐km radius. Vaccine efficacy should be monitored and complement other surveillance and preventive efforts.
Keywords: Highly pathogenic avian influenza (HPAI), poultry, vaccines, vaccine efficacy, avian influenza transmission, vaccination strategies
Short abstract
This publication is linked to the following EFSA Journal article: https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2023.p211001
Summary
Background and Terms of Reference
Observation of the past three seasons of highly pathogenic avian influenza (HPAI) has led to finding that multiple HPAI virus subtypes have co‐circulated in the same epidemic season and the virus has been increasingly detected in migratory waterfowl birds, amplifying thereby the risk of infection in poultry. Therefore, it is necessary to explore the potential of the application of vaccination of poultry in addition to the already applied measures to prevent and control HPAI infections. Given this situation, the Commission asked EFSA to:
ToR1 ‐ Update on available HPAI vaccines for poultry, identifying and describing the vaccines that may be available for vaccination of poultry against HPAI, including experiences with their use under laboratory conditions and in the field, as well as future perspectives indicating the cost‐effectiveness aspects, and the scientific and practical advantages and disadvantages of the identified vaccines of different technologies. In addition, EFSA was requested to provide a description of any possible aspects related to the use of different types of vaccines that may jeopardise a swift eradication of the virus in a vaccinated area.
ToR2 ‐ Assess the suitable vaccination strategies to be implemented (emergency suppressive, emergency protective, preventive) considering their objectives and risk factors, and recommending on vaccination zones and minimum required vaccination coverage, type of vaccine and frequency of vaccination.
Data and Methodologies
ToR 1 – Information about available vaccines for HPAI and their characteristics was collected via a literature review and completed via EMA, WOAH and the EURL and NRLs consultation.
Retrieved vaccines were described according to different characteristics that included virus subtype, replication competence, vaccine development method, production technology, GMO status and maternal immunity interference. Each vaccine was then described more in depth providing the technology used for its production, stage of authorisation if applicable, administration protocol, vaccine efficacy parameters and antigenic matching and, where possible, onset and duration of immunity.
Antigenic distance between vaccine seed strains and currently circulating strains was analysed with an in‐silico methodology introduced by Peeters et al., 2017. This was done by determining an HA consensus sequence of H5Nx HPAIV of the clade 2.3.4.4b (done by aligning sequences from GISAID database). Amino acids at the 27 positions identified as determinant for protective immunity were extracted from HA sequences of vaccines that were available in public databases. The genetic distance from the consensus sequence was calculated as the proportion of the amino acid residues that differ between the two viruses on the total extracted at each of the 27 residues. Linear regression was then used to calculate the antigenic distance values of vaccine seed strains from the currently circulating H5Nx HPAI strains.
Vaccine efficacy (VE) was defined as the measure of a vaccine to prevent infection in a given population. Focus of this opinion was investigation of the efficacy of a vaccine to stop sustained HPAIV transmission in a vaccinated population (VET). The parameter R was used to assess transmission, where R is the number of secondary infections caused by one single infected individual; therefore, when R < 1 transmission fades out, when R ≥ 1 extensive transmission can occur. Since most studies retrieved from the literature do not assess VET directly, secondary VE parameters were calculated and used to infer VET as follows.
Firstly 12 studies performing transmission experiments to assess the efficacy of vaccination to stop transmission were selected and used for data extraction. From these data secondary VE parameters were calculated for each vaccine. These parameters were: VE to decrease the susceptibility of vaccinated birds VEs, VE to decrease mortality in vaccinated infected birds VEm, VE to decrease virus shedding in vaccinated infected birds VEsh and VEs,sh, which expresses the combined effect of the vaccination on reduction of susceptibility and shedding (assumed to be an indicator of infectiousness). Additionally, the R values (as reported in the selected studies) for vaccinated and unvaccinated groups were extracted. The R values for the vaccinated groups were then used to create a binomial variable indicating whether a vaccine could or not stop transmission (R < 1, R ≥ 1). This variable was used as the response variable to assess the association between VEs,sh and the probability of stopping sustained transmission (R < 1) by fitting a univariable logistic regression model.
Secondly, data from 28 studies reporting challenge experiments (animals challenged by direct inoculation without assessment of transmission) to assess vaccines against infection with H5Nx HPAIV of the clade 2.3.4.4 were retrieved. VEs, VEm, VEsh and VEs,sh for each of the assessed vaccines were quantified. The estimated VEs,sh values were then used to predict the probability of each of these vaccines to stop sustained transmission in vaccinated animals (VET) by using the above mentioned logistic regression model.
Reviewed studies gave information mainly on the humoral immune response and attention was placed on antibody titres measured by HI, in the attempt to establish an HI threshold representative of protection to stop transmission.
ToR 2 ‐ Three vaccination strategies (emergency suppressive, emergency protective and preventive) with their final foreseen outcome (freedom from disease, rapid eradication or minimising losses) and risk factors were characterised, resulting in different vaccination scenarios. Then, the spatio‐temporal spread of AIV infection after the introduction of the virus in areas with the highest risk of between‐farm transmission was assessed for specific vaccination scenarios by simulating epidemics where the probability of between farm transmission is given by a spatial transmission kernel function. The kernel was parametrised using data from HPAI epidemics observed in Italy, France and the Netherlands. These countries were selected as case studies due to their recent experiences with major HPAI epidemics involving the currently circulating virus strains and their specific affected poultry production systems and species of interest (ducks, turkeys and chicken layers, respectively). For this simulations, hypothetical vaccination scenarios were built by the WG keeping in consideration vaccination strategy, target population, production system, timing and size of vaccination zone. Two scenarios (S0 and S1) did not involve vaccination and were used as baseline for comparisons. S0 included culling of all infected farms and S1 included culling of all infected farms as well as those within a 1‐km radius of infected farms. Three scenarios (S2, S3 and S4) applied emergency protective vaccination, and considered ring vaccination of all poultry species within a 1‐km, 3‐km and 10‐km radius, respectively, of infected poultry farms. The last scenario (S5) applied preventive vaccination, and considered vaccination of only targeted poultry species with higher susceptibility and/or spreading potential, which were expected to contribute the most to secondary virus transmission (e.g. duck farms in France, turkey farms in Italy and layer chicken farms in The Netherlands) and that are located in areas with the highest risk of between‐farm transmission. Emergency suppressive vaccination was not modelled as, due to the time needed to develop immunity, varying VE and the potential for virus transmission during vaccination practices, immediate culling of poultry is more effective to eradicate the outbreaks and control secondary spread. Epidemic simulations were done for each of the selected case studies and the different vaccination scenarios were assessed and compared at the end of the epidemic based on number of infected farms (i.e. virus is transmitting among poultry within the farm), duration of the epidemics, number of culled farms (i.e. farms within which all birds have been culled) and number of vaccinated farms (i.e. farms within which birds have been vaccinated).
Assessment
ToR 1 ‐ Inactivated and adjuvated whole virus AIV vaccines are by far the most frequently commercially produced, as virus amplification, inactivation and adjuvantation is a well‐established procedure. To overcome the biosafety level 3 handling of HPAIV for vaccine production, its pathotype can be changed from level 3 to level 2 or its HA and NA proteins expressed in recombinant systems. Vectored vaccines also were found to be widely used in the poultry sector. Nucleic acid vaccines are being given consideration for the poultry sector, following their success in the COVID‐19 epidemics. Two vaccines based on nucleic acid technology are available. Most vaccines are specific for use in chickens, some of which have indication for ducks and geese, while very few are specific for turkeys and ducks. Vaccines are mainly administered subcutaneously by injection with administration age varying widely, but most are recommended at 2 weeks of age. Very few can be administered earlier or in ovo. Again, number of doses varies greatly, with most vaccines requiring one dose, but a large number also requiring a booster dose. Limited information was retrieved on onset of immunity and its duration; data retrieved suggested 2–3 weeks after primary vaccination to achieve protection, with some HVT‐vectored vaccines requiring up to 4 weeks. Available H7 vaccines contain inactivated whole live AIV, are indicated for use mainly in chicken, and are administered via injection. Data on age of administration was very limited, as was that on number of doses and duration of immunity. Assessment of H5 + H7 vaccines showed that all vaccines contain inactivated whole live AIV, they are indicated for use in chicken, three vaccines are indicated for ducks and two of the latter also for geese. They are administered through injection, and information on age of administration and number of doses is limited, although most vaccines require more than a single dose.
Examples of application of vaccination against HPAI in three different countries outside the EU are reported; these experiences triggered the development of vaccine products that could fit a country's specific epidemiological needs (China in 2004, Mexico in 1995 and Egypt in 2005).
To assess vaccine efficacy a logistic regression model was developed that uses estimated values of VEs,sh to predict the probability that a vaccine would stop sustained transmission (Rv <1). For example, using this model, VEs,sh values that would predict probabilities of protection against transmission of 50%, 80% or 90% are 0.41, 0.67 and 0.82, respectively. This model was then applied to data derived from studies assessing vaccines by performing challenge studies. Results suggest that most assessed vaccines may reduce transmission of H5Nx HPAIV of clade 2.3.4.4 in chickens under experimental conditions. It should be mentioned that the model was developed using data from transmission experiments done with chickens only. Hence predictions for experiments done in turkeys or ducks must be interpreted with care.
A small antigenic distance, i.e. an optimal antigenic match, between the vaccine strain and the circulating field virus was found to be important for VE. Using an in silico approach to calculate antigenic distances, only a few H5 vaccines with a small distance to the clade 2.3.4.4b viruses of HPAI H5N1 currently circulating in Europe were identified.
Most of the data regarding serological response was limited to humoral immunity measured with the HI test; significant heterogeneity between studies was observed with respect to the virus strain used as antigen for the HI test. The data suggest an association between HI titres and protection against transmission, especially in chicken immunised with inactivated vaccines. This relationship is less clear in ducks and not present in turkeys.
Based on data on transmission experiments on chicken, full protection could be expected 2 weeks after vaccination, but this is based on the assumptions of good vaccination coverage and good antigenic match. At the same time evidence on duration of immunity is scarce, and although sequential challenge experiments can give more precise information on protection over time, experimental setups provide limitation to data interpretation. One study suggests that immunity to stop transmission in chicken could last less than 6 months, while in turkeys and ducks the duration of immunity has not been fully addressed.
Application of vaccination programmes for complementing HPAIV control and eradication could lead to different drawbacks that may put at risk timely eradication of the virus. The main drawbacks identified were (i) low VE to fully protect vaccinated birds and prevent new outbreaks, (ii) host‐specific factors that could lead to vaccine failure due to hampered immune response of vaccinated birds, (iii) inadequate vaccine coverage to stop virus circulation and (iv) inefficient surveillance that may lead to the inability to detect field virus in vaccinated flocks, resulting in clinically silent circulation of HPAIV.
Limitations and practical considerations – In the choice of a suitable vaccine, the main limitation is that there is no one (or best) solution for a given situation, due to the great number of different factors that are interconnected, in such a way that a decision tree solution is not possible at the moment. Most retrieved vaccines are based on whole inactivated virus, using a reverse genetics technology that allows handling in BSL‐2 laboratories and rapid update of vaccine seed strains. At the same time though full immunity or long term immunity for different production types may require more than one dose, and vaccines should be compatible with DIVA strategy for surveillance. Recombinant technology and tools for nucleic acid synthesis allow smooth adaptation to the circulating strains and require only information related to the genetic sequence of circulating strains, only two vaccines are based on nucleic acid technology (RNA replicon and DNA) but the ease for antigenic update for these vaccines is a major advantage and therefore it is likely that more will become available.
Most of the available vaccines are for use in chicken; off label use of vaccines in species other than chicken may be a solution, provided that the vaccine has the characteristics to be used in different poultry species, but lack of data on efficacy in minor poultry species hampers the prediction of possible outcomes of use in those species. Most vaccines are administered by injection, requiring manipulation of birds that can result in stressful situations for animals, bird size can result in difficulty in managing vaccine delivery, certain housing systems make individual administration challenging. There are no available vaccines that can be delivered through drinking water and only one through aerosol/spray. Data retrieved showed high variability in the age of first administration, ranging from 1 day to 6 weeks. It should be noted that interference of maternal immunity could reduce efficacy of vaccination and should be considered in age of administration, in particular for inactivated vaccines, whilst live vectored vaccines are less susceptible to maternal immunity and can be administered at early stages. Limited information on onset and duration of immunity is available; studies show onset after 2 to 3 weeks after vaccination. Study of onset and duration is of crucial importance not only to ensure adequate protection for the entire production cycle in each species and production type, but also to understand time needed to have a population protected after initiation of a vaccination campaign and to determine the ‘open window’ between vaccination and protective immunity. For live‐vectored vaccines compared with inactivated vaccines, the antigenic distance may have less impact on the VE. At the same time, it is also difficult to assess the antigenic match between the immunity provided by vaccines that contain multiple antigens or the effect of prime–boost immunisation schemes involving heterologous vaccines.
Vaccine efficacy studies showed that some vaccines can stop transmission under experimental conditions. However, these measures may not always match with effectiveness in the field and there may be also differences in different experimental trial layouts. Most of the studies that estimated vaccine efficacy only examined infection and shedding as parameters to express efficacy, although we show association between these factors, this association may be prone to bias for individual vaccines.
TOR 2 ‐ To define effective vaccination scenarios and to achieve effective prevention, control and eradication of HPAI outbreaks in poultry populations, both intrinsic factors related to the type of vaccine chosen and extrinsic factors about policy priorities and operational factors have to be evaluated. Several conditions might trigger the initiation of vaccination and the objectives of the vaccination programme should be defined accordingly to support rapid eradication or protection of poultry farms.
Factors that trigger the initiation of HPAI vaccination in poultry depend on the prevailing epidemiological situation (e.g. high number of poultry outbreaks, infected wild bird populations, high risk of virus introduction from neighbouring zones, etc.); the characteristics of the susceptible poultry population (e.g. high density of poultry farms – there is correlation between poultry density and poultry outbreaks, intense trading activities, presence of species not showing clinical signs of infection, presence of high‐genetic value species); and relevant environmental (e.g. farms in proximity to wetlands, areas with high density of migratory wild birds); and social factors (the public may not support culling as a preventive measure).
Formulation of vaccine scenarios will depend on the corresponding vaccination strategy:
Vaccination of poultry in affected establishments to obtain rapid eradication: this would lead to implement an emergency suppressive vaccination, which aims at a short, temporary containment of the disease.
Vaccination of poultry in case of a change in the risk of HPAIV infection to prevent disease introduction and spread/to maintain freedom from disease/to prevent economic losses: this would lead to implement emergency protective vaccination
Vaccination of poultry in the absence of a change in the risk of HPAIV infection to prevent disease introduction and spread/to maintain freedom from disease/to prevent economic losses: this would lead to implement a preventive vaccination
Using the transmission‐kernel model, risk maps were built: areas with a high risk of between‐farm transmission were characterised by farms with estimated reproduction number > 0.8.
Farm densities in areas with > 0.8 were > 0.54 farms/km2, > 0.52 farms/km2 and > 0.84 farms/km2 for France, Italy and The Netherlands, respectively. This means that within a 5‐km radius, a high‐risk area would have, on average, 43, 41 and 66 farms 1 in France, Italy and The Netherlands, respectively; these density values, corresponding to areas with high‐risk for transmission, were considered as high‐density poultry areas (HDPA).
In all three countries, the culling of infected farms only (S0) is the scenario involving the highest number of infected farms and the longest epidemic duration; concerning emergency vaccination scenarios, the 3‐km ring‐vaccination (S3) is the one that shows lowest numbers of infected, culled and vaccinated farms, and shorter epidemic duration. The preventive vaccination scenario (S5) overall resulted in the lowest number of infected farms and shortest epidemic duration in all case studies. It should be considered that these results rely on the accuracy of the model's assumptions and parameter values. The parameters are based on data provided by the studied countries and the model is a simplification of the real‐word complexity. The scenarios tested are hypothetical and do not necessarily cover all possible scenarios, whose choice and tailoring can vary based on each MS.
Logistic constraints, e.g. administration of vaccine injecting single individuals in a short period of time, that could affect vaccination delivery should be taken into consideration.
Conclusions and recommendations
Although large number of vaccines with different technologies are produced, few are applied commercially. Therefore, further research and development of AIV vaccines is required, and their effectiveness should be preferably tested in real life scenarios.
Since there is only a single authorised vaccine against AI currently in the EU, licensing of further vaccines is required. Standardised data and protocols should concentrate efforts to generate harmonised data by conducting standardised trials. Harmonised studies should also be conducted to provide data on onset and duration of immunity, maternal immunity effects and indications for species other than chickens. The concept of antigenic distance should be given consideration especially when using inactivated vaccines. Reference strains and sequences of recommended vaccine viruses should be made readily available and authorised vaccines should be rapidly adaptable to changing circulating viral strains. VE to bring virus transmission to R0 < 1 should be assessed also in field trials, taking into account real life limitations.
Based on model evaluations of hypothetical scenarios in three EU member states, preventive vaccination could be considered in high‐risk areas to minimise number of infected and culled farms and to decrease epidemic duration. In case of HPAIV outbreaks in high‐risk areas for between farm transmission, emergency protective vaccination in a 3‐km radius is recommended. The vaccine used should be selected according to the vaccination strategy. Rapid onset of protection, independence from species restrictions and mass applicability are hallmarks of vaccines most suitable for emergency protective vaccination strategies.
VE should be monitored over time. Vaccination should not be considered as a replacement for biosecurity and surveillance, instead it should complement these approaches.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
Regulation (EU) No 2016/429 2 on transmissible animal diseases (‘Animal Health Law’ ‐ AHL) lays down the rules for disease awareness, preparedness and control. Title I, Chapter 2 of Part III in the AHL lays down provisions for the use of veterinary medicinal products for disease prevention and control. In addition, Article 69 of AHL provides for the possibility of using emergency vaccination for the effective control of category A diseases.
The Animal Health Law empowers the Commission to adopt delegated acts supplementing the rules on that field laid down in that Regulation. Therefore, the Commission adopted Delegated Regulation (EU) No 2020/687 3 laying down rules for the prevention and control of certain listed diseases, in particular Category A diseases, including HPAI. Delegated Regulation (EU) No 2020/687 do not provide any specific rules regarding vaccination. Consequently, the Commission developed a Delegated Regulation providing for detailed and specific rules on the use in the Union of veterinary medicinal products for the prevention and control of certain diseases, including HPAI (in the specific Annex): Delegate Regulation (EU) 2023/361 4 .
More specifically, for the use of vaccines against HPAI, the specific Annex of the Delegated Regulation lays down:
specific conditions for the implementation of emergency protective vaccination for prevention and control of HPAI (Part 1);
reinforced clinical and laboratory surveillance to be implemented in the vaccination and peri‐vaccination zones during emergency protective vaccination (Part 2);
animals and products subject to prohibition of movements and conditions for granting a derogation in a vaccination zone where emergency protective vaccination for prevention and control of HPAI is carried out (Part 3);
recovery periods for HPAI following emergency protective vaccination (Part 4);
specific conditions for preventive vaccination of HPAI (Part 5).
Before the date of application of AHL, as general rule, vaccination against HPAI in EU was prohibited in accordance with the provisions laid down in Chapter IX of Directive 2005/94/EC 5 . However, the Member States following approval by the Commission of a vaccination plan could introduce emergency or preventive vaccination.
Only a limited number of Member States have implemented, for a short period, emergency or preventive vaccination against Avian Influenza. Most of the vaccination programmes against Avian Influenza had their validity expired in the years before 2010. Only one establishment with high value breeding mallard ducks in Portugal continued to implement preventive vaccination against avian influenza based on a vaccination programme approved until the end of 2020.
In the last three epidemic seasons of HPAI, the EU faced a constant increase in the detection of the virus in wild birds, in particular in migratory waterfowl birds. Multiple serotypes of the HPAI virus, consequence of multiple reassortant events, have co‐circulated during the same epidemic seasons.
Areas with high concentration of poultry have faced serious challenges to prevent the introduction and spread of the virus within establishments. Clusters of outbreaks have occurred in every epidemic season, in particular in the areas with intensive production of certain type and category of poultry.
Consequently, the Member States are looking for solutions to increase their ability to prevent and control HPAI by using vaccination as an additional tool to the already available preventive and control measures.
The latest scientific opinion on vaccination against avian influenza of H5 and H7 subtypes in domestic poultry and captive birds has been adopted by EFSA on 2007. That scientific opinion focused on: updating on the vaccines against avian influenza, including experiences with their use under laboratory conditions and in the field, as well as future perspective; evaluating laboratory testing methods for surveillance of vaccinated flocks in particular discriminatory tests used in the context of DIVA (Differentiating Infected from Vaccinated Animals).
Since 2007, new vaccines against avian influenza may have been developed, taking advantage of the scientific and technological progress in this domain. Therefore, a new updated overview is needed in this respect.
In the lack of recent scientific knowledge and of sufficient experience in EU with the implementation of vaccination against avian influenza, the conditions laid down in specific Annex for HPAI to the Delegated Regulation (EU) No 2023/361 are largely replicating the ones required in accordance with Directive (EC) No 2005/94 and other specific Implementing Decisions regarding approval of vaccination programmes against avian influenza in certain Member States.
However, the concept of vaccination against HPAI is complex when considering different epidemiological situations each year and differences existing in poultry species and poultry sectors. Therefore, there is no simple solution for vaccination against HPAI and for the measure to be implemented following vaccination that could fit in all epidemiological circumstances and for all poultry production sectors.
In order to obtain a better overview on the latest scientific developments, an update of the previous opinion on the aspects of vaccines and vaccination against HPAI is crucial. It is expected that this opinion will provide an important input for the Commission's overall approach on vaccination against HPAI. In addition, it will be a source of information to Member States when deciding on the vaccination strategies, as and when they become necessary.
Due to the characteristics of the requests in relation with the vaccines, we suggest European Medicines Agency (EMA) and Reference Laboratory of the European Union (EURL) for Avian Influenza being associated as appropriate to this mandate.
1.1.2. Terms of References
In view of the above, and in accordance with Article 29 of Regulation (EC) No 178/2002 6 , the Commission asks EFSA to provide scientific advice in the light of the new scientific data that has become available since the adoption of the above scientific opinion and other developments in the field. In particular, the Commission is requesting EFSA to:
- Update on the available vaccines against HPAI for poultry
-
1.1)Identify and describe the vaccines that may be available for vaccination of poultry against HPAI, including experiences with their use under laboratory conditions and in the field, as well as future perspectives indicating the cost‐effectiveness aspects, the scientific and practical advantages and disadvantages of the identified vaccines of different technologies, in particular against their:
- suitability to be used for different species of poultry (e.g. chickens, turkey, geese, ducks);
- capacity to protect against the currently circulating strains of HPAI virus, to be adjusted to the future strains of HPAI virus, to protect against multiple strains and to be used in the context of DIVA (Differentiating Infected from Vaccinated Animals) strategy using different surveillance diagnostic approaches/methods (serological, virological);
- effectiveness in preventing the infection with HPAI viruses;
- potential drawbacks, such as shedding of live attenuated strains.
-
1.2)Describe any possible aspects related to the use of different types of vaccines that may jeopardise a swift eradication of the virus in the vaccinated area.
-
1.1)
Vaccination strategies
-
2.1)Assess the suitable vaccination strategies to be implemented (emergency suppressive, emergency protective, preventive) taking into account:
- objectives of such strategy (i.e. maintain freedom of disease status, rapid eradication of HPAI virus in poultry including recovery of the free status or minimise losses with the eradication of outbreaks);
- risk factors that would trigger the need for vaccination to complement the preventive or control tools (e.g. high‐risk areas for the introduction and spread of HPAI viruses, type of production and industry practices, density of poultry establishments).
-
2.2)For the different vaccination strategies assess and recommend on the:
- suitability of establishing vaccination or peri‐vaccination zones, on the criteria to be considered when establishing such zones and on the minimum size of those zones;
- minimum coverage to be ensured in the vaccinated flock, establishment and zone in order to:
- reach the objective of the vaccination strategy, in particular in areas with specific risks such as high density of different poultry species, proximity to high risk wild bird habitats or type of production,
- prevent mutations of the HPAI viruses following circulation in an environment with insufficient immune response (vaccine escapes);
- the type of vaccine that might be used, considering the species and category or types of production (such as hatching eggs, day‐old chicks, laying hens, broilers, fattening/slaughter ducks and turkeys, ducks/geese for production of foie gras, breeding poultry of target species), including when targeting multiple species and category of production in the same area;
- the frequency of vaccination to ensure protection of the vaccinated birds, taking into account relevant factors such as the length of the risk period of infection with the HPAI viruses from wild birds, the level and duration of antibodies following vaccination with different type of vaccines as recommended in point c).
-
3
Surveillance in the vaccinated zone and/or vaccinated establishments
-
3.1)
Assess the suitability and effectiveness of the reinforced surveillance set out in Part 2 and in point 2 of Part 5 of HPAI Annex to the Delegated Regulation (EU) No 2023/361 to early detect infection in the vaccinated flocks and to prevent spread of the HPAI virus by movement of birds and their products from vaccinated establishments/flocks.
The above assessment should include scenarios depending on different vaccination strategies.
-
3.2)Taking into account the possible different surveillance approaches (e.g. serology, virology, conventional, DIVA, passive/active surveillance of vaccinated birds, use of sentinels) explore and provide for alternative suitable surveillance approaches/strategies indicating the minimum level and duration of surveillance required in a vaccinated establishment, including sampling schemes and testing procedures, to:
- ensure early detection of infection with HPAI viruses;
- be implemented as additional guarantees to authorise the movement of vaccinated and non‐vaccinated poultry and poultry products within the vaccinated zone and from the vaccinated zone or establishment to outside that zone or establishment;
- be implemented after cessation of vaccination as necessary risk mitigation measure to authorise movement of birds and their products from those establishments, taking into account:
the relevant factors to influence the level and duration of protection following vaccination with different type of vaccines,
simultaneous presence in the establishment of vaccinated and unvaccinated animals.
-
3.3)
Taking into account the WOAH standards (i.e. surveillance to be carried out in all vaccinated flocks) explore and provide for alternative suitable surveillance approaches/strategies to be implemented in a vaccination zone to demonstrate freedom from HPAI based on representative sampling of the vaccinated (and not vaccinated) establishments within a vaccination zone.
-
4
Restrictions and risk mitigation measures to be applied in a vaccinated establishment or a vaccination zone
-
4.1)
Assess the suitability of the restrictions and risk mitigation measures set out in Part 3 and in points 3 to 5 of Part 5 of HPAI Annex to the Delegated Regulation (EU) No 2023/361 to prevent the spread of HPAI viruses, enabling safe movement of poultry and their products following emergency and preventive vaccination, respectively.
-
4.2)
Explore and provide for alternative (to those referred in 4.1.) suitable movement restrictions and risk mitigation measures required to prevent the spread of virus by movement of birds or their products from a vaccinated establishment/vaccination zone.
1.2. Interpretation of the Terms of Reference
The available poultry vaccines against HPAI virus (HPAIV) considered in this work include inactivated vaccines or vaccines based on technologies other than live attenuated avian influenza virus, owning a marketing authorisation from a competent authority, used in the field or being in their later stage of development, for which laboratory or field experiments have been already performed and data on their performance are available. Prototypes of vaccines still in the pipeline will only be mentioned when pertinent as potential future solutions, but not assessed in detail.
1.2.1. Term of Reference 1 – Update on the available vaccines against HPAI for poultry
1.1 The ToR 1 was understood as the identification and assessment of the available poultry vaccines and their type (defined by the technology used to produce the vaccine, e.g. inactivated, subunit vaccines, recombinant vector vaccines) against HPAIV worldwide, which provides useful information for the assessment of further ToRs. Specifically, information on available vaccines and their characteristics (e.g. type of vaccine and the set of criteria listed below) are collected primarily by reviewing scientific literature. In addition, in collaboration with EMA, WOAH and the European and National Reference Laboratories (EURL and NRL), data from ongoing field and laboratory vaccination studies assessing vaccine efficacy against currently circulating HPAIV strains are collected. Each vaccine is assessed against a common set of criteria as mentioned in the ToR, including the possibility of using them in several poultry species, their protection capacity (against current strains, future strains and multiple strains), dose and administration route, the possibility of using them as DIVA, their effectiveness in preventing infection and risks of potential drawbacks such as asymptomatic infection, shedding of virus and transmission as a result of not having reached full immunity. This potentially broad list of available vaccines will be narrowed down to those that, according to their characteristics, would be considered suitable to reduce or stop transmission and allow the implementation of a suitable surveillance system.
An overview of vaccine types and technologies available or under development for HPAI vaccine production will be provided. This overview, combined with the assessment done on the available vaccines, will guide the identification of the best technologies for vaccine development for future circulating HPAIV strains.
1.2 According to the different types of vaccines available, a summary of the practical aspects that could jeopardise effective eradication and control will be given, e.g. shedding and silent circulation of HPAIV in vaccinated population, and limitations on applying a DIVA strategy.
1.2.2. Term of Reference 2 – vaccination strategies
The ToR 2 was interpreted by the working group (WG) as the assessment of different vaccination scenarios describing the application of the vaccination strategies in different conditions, including the parameters required to reach the most effective strategy to control within‐farm and between‐farm virus transmission.
2.1 A list of different vaccination scenarios are defined by reviewing scientific literature and complemented by new approaches/alternatives suggested by the WG. The three possible vaccination strategies detailed in the Regulation, i.e. preventive, emergency suppressive and emergency protective, will be characterised based on their objectives (freedom from disease, rapid eradication or minimising losses) and risk factors that would trigger as well as hamper their implementation (conditions that indicate the need to apply vaccination, immediate availability of a suitable vaccine and available vaccination capacity). This assessment will result in a set of scenarios (e.g. ‘preventive vaccination’ to ‘maintain freedom of disease status’ due to a ‘high risk of introduction of HPAIV’ in a ‘densely populated area’) that will be the basis for the assessment of ToR 2.2.
2.2 The vaccination scenarios defined in 2.1. are assessed using a between‐farm transmission model of HPAI (Boender et al., 2007), characterised by a spatial kernel describing how infectivity scales by geographical distance between farms (the smaller the distance, the higher the infection pressure, the higher the infection probability). To parametrise the model, epidemic and poultry holdings data from three European countries that were severely affected during the recent HPAI epidemic waves (i.e. France, Italy and The Netherlands) and including different poultry production systems (duck, turkey and chicken layers farms, respectively) are used. Then, the model simulates the spread of HPAI among poultry farms in these countries and the vaccination scenarios previously listed are compared to identify which one (considering poultry species, geographical zone and timing of vaccination) proves to be the most effective to reach the control objectives previously defined.
1.2.3. Term of Reference 3 – surveillance in the vaccinated zone and/or vaccinated establishments
ToR 3 was interpreted by the WG as the detailed assessment of different surveillance approaches following poultry vaccination. Following the same principles as in ToR 2, firstly, a list of different surveillance approaches are defined including the reinforced surveillance laid down in the Delegated Regulation (EU) No 2023/361 and the WOAH standards of surveillance of all the vaccinated flocks, complemented by new approaches/alternatives suggested by the WG. Afterwards, the effectiveness of these surveillance approaches for the different vaccination strategies will be assessed using suitable models. For example, compartmental models could be used to assess surveillance approaches for early detection during emergency response or scenario tree modelling could be used to assess freedom from disease once an epidemic has been controlled.
1.2.4. Term of Reference 4 – restrictions and risk mitigation measures to be applied in a vaccinated establishment or a vaccination zone
ToR 4 was interpreted by the WG as the detailed assessment of the impact on HPAI transmission of adding risk mitigation measures that encompass the use of vaccination against HPAI in poultry farms. As done for the surveillance approaches, the risk mitigation measures laid down in the Delegated Regulation (EU) No 2023/361 will be assessed together with new approaches suggested by the WG.
2. Data and methodologies
The methodological approach adopted to address the ToRs is described in the Protocol reported in Appendix A. The protocol was developed upfront of the initiation of the risk assessment. In this section, a more detailed description of the specific methodology used for each ToR is provided.
2.1. ToR 1 – Available vaccines
The aim of ToR 1 was to identify and describe the vaccines that are available for vaccination of poultry against HPAI. The term ‘available’ refers to inactivated vaccines or vaccines based on technologies other than live attenuated avian influenza virus that have been evaluated by a regulatory authority and have obtained a marketing authorisation, or that have been used inside or outside the EU, or that are in a late development stage with laboratory or field data on their performance already available. Prototypes of vaccines still in an early stage of development have been only mentioned when relevant, as they represent potential future solutions, but have not been assessed in detail. For this ToR, information about the available vaccines and their characteristics have been collected through literature review, pharmaceutical companies' websites, responses to a survey launched through EMA, WOAH and EURL, and NRL network consultation. In case of incomplete information from the literature search or the survey, companies were contacted to follow‐up and obtain more specific information. Note that the literature search has focused on vaccines for HPAIV regardless of the subtype (i.e. H5 and/or H7), whilst vaccine efficacy and antigenic matching have been estimated on H5 due to its predominant circulation in Europe in recent years.
2.1.1. Literature search on available vaccines for HPAI in poultry
To identify publications on HPAI in poultry that described the use of vaccines, a literature search was performed. It was conducted on 31 January, 2023, and the publication date was restricted from January 2018 to January 2023 to supplement a prior literature review carried out by EFSA on January 2022 where no time limit was retrospectively applied and which focused on commercial vaccines. The search was conducted in English and no restrictions were imposed on publication language and study location. Search terms were agreed by experts in the following Boolean query: “highly pathogenic avian influenza” AND “vaccin*” and were searched in all fields for CAB Abstracts, Web of Science and Scopus. Retrieved papers from those databases (n = 240, n = 352 and n = 294, respectively) were merged to those identified by the previous literature review performed by EFSA (n = 95) (EFSA, online). Following removal of duplicates, inclusion criteria for the primary (based on abstract and title) and secondary (based on full text) screenings of the articles included: (i) the topic of experimental studies of vaccine efficacy against HPAI and (ii) reference to a vaccine seed and challenge virus. Exclusion criteria for the primary and secondary screenings of the articles consisted of the following: the full text was not available in English, the study involved the use of live attenuated vaccines only, the study used a non‐poultry animal model (such as ferret or mouse), the study involved low pathogenic avian influenza (LPAI) viruses only. Discussions among experts occurred at all stages until a consensus on the studies to be included was reached based on the above‐described criteria.
The resulting literature selection cascade is depicted in Figure 1. In total, 95 articles were kept for data extraction on characteristics of the vaccines. Information collected from each paper included details about the vaccines and challenge strains. The complete set of information extracted is available in Table A1 (Annex A); the summarised results are presented in Section 3.1.2.
Figure 1.
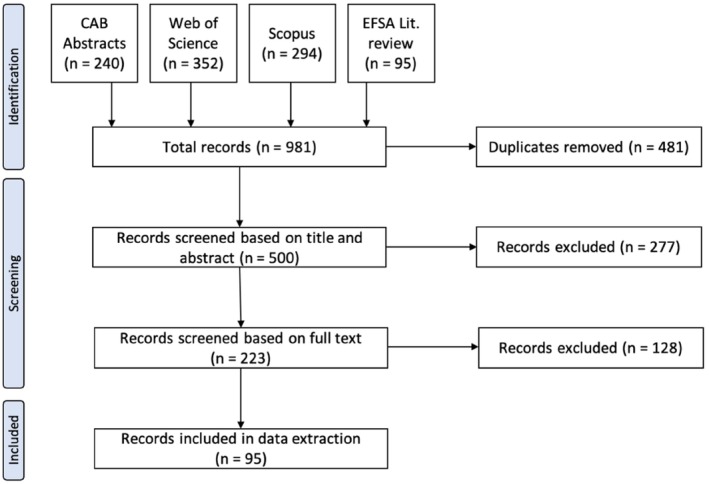
Flow diagram of the literature search
- A previous EFSA literature review (EFSA, online) retrieved 95 references. Papers published between 2018 and 2023 that were retrieved from CAB Abstracts, Web of Science and Scopus, using the same search terms were added to those initial 95 for a total of 981 records in the identification step that were subjected to further screening.
Websites of pharmaceutical companies that produce and commercialise the vaccines that were retrieved via the literature search were also consulted. These websites provided access to the product leaflets containing detailed information about the vaccine characteristics, including their composition, recommended dosages and potential side effects. Information retrieved from this source on the vaccine characteristics was also added in Table A1 (Annex A).
2.1.2. Surveys on available vaccines for HPAI in poultry
Information about the available vaccines and their characteristics, i.e. those reported in Table A1 (Annex A), were also collected through responses to a survey launched through EMA, WOAH, EFSA and EURL, and NRL network consultation. Pharmaceutical companies were directly consulted by EMA or EFSA in case of incomplete information from the literature search or the survey.
2.1.2.1. Consultation of EMA network
In February 2023, EMA launched a survey to collect information on existing or under development HPAI poultry vaccines. The survey targeted EU pharmaceutical companies and was conducted through the EU Survey platform, including 14 open‐ended and close‐ended questions. The aim of the survey was to determine whether the companies had or did not have such vaccines in their portfolio. Additionally, the survey provided an opportunity for companies to express their willingness to discuss detailed information with EMA in an online meeting.
EMA distributed the survey within their network via AnimalhealthEurope (the association representing the manufacturers of animal medicines, vaccines and other animal health products in Europe), on the official EMA LinkedIn profile, on the ‘Pharmaceutical industry’ landing page of the EMA's corporate website, and to the veterinary industry stakeholder's distribution list (which includes, among others, the association representing small to medium enterprises).
After a 15‐day response period, in total 18 responses were received and screened by the EMA staff to exclude irrelevant responses and to evaluate the information provided. Relevant responses with available contact details were selected for further follow‐up. This led to three follow‐up meetings and two additional companies that provided more detailed information via electronic communication.
Non‐commercially confidential data provided by the companies are reported in Table A1 (Annex A) together with the data from the literature search.
2.1.2.2. EFSA follow‐up
Since the EMA network covered mainly European companies or companies with a significant European market, EFSA further distributed the survey, and in particular the prototype of Table A1 (Annex A) to be compiled, to a number of additional companies outside of the EU. These companies were selected based on the results of the literature review. Specifically, companies outside the EU identified from the scientific papers as having a vaccine production relevant for the scope of this assessment were contacted by EFSA to collect more detailed information. This resulted in three additional answers with all the information collected being reported in Table A1 (Annex A).
2.1.2.3. World Organisation for Animal Health network consultation
WOAH forwarded the survey to members of the Global Framework for the Progressive Control of Transboundary Animal Diseases (GF‐TADs) 7 regional steering committee of Asia‐Pacific where avian influenza vaccination is practised and to WOAH reference laboratories, but no contribution was received from this channel.
2.1.2.4. Consultation with EURL and NRLs network
The European Reference Laboratory (EURL) for Avian Influenza and Newcastle Disease was consulted to provide information about the available vaccines and their characteristics: it participated in the literature search and assessment of available HPAI vaccines, provided information obtained from the NRL network on current vaccination trials being performed in the EU and analyses to assess the antigenic match between currently circulating strains and available vaccines.
2.1.3. Parameters to describe the characteristics of available vaccines
2.1.3.1. Type, technology and available vaccines
The vaccines were described based on a two‐stage categorisation process. First, they were categorised based on general information, such as the vaccine virus subtype (H5, H7, etc.), replication competence of the vaccine virus (replication defective, competent or compromised), the principle of vaccine development (inactivated, vectored, etc.), the production technology (recombinant, synthetic, plasmid, etc.), genetically modified organism (GMO) status and its interference with maternal immunity (see Table 3). Secondly, detailed information for each vaccine was provided, such as the technology used (e.g. inactivate whole avian influenza virus (AIV), virus‐like particles, etc.), the vaccine development status (e.g. authorised, experimental, etc.), the vaccination protocol (administration route, age of first dose, dosage, number of doses, etc., the vaccine efficacy parameters and antigenic matching with currently circulating strains (clade 2.3.4.4.b), and the onset and duration of immunity (see Table A1 in Annex A).
Table 3.
Vaccine types and production technology
| Type | Principle | Technology | GMO (a) | Genetic engineering technology (b) | Maternal immunity interference | Number of identified vaccines |
|---|---|---|---|---|---|---|
| Replication defective | Inactivated full virus | Virus amplification, adjuvantation | No | Possible | Yes |
H5 subtype = 46; H7 subtype = 11; H5 + H7 vaccines = 6 |
| Inactivated vector vaccine | Recombinant expression of major immunogenic proteins using replication‐deficient vectors for vaccination | No | Yes | Yes | 1 H5 vaccine | |
| Split vaccine | Virus amplification, purification, adjuvantation | No | Possible | Yes | None met criteria for inclusion | |
| Subunit vaccine | Recombinant expression of 1–3 proteins (e.g. bacteria, yeast, plant, fungi, insect, avian and mammalian cells), purification, adjuvantation or incorporation into virus‐like particles | No | Possible | Yes | H5 subtype = 1 | |
| Nucleic acids | Synthetic mRNA | No | Yes | Unknown | None met criteria for inclusion | |
| Plasmid DNA | No | Yes | Unknown | 1 H5 subtype = 1 | ||
| Replication‐competent | Live vector vaccine | Recombinant expression of major immunogenic proteins using replication‐competent vectors for vaccination (e.g. adenovirus, duck enteritis virus, herpesvirus of turkey, Newcastle disease virus, baculovirus, Fowlpox virus, infectious laryngotracheitis, Salmonella, lactobacilli) | Yes | Yes | Depending on vector | H5: live vector = 7; H7: live vector = 3 |
| Native H5/H7 AIV | Live attenuated vaccines not feasible due to high risks of reversion to virulence | No | Possible | n.a. | – | |
| Replication compromised | Replicons | Recombinant replication compromised RNA (i.e. alpha virus replicon, VSV replicons) | Yes | Yes | Yes | H5 subtype = 1 |
| Codon de‐optimisation | Recombinant (synthetic) attenuated influenza virus | Yes | Yes | Yes | None met criteria for inclusion |
According to Directive (EC) No 2001/18 (Directive 2001/18/EC of the European Parliament and of the Council of 12 March 2001 on the deliberate release into the environment of genetically modified organisms and repealing Council Directive 90/220/EEC OJ L 106, 17.4.2001, p. 1–39.) genetically modified organism (GMO) means an organism (any biological entity capable of replication or of transferring genetic material), with the exception of human beings, in which the genetic material has been altered in a way that does not occur naturally by mating and/or natural recombination (e.g. vaccines containing a herpesvirus of turkey [HVT] vector are considered GMO).
Genetic engineering technology allows the modification of genomes by inserting, deleting and modify DNA at multiple levels. This technology is used to produce a product that itself could be considered a GMO or not considered GMO (e.g. a whole inactivated H5 vaccine with a LP cleavage site that has been engineered by reverse genetics).
2.1.3.2. Antigenic distance as an in silico measurement for vaccine strain selection
Selection of vaccine strains is pivotal for a successful vaccination programme, as vaccine protection is most likely to be achieved when the vaccine strain is closely related to those antigenic variants that would likely cause future outbreaks. Antigenic match of the field and vaccine strain can be quantified by a haemagglutination inhibition (HI) titre using animal sera raised against the vaccine viruses that prevent agglutination of red blood cells by a standardised amount of an influenza field virus. This in‐vitro measure, coupled by phylogenetic analyses, notably of the HA gene, is used to describe and map the appearance of new virus strains or antigenic‐escape mutants. As the hemagglutinin protein (HA) of influenza viruses changes continuously, great attention must be devoted to measure the antigenic distance between circulating viruses and the vaccine strains. However, determining the antigenic distance is methodologically demanding, as it is based on an explicit and robust measurement. Furthermore, it is limited by the availability of seed strains, field viruses and corresponding reference sera by the laboratories performing this type of analysis and on continuous monitoring of the virology and epidemiology of influenza viruses.
OFFLU, WOAH and the FAO network of expertise on animal influenza were contacted to gather information on the work conducted on antigenic cartography through the Avian Influenza Matching project (OFFLU AIM). However, the first report of this project was not finalised at the time of writing this Scientific Opinion and could not be used in the current assessment.
To quantify the antigenic distance between vaccine seed strains and the currently circulating HPAI viruses in Europe, an in silico analysis was conducted, based on the methodology described by Peeters et al. (2017). First, a consensus sequence of the HA gene segment of H5Nx clade 2.3.4.4b virus genomes circulating in Europe between 1 October 2022 and 14 February 2023 was determined by downloading and aligning sequences available from the GISAID (GISAID, online) online database (n = 17,451, accessed on 14 February 2023) using MAFFT v7 (Katoh and Standley, 2013). Figure 2 provides information on the geographical sources of the virus genomes sequenced that were used to determine the consensus sequence. The consensus amino acid sequence of the HA protein was generated from this alignment with the EMBOSS Cons online tool (EMBL‐EBI, online), with an identity threshold of 70%. In addition, HA sequences of vaccines listed in Table A1 (Annex A) were collected, when available, from public databases. Vaccines with partial HA sequence data, particularly in the examined region, were excluded from this analysis. Secondly, the amino acid residues were extracted for this set of HA sequences at the following 27 positions: 53, 72, 97, 115, 124, 129, 133, 136, 138, 140, 141, 144, 151, 154, 162, 163, 165, 183, 184, 185, 188, 189, 190, 194, 212, 226 and 236. These positions have been identified as pivotal in defining antigenic epitopes associated with resilient protective immunity (Peeters et al., 2017). Notably, it has been shown that the genetic and antigenic differences correlated better in those 27 selected residues than in the complete HA1 domain.
Figure 2.
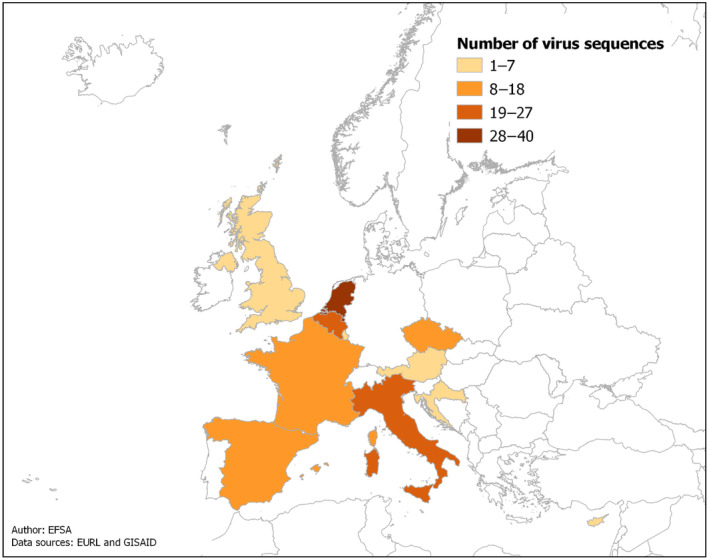
Geographical source of the H5Nx clade 2.3.4.4b virus genomes sequenced in Europe between 1 October 2022 and 14 February 2023 and analysed to determine the consensus sequence
A linear regression model was built to quantify the antigenic distance as a function of genetic differences at the 27 selected positions and fitted to the data provided by Peeters et al. (2017) in the supplementary material. For each vaccine strain considered in this opinion and for which the genetic sequence of the HA gene was available in public repositories, the genetic distance from the consensus sequence was calculated as the proportion of the amino acid residues that differed between the two viruses on the total extracted at each of the 27 selected residues. The fitted regression equation was then used to estimate the antigenic distance values (i.e. the number of antigenic units (AU) of distance, where one unit is equivalent to a two‐fold dilution in HI or virus neutralisation assay data) for the vaccine seed strains listed in Table A1 (Annex A).
Note that attempts to perform a similar distance analysis for European H7 viruses were unsuccessful due to the limited circulation of H7 subtype avian influenza (AI) viruses in Europe in the recent years.
2.1.3.3. Vaccine efficacy parameters
Generally speaking, vaccine efficacy (VE) is a measure of the effect of a vaccine on preventing disease/infection in a population. For this assessment, however, our primary interest was the assessment of the efficacy (VET) of the vaccine to stop sustained HPAIV transmission in a vaccinated population. To assess sustained HPAIV transmission, the parameter used as reference was R, which is the mean number of secondary infected individuals in a naïve (or uninfected) population caused by a single infected individual during the period of infectiousness. If R ≥ 1, sustained transmission can occur; when R < 1, transmission fades out.
Ideally, VET would be assessed by performing transmission experiments where transmission among vaccinated birds is quantified (Rvac) and compared with transmission among unvaccinated birds (Runv) (Sitaras et al., 2016; Palya et al., 2018; Germeraad et al., 2019). However, most of the published studies available in the literature review did not explicitly assess the efficacy of a vaccine to stop transmission. In these studies, assessment of efficacy was made based on challenge experiments in which all animals were exposed to virus by direct inoculation and no sentinel birds either vaccinated or not were included to monitor transmission. However, from this type of experiments, secondary VE parameters can be estimated: (a) VE to decrease the susceptibility of vaccinated birds (VES), (b) VE to decrease mortality in vaccinated infected birds (VEm) and (c) VE to decrease virus shedding in vaccinated infected birds (VESh). The latter can be used as a proxy measure for the reduction in infectiousness. Also, VES and of VESh were combined to estimate the decrease of the susceptibility and shedding in vaccinated infected birds (VEs,sh). In Table 1, information on the VE parameters considered and how they were retrieved or estimated is presented.
Table 1.
Vaccine efficacy (VE) parameters quantified for each of the vaccines evaluated within a selected study using data from vaccinated and unvaccinated control groups within a study. These parameters were quantified from data extracted from selected transmission and challenge experiments
| Parameter | Estimation | Description | |
|---|---|---|---|
| VE to stop transmission in vaccinated infected birds (VE T ) | Based on the value of that was directly retrieved from the studies. For a vaccine to be effective to stop transmission | is the reproduction number estimated in both the vaccinated and unvaccinated groups . This parameter was obtained only from studies performing transmission experiments. For the study to be valid for data extraction had to be > 1 | |
| VE to reduce susceptibility to infection in vaccinated birds (VE s ) |
|
is the proportion of infected animals following direct inoculation of the virus estimated for the vaccinated and unvaccinated groups | |
| VE to reduce mortality in vaccinated infected birds (VE m ) |
|
is the proportion of birds dying following infection estimated for the vaccinated and unvaccinated groups | |
| VE to reduce virus shedding in vaccinated infected birds (VE sh ) |
|
is the level of virus shed (log10 transformed) in the vaccinated and unvaccinated groups | |
| VE to reduce susceptibility and shedding in vaccinated infected birds (VE s,sh ) |
|
According to the above‐described VEs and VEsh |
Whilst the combined effect of the secondary VE parameters, collected from challenge experiments, contribute to the potential reduction in transmission, we still cannot infer whether the observed effects could lead to R < 1. Hence, we performed the literature review and quantitative analyses in two steps:
Review of transmission experiments: Twelve studies were selected (nine studies using chickens and three using mule and Pekin ducks) and data were extracted from the unvaccinated and vaccinated groups to quantify VES, VEm, VEsh and VEs,sh (Table A3 in Annex A) for each vaccine assessed within each study. Note that these VE parameters are estimated using as reference the unvaccinated (control) group within the same study. Therefore, the influence of different experiment procedures (e.g. inoculation dose, inoculation route, etc) between studies is minimised. In addition, the estimated R values for the unvaccinated () and vaccinated () groups were also extracted. The R value of the vaccinated group was used to create a binomial variable classifying whether the vaccine could stop transmission (R < 1) or not. This variable was then used as response variable to assess the association of the parameters VEs,sh with the probability of sustained transmission by fitting univariate logistic regression models. VEs,sh was chosen because it shows the combined effect of the vaccination on susceptibility and infectivity (using virus shedding as indicator).
Review of challenge experiments: Data from the 28 selected studies (which assessed one or more vaccines and species) were extracted (23 used chickens, six Pekin, mule and Muscovy ducks and one turkeys) from the unvaccinated and vaccinated groups to quantify VES, VEm, VEsh and VEs,sh (Table A2 in Annex A) for each vaccine assessed within each study. Next, using the outcome from the logistic model fitted in step 1, for each value of VEs,sh the probability Rvac < 1 was estimated to infer the probability of the vaccine to potentially stop transmission in vaccinated birds (VET).
2.1.3.4. Correlates of protection
Vaccination will induce an immune response (humoral and cellular), which in turn is responsible for the different effects previously described and quantified as VE parameters (see Section 2.1.3.3). From the reviewed studies (Table A1 in Annex A), there were data, at animal level, only for the humoral response. From the latter we were particularly interested in the antibody titre measured using the HI test, which is a surrogate test for the neutralisation assay. These data were extracted from transmission and challenge experiments to try identifying an HI threshold value that could be considered a correlate of protection to stop transmission, using a methodology described by Sitaras et al. (2016), or protection from infection as assessed by fitting dose–response models.
2.1.4. Undesired vaccine‐related aspects that could jeopardise eradication and control
Undesired characteristics of vaccines and vaccination strategies that could jeopardise the eradication and control of HPAI in poultry were identified by the experts of the WG via group discussions and described in the relevant section (Section 3.2). A causal diagram describing the possible causal paths leading to failure of eradication of HPAI in vaccinated areas was compiled using the R package ‘dagitty’ (Textor et al., 2016).
2.2. ToR 2 – vaccination scenarios
The aim of ToR 2 was to identify effective vaccination scenarios to control within‐ and between‐farm HPAIV transmission. General key factors to build a vaccination scenario, including policy priorities and operational factors, were first identified. Specific scenarios were then developed and compared for three European countries (France, Italy and The Netherlands) with recent HPAI outbreaks in specific poultry species (duck, turkey and chicken farms, respectively) using a kernel model. The aim was to identify the most effective vaccination scenarios to complement country‐specific control measures.
In the present assessment, a ‘farm’ was considered to be characterised by unique location (XY coordinates), owner and poultry species.
2.2.1. Definition and assessment of vaccination scenarios for HPAI in poultry
Building a successful vaccination scenario to control HPAI in poultry requires a systematic approach that considers multiple factors. Based on the definitions of vaccination strategies provided by the Delegated Regulation (EU) No 2020/687 and complemented by group discussions, the experts of the WG identified several key factors to consider when building such scenarios. This includes factors that trigger the initiation of vaccination such as the prevailing epidemiological situation, the characteristics of the susceptible poultry population, and relevant environmental and social factors. Additionally, the objectives of the vaccination programme, which significantly shape the design of the vaccination scenarios, were taken into account.
Based on these key factors and via group discussions, the experts of the WG defined a number of specific (hypothetical) vaccination scenarios to be assessed using the selected three European countries as case studies: France, Italy and The Netherlands. These scenarios accounted for the vaccination strategy according to Regulation (EU) No 2023/361, the target poultry species and their production systems, the timing of vaccination and the geographical size of the vaccination zones. France, Italy and The Netherlands were chosen as case studies due to their recent experiences with major HPAI epidemics involving the currently circulating virus strains and their specific affected poultry production systems and species of interest (ducks, turkeys and chicken layers, respectively). It should be noted that these assessment does not aim at identifying specific strategies for each of these countries but to assess the overall effect of vaccination under different poultry production conditions.
In each country, the virus was assumed to be introduced via wild birds into densely populated poultry areas, where the risk of between‐farm transmission is the highest (Boender et al., 2007; Dorigatti et al., 2010; Backer et al., 2015). While other introduction scenarios, like multiple virus introductions via wild birds, are possible, exploring them would need more time, which was limited for this assessment.
The between‐farm transmission and the impact of the vaccination scenarios were then investigated using the methodology described by Boender et al. (2007) and Backer et al. (2015), respectively. An overview of the method's key aspects is given below, while more details can be found in Boender et al. (2007) and Backer et al. (2015).
Briefly, we used a Susceptible‐Exposed‐Infectious‐Removed (SEIR) model framework (see point [a] and [b] below), incorporating a spatial kernel for between‐farm transmission dynamics based on Boender et al. (2007). This model was extended to allow for poultry production and species differences as well as for farm size (total number of susceptible birds housed) in the quantification of transmission probabilities (equation 2). Within this model, the likelihood of virus transmission between farms is dependent on the distance between the source farm i and the destination farm j and the corresponding poultry type, with all possible infection routes being represented by a single probability function (equation 1).
| (1) |
where is the infectious period (in days) of farm and is a transmission kernel (equation 2)
| (2) |
where parameter determines the maximum value of the kernel (amplitude), is the distance between the source and the destination farm, parameter is the between‐farm distance for which the kernel value is at half of its maximum and parameter α determines the shape of the kernel (more precisely, the rate of decline at long distances). and represent the total number of birds in the source and destination farm and is a parameter that describes the dependency between farm size and transmission. represents an element of a mixing matrix which considers the relative infectiousness of a source farm with poultry type (e.g. layers) and the relative susceptibility of a destination farm with poultry type (e.g. fattening turkeys).
For this assessment, the following steps were followed:
1) The kernel parameters, which describe the transmission dynamics in a non‐vaccinated population, were quantified for each of the three countries by fitting the model to available data from H5N1 epidemics that occurred between 2021 and 2022 in France and Italy. As for the Netherlands, because limited between‐farm transmission of H5Nx viruses has been observed since 2014, the kernel parameters were based on the 2003 H7N7 HPAI epidemic updated to the 2022 situation of the poultry sector (numbers of farms, spatial distribution of farms, farm sizes and farm types) and validated against the H5N1 outbreaks observed in 2021–2022 (Hagenaars et al., 2023). In summary, the analyses relied on the availability of the following information, within the study period:
a) Information about poultry population data: spatial location of poultry farms during the study (epidemic) period, species, number of birds present and type of production. Because including all types of production systems (e.g. fattening turkeys, turkey breeders, layer breeders, broiler breeders, fattening ducks, fattening geese, duck/geese for production of foie grass, etc) would result in a large number of variables included in the model which would make the fitting process complex and reduce the power to identify variables (infectiousness or susceptibility of the different production systems) that significantly contributed to transmission during the epidemics, we reduced the number of variables by grouping them in five poultry types: chicken layers & breeders, broilers, turkeys, ducks & geese and others (e.g. quails, pheasants, etc.).
For this assessment it was assumed that all poultry farms were active (meaning that they housed susceptible poultry) at the beginning of the study period (Susceptible). It is also assumed that positive/pre‐emptively culled farms (i.e. farms within which birds have been culled) were no longer populated/active during the course of the epidemic. The latter is likely to be the case since in affected areas no repopulation is allowed until the outbreak(s) are considered eradicated and after a risk assessment by the CA, and the requirements of the Regulation (EU) No 2020/687 are fulfilled.
b) Information about HPAI outbreaks: spatial location of affected establishments, dates of expected infection with an assumed 1 day latent period (Exposed) and duration of infectious period (Infectious). The date of expected infection for each infected farm in France and Italy was estimated based on previous studies: in France and Italy, each infected farm was assumed to have been infected ~ 7 days (irrespective of farm size, poultry species and production type) before the date of reported suspicion, based on estimates made by Hobbelen et al. (2020) and Lambert et al. (2023). Each farm has a different reported suspicion date and therefore a different date of infection was estimated. In The Netherlands, there were data available to estimate day of infection for most of the infected farms (i.e. virus is transmitting among poultry within the farm). The duration of the infectious period for each infected farm was assumed to start from day 1 post‐expected infection (1 day latent period was assumed) and to end on the day when the infected farm was culled (Removed) (Hobbelen et al., 2020). Then the median duration of the infectious period was calculated from these data (Table B.2 in Appendix B).
Table B.2.
Estimated median length of the infectious period for each of the poultry types (species) considered in the estimation of the kernel parameters and simulations to assess the vaccination scenarios
| Country | Infectious period (days) | ||||
|---|---|---|---|---|---|
| Layer and breeders | Broiler | Turkey | Duck and geese | Others | |
| France | 13 | 13 | 13 | 10 | 12 |
| Italy | 26 | 16 | 16 | – | 12 |
| The Netherlands | 12 | 12 | 12 | 12 | – |
c) Information about pre‐emptively culling: date and spatial location of pre‐emptively culled farms.
Once the transmission kernel model was fitted to the epidemic data, the between‐farm reproduction number for each individual farm was calculated and then used to generate transmission‐risk maps where regions with a high risk of between‐farm transmission were identified using the method described in Boender et al. (2007). To express the transmission risk in terms of farm density instead of , we explored the relationship between each farm (response variable) and their corresponding farm density (explanatory variable) by fitting linear regression models. Farm density was calculated as the number of farms within a 1‐, 3‐, 5‐ or 10‐km radius of each farm. A model was fitted for each of these densities (calculating the radius) and the best fitting model was selected.
2) Control measures included in the model (vaccination and culling): The estimated kernel parameters for each country were used to perform epidemic simulations and compare the following outcomes resulting from the implementation of the intervention scenarios, with and without vaccination: duration of the epidemic, the number of infected farms, the number of culled farms (either because detected as infected or because pre‐emptively culled) and the number of vaccinated farms (i.e. farms within which birds have been vaccinated) for strategies involving vaccination.
Table 2 presents the list of specific vaccination scenarios within the framework of emergency protecting and preventive vaccinations, to be assessed for three European MS: France, Italy and The Netherlands.
Table 2.
Description of the specific vaccination scenarios to be assessed for three European countries (France, Italy and The Netherlands). All scenarios are in addition to compulsory measures like establishing a 3 and 10 km zone for surveillance and movement control
| Scenario | Description |
|---|---|
| Scenario 0 (S0) |
No vaccination Culling in all infected poultry farms |
| Scenario 1 (S1) |
No vaccination Culling in all infected poultry farms Preventive ring culling in all poultry farms within 1‐km radius of infected poultry farms |
| Scenario 2 (S2) |
Emergency protective vaccination of all poultry farms (except broiler farms) within a 1‐km radius of infected poultry farms Culling in all infected poultry farms |
| Scenario 3 (S3) |
Emergency protective vaccination of all poultry farms (except broiler farms) within a 3‐km radius of infected poultry farms Culling in all infected poultry farms |
| Scenario 4 (S4) |
Emergency protective vaccination of all poultry farms (except broiler farms) within a 10‐km radius of infected poultry farms Culling in all infected poultry farms |
| Scenario 5 (S5) |
Preventive vaccination of only duck farms (France), turkey farms (Italy) or layer chicken farms (The Netherlands) located in the high‐risk transmission area (Rh ≥ 0.8) Culling in all infected poultry farms |
The scenarios presented in Table 2 are hypothetical and are not based on the intentions of the MSs to apply vaccination against HPAIV in poultry, as assessed in the current opinion. These scenarios were selected by the WG to assess specific cases of vaccination programmes that could serve as example for other MS. They do not necessarily cover all possible scenarios, the choice and tailoring of vaccination scenarios can vary based on each MS.
The scenarios are focussing on the main domestic poultry species (chickens, domestic ducks and turkey) reared in areas that have been heavily affected by HPAIV in recent years.
Emergency suppressive vaccination strategy was not considered in this assessment as it involves vaccinating poultry in an infected farm and subsequently culling them to reduce virus spread. However, due to the time needed to develop immunity, varying VE and the potential for virus transmission during vaccination practices, immediate culling of poultry is more effective to eradicate the outbreaks and control secondary spread. Also, backyard farms were not considered in this assessment (see Section 3.5).
Two scenarios (S0 and S1) were used as baseline for the comparison of the vaccination scenarios. Scenario S0 consisted only of culling of infected poultry as the minimal control measure required by the Regulation (EU) No 2020/687, and S1 additionally included preventive ring culling within a 1‐km radius of infected poultry farms. Both scenarios did not include vaccination.
Three scenarios (S2, S3 and S4) involved emergency protective vaccination, in which vaccination of poultry is assumed to be implemented upon detection of an outbreak to control disease spread. Given the time required for onset of immunity (as indicated in the literature review in Section 3.3), poultry farms were assumed to be protected 3 weeks following vaccination. Scenarios S2, S3 and S4 considered vaccination of all poultry species within a 1‐km, 3‐km and 10‐km radius of infected poultry farms, respectively (ring vaccination). However, chicken broilers were excluded from vaccination considering their short life span compared to other poultry production systems and the results of the kernel analysis, which showed for all three countries that broilers farms were significantly less susceptible to HPAIV infection than layers and breeders.
Scenario S5 involved preventive vaccination, in which vaccination of poultry is completed before potential exposure to the virus, aiming at pro‐actively protecting poultry against future outbreaks. Consequently, vaccination should align with the natural infection cycle of the virus to ensure maximum for optimal effectiveness. In this scenario, poultry were assumed to be fully protected before the occurrence of the first outbreak. Scenario S5 considered vaccination of poultry located in areas with the highest risk of between‐farm transmission (identified in Section 4.2), which corresponded to regions with the highest poultry density (see Section 4.2 for the density threshold). S5 targeted only poultry species with higher susceptibility and/or spreading potential, which were expected to contribute the most to secondary virus transmission (e.g. duck farms in France, turkey farms in Italy and layer chicken farms in The Netherlands; Table B.1. in Appendix B). Note that for France, duck farms included duck farms, geese farms and duck breeder farms; in Italy, turkey farms included meat turkey and turkey breeder farms; in The Netherlands, chicken layers farms included also breeders and rearing farms. An additional scenario was considered in which broilers were also vaccinated alongside with the specific targeted poultry species. However, this inclusion of broilers did not lead to significant changes in the conclusions of the outcomes of the scenarios (data not shown).
Table B.1.
Maximum likelihood estimates of the between‐farm transmission kernel parameters. Values presented for each country are the mean value (95% confidence intervals)
| Parameter | Description | The Netherlands | Italy | France | |
|---|---|---|---|---|---|
|
|
Amplitude (height) | 0.0022 (0.0012–0.0041) | 0.0005 (0.0003–0.0011) | 0.0004 (0.0003–0.0006) | |
|
|
Shape | 2.20 (1.87–2.63) | 2.28 (2.05–2.54) | 2.29 (2.19–2.39) | |
|
|
Half kernel distance | 2.50 (1.47–3.89) | 2.35 (1.78–3.10) | 4.38 (3.91–4.91) | |
|
|
Farm size dependence | 7,490 (4,510–11,900) | 6,364 (5,482–7,289) | ||
| g L I L (a) | Layers/breeders (reference) | 1 | 1 | 1 | |
| g B (a) | Relative susceptibility broilers | 0.134 (0.041–0.322) | 0.452 (0.328–0.625) | 0.250 (0.217–0.275) | |
| g D (a) | Relative susceptibility ducks/geese | 0.377 (0.093–0.999) | – | – | |
| g T (a) | Relative susceptibility turkeys | 3.00 (1.82–4.67) | 1.27 (0.96–1.69) | 2.59 (2.30–2.96) | |
| g o (a) | Relative susceptibility Other poultry types | – | – | 0.123 (0.104–0.145) (c) | |
| I D (a) | Relative infectiousness ducks/geese | – | 7.27 (2.87–9.84) (b) | 19.66 (15.39–25,56) | |
| I T (a) | Relative infectiousness turkeys | – | 5.34 (2.76–9.84) | – |
These parameters represent the relative susceptibility or infectiousness of these poultry species relative to chicken layers/breeders.
This parameter (for Italy) also include other poultry types (e.g. quails, pheasants). These population consisted of 158 duck farms and 18 farms (others).
This category included the following poultry species/production type: pigeon, quails breeders, quails fattening, game birds, guinea fowls breeders, guinea fowls fattening, multispecies with palmiped in the pregavage stage, multispecies with palmiped in the gavage stage, multispecies with palmiped layers, multispecies with palmiped fattening, multispecies with no palmiped fattening, multispecies with no palmiped layers, multispecies breeders, multispecies fattening.
In all vaccination scenarios, it was assumed that only 70% of the vaccinated farms would be effectively protected. This percentage, estimated by expert knowledge, takes into account different aspects that could limit reaching 100% protection (e.g. concurrent infections, flaws in the vaccination process, etc.). Moreover, particularly when considering preventive vaccination, immune waning over time may result in the loss of protection in some farms. A lower value of level of protection of 50% was also tested, and did not lead to changes in the order of magnitude of the outcomes in the different scenarios (data not shown), hence leading to the same conclusions.
In this analysis, a farm was considered to be characterised by a unique location (XY coordinates), owner and poultry species. When a farm was detected as infected, it was considered that poultry of this farm and all the other farms having the same location but with different poultry species or owner, would be culled.
For scenarios involving vaccination as a control measure (emergency vaccination), the time from vaccination to full protection was considered to be 3 weeks, as derived from the literature review performed in ToR 1 (see Table 9). However, available data within ToR 1 lacked sufficient information to assess the level and duration of immunity following vaccination, preventing us to assess those parameters. The frequency of vaccination to ensure protection was also not considered due to the same reasons. The detailed characteristics of the vaccine product administered to the poultry were not considered in model. However, an overview of vaccine types, characteristics and frequency of vaccination was reported in Section 3.4.1.2 according to the vaccination strategy, given the poultry species and the age. In the model, it was assumed that the vaccine(s) chosen aligned with the poultry species and the objective of the vaccination programme to obtain optimal performance in terms of efficacy and level of protection at both animal and population levels.
Table 9.
Response to vaccination at 7, 14 and 21 days post HPAI vaccine administration
| Species, Study | Vaccine name | Virus | Vaccine (number of doses) | Time (days) challenge post vaccination | HI (log2) | Beta | Infectious period (days) | R (95%UCL) |
|---|---|---|---|---|---|---|---|---|
| Chickens | ||||||||
| van der Goot et al. ( 2005 ) (a) | H7N7 | – | – | 0 | 33 | 6.3 | 208 | |
| A/Chicken/Italy/99 | H7N1 (1) | 7 | 0.25 (0, 0.52) | 0.03 | 1 | 0.03 (1.2) | ||
| A/Chicken/Pakistan/95 | – | H7N3 (1) | 7 | 0 | 0.3 | 3.7 | 1.1 | |
| A/Chicken/Italy/99 | – | H7N1 (1) | 14 | 5.8 (5.5, 6.1) | – | – | < 0.7 | |
| A/Chicken/Pakistan/95 | – | H7N3 (1) | 14 | 3.6 (3.3, 4.1) | – | – | < 0.7 | |
| Ducks Pekin | ||||||||
| van der Goot et al. ( 2008 ) | A/Chicken/Mexico/232/94/CPA H5N2 | H5N1 | – | – | 0 | 4.7 | 4.3 | 20 |
| – | – | H5N2 (1) | 7 | 0.14 | 2.7 | 3.4 | 9.2 | |
| – | – | H5N2 (1) | 14 | 0.19 | 0.23 | 3.3 | 0.76 (2.2) | |
| – | – | H5N2 (2) | 14 | 3.1 | – | – | < 1* | |
| Tatar‐Kis et al. ( 2019 ) (b) | H5N8 2.3.4.4 | – | – | – | 3.4 | 4.9 | 16.5 | |
| VLP‐based vaccine | pH5N8(2) | 21 | 8.8 | – | – | < 1 | ||
| Ducks mule | ||||||||
| Tatar‐Kis et al. ( 2019 ) (b) | VLP‐based vaccine | H5N8 2.3.4.4 | – | – | 0 | 4.02 | 5.1 | 20 |
| – | pH5N8 (1) | 21 | 7.3 | – | – | < 1 | ||
| – | pH5N8 (2) | 21 | 10.6 | – | – | < 1 | ||
| Turkeys | ||||||||
| Bos et al. (2008) | A/Chicken/Italy/99 | H7N7 | – | – | 0 | 6.6 | 1.3 | 8 |
| – | – | H7N1 (1) | 14 | 0.5 (0–4) | – | – | < 1 | |
| – | – | H7N1 (2) | 14 | 9 (7–11) | – | – | < 1 | |
These studies used inactivated vaccines and reported HI titres are heterologous titres.
This study used a virus‐like particle vaccines homologous to virus used for challenge.
Other relevant parameters such as culling capacity (number of farms/day) and vaccination capacity (number of farms/day), were obtained from the literature, from the data (median number of farms culled per day during the analysed epidemic) or the experience of the WG experts from the three selected countries. For the three countries, the vaccination capacity in the emergency vaccination scenario was limited to 20 farms/day (Backer et al., 2015). The reason for this limitation is that vaccines suitable for emergency vaccination require injection in individual birds. On a specific day, each unvaccinated farm within the vaccination area around an infected farm had the same probability of being selected for vaccination. For example, if on a given day, two farms were infected, each having 20 farms (40 in total) within their corresponding control zones (ex. 3 km vaccination radius), 20 farms/day were randomly selected and vaccinated from these 40 farms. The culling capacity was limited to two farms/day in The Netherlands, to one farm/day in Italy (both based on the information of the respective veterinary authorities) and to six farms/day in France (calculated from the data of the analysed epidemic). Farms to be culled on each day of the simulated epidemic were randomly selected, however, infected farms were always prioritised. In total, 10,000 simulations were conducted for each assessed scenario. To initiate each simulation, one index case was randomly selected among the active farms located within the identified areas with high‐risk of between‐farm transmission (see step 1).
2.2.2. Uncertainty analysis
The uncertainty analysis was performed in a qualitative manner following the procedure detailed in the EFSA guidance on uncertainty analysis in scientific assessments (EFSA Scientific Committee, 2018). The sources of uncertainty associated with the methodology used are listed and discussed in Section 3.5.
3. Assessment
3.1. Update on the available poultry vaccines against HPAI (ToR 1)
3.1.1. Available vaccine types and technologies targeting HPAI virus
The essential aim of HPAI vaccination in poultry is to generate effective and resilient protective immunity against HPAIV infection but also to stop sustained HPAIV transmission. The selection of viral antigens for vaccine development must consider: (i) the use of viral proteins or parts thereof that carry antigenic epitopes, which are relevant targets of protective immune effectors, (ii) their presentation in an antigenically authentic form and (iii) their final formulation into a highly immunogenic product. Ideally, vaccination‐induced immunity should replicate that induced by natural infection and activate both humoral and cellular immune responses. In addition, ideal vaccines can be modified to allow discrimination between vaccinated and infected birds by serological diagnostic means (DIVA principle – differentiate infected from vaccinated animals).
Several approaches have been explored to generate AIV vaccines. Whilst the largest experience with influenza vaccination stems from vaccines developed for human seasonal influenza, influenza vaccines have also been widely used for decades in horses, pigs and poultry. Historically, from the industry perspective, influenza vaccines for use in animals, and livestock in particular, are under heavy economic constraints. Since vaccinations are often not done routinely or continuously (for example due to the applicable legislation in the EU), non‐return of investments is a constant looming risk considering the costs associated with their development, demonstration of efficacy and possible adaptation to circulating strains. From the poultry holder perspective, in turn, in addition to having the desired immunogenic effects, vaccination must also be economically sustainable to be acceptable. These constraints have shaped the type of AIV vaccines and their production. Table 3 provides an overview of the various technologies that have been used so far. Whilst various molecular engineering methods have led to many promising vaccine candidates in recent years, only a small number of these principles and candidates has been exploited for commercial production and authorisation of vaccines.
Most commercially produced AIV vaccines are based on inactivated and adjuvanted whole virus. This is mainly due to the straightforward and cost‐effective technology of virus amplification, inactivation and formulation using oil or carbomer adjuvants. Replication‐competent virus isolates of low pathogenicity AIV must be handled under biosafety level 2 (BSL2) regulations. Therefore, this technology cannot be easily adapted to HPAIV due to the associated biosafety concerns since HPAIV is considered a biosafety level 3 (BSL3) infectious agent and its handling requires enhanced and expensive biosafety measures. There are several strategies to overcome the problem of handling HPAIV in vaccine production:
-
Changing the pathotype from highly pathogenic (HP) (BSL3) to low pathogenic (LP) (BSL2) without affecting antigenicity.
A key molecular marker located in the haemagglutinin (HA)‐encoding gene distinguishes HP from LP phenotypes: the HA protein harbours an essential endoprotease cleavage site required for activation of virus infectivity by interaction with host cellular proteases. In HP viruses, this cleavage site is represented by a stretch of basic amino acids (i.e. polybasic) whilst LP viruses express one or two basic amino acids at most (i.e. monobasic). By using reverse genetics to change the polybasic cleavage site to a monobasic one, the HP phenotype can be transformed to a LP phenotype, and the resulting virus be handled as a BSL2 agent and used for vaccine production in a standard biosafety level 2 environment. This technique has been used to produce most vaccines used in nationwide HPAI vaccination programmes in China and other vaccinating countries (Qiao et al., 2006).
Using recombinant expression systems. The immunogenic viral proteins such as the HA and/or the neuraminidase (NA) have been expressed from a huge variety of recombinant vectors as shown in Table 3. The proteins or parts thereof can be purified and then formulated into adjuvanted inactivated vaccines. Alternatively, if the vectors are replication‐competent in the targeted vaccine species, they can be used to inoculate the vaccines for in vivo expression of the recombinant influenza virus proteins.
More recently, as a side phenomenon of the proven effectiveness of vaccines used in vaccination campaigns against the human COVID‐19 pandemic, direct use of chemically packaged engineered nucleic acids are given consideration for use in poultry. Basically, two strategies can be followed: (i) Recombinant RNA replicons are capable of providing single‐cycle replication in vivo; this restricts spread of the vaccine replicon between vaccinated birds and compromises the replicon's ability to cause diseases, thereby providing a safe source of selected influenza virus target proteins to the vaccinee. Similarly, (ii) specially engineered and packaged mRNA can also be used for this purpose in poultry. These approaches can circumvent the species‐specificity of some viral vectors and therefore allowing vaccination of multiple poultry species. The use of pure mRNA can also prevent the induction of immunity against replicon components that could interfere with booster applications.
It should be emphasised that the use of attenuated live influenza A virus isolates as vaccines is not feasible for subtype H5 and H7 influenza viruses, even if LP phenotypes would be used: during replication activity, insertional mutations can be introduced de novo into the monobasic HA cleavage site of LP phenotype viruses. These insertions lead to the coding of additional basic amino acids, which subsequently causes a reversal of virulence from LP to HP. This phenomenon has been repeatedly described in vivo and in vitro. Moreover, reassortment of vaccine strains with field circulating AIV poses unpredictable animal and public health risks. Very recent developments have provided new solutions to prevent reassortment of live vaccine viruses with field strains; such vaccines have been based on chimeric bat influenza viruses.
3.1.2. Overview of the available vaccines
The term ‘available vaccines’ refers to inactivated vaccines or vaccines based on technologies other than live attenuated AIV that have been evaluated by a regulatory authority and have obtained a marketing authorisation, or that have been used inside or outside the EU, or that are in a late development stage with laboratory or field data on their performance already available. Prototypes of vaccines still in an early stage of development have been only mentioned when relevant, as they represent potential future solutions, but not assessed in detail.
Based on the information retrieved by the literature review, pharmaceutical company websites, responses to the survey and network consultation, several available HPAIV vaccines have been identified. The complete list and all the information available on those vaccines are reported in Table A1 (Annex A).
In this section, we provide a descriptive summary of the characteristics of some of the identified vaccines, by vaccine virus subtype, including those currently being tested in Member States (France, Hungary, Italy, The Netherlands), vaccines for which information was directly provided by pharmaceutical companies contacted by EMA or EFSA, vaccines commercially available in non‐member countries, and vaccines that have been used in the EU or in non‐member countries during vaccination campaigns (Tables 4, 5–4, 5). This descriptive summary excludes other vaccines identified with the literature review, either due to insufficient data retrieved for a comprehensive description or a lack of evidence of possible further development, possible marketing authorisation or use in EU.
Table 4.
Available vaccines for H5 subtype
| Technology | Poultry species (a) (experimental data) | Administration route | Vaccine name | Seed strain (or HA gene source) | Estimated antigenic distance (AU) (b) | Lineage, clade (g) | Predicted VET (i) |
|---|---|---|---|---|---|---|---|
| Inactivated full virus | Chickens (c) (Pekin ducks, turkeys) | Subcutaneous (from 8 days onwards) or intramuscular (from 14 days onwards) | Nobilis Influenza H5N2 (f) | H5N2 A/duck/Potsdam/1402/86 | 4.37 | Eurasian H5 | < 0.5 in chickens after 1 dose |
| Inactivated full virus | Chickens (ducks) | Subcutaneous | POULVAC Flufend H5N3 rg vaccine | H5N1 A/chicken/Vietnam/C58/04 | 4.37 | 1 | – |
| Inactivated full virus | (chickens) | Subcutaneous | POULVAC Flufend H5N1 rg vaccine | H5N8 A/Gyrfalcon/WA/41088–6/2014 | 2.51 | 2.3.4.4a | – |
| Inactivated full virus | Chickens | Subcutaneous | CEVAC Flu‐Kem H5N2 | H5N2 A/Chicken/Mexico/232/94/CPA | 4.74 | American H5 | – |
| Inactivated full virus | Chickens | Intramuscular | GALLIMUNE FLU H5N9 | The H5N9 strain is either A/chicken/Italy/22A/98 or A/turkey/Wisconsin/1/1968 depending on the country where it was applied |
4.56 4.56 |
American H5 | – |
| Inactivated full virus | Chickens and turkeys | Subcutaneous or intramuscular | Mefluvac H5 + ND7 |
A/Chicken/Egypt/ RG‐173 CAL/2017 rgA/CK/Egypt/ME1010/2016 rgA/chicken/ME‐2018/H5N8 |
4.18 5.30 2.32 |
2.2.1.2 2.2.1.1 2.3.4.4b |
– |
| Inactivated full virus | Chickens, turkeys and ducks | Subcutaneous or intramuscular | Mefluvac H5 PLUS 8 |
A/Chicken/Egypt/ RG‐173 CAL/2017 rgA/CK/Egypt/ME1010/2016 rgA/chicken/ME‐2018/H5N8 |
4.18 5.30 2.32 |
2.2.1.2 2.2.1.1 2.3.4.4b |
– |
| Inactivated full virus | Chickens | Subcutaneous or intramuscular | Mefluvac H5 + H9 + ND7 |
rgA/CK/Egypt/ME1010/2016 rgA/chicken/ME‐2018/H5N8 A/Chicken/Egypt/ RG‐173 CAL/2017 |
5.30 2.32 4.18 |
2.2.1.1 2.3.4.4b 2.2.1.2 |
– |
| Inactivated full virus | Chickens | Subcutaneous or intramuscular | Mefluvac H5 + H9 |
A/Chicken/Egypt/ RG‐173 CAL/2017 rgA/CK/Egypt/ME1010/2016 rgA/chicken/ME‐2018/H5N8 |
4.18 5.30 2.32 |
2.2.1.2 2.2.1.1 2.3.4.4b |
– |
| Inactivated full virus | (chickens) | Subcutaneous or intramuscular | Mefluvac H5N8 | rgA/chicken/ME‐2018/H5N8 | 2.32 | 2.3.4.4b | – |
| Inactivated full virus | Chickens, turkeys, ducks, geese and guinea fowl | Subcutaneous or intramuscular | SER‐VACCFLU | A/Chicken/Egypt/Q1995D/2010 and A/Duck/EGYPT/M2583D/2010 |
5.30 4.37 |
2.2.1.2 2.2.1.2 |
– |
| Inactivated full virus | Chickens | Subcutaneous | Volvac AI + ND KV | H5N2 A/Chicken/Mexico/232/94 | 4.74 | American H5 | – |
| Inactivated full virus | Chickens | Subcutaneous | Volvac AI KV | H5N2 A/Chicken/Mexico/232/94 | 4.74 | American H5 | – |
| Inactivated full virus | Chickens, ducks and geese | Subcutaneous or intramuscular | Yeflu H5 | H5N1 A/duck/Guangdong/S1322/2006 | 4.56 | 2.3.2.1b | – |
| Inactivated full virus | Chickens, ducks and geese | Subcutaneous or intramuscular | Yeflu (H5) Su |
H5N6 A/duck/Fujian/S1424/2020 H5N8 A/whooper swan/Shanxi/4–1/2020 |
4.00 2.32 |
2.3.4.4 h 2.3.4.4b |
– |
| Inactivated full virus | Chickens, ducks and geese | Subcutaneous or intramuscular | Yeflu H5(6 + 8) |
H5N1 A/duck/Guangdong/S1322/2006 H5N1 A/chicken/Guizhou/4/13 |
4.56 3.07 |
2.3.2.1b 2.3.4.4 |
– |
| Inactivated full virus | Chickens | n/a | BioTek | H5N2 A/Turkey/England/N‐28/73 | 4.18 | Eurasian H5 | – |
| Inactivated full virus | Chickens | n/a | Bird CLOSE 5.1 | H5N1 A/chicken/Legok/2003 | 4.37 | 2.1.1 | – |
| Inactivated full virus | Chickens | Subcutaneous and intramuscular | Caprivac AI‐K | H5N1 A/chicken/West Java/Pwt‐Wij/2006 | 4.37 | 2.3.1.2 | – |
| Inactivated full virus | Chickens, quails, ducks, swans and geese | Subcutaneous | NAVET‐VIFLUVAC | H5N1 A/Vietnam/1194/2004 | 4.37 | 1 | – |
| Inactivated full virus | Chickens, quails, ducks, mallards, geese | Subcutaneous | NAVET‐FLUVAC 2 | H5N1 A/Vietnam/1194/2004 and H5N1 A/Hubei/1/2010‐PR8‐IDCDC‐RG30 |
4.37 4.18 |
1 2.3.2.1 |
– |
| Inactivated full virus | (chickens,ducks, geese and turkeys) | Subcutaneous | H5N1 Re‐1 | H5N1 A/Goose/Guangdong/96 | 4.37 | 0 | – |
| Inactivated full virus | (chickens) | Subcutaneous | H5N1 Re‐4 | H5N1 A/Chicken/Shanxi/2/2006 | 5.30 | 7 | – |
| Inactivated full virus | (chickens) | Subcutaneous | H5N1 Re‐5 | H5N1 A/Duck/Anhui/1/2006 | 4.18 | 2.3.4 | – |
| Inactivated full virus | (chickens, quail, (Pekin ducks) | Subcutaneous | H5N1 Re‐6 | H5N1 A/duck/Guangdong/S1322/2010 | 4.56 | 2.3.2.1b | – |
| Inactivated full virus | (chickens) | Subcutaneous | H5N1 Re‐7 | H5N1 A/Chicken/Liaoning/S4092/2011 | 6.05 | 7.2 | – |
| Inactivated full virus | (chickens) | Intramuscular | H5N1 Re‐8 | H5N1 A/chicken/Guizhou/4/2013 | 3.07 | 2.3.4.4 | ≤ 0.5 |
| Inactivated full virus | (chickens) | Subcutaneous | H5N1 Re‐10 | H5N1 A/duck/Anhui/SI246/2014 | 4.74 | 2.3.2.1e | – |
| Inactivated full virus | (Chickens, (ducks) | Subcutaneous | H5N1 Re‐11 | H5N6 A/duck/Guizhou/S4184/2017 | 3.44 | 2.3.4.4d | > 0.9 in chickens and ducks |
| Inactivated full virus | (chickens) | Subcutaneous | H5N1 Re‐12 | H5N8 A/chicken/Liaoning/SD007/2017 | 4.18 | 2.3.2.1d | – |
| Inactivated full virus | (chickens) | Subcutaneous | H5N6 Re‐13 | H5N6 A/duck/Fujian/S1424/2020 | 4.00 | 2.3.4.4 h | – |
| Inactivated full virus | (chickens) | Subcutaneous | H5N8 Re‐14 | H5N8 A/whooper swan/Shanxi/4–1/2020 | 2.32 | 2.3.2.1d | – |
| Inactivated full virus | (chickens) | Intramuscular | ES2/2.3.4.4c | H5N6 A/duck/Korea/ES2/2016 | 3.25 | 2.3.4.4 | > 0.9 |
| Inactivated full virus | (chickens) | Intramuscular | KA435/2.3.2.1c | H5N1 A/chicken/Vietnam/NCVD‐KA435/13 | 4.37 | 2.3.2.1c | – |
| Inactivated full virus | Chickens | Subcutaneous | Emulmax – C AI | H5N2 A/Chicken/Mexico/232/94/CPA | 4.74 | American H5 | – |
| Inactivated full virus | Chickens | Subcutaneous | Emulmax – C AI G15 | H5N2 A/chicken/Guanajuato/CPA‐20966‐15‐VS/2015 | 5.30 | American H5 | – |
| Inactivated full virus | Chickens | Subcutaneous | Emulmax – C AI + ND | H5N2 A/Chicken/Mexico/232/94/CPA | 4.74 | American H5 | – |
| Inactivated full virus | Chickens | Subcutaneous | Emulmax – C AI + N5 | H5N2 A/Chicken/Mexico/232/94/CPA | 4.74 | American H5 | – |
| Inactivated full virus | Chickens | Subcutaneous | Emulmax AI + N5 | H5N2 A/Chicken/Mexico/232/94/CPA | 4.74 | American H5 | – |
| Inactivated full virus | Chickens | Subcutaneous | Emulmax‐C AI + N5 G15 | H5N2 A/chicken/Guanajuato/CPA‐20966‐15‐VS/2015 | 5.30 | American H5 | – |
| Inactivated full virus | Chickens | Subcutaneous | Emulmax AI + N5 G15 | H5N2 A/chicken/Guanajuato/CPA‐20966‐15‐VS/2015 | 5.30 | American H5 | – |
| Inactivated full virus | Chickens | Subcutaneous | Emulmax AI + ND | H5N2 A/Chicken/Mexico/232/94/CPA | 4.74 | American H5 | – |
| Inactivated full virus | Chickens | Subcutaneous | OTTO FLUPLUS VAC | Local (Pakistan) strain of H5 | – | – | – |
| Inactivated full virus | Chickens | Subcutaneous | OTTO FIGHT FLU VAC | Local (Pakistan) strain of H5 and H9 | – | – | – |
| Inactivated full virus | Chickens | Subcutaneous | Newcastle Influenza killed virus vaccine | H5N2 A/Chicken Mexico/232/CPA/94 | 4.74 | American H5 | – |
| Inactivated full virus | Poultry (Muscovy ducks) | Subcutaneous | Vaxigen Flu H5N8 (e) | A/green‐winged teal/Egypt/877/2016 | 2.32 | 2.3.4.4b |
in chickens > 0.9; in Muscovy ducks < 0.5 after 1 dose, > 0.9 after 2 doses |
| Subunit | Chickens (Muscovy, Pekin, mule ducks, turkeys) | Subcutaneous | Volvac B.E.S.T. AI + ND (d) , (e) | H5N1 A/duck/China/E319‐2/2003 | 4.18 | 2.3.2 |
In mule duck > 0.9 (after 2 doses); in Muscovy ducks 0.8–0.9 after 1 dose, > 0.9 after 2 doses; in Pekin ducks and turkeys > 0.9 |
| Inactivated vector | Chickens | n/a | Vaxigen K‐NewH5 | n/a | – | – | – |
| Live vector | Chickens | Spray | Vaxigen NewH5 | n/a | 2.51 | – | – |
| Live vector | Chickens | In ovo | Vaxigen Ad‐H5 | n/a | – | – | – |
| Live vector | (chickens) | Subcutanous or intramuscular | rHVT‐H5 | A/Avian/Netherlands/H5N1/2008 | 4.18 | – | – |
| Live vector | Chickens (ducks, turkeys) | In ovo or subcutaneous | Vectormune AI (e) , (f) | A/swan/Hungary/4999/2006 (modified) | 4.18 | 2.2 |
in chickens > 0.9; in turkeys 0.5–0.8 |
| Live vector | Chickens | Subcutanous | Trovac‐AIV H5 | H5N8 A/turkey/Ireland/1378/83 | 4.18 | Eurasian H5 | – |
| Live vector | Chickens | Subcutaneous | Trovac‐H | H5N2 A/chicken/Mexico/P‐14/2016 | 4.93 | Eurasian H5 | > 0.9 |
| Live vector | (chickens) | In ovo or subcutaneous | HVT‐IBD‐AIV‐H5 haemagglutinin ‘COBRA’ (computationally optimised broadly reactive antigen) (f) | H5 insert codon‐optimised with computer models | 4.97 (i) | 2.3.2 (i) | > 0.9 |
| Replicon | (ducks, geese, chickens, zoo birds) | Intramuscular | Duck H5‐SRV vaccine® (d) , (h) | A/duck/France/161108 h/2016 | 2.32 | 2.3.4.4b | > 0.9 in mule ducks |
| Nucleic acids (DNA) | (chickens, turkeys) | Intramuscular | ExactVac – Vaxliant ENABLE adjuvant (e) , (f) | A/gyrfalcon/Washington/41088–6/2014 | 2.51 | 2.3.4.4a | < 0.5 in chickens after 1 dose |
When a target species is listed without parentheses, it means that the product is, somewhere in the world, according to the information retrieved, either authorised for use (e.g. in emergency), or fully authorised (marketing authorisation) or with experiences of use in the field. In parentheses are poultry species for which only experimental data were retrieved, or where a licence existed in the past, but it is not valid anymore.
Antigenic distance expressed here in arbitrary antigenic units (AU) between vaccine and the H5N1 consensus strain of clade 2.3.4.4b as described in Section 3.1.3.
Authorised in the EU.
Tested at ANSES, FR in ducks.
Tested at EURL, IT in turkeys (ExactVac – Vaxliant ENABLE adjuvant, Vectormune AI, Volvac B.E.S.T. AI + ND), Muskovy ducks (Vaxigen FLU H5N8), chickens (Vaxigen FLU H5N8)
Tested at Wageningen Bioveterinary Research, NL in chickens.
Lineage refers to phylogenetic origin of the strains as Eurasian or American; all clade designation given refer to the Eurasian goose/Guangdong lineage.
Tested in geese in the field in Hungary.
Table 5.
Available vaccines for H7 subtype
| Technology | Poultry species (a) (experimental data) | Administration route | Vaccine name | Seed strain |
|---|---|---|---|---|
| Inactivated full virus | Chickens | Subcutaneous | Cevac FLU H7 K | H7N3 A/pato/2817/2006 |
| Inactivated full virus | Chickens | Subcutaneous | Emulmax H7 | H7N3 A/pato/2817/2006 |
| Inactivated full virus | Chickens | Subcutaneous | Emulmax GR7 | H7N3 A/Chicken/Synthetic/México/CPA‐07669/16 |
| Inactivated full virus | Chickens (ducks) | Subcutaneous or intramuscular | Nobilis Influenza H7N1 | H7N1 A/CK/Italy/473/99 |
| Inactivated full virus | Chickens | Subcutaneous or intramuscular | Nobilis Influenza H7N7 | H7N7 A/duck/Potsdam/15/80 |
| Inactivated full virus | Chickens | Subcutaneous or intramuscular | H7N9 Re‐1 Oil adjuvanted inactivated AI vaccine | H7N9 A/pigeon/Shanghai/S1069/2013 |
| Inactivated full virus | Chickens | Subcutaneous or intramuscular | H7N9 Re‐2 Oil adjuvanted inactivated AI vaccine | H7N9 A/Chicken/Guangxi/SD098/2017/2013 |
| Inactivated full virus | (chickens, ducks, geese) | Subcutaneous or intramuscular | H7N9 Re‐3 Oil adjuvanted inactivated AI vaccine | H7N9 A/CK/IM/SD010/19/2017/2013 |
| Inactivated full virus | Chickens | Subcutaneous or intramuscular | H7N9 Re‐4 Oil adjuvanted inactivated AI vaccine | H7N9 A/CK/YN/SD024/21/2013 |
| Inactivated full virus | Chickens | Subcutaneous or intramuscular | Vaxigen® Flu‐H7 | – |
| Inactivated full virus | (chickens) | Subcutaneous or intramuscular | H7N8 vaccine | A/turkey/IN/16–01571‐6/2016 |
| Live vector | Chickens | Subcutaneous | Trovac Prime 7 (rFPV‐H7) | H7N3 A/chicken/Guanajuato/07437–15/2015 |
| Live vector | Chickens | Subcutaneous | Vectormune H7 (rHVT‐H7) | H7N3 A/chicken/Guanajuato/15 |
| Live vector | Poultry (b) | In ovo or subcutaneous | Vaxigen Ad‐H7 | H7N3 A/chicken/Jalisco CPA1/2012 |
When a target species is listed without brackets, it means that the product is, somewhere in the world, according to the information retrieved, either authorised for use (e.g. in emergency), or fully authorised (marketing authorisation), or with experiences of use in the field. In brackets are poultry species for which only experimental data were retrieved, or where a licence existed in the past, but it is not valid anymore.
Specific information on the poultry species was not available from the original source.
3.1.2.1. Descriptive summary of available vaccines for H5 subtype
The descriptive summary includes information related to the technology used, the target poultry species, the administration route, the vaccine name and the seed strain, for H5 HPAIV subtype (Table 4).
Technology
Most available vaccines contain inactivated whole AIV in an oil‐emulsion formulation, reflecting the traditional manufacturing process. Most of the more recent vaccines are based on strains which have been created by reverse genetics technology using an egg‐adapted H1N1 human influenza virus as backbone (A/Puerto Rico/8/1934 or PR8) with the HA and NA genes of an HPAIV strain.
Few other technologies have reached the market or later stages of technology readiness level (TRL). Vectored vaccines based on Fowlpox virus (FPV), Herpesvirus of turkeys (HVT) or Newcastle Disease Virus (NDV) as vectors of the AI HA represent a consolidated technology within the poultry sector for active immunisation against several diseases, including AI.
Another vaccine based on a recombinant baculovirus propagated in insect cells and expressing an H5 protein (Volvac B.E.S.T. AI + ND) has been used extensively in many endemic countries outside the EU for AI vaccination.
In addition, two vaccines that are based on nucleic acid technology (RNA replicon and DNA) are currently available (Duck H5‐SRV vaccine® and ExactVac – Vaxliant ENABLE adjuvant).
Target species
Most H5 vaccines are indicated for use in chickens. However, it should be noted that the data collected for this opinion include not only vaccines authorised for this target species but also vaccines only experimentally administered to chickens.
There are a few vaccines intended for turkeys, with a limited number of them currently holding a marketing authorisation. For example, two inactivated vaccines, Mefluvac range, are authorised in Egypt, some Middle East countries and Vietnam (Table 3). One inactivated vaccine, SER‐VACCFLU, produced by the Veterinary Serum and Vaccine Institute, Cairo, Egypt, is indicated for chickens and turkeys but also ducks, geese and guinea fowl. More information on other inactivated vaccines used in turkeys can be found in Table A1 (Annex A). In the United States, two vaccines, one based on plasmid DNA technology (ExactVac) and the other one based on RNA particle technology (RP‐H5 vaccine manufactured), hold an emergency authorisation by the United States Department of Agriculture (USDA) for use in chicken (both) and for the use in turkeys (the RNA one). The ExactVac DNA vaccine, Volvac B.E.S.T. AI + ND and Vectormune AI have been tested in turkeys against clade 2.3.4.4b in vaccine trials performed in Italy aimed to assess the efficacy of these vaccines, alone or in combination, for the clinical protection and reduction of viral shedding. Further trials are planned in Italy to understand immunity provided by other vaccines or vaccine combinations and to evaluate the duration of immunity offered by the different vaccination protocols.
For geese and ducks, there are several vaccines available, which are primarily authorised for use in chickens (see also SER‐VACCFLU above), but they also have claims for ducks or geese. Two inactivated vaccines (Poulvac Flufend H5N3‐ initially authorised in the EU but with the licence subsequently withdrawn ‐ and Mefluvac) are indicated for ducks. Furthermore, three inactivated vaccines (Yeflu, with different strains) can be used for geese and ducks. Additionally, two inactivated vaccines (Navet‐VIFLUVAC and Navet‐Fluvac 2 containing different H5N1 strains), are indicated for geese (both vaccines) but also quails (both) and mallards (Navet‐Fluvac 2). Testing has been conducted for an RNA Replicon vaccine (Duck H5‐SRV vaccine®) specifically designed (codon‐optimised) for use in mulard ducks in France and has also undergone a field trial in geese in Hungary.
Route of administration
Most available vaccines are administered by injection, either subcutaneously or intramuscularly. A few vaccines can be administered by injection in‐ovo or at 1 day of age in the hatcheries (e.g. Vaxigen Ad‐H5 and Vectormune HVT‐AIV). Only one vaccine (Vaxigen NewH5 – an NDV‐vectored vaccine) allows spray vaccination.
Age at first administration
There is a wide variability in the recommended age for vaccination across different vaccines. Nevertheless, from the data collected, most vaccines are recommended to be administered at a minimum of 2 weeks of age, with some indicating first administration up to 6 weeks of age. This is particularly valid for inactivated vaccines, which have generally less age‐related restrictions unless maternal immunity is present. For subunit vaccines and vaccines based on nucleic acid technology, the recommended age for first administration typically falls from 1 to 2 weeks of age, thus providing opportunity for early onset of immunity in the production life‐cycle. Some vaccines, in particular the recombinant HVT ones, are recommended for in‐ovo use or in day‐old chicks.
Number of doses
There is some variability regarding the number of doses administered to achieve the immunisation goal. Also, this specific information could not be retrieved for all vaccines due to limited data availability. From the data collected, most available vaccines presented in Table 4 were intended by the manufacturer to be administered in a single dose administration. However, a notable number of vaccines still demands a prime‐boost scheme with at least two doses. Further booster doses may be required in birds that have a longer production cycle (e.g. turkeys, layers, breeders). There are a few vaccines that claim to require three doses to achieve the basic immunisation goal (e.g. BioTek, Caprivac, rgEsS2 and rgKA435). For few vaccines, indications regarding the number of boosts required for longer lived birds (e.g. layers or breeders) were retrieved. Trials performed in Italy in turkeys have showed that to reach 100% survival after a challenge at 50 days with a recent 2.3.4.4b isolate following a priming with HVT‐AI (Vectormune AI) boosting with a DNA (ExactVac) or subunit vaccine (Volvac B.E.S.T. AI+ND) was needed. Single immunisation with HVT‐AI (Vectormune AI) or homologous prime‐boost protocols (with either the DNA or subunit vaccine) lead to unsatisfactory results (60%, 25% and 38% survival rate, respectively) (EURL, personal communication).
Onset and duration of immunity
Onset of immunity is a poorly investigated aspect; however, most of the available vaccines report 2–3 weeks after the completion of the immunisation scheme as the period to achieve protection. HVT‐vectored vaccine vaccinated birds, in some of the studies retrieved, have shown an even slower onset of immunity in both chickens and turkeys, with antibodies being detectable in most birds approximately from 4 weeks onwards after vaccination. Specific information on the duration of immunity could not be retrieved for all vaccines due to limited data availability. Also, instead of providing a specific duration, the available information indicated a time step at which antibodies were still detected following vaccination (mostly 40–50 days after vaccination), however with varying percentages of birds showing this response. Only very few studies investigating this aspect in birds, despite a long production cycle (e.g. chicken layers and breeders), have been retrieved by the literature review. Earlier onset of immunity has been described under laboratory conditions for Trovac AI, with protection starting from 1 week of age, however available vaccines using FPV as vector express an H5 HA protein with a considerable antigenic distance from currently circulating strains.
3.1.2.2. Descriptive summary of available vaccines for H7 subtype
The descriptive summary includes information related to the technology used, the target poultry species, the administration route, the vaccine name and the seed strain, for H7 HPAIV subtype (Table 5).
Technology
Most available vaccines contain inactivated whole live AIV, either using LPAI strains or built by reverse engineering (such as H7 Re‐1 to Re‐4 seed strains). Three vaccines are based on live vectored technology (Trovac Prime 7, Vectormune H7 and Vaxigen Ad‐H7) with the recombinant vector being a FPV, an HVT or a Fowl adenovirus 9 (FAdV‐9), respectively.
Target species
Most H7 vaccines are indicated for use in chickens. There was one vaccine intended for ducks (Nobilis Influenza H7N1) authorised in the EU. However, the licence has been withdrawn by the marketing authorisation holder and it is therefore not valid anymore. Four inactivated vaccines (H7N9 Re‐1, Re‐2, Re‐3, Re‐4), indicated for chickens, ducks and geese, have been used in China during vaccination campaigns.
Route of administration
Most available vaccines are administered through injection, either subcutaneously or intramuscularly. However, HVT‐vectored vaccines (based on the same technology of Vectormune H7) have been used also in‐ovo. Vaxigen Ad‐H7 holds an authorisation in Mexico for in‐ovo or subcutaneous administration.
Age at first administration
Information about the age at first administration was very limited and could only be retrieved for two inactivated vaccines (Emulmax H7 and Emulmax GR7) for which the estimated age at first administration is 8–10 days. A vectored vaccine is administered at 1 day of age or in‐ovo (Vaxigen Ad‐H7).
Number of doses
The available data regarding the number of doses required for vaccination were limited. It appears that most vaccines require a single dose for administration, whilst some vaccines require two doses, depending on the targeted species. The H7 vectored vaccines identified are intended for a single administration.
Onset and duration of immunity
Specific information on the onset and duration of immunity could not be retrieved due to limited data availability.
3.1.2.3. Descriptive summary of available vaccines for H5 + H7 subtypes
The descriptive summary includes information related to the technology used, the target poultry species, the administration route, the vaccine name and the seed strain, for H5 + H7 HPAIV subtypes (Table 6).
Table 6.
Available vaccines for H5 + H7 subtypes
| Technology | Poultry species (a) (experimental data) | Administration route | Vaccine name (seed strain(s)) | Seed strain(s) | H5 clade |
|---|---|---|---|---|---|
| Inactivated full virus | Chickens and turkeys | Subcutaneous | BioFlu H7N1 + H5N9 |
A/chicken/Italy/1067/99 (H7N1) A/chicken/Italy/22A/98 (H5N9) |
Eurasian H5 |
| Inactivated full virus | (Pekin ducks) | Subcutaneous | Poulvac i‐AI H5N9 + H7N1 | A/chicken/Italy/1067/99 (H7N1) A/chicken/Italy/22A/98 (H5N9) | Eurasian H5 |
| Inactivated full virus | Chickens | Subcutaneous or Intramuscular | OTTO FLUPLUS + VAC | (seed strains unknown) | – |
| Inactivated full virus | Chickens, ducks and geese | Subcutaneous or Intramuscular | Yeflu H5 + H7 V3 | H5N1 Re‐11 + Re‐12 strains; H7N9 H7‐Re3 strain |
2.3.4.4d, 2.3.2.1d |
| Inactivated full virus | Chickens, ducks and geese | Subcutaneous or Intramuscular | Yeflu H5 + H7 V5 | H5N6 H5‐Re13 strain + H5N8 H5‐Re14 strain + H7N9 H7‐Re4 strain |
2.3.4.4 h 2.3.2.1d |
| Inactivated full virus | Chickens and ducks | Intramuscular | Harbin Trivalent AI Vaccine | H5N6 H5‐Re13 strain + H5N8 H5‐Re14 strain + H7N9 H7‐Re4 strain |
2.3.4.4 h 2.3.2.1d |
When a target species is listed without parentheses, it means that the product is, somewhere in the world, according to the information retrieved, either authorised for use (e.g. in emergency), or fully authorised (marketing authorisation), or with experiences of use in the field. In parentheses are poultry species for which only experimental data were retrieved.
The use of bivalent or multivalent vaccines becomes relevant when multiple strains co‐circulate, particularly if they are antigenically distinct such as different subtypes, such as H5 and H7. This situation has been observed in a few countries, with China being a major example, where co‐circulation of gs/GD HP H5 strains of different clades and HPAI H7N9 viruses have been observed. Region‐specific vaccines have been developed to specifically target these co‐circulating strains.
Technology
All vaccines contain inactivated whole live AIV.
Target species
Most of the few H5 + H7 vaccines are indicated for use in chickens, whist only one vaccine is specifically indicated for turkeys as well (BioFlu H7N1 + H5N9). Three vaccines are indicated for ducks (Harbin trivalent, Yeflu H5 + H7 V3 and Yeflu H5 + H7 V5 – with these two latter also indicated for geese). One vaccine (Poulvac i‐AI H5N9) has been also used to generate experimental data in Pekin ducks.
Route of administration
All vaccines are administered through injection, either subcutaneously or intramuscularly.
Age at first administration
Information about the age at first administration was very limited and could only be retrieved for two vaccines used experimentally. Poulvac i‐AI H5N9 was experimentally administered to pecking ducks at 1 day of age, with a booster dose given at 3 weeks of age. BioFlu H7N1 + H5N9 was administered as follows: in turkeys, primary vaccination between 5 and 20 days of age, second vaccination between 40 and 45 days of age, third vaccination between 70 and 75 days of age; in pullets primary vaccination between 30 and 45 days of age, second vaccination between 105 and 120 days of age for which the estimated age at first administration is 8–10 days.
Number of doses
The available data regarding the number of doses required for vaccination were limited. It appears that most vaccines required more than a single dose.
Onset and duration of immunity
Specific information on the onset and duration of immunity could not be retrieved due to limited data availability.
3.1.2.4. Description of application of authorised vaccine in the EU
Currently, there is only one vaccine against HPAIV with a valid marketing authorisation in the EU: Nobilis Influenza H5N2. The authorisation was obtained through the centralised procedure, making it valid in all EU Countries, as well as Iceland, Liechtenstein and Norway. Nobilis Influenza H5N2 is a whole H5 virus inactivated vaccine (technology replication defective, Table 3) (European Union, online) This vaccine is specifically intended for chickens (although it has been used off‐label also in other species) and can be administered subcutaneously from 8 days of age. From 14 days onwards, it can be administered either subcutaneously or intramuscularly. In long‐living birds, such as future laying hens and breeders, a second dose can be administered after 4–6 weeks to provide extended protection. Clinical studies conducted during the marketing authorisation procedure, with a classical H5 HPAI challenge strain, demonstrated a reduction in clinical signs, mortality and viral excretion 3 weeks after vaccination. The duration of immunity has not been established. However, according to the authorised product information, serum antibodies are expected to persist for at least 1 year after the administration of two vaccines. Previous use of this vaccine in zoo birds, following special derogation, has been reported annually to the European Commission by the MSs.
More details about Nobilis Influenza H5N2, its product information and public assessment report can be found on the Veterinary Medicines information website (European Union, online). Each new product that receives a marketing authorisation in the EU will be uploaded on that portal, which can be used to monitor any new vaccine, including AIV ones. For products that have received a positive opinion from the EMA ‐ Committee for Veterinary Medicinal Products (CVMP) which are still awaiting for the European Commission Decision on the marketing authorisation, the CVMP minutes and reports can be consulted (EMA, online). Because the assessment of the product has not been concluded and an opinion issued, no information on new, ongoing product evaluation is disclosed.
3.1.2.5. Examples of applications of authorised vaccines outside the EU
Several vaccination strategies against HPAI have been implemented in countries outside the EU, leading to the development of a wide range of vaccine products, often tailored to the specific epidemiological situation in each country. The following examples serve as key illustrations of large‐scale vaccination programmes implemented in response to HPAI outbreaks in three different continents with different epidemiological situations.
In China, vaccination against H5 viruses was initiated in 2004 as a response to a large‐scale outbreak in domestic poultry. The initial vaccine used in the campaign was an oil‐emulsified inactivated AI vaccine of low pathogenicity, specifically featuring the H5N2 subtype virus A/turkey/England/N‐28/73. This vaccine has been reported to play a crucial role in containing the spread of the virus within the affected provinces, with ~ 2.5 billion doses administrated in 2004 alone (Chen and Bu, 2009). Due to poor antigenic match between the seed strain and the circulating viruses of the goose/Guangdong lineage and limited replication capability in eggs, researchers at the Harbin Veterinary Research institute developed a series of reassortant viruses (‘Re’) based on the high‐replication egg‐adapted A/Puerto Rico/8/34 (PR8) virus and the haemagglutinin (HA) and neuraminidase (NA) genes of H5N1 gs/GD HPAI viruses in which the multiple basic amino acid cleavage site motif had been modified to the LP phenotype. Several reassortant viruses (referred to as Re‐ followed by a progressive number) were generated since then to adapt the vaccine to the changing antigenic diversity of circulating strains. These viruses were used alone or in combination based on the epidemiological situation (e.g. introduction of a Re‐ H7N9 virus in the vaccine composition since September 2017 to contain the H7N9 epidemic and reduce spill over transmission to humans). The bivalent vaccination programme has been effective in protecting domestic poultry production (Zeng et al., 2016; Wu et al., 2019), with no further large‐scale epidemics reported since the start of the strictly conducted, continued nationwide vaccination campaigns in 2017. However, surveillance programmes have identified low prevalence of gs/GD H5 and H7N9 virus circulation in vaccinated domestic birds, showing that vaccination has grossly reduced but not entirely eliminated viral circulation in domestic birds (Chen et al., 2023).
In Mexico, the first vaccination programme using an inactivated vaccine based on a local H5N2 LPAI isolate (A/chicken/Mexico/CPA‐232/94) was implemented in 1995 to control the spread of an H5N2 HPAIV. The elimination of the H5N2 HPAIV was successfully achieved, leading to the declaration of disease freedom in December 1995. Vaccination is still used in Mexico to protect commercial poultry flocks from LPAI H5N2 virus outbreaks. However, due to viral evolution, updates of the vaccines have been necessary due to reported lack of protection. Despite extensive vaccination efforts, the implementation of biosecurity measures and surveillance activities, H5N2 LPAI remains enzootic in Mexico. The experience gained with the H5N2 epidemic was of paramount importance for a rapid response to another HPAIV incursion from the wild reservoir. In June 2012 after the detection of an H7N3 HPAIV in three chicken farms in the State of Jalisco, which spread rapidly and infected 36 farms by 24 July, a vaccination campaign begun on the 27 July, less than 45 days after the initial outbreaks. Since no commercial vaccine was available at that time, a recent H7N3 LPAI wild bird isolate from Mexico (A/cinnamon teal/Mexico/2817/2006) was selected and used as the vaccine seed strain. Although the vaccine was proven initially to be effective, a rapid evolution of the H7N3 HPAIV was observed with additional N‐glycosylation sites contributing to the escape of 2015 Mexican H7N3 HPAI viruses from vaccine‐induced immunity, which prompted the update of vaccine seed strains. Sporadic outbreaks have been detected under surveillance activities over the years triggering vaccination policy to remain in place.
In Egypt, vaccination began in 2006 as an emergency measure in response to the introduction of HPAI H5N1 virus in the country. The decision to implement vaccination was based on the positive results obtained in other countries (e.g. China). Several inactivated AIV vaccines were used since the beginning of the vaccination campaign. However, the vaccines used initially showed limited efficacy in preventing the spread of the epidemic in domestic poultry. As a result, in 2008, HPAIV H5N1 was still actively circulating in Egypt. In addition, the vaccination campaign that was initially led by the Government was largely discontinued and efforts handed over to the poultry industry. The reasons for the failure of vaccination have been extensively investigated by several authors and several main causes have been identified: low VE offered by some of the vaccines (possibly as a result of antigenic distance between seed strain and field viruses circulating in Egypt), lack of control of Good Manufacturing Practices (GMP) production conditions of vaccines, maternal immunity interference, poor biosecurity measures, inadequate protection of poultry population and insufficient surveillance. Furthermore, the introduction of H5 viruses belonging to different clades since 2006 made the epidemiological situation more complicated. As a response, the seed strains of vaccines were adapted to match the changes in the epidemiological situation, with more recent vaccines including three different H5Nx viruses and one H9N2 AIV seed strains (Kim et al., 2010).
3.1.3. Antigenic distance
The results of the antigenic distance analysis are reported in Table 4 for each vaccine strain for which the sequence was available and summarised in Figure 3. The values obtained indicate that most of the vaccine virus strain sequences showed a considerable antigenic distance (4–5 AU) from the consensus sequence of the AI virus genomes circulating in Europe between 1 October 2022 and 14 February 2023. Only few vaccines (e.g. Duck H5‐SRV vaccine®, MEFLUVAC H5N8, etc.) showed a smaller antigenic distance (2–3 AU) due to the presence of an H5 of the clade 2.3.4.4b in the vaccine composition (Table 4).
Figure 3.
Boxplots of the estimated antigenic distance, expressed in antigenic units (AU), according to different vaccine technologies considered
- The category ‘other technology’ includes live vectored, subunits and nucleic acids H5 vaccines.
3.1.4. Vaccine efficacy
3.1.4.1. Analysis of transmission experiments
In total, 12 studies performing transmission experiments using HPAI H5Nx viruses were identified (Table A3 in Annex A). Nine of these studies assessed VE to stop transmission in chickens and three in mule and Pekin ducks. From the identified studies, one conducted with chickens was not included in the assessment because it assessed the effect of maternal immunity on transmission rather than vaccination‐induced immunity. In the remaining 11 studies, in total, 53 experimental groups were assessed, consisting of 36 vaccinated groups and 17 unvaccinated control groups. The evaluated vaccines were inactivated vaccines (number of groups = 24), vectored vaccines (n = 7), DNA vaccines (n = 4) and RNA vaccines (n = 1). Two studies assessed the efficacy of vaccines against H5Nx virus belonging to the clade 2.3.4.4.b in vaccinated (single dose) chickens. One of these studies evaluated two vector (HVT‐H5 (COBRA) and Vectormune®), one DNA (ExactVac) and one inactivated (Nobilis® AI H5N2) vaccines against an H5N1 virus (Germeraad et al., 2023) whilst the other study assessed a vector vaccine (Vectormune® AI) against an H5N8 virus (Palya et al., 2018). Both studies concluded that the HVT vector vaccines reduced transmission to Rvac < 1, whereas the inactivated and DNA vaccine did not. One study (Grasland et al., 2023) assessed the efficacy of an RNA vaccine (Duck H5‐SRV vaccine®) and a subunit vaccine (Volvac B.E.S.T. AI+ND®) against transmission of an H5N1 (clade 2.3.4.4.b) virus in mule ducks. Both showed the ability to reduce direct transmission in 7‐week‐old mule ducks.
Using data from the eight transmission studies done in chickens, the VE efficacy parameters VEs, VEsh, VEm and VEs,sh were derived. Figure 4 shows the distribution of these parameters for the experimental groups where vaccination stopped sustained transmission (R < 1) and those where sustained transmission was not stopped (R > 1). Logistic regression analysis showed a significant association (p < 0.05) between these VE parameters and the probability of stopping transmission. Because we were interested in the combined effect of the reduction in susceptibility and infectiousness on transmission, a curve was fitted showing the predicted association between VEs,sh on the one hand and transmission on the other hand.
Figure 4.
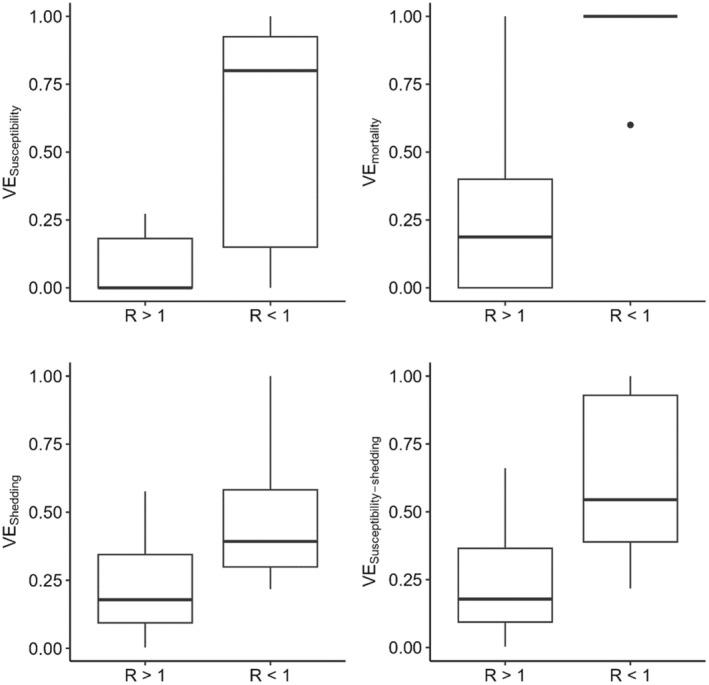
Boxplots of estimated vaccine efficacy (VE) parameters for vaccines that stopped sustained transmission between vaccinated chickens (R < 1) or did not (R > 1)
This predicted relationship between VEs,sh and transmission is shown in Figure 5. From this curve the probability that a certain VEs,sh value is associated with Rv < 1 can be derived. For example, the VEs,sh values that would predict probabilities of protection against transmission of 50%, 80% or 90% are 0.41, 0.67 and 0.82, respectively. VEm was not considered in this analysis, because mortality is associated with clinical protection and our interest here is blocking transmission. The predicted probabilities of protection against transmission (VET) are presented in Table A2 in Annex A and in Table 4 for the H5 vaccines for which the available data allowed the estimations.
Figure 5.
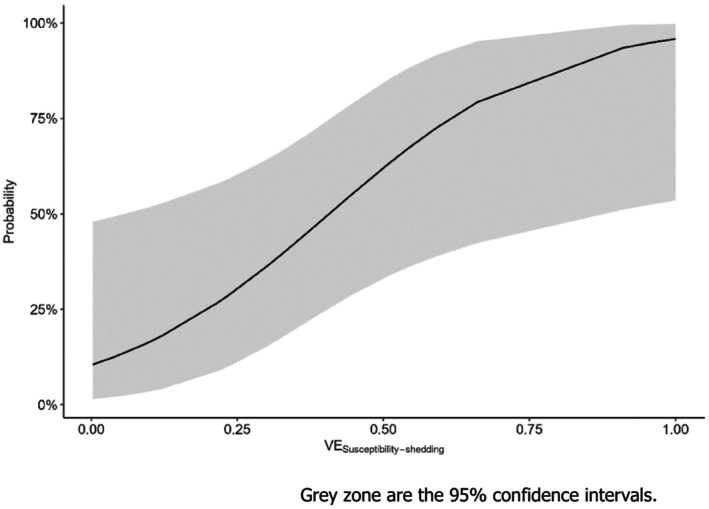
Relationship between vaccine efficacy parameter for infection and shedding VEs,sh and the probability of protection against transmission VET (R < 1)
Grey zone are the 95% confidence intervals.
Differences in challenge doses might create uncertainty in this assessment because it is likely associated with the probability of infection. However, from the review it was not possible to examine a relation between the challenge dose and VE. Nevertheless, because the corresponding unvaccinated control groups showed extensive transmission and 100% infection and mortality in the inoculated donors, this uncertainty is not expected to impact the conclusion. In addition, even though no data were retrieved for turkeys, it is assumed that the model developed for chickens would be also suitable for turkeys because they are both gallinaceous poultry.
A summary of the VE parameters' values for the studies conducted in mule and Pekin ducks is presented in Table 7. The small number of studies in ducks did not provide sufficient power to test the associations between transmission and the VE parameters like performed for chickens.
Table 7.
Summary of VE parameters quantified from data retrieved from studies assessing vaccine efficacy to stop transmission (Rvac < 1) in vaccinated ducks
| Number of vaccinated groups | HI Hom. Median (IQR) (a) , (b) | HI Het. Median (IQR) | VEs Median (IQR) | VEsh Median (IQR) | Ves,sh Median (IQR) | Rvac |
|---|---|---|---|---|---|---|
| 7 | 8.1 (7.45–9.25) | 0.09 (0–0.92) | 0.6 (0.3–0.95) | 0.39 (0.27–0.53) | 0.69 (0.53–0.71) | R < 1 |
| 1 | – | 0.14 | 0 | 0.15 | 0.15 | R ≥ 1 |
Haemagglutinin inhibition (HI) titres (log2 transformed) measured using the vaccine virus strain as antigen [homologous (Hom)] or the challenges virus strain [heterologous (Het)].
IQR: interquartile range. The 25% and 75% quantiles are presented.
Even though the associations could not be examined for ducks (see above), the association between the VE parameters and transmission seems also present for ducks (see Table 6).
3.1.4.2. Analysis of challenge experiments to estimate transmission
In total, 28 studies with 67 vaccines (47 inactivated, 18 vector, 1 subunit and 1 RNA vaccine) were included in the analysis. These studies did not assess transmission. When considering all studies, in total, 126 vaccination experiments (i.e. 126 groups characterised by a different number of doses–poultry species–vaccine type combination that were assessed against unvaccinated control groups) were performed. Among these experiments, 92 vaccinated groups included chickens, 25 ducks (10 Muscovy ducks, 11 Pekin ducks and 4 mule ducks) and 9 turkeys. The number of experiments evaluating VE after one dose were 73, after two doses were 52 and only one study assessed the VE after three doses. The time of challenge post vaccination, for the studies assessing one dose, ranged from 14 to 66 days. Two studies assessed VE 14 days post vaccination, 57 between 21 and 30 days and 14 studies later than 30 days.
For each study the VE parameters were derived for the tested vaccine. These are presented in Table A2 in Annex A. The probability of each specific vaccine to reduce Rvac < 1 was estimated using VEs,sh. We chose the combination of VEs and VEsh instead of only one of those two parameters because transmission can be considered as the result of susceptibility and shedding (indicator of infectiousness) and VEs,sh combines the effect of vaccination on susceptibility and shedding. Table 8 shows the predicted numbers of vaccines that have a probability > 50% to have Rvac < 1 based on the effect of vaccination on susceptibility and shedding combined (VEs,sh). According to this table, 31/46 (67%) and 14/15 (93%) of the assessed vaccines in chickens experiencing a homologous or heterologous challenge, respectively, are predicted to have a > 90% probability of Rvac < 1. For the duck experiments it was predicted that five out of the six assessed vaccines with a homologous challenge would have a > 90% probability of Rvac < 1, whilst only 1/3 vaccines with a heterologous challenge predict a probability between 80% and 90%. For turkeys, only experiments with heterologous challenge were available and two vaccines were assessed. Both vaccines had a probability of Rvac < 1 between 80% and 90%.
Table 8.
Number of vaccines predicted to have a probability > 0.5, > 0.8 or > 0.9 to have a Rv < 1 based on the effect of vaccination on susceptibility and shedding combined (VEs,sh). The number of experimental groups that provided the information are between parentheses
| Species (no. vaccines tested) | No. of vaccines tested | Type of challenge | No. of vaccines (no. of experiments) | Prob (R < 1) > 0.5; no. of vaccines (no. of experiments) | Prob > 0.8; no. of vaccines (no. of experiments) | Prob (R < 1) > 0.9; no. of vaccines (no. of experiments) |
|---|---|---|---|---|---|---|
| Chicken | 58 | Homologous | 46 (64) (a) | 36 (45) | 33 (41) | 31 (39) |
| Heterologous | 15 (27) | 15 (27) | 15 (27) | 14 (25) | ||
| Ducks | 7 | Homologous | 6 (13) | 6 (13) | 6 (13) | 5 (11) |
| Heterologous | 3 (12) | 1 (5) | 1 (5) | – | ||
| Turkeys | 2 | Homologous | – | – | – | – |
| Heterologous | 2 (9) | 2 (9) | 2(9) | – |
Number of vaccines are provided in decreasing (cumulative) values.
The results suggest that most assessed vaccines may effectively reduce transmission in chickens under experimental conditions. The predicted results for ducks and turkeys have to be considered with extra care because the prediction model is based on vaccination (transmission) studies in chicken.
3.1.5. Correlates (HA) of protection, onset and duration of immunity
3.1.5.1. Relationship between HI titre and transmission
Most of the data retrieved regarding serological response upon vaccination were limited to humoral immunity, which was mostly measured using the HI test. Among the different studies assessed, there was heterogeneity in the virus strain used as antigen for the HI test. In some studies, HI titres in vaccinated birds were measured using either the vaccine strain (homologous titres) or the challenge strain (heterologous titres) as test antigens. Other studies used both, whilst some studies used not the same but a similar strain to the challenge virus. When looking at the relationship between antibody titres and protection against transmission, particularly for chickens vaccinated with inactivated vaccines, there appears to be an association between HI titres and protection against transmission. However, this association was less clear for ducks and was absent for turkeys (Figure 6). The retrieved data on transmission experiments show that vaccinated ducks were protected against transmission whilst having very low levels of heterologous HI antibodies (Table 6). Despite the apparent association between antibody titres and protection observed for chickens, it was not possible to derive a uniform antibody threshold that would signal protection against transmission. This could in general be attributed to the heterogeneity in the HI assay procedures (difficult to harmonise between laboratories) from the retrieved studies, the virus antigen used for the assays and differences in response to vaccination (e.g. live vectored vs. inactivated).
Figure 6.
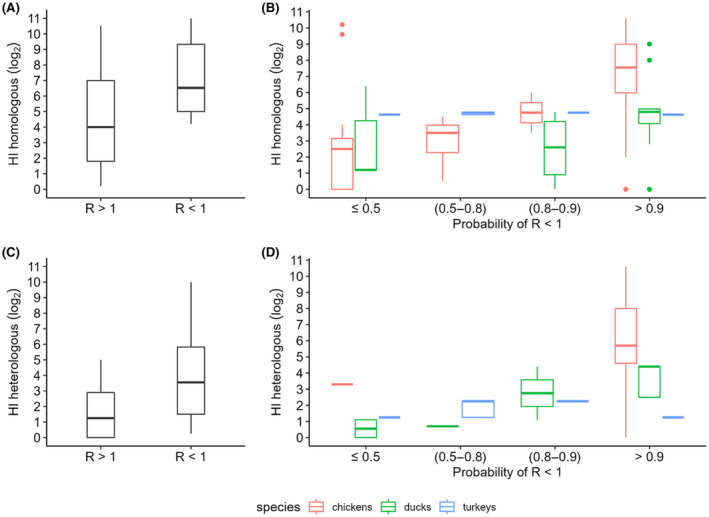
Distribution of geometric mean haemagglutinin inhibition (HI) titres for vaccines which stopped sustained transmission between vaccinated chickens (R < 1) or did not (R > 1)
- Panels (A) and (C) show the distribution of homologous (A) or heterologous (C) HI titres reported in the reviewed transmission experiments. Panels (B) and (D) show the distribution of HI titres from the data obtained from the challenge experiments. Probability (R < 1) classes in these panels were derived using the model presented in Figure 5.
As for vaccine technologies such as live vectored vaccines, heterologous humoral response may have less predictive value than for inactivated vaccines, as observed in the study of Germeraad et al. (2023) where vaccinated groups with HVT vaccines were protected against transmission despite showing very low heterologous HI titres against the challenge virus.
3.1.5.2. Time to reach protection post vaccination (onset of immunity)
Table 9 summarises transmission experiments carried out in chickens, ducks and turkeys that were single or double (first dose + booster) vaccinated and challenged either at 7, 14 or 21 days post vaccination. Based on these experiments one could expect – assuming a good vaccination coverage/immunity in the flock and close matching between circulating virus and the vaccine strain – that at 2 weeks post vaccination all these three species would be protected against virus transmission.
3.1.5.3. Duration of immunity
Evidence on duration of immunity in the retrieved literature was scarce. The difficulty in identifying correlates of protection makes this assessment also difficult. Challenge studies allow more precise information about protection over time in vaccinated poultry compared with measuring (humoral) immune responses alone. Rudolf et al. (2010) conducted a challenge study to overcome this problem. Commercial layers (20 weeks old, n = 3,600) kept under field conditions were vaccinated following different vaccination schedules and monitored during a 2‐year period. The vaccine used was an authorised inactivated vaccine based on an LPAIV H5N2 strain A/duck/Potsdam/1402/86 and the virus used for the challenge study was an HPAIV H5N1 clade 2.2 (antigenic distance around six AUs). Groups of vaccinated (one group per vaccination schedule tested) and not vaccinated layers were randomly selected at different time points and used to assess VE to reduce susceptibility to infection (VES) and for some groups also assessed the efficacy to stop transmission to unvaccinated contacts. Three weeks following prime–boost vaccination, around 70% of chickens in the flock had heterologous HI titres ≥ 5 log2 and four out of eight challenged layer chickens were protected against infection, with infected layers being able to transmit infection to unvaccinated contacts (n = 5). Six months later, less than 50% of the vaccinated layers had heterologous HI titres ≥ 5 log2, and all challenged layer chickens (n = 8) became infected and transmitted infection to unvaccinated contacts (n = 5). Because of the group experimental set up, it is not possible to discern how many contacts were actually infected by the vaccinated (inoculated) layers. Inference about the level of protection against transmission cannot be made because the contact birds were unvaccinated, resulting to overestimation of transmission. Based solely on the results of the challenge experiments of this study, the results of this study indicate that immunity able to stop transmission is likely to last less than 6 months in chickens.
In turkeys and ducks, duration of immunity following vaccination has not been fully addressed.
Santos et al. (2017) tested the efficacy of different vaccination protocols in turkeys challenged at 6 and 16 weeks of age with an HPAIV H5N2 virus isolate of the 2.3.4.4 lineage. All tested vaccination protocols conferred significant protection and reduction of viral shedding when birds were challenged at 6 weeks of age. In contrast, challenge at 16 weeks of age demonstrated that protection was incomplete with a lower reduction of mortality and of viral shedding for all the protocols and that inter‐protocol variation was higher. This study, together with the preliminary results obtained from the turkey vaccination trials performed at the EURL that also are exploring protection later in the productive cycle (i.e. 100 days of age), show that duration of immunity following a primary course of vaccination depends on the vaccines used, dosage and vaccination schedule and that additional booster doses might be needed to ensure protective immunity for the entirety of the productive cycle.
In ducks, as reviewed by Pantin‐Jackwood and Suarez (2013), very little information is known regarding duration of immunity and even less regarding protection since the few studies that investigated these aspects only obtained serological data at 28–40 weeks post vaccination, without performing challenge experiments. However, it has been observed that antibody levels in Pekin ducks can diminish significantly at 28 weeks of age after having obtained high‐antibody levels 20 days after a booster dose of an LPAI H5N2 inactivated vaccine (Beato et al., 2007). Moreover, in ducks the response to vaccination might differ between species: Muscovy ducks showed lower antibody titers than Pekin ducks when vaccinated with the same vaccine, and they also presented higher morbidity and mortality (Cagle et al., 2011).
3.2. Drawbacks of a vaccination programme that may jeopardise a swift eradication of the virus and possible solutions (ToR 1)
There are several possible drawbacks related to the application of vaccination for complementing HPAIV control and eradication programmes that may jeopardise a swift eradication of the virus. An overview of most likely possible causal paths leading to failure of eradication of HPAI in vaccinated areas is described in Figure 7. Selected details of these factors and suggested corrective measures are listed and described in Table 10.
Figure 7.
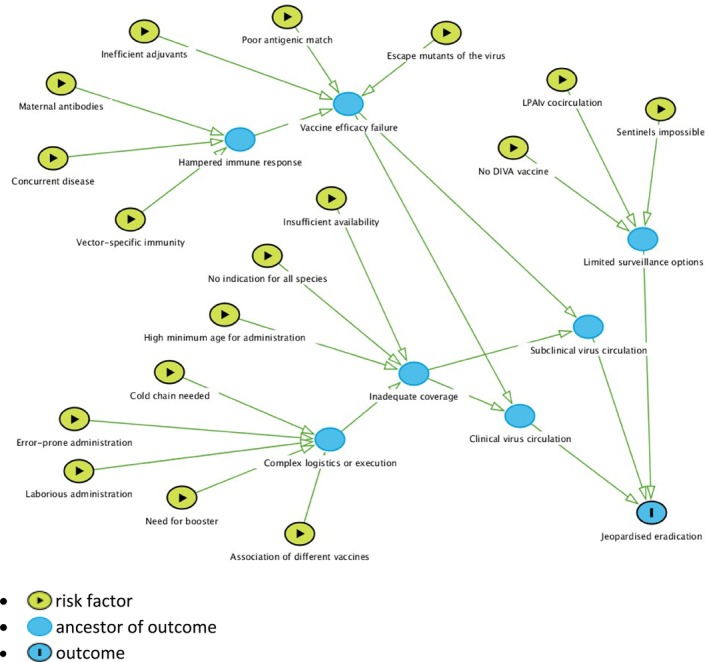
Overview of potential factors and possible causal paths that could compromise the eradication of HPAI in vaccinated areas
Table 10.
Aspects related to the use of HPAI vaccines that can jeopardise the HPAIV control and eradication programmes
| Possible drawbacks in the control and eradication of HPAI by vaccination | Potential causes of listed drawbacks | Vaccine type associated with the cause | Corrective options |
|---|---|---|---|
| Insufficient intrinsic vaccine efficacy to fully protect birds | Poor antigenic match (no threshold for distance with regard to antigenic units available due to different types of vaccines, i.e. adjuvant, live vectored etc.) | All types | Conduct sufficient antigenic matching a priori |
| Selection or new incursion of escape mutant strains (several examples from the field, e.g. in China, required the consecutive replacement of at least 14 different vaccine strains: Re‐0 to Re‐14) | All types | Ensure sufficiently effective vaccination to prevent the rise of virus variants escaping vaccine‐induced immunity in the vaccine region and prevent virus incursions from outside (i.e. aim should be sterilising immunity) | |
| Ineffective adjuvants (scarcely stimulating immune response) | Inactivated | Ensure GMP vaccine production | |
| Host‐specific factors leading to extrinsic vaccine failure | Hampered immune response of the host due to: | ||
| Interference by maternal antibodies (target antigen: H5‐specific; vector antigens, e.g. NDV specific) | All types (excl. HVT vaccines administered at day 1) | Start vaccination when yolk‐derived antibodies (if present: measure!) no longer interfere (depending on maternal immunity levels, usually > 14 day of age) | |
| Concurrent immunosuppressive disease (e.g. IBD, HE) | All types | Health checks of the flock to be vaccinated should exclude such diseases; else, such diseases must be managed as well | |
| Interference by vector‐specific immunity (e.g. NDV specific) | Vectored vaccines | Ensure absence of vector‐specific immunity (vaccination records, measurements) | |
| Inadequate vaccine coverage within farms and/or regions | Insufficient availability of the vaccine | All types | Ensure sufficient supply before start of a campaign |
| Vaccine inadequacy for some species reared in affected area | HVT or FPV vector based | Ensure vaccine is licensed for species targeted | |
| High minimum age for administration (e.g. > 14 days of age) and/or the need for booster(s) to have sufficient protection | Inactivated, RNA | Time may not be sufficient to induce effective protection of birds before slaughter (e.g. chicken broilers) | |
| Complexity in the logistics or the execution due to: | |||
| Low thermostability of the vaccine (i.e. HVT: to be kept in liquid nitrogen; future mRNA vaccines) | Cell‐associated (HVT), RNA | Ensure cold chain are maintained before campaign starts | |
| Error‐prone application technique (e.g. no mass administration available) | All injectable | Ensure vaccination teams are large enough (and experienced) to conduct herd vaccination thoroughly and timely | |
| Need for booster vaccination | Depending on species and length of production cycle | Adjust vaccinations scheme with rearing phases of poultry; use one‐shot vaccines if appropriate | |
| Need for association of different vaccines to increase protection or to increase duration of immunity | Related to individual vaccines | High potency vaccines, repeated doses as well as cross‐boosting may be helpful | |
| Limited surveillance options | Lack of compatibility with a serological DIVA strategy (e.g. no suitable NA‐antibody assays available) | Inactivated whole virus vaccines | Can be compensated by using virological DIVA (e.g. PCR testing) |
| No possibility to use sentinels in some species | Live vectored | Can be compensated by using virological DIVA (e.g. PCR testing) | |
| LPAIV co‐circulation hampering/interfering with the assessment of the immunity and limiting the DIVA strategies | Serological DIVA compatible vaccines | Can be compensated by using virological DIVA (e.g. PCR testing) |
The main drawbacks that could hamper the success of a vaccination programme identified by the experts of the WG were (i) insufficient intrinsic efficacy of the vaccine to fully protect vaccinated birds and prevent new outbreaks, (ii) host‐specific factors (including external factors that adversely affect the host's immune system, such as immunosuppressive diseases) leading to extrinsic vaccine failure due to hampered immune response of vaccinated birds, (iii) inadequate vaccine coverage within farms and/or regions which could prevent the achievement of sufficient herd immunity to stop virus circulation and (iv) inefficient surveillance that may lead to the inability to detect field virus in vaccinated flocks, resulting in clinically silent circulation of HPAIV (Table 8).
Factors causing insufficient VE in general were not related to specific vaccine types with exception of two – ‘inefficient adjuvants’ related to inactivated vaccines and ‘interference by vector‐specific immunity’ that is associated only with vector‐based vaccines.
Insufficient VE due to poor antigenic match between the vaccine and field strain caused by in situ emergence of an escape mutant virus or the incursion of a new virus clade would require a swift update of available vaccines. In this regard, vaccines based on rapidly updatable vaccine platforms (e.g. nucleic acids‐based vaccines) offer advantages in terms of preparedness. In addition, it is important to start any vaccination campaign by selecting vaccines based on documented evidence, like transmission trials, that indicate their effectiveness stop transmission of the circulating field strain.
Host‐related factors that could lead to extrinsic vaccine failure are due to hampered immune responses of the host. A likely cause is interference between vaccines and maternal antibodies. In case of emergency vaccination, this should not become an issue, as the vaccinated population should be immunologically naïve to HPAIV. However, in case of preventive vaccination, this has to be considered relevant since grandparent and parent flocks being the most valuable breeding stock likely will be vaccinated. Interference by vector‐specific immunity either maternally derived or acquired through vaccination against other diseases (e.g. NDV ‐ specific) likewise need to be considered when vectored vaccines are going to be applied. Review of the available literature has gathered substantial evidence on the detrimental effects of maternal immunity against AIV on VE as a function of age of the birds at application. Only a few vaccines (e.g. HVT‐vectored) seem not to be affected in this respect. This is of particular relevance in vaccination programmes including breeders as in that situation progeny with high‐antibody titres against AIV might be produced.
Continuous monitoring of immunity in longer‐lived poultry populations might be needed to ensure protection during the whole production cycle or else indicate the need for a booster. An approximation of the duration of protective immunity after basic immunisation can be derived from information obtained in laboratory trials investigating this aspect. However, serologic monitoring of the vaccinated flock is highly recommended in the field to choose the optimal time point for possible booster applications. Concurrent immunosuppressive diseases (e.g. IBD) in the vaccinated flock could likewise hamper vaccination efficacy. Monitoring the uptake of vaccination should therefore be implemented to help identify flocks with inadequate mounting of immunity.
Identified factors leading to inadequate vaccine coverage within farms and/or regions were not related only to certain vaccine types but also to specific vaccine preparations or species and production types of birds. Vaccine types related to specific factors often were vector‐based vaccines (no indication to all species), cell‐associated (HVT) or based on RNA (low thermostability requiring storage and transportation of vaccines at −20°C or lower temperatures). The general scarcity of mass administrable vaccines is a major limitation for immunisation of large bird populations in particular for emergency preventive vaccination when high number of birds need to be immunised in an as short as possible time to ensure rapid onset of immunity. Alternatively, a large amount of trained workforce is required that, in turn, might increase fomite‐based virus transmission between holdings. In a preventive vaccination scenario, this is less likely to represent a limitation since vaccination of the population can be planned in advance and tolerates longer times to be completed. However, movement of (vaccination) personnel between farms represents a risk for agent transmission under all circumstances. Limited options of surveillance would likely restrict the capability of detecting field virus in vaccinated flocks, may delay the discovery of vaccination programme failure, and allow time for the virus to form escape mutants, which would further jeopardise virus control. Establishment of an endemic status of the disease within the poultry population is a likely outcome of inefficient surveillance. Implementation of a robust surveillance system alongside the vaccination campaign is a pivotal prerequisite. Balancing of the costs and the sensitivity of the surveillance, however, can be a delicate task as surveillance for HPAIV circulation in vaccinated birds cannot rely on passive surveillance alone. Instead, a multi‐layered surveillance approach combining and integrating passive and active components is required. Mixed experiences with the use of unvaccinated sentinels as an attempt to enhance the sensitivity of the passive surveillance component has given rise to reservations regarding the sentinel strategy. This relates to logistic problems of ensuring sufficient contact of susceptible sentinel poultry with vaccinated birds and also refers to the use of live vectored vaccines since the replicating vaccine vector virus might infect the unvaccinated sentinel birds if spread from vaccinees.
3.3. Practical considerations and limitations on available vaccines (ToR 1)
The most obvious practical utility of the data reviewed above would be a simple decision tree, allowing the user to input data and the resulting output being a suggestion of certain vaccine(s) assigned to the user's particular epidemiological situation. However, due to the plethora of factors that are intricately interwoven with each other, too many chance nodes, alternative branches and optional features would result that render such a tree unmanageable. Thus, there is no single option/solution for a given situation.
In addition to the assessment of the data retrieved from the literature or provided by the producers, there are general considerations and limitations that can be inferred for the available vaccines. These general considerations and limitations are quite similar between those for H5, H7 and H5–H7 subtypes.
3.3.1. Technology
Most of the identified vaccines are based on whole inactivated virus. Most of the more recent vaccines are based on strains which have been created by reverse genetics technology using an egg‐adapted H1N1 human influenza virus A/Puerto Rico/8/1934 (PR8) as backbone with the HA and NA genes of an HPAIV strain. This allows production under BSL‐2 conditions and increases safety of the vaccine strains; in addition, production yields increase grossly when using the PR8 backbone. This technology, being widely available, allows rapid update of vaccine seed strains. Immunogenicity of inactivated vaccines is highly dependent on the adjuvants used, with some variability observed between the different poultry species. Moreover, the immunity that is provided by inactivated antigens is usually short lived following a single dose (usually few weeks) and therefore, multiple vaccinations are usually required to provide immunity for the duration of the entire production cycle of longer living birds. Also, effectors of immunity are strictly skewed to the humoral side. It is also worth to notice that, despite widespread use, these vaccines should be compatible with a serological DIVA strategy. Consequently, they need to be coupled with an ad hoc serological test able to detect the specific antibodies against the NA of the circulating strain that needs to differ from the one of the vaccine seed strain. However, reassortment with viruses with a different NA protein or co‐circulation of viruses with different genetic composition might hamper such a DIVA strategy. Recombinant technology and tools for nucleic acid synthesis allow smooth adaptation to the circulating strains compared with whole virus vaccines without the need for virus isolation, but requiring only information related to the genetic sequence of circulating strains.
In this regard, the new Veterinary Medicines Regulation (EU) No 2019/6 8 , has introduced the concept of a vaccine platform technology master file (vPTMF), for which the main aim is to avoid unnecessary re‐submission and re‐evaluation of data in the EU. Once a vPTMF for a given platform technology has been approved for the first time, after scientific and technical evaluation of its documentation, re‐submission and re‐assessment of data included in a certified vPTMF will not be necessary for other products using the same platform for a different gene of interest and intended for target species and for the route(s) of administration already accepted for the vPTMF. This will result in less documentation required to be provided by a company and less data to be evaluated by the authorities with a consequent reduction of the time needed (from development to authorisation). This could be used, for example, for vector vaccines to exchange the expressed AI specific strain gene.
Whilst some recombinant technology vaccines have been identified, currently, only two vaccines are based on nucleic acid technology (RNA replicon and DNA). However, the flexibility for antigenic update offered by these technologies is a major advantage and therefore it is likely that more will become available, if the costs will be sustainable. The amount of immunogenic antigen administered (i.e. the antigen mass) plays a pivotal role in the final titres reached against the vaccine antigen. To compare different inactivated vaccines, based on whole virus or recombinantly expressed proteins, the antigen mass per dose should be specified. However, the choice of adjuvants may potentiate the antigen mass effect in a way that is difficult to quantify. For replication‐competent vaccines, e.g. recombinant HVT vector vaccine, the amount of antigen presented to the immune system depends on the replication efficacy of the vaccine.
3.3.2. Target species
Most of the available vaccines are indicated for use in chickens. For species other than chicken there is a lack of authorised indications for use and of associated experimental trials to prove safety and efficacy in each species, which is generally required by regulatory authorities before authorisation. Off‐label use of vaccines in species other than chicken could be a potential solution, provided that the vaccine has the characteristics to be used in different poultry species (depending on the technology, see examples and explanation below, e.g. for all AI inactivated vaccines), although the efficacy of vaccines can vary between species due to differences in vaccine formulations and adjuvants used. Some vaccines have intrinsic technological characteristics that limit their use to specific poultry species or pose specific limitations (e.g. inefficient replication of HVT in ducks does not allow use of HVT‐vectored vaccines in this species). Given the lack of applications for a specific marketing authorisation for minor poultry species reared in Europe (e.g. geese, guinea fowl, pheasant, etc.), it must be considered that, in case of need, and according to the provisions outlined in Regulation (EU) No 2019/6 on veterinary medicinal products, vaccines registered for the disease in a different poultry species could be used in the absence of specific products. However, general lack of experimental evidence of immunogenicity and efficacy in minor poultry species hampers the prediction of possible outcomes of use in those species.
3.3.3. Route of administration
Most of the available vaccines are administered by injection, either subcutaneously or intramuscularly. Individual injection requires manipulation of birds, a procedure that can cause stress in birds and affect production. For heavier birds (e.g. turkeys late in the production cycle), individual manipulation can be highly labour intensive, and thus impractical for a rapid immunisation of large poultry population size. In addition, in certain housing systems (e.g. laying hens in aviaries where birds roam free), individual administration is complicated. Furthermore, the need of a large number of personnel entering the farms (i.e. vaccinating crews) increases the risk of potential virus spread among different poultry farms. Currently, there are no available vaccines that can be applied through drinking water. Only one vaccine (Vaxigen NewH5 – a NDV‐vectored vaccine) allows for spray vaccination, which represents a less labour‐intense alternative to individual immunisation and could allow immunisation of several flocks within a shorter period of time. However, the presence of pre‐existing immunity against NDV might interfere with vaccination efficacy (Lardinois et al., 2016; Bertran et al., 2018; Kim and Samal, 2019). Live vectored vaccines based on HVT or FPV as vectors are suitable for mass administration only in the hatchery (day‐old chicks or in‐ovo). This represents a limitation when large poultry populations of different ages need to be immunised over a short period of time (i.e. emergency preventive vaccination).
Vectored vaccines are widely used in many European countries for immunisation of chickens for other poultry diseases and have already proven to be time and cost efficient and able to provide homogeneous immunisation levels in chickens. Vaccination in‐ovo or at 1 day of age, however, is not commonly used in poultry species other than chickens and therefore the purchase of specific equipment and training in hatcheries would be required before initiation of vaccination.
3.3.4. Number of doses
According to Table 7, vaccination can result in good protection in a naïve population after a single dose, thus allowing a fast immunisation of birds during emergency vaccination. It has to be noted, however, that protection obtained in the field can be lower than in the laboratory (Poetri et al., 2014) due to less accurate vaccination or vaccination efficacy affected by concurrent infections or other immunosuppressive diseases, and breed differences in response have also been observed, thus increasing the possibility of additional booster needed under field conditions. Replication‐competent live vectored vaccines instead could provide longer immunity after a single shot but do not allow boosting with the same vaccine, because they stimulate immunity also against the vector, which may prevent successful replication. Therefore, boosting with different vaccines based on different technology (e.g. inactivated vaccine) would be required if immunity wanes over time.
3.3.5. Age at first administration
There is wide variability in the recommended age at the first administration of the different vaccines, ranging from 1 day to 6 weeks of age. However, this information was often lacking. The age at first administration also depends on the interference of maternal immunity (of interest in case of preventive vaccination), which can reduce the efficacy of vaccination, especially for inactivated vaccines. Some live vectored vaccines (i.e. HVT vectored) have been proven to be less affected by maternal immunity and could be administered early even in presence of maternally derived antibodies. However, other vectored vaccines (i.e. NDV‐vectored) are more likely to be impacted by pre‐existing immunity against the vector (Lardinois et al., 2016; Bertran et al., 2018; Kim and Samal, 2019). If vaccination plans include breeders, the presence of maternal immunity in chicks could require the postponement of the vaccination schedule to reduce interference.
It is important to notice that it is common practice to use specific pathogen‐free (SPF) or specific antibody‐negative (SAN) birds for immunisation or efficacy trials and therefore interference of maternal immunity has not been studied extensively for many available vaccines, with possible repercussions on the number of doses administered or on the vaccination schedule.
3.3.6. Onset and duration of immunity
There was a limited amount of information available on the onset and duration of immunity. Among the studies that investigated this aspect, it was found that the onset of immunity occurred ~ 2–3 weeks after completion of primary vaccination. Also, a certain level of immunity was still observed in a proportion of birds for up to 40–50 days after vaccination. The assessment of the onset and duration of immunity is of extreme importance to ensure adequate protection for the entire production cycle in each species and production type. For example, vaccination of short‐lived poultry, such as chicken broilers with generally a lifespan of 28–42 days, is not expected to provide any significant protective value due to the limited duration of their lives and immunity being protective only for the last few days of the production cycle. A rapid onset of immunity is a desirable characteristic as it can reduce the window of susceptibility in vaccinated birds. Knowing precisely the time between vaccination and protection can help in the understanding the time required to have a target poultry population protected after the initiation of a vaccination campaign and to understand the duration of the ‘open window’ between vaccination and protective immunity.
3.3.7. Antigenic distance
Studying antigenic distance is of significant interest as it provides information on the antigenic match, which is pivotal in determining the most effective vaccine antigens. Antigenic distance is commonly used as an indicator of VE for inactivated, non‐adjuvanted human seasonal influenza vaccines. A shorter antigenic distance is likely to indicate a higher antigenic match, suggesting that the vaccine is likely to induce strong protective immune responses. Antigenic cartography has been successfully used to identify antigenic clusters likely to escape immunity induced by non‐adjuvanted seasonal influenza vaccines in the human host, with a close monitoring of poorly reacting strains and genetic data to assess the needs for vaccine composition updates. For AIV vaccine seed strains updates in vaccinating countries have been mainly driven from field observation of vaccine failures and supported by laboratory testing (Cagle et al., 2011).
Mapping approaches similar to the ones conducted for seasonal influenza have also been applied for HPAIV (Smith et al., 2004; Sitaras, 2017, 2020; Isoda et al., 2020). However, providing data to create antigenic cartography remains cumbersome and requires the availability of specific antisera and harmonised analytical pathways, which can be difficult to achieve ad hoc. In contrast, rapid in silico comparisons of antigenicity indices, as exemplified in this assessment, rely on more easily accessible nucleotide sequence information of the haemagglutinin gene. However, information drawn from this analysis remains limited and, at best, can provide a rough overall comparison of antigenicity similarities.
For live‐vectored vaccines, the antigenic distance might have less impact on the VE compared with inactivated vaccines, which allow a higher degree of cell‐mediated immunity stimulation (Zhao et al., 2018). It is also difficult to assess the antigenic match between the immunity provided by vaccines that contain multiple antigens or the effect of prime–boost immunisation schemes involving heterologous vaccines. Experimental vaccination–challenge data in the targeted poultry species for these types of vaccines, or experiments with specific immunisation protocols, would be required for a more reliable assessment. However, as a general rule, repeated exposure to slightly different antigens could increase protection by preferentially stimulating immune cells that produce cross‐reacting antibodies.
In addition, it should be acknowledged that antigenic escape can occur with the exchange of just a few amino acids in the HA gene (Cattoli et al., 2011a,b) and that such changes may not be captured by antigenicity index comparisons unless more advanced weighted methods are used.
3.3.8. Vaccine efficacy
The assessment showed that vaccination can stop transmission under experimental conditions (Rvac < 1). In naïve birds, protective immunity is obtained 2–3 weeks after a single vaccination. Vaccine efficacy measured under controlled conditions may not always correlate with the effectiveness in the field, as demonstrated by Poetri et al. (2014) and Koch et al. (unpublished). In addition, circumstances between experimental trials may differ with regard to housing of the animals, ventilation and density that may influence the contacts between the birds and thus the virus transmission. Furthermore, most studies estimating VE only examined infection and shedding as parameters to express efficacy. Although this assessment showed the association between these VE parameters and transmission, this association may be prone to bias for individual vaccines, e.g. factors like challenge dose and route.
3.3.9. Correlates of protection
In most vaccines assessed, HI antibody titres, a surrogate of neutralising antibodies, are used as a correlate of protection. Defining a reliable threshold indicating protection, however, remains elusive. This is due to (i) species‐specific responses of immune response dynamics and (ii) features of the vaccine employed (live vector vs. inactivated‐adjuvanted). Often, homologous HI titres, i.e. measured against the vaccine HA antigen, are indicated but these may differ substantially from heterologous ones obtained against a current circulating field virus in case of a poor antigenic match between the two. Cellular immune response contributing to protection induced by live‐vectored vaccines cannot currently be judged in routine vaccine assessment procedures, due to a lack of scalable methods for assessment of this immunity component.
For inactivated vaccines the heterologous HI titre is clearly associated with protection against transmission. This proved to be less clear for the vectored vaccines, probably because the cell–mediated immunity induced by these vaccines adds to protection. In addition, in contrast with Rudolf et al. (2010), Koch (unpublished) and Poetri et al. (2014) demonstrated that vaccination–induced HI titres may be lower in the field than in the laboratory. The reason is unclear, but could be due to concurrent infections or flaws in the vaccination process. This implies that, in addition to establishing efficacy under controlled conditions, VE should also be tested in birds vaccinated under field conditions.
3.4. Definition and assessment of vaccination scenarios (ToR 2)
To define vaccination scenarios in order to achieve effective prevention, control and eradication of HPAI outbreaks in poultry populations, both intrinsic factors related to the type of vaccine chosen (see Sections 3.1–3.3 related to ToR 1) and extrinsic factors about policy priorities and operational factors have to be considered.
3.4.1. Key factors to consider when building vaccination scenarios
In this section, the extrinsic aspects to be considered when planning a vaccination campaign are illustrated and discussed. It is assumed that for vaccination purpose the selection of the suitable vaccine to be used in the programme has been done according to the vaccine type and characteristics as described in ToR 1.
3.4.1.1. Factors triggering the initiation of vaccination against HPAI
Factors that trigger the initiation of HPAI vaccination in poultry depend on the prevailing epidemiological situation, the characteristics of the susceptible poultry population, and relevant environmental and social factors. Note that ‘poultry’ refers to the definition given by the Regulation (EU) No 2016/429 of the European Parliament and of the Council of 9 March 2016, point (9): ‘poultry’ means birds that are reared or kept in captivity for (a) the production of meat, eggs for consumption, other products; (b) restocking supplies of game birds; (c) the purpose of breeding of birds used for the types of production referred to in points (a) and (b).
Epidemiological situation and animal welfare concerns
The main epidemiological factors that would trigger the initiation of HPAI vaccination in poultry include: (expected) extensive numbers of poultry outbreaks resulting in insufficient culling and disposal capacity or derived from poor early detection systems; infected wild bird populations, leading to repeated primary incursions of HPAIV; high risk of virus introduction from a neighbouring affected country/zone. Also, the circulation of virus strains with a high‐zoonotic potential might trigger vaccination in poultry to reduce the risk of human exposure to infected poultry with zoonotic virus which poses a threat to public health (Sims and Peiris, 2013; Shi et al., 2018; Wu et al., 2019).
Expected animal welfare consequences is another important factor that can trigger the need for vaccination. The suffering of birds from clinical signs of infection, in addition to specific measures like the confinement of poultry that are typically raised outdoor during high‐risk period of virus introduction, have negative impacts on the welfare of the poultry population. Using vaccination as a control measure, it is possible to mitigate these welfare impacts. In addition, vaccination serves as a mean to reduce the number of outbreaks thus reducing the overall number of culled birds, including pre‐emptive culling measures. The recent unprecedented number of culled poultry has raised ethical questions, since vaccination could be used as an alternative.
Characteristics of the susceptible poultry population
The initiation of HPAI vaccination in poultry is also influenced by the characteristics of the susceptible poultry population. Factors such as a high density of poultry farms, which can lead to multiple poultry outbreaks caused by secondary spread, play a significant role (Boender et al., 2007; Dorigatti et al., 2010; Lambert et al., 2022; Bauzile et al., 2023). The spatial distribution of poultry in Europe is presented in Figure 8 and highlights the regions with the highest densities of chickens (scattered relatively across Europe) and ducks (in particular France and Hungary). The spatial distribution of HPAI poultry outbreaks is presented in Figure 9 suggests a correlation between poultry density and the occurrence of poultry outbreaks. Other factors that could trigger the initiation of HPAI vaccination are the structural characteristics of the poultry production systems such as outdoor poultry flocks, i.e. premises in which poultry are more likely to be in contact with wildfowl; poultry production systems with a high level of trading activities (Guinat et al., 2019, 2020a,b), leading to intense movements of live poultry, vehicles, equipment and persons; presence of highly susceptible poultry species: meat turkey, laying hens; presence of susceptible species not showing clinical signs of infection: ducks, geese; presence of long productive life poultry species, multi‐age or multispecies poultry farm holdings; presence of poultry with high‐genetic value/expensive/rare breeds; poultry farms with low level of biosecurity (Guinat et al., 2020a,b; Delpont et al., 2021), likely to be associated with a high risk of HPAIV introduction; and low level of immunity of poultry population in case of previous vaccination.
Figure 8.
Density (animals per km2) distribution of chickens (left panel) and ducks (right panel) from Gridded Livestock of the World −2015 (GLW4) by Gilbert et al. (2022)
Figure 9.
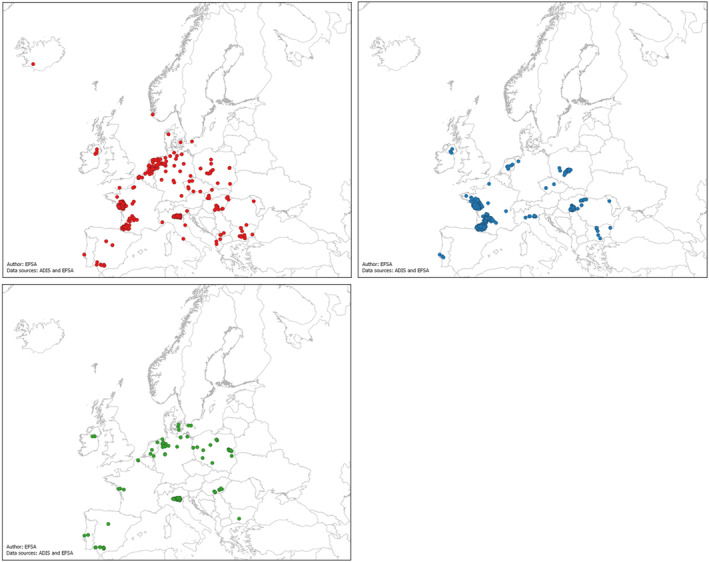
Spatial distribution of HPAIV outbreaks in chickens (n = 652) (left upper panel), in ducks (n = 1,059) (right upper panel) and in turkeys (n = 235) (lower panel) from October 2021 to September 2022
Environmental factors
The initiation of vaccination could be prompted by environmental factors that influence the occurrence of HPAIV outbreaks. Examples are proximity of poultry holdings to wetlands or areas with high density of migratory wild birds (Scolamacchia et al., 2021; Schreuder et al., 2022) along with climatic factors that influence the persistence of HPAIV in the environment HPAIV (e.g. temperatures, humidity, rainfall) (Si et al., 2010; Henning et al., 2019; Elsobky et al., 2020; Gass et al., 2023).
Social factors
The public or industry stakeholders might not fully support culling as a control measure for HPAI (Stokstad, 2022; Cohen, 2023). This could be due to various reasons such as the negative impact on local economies or on local rare breeds, ethical reasons or simply a lack of understanding of the risks posed by HPAI. In addition, large–scale culling of poultry results in wasting animal sourced proteins, which conflicts with the EU policy to increase sustainability of farming practices. In these cases, vaccination can be seen as a more acceptable alternative, as it allows for the preservation of flocks whilst reducing the spread of the disease.
3.4.1.2. Objectives of the vaccination and corresponding strategy
The formulation of vaccination scenarios will depend on the defined objectives and the corresponding vaccination strategy, as defined in Article 7 of the Regulation (EU) No 2023/361:
Vaccination of poultry in affected establishments to obtain rapid eradication: this would lead to implement an emergency suppressive vaccination , which aims at a short, temporary containment of the disease. This vaccination strategy would be implemented following incapacity to timely cull affected flocks due to e.g. shortage of personnel or means to cull the animal (shortage of gas, inaccessibility/overload of rendering plants, etc.). In such cases, vaccination would help ‘to buy time’. Nevertheless, all vaccinated poultry from affected premises are destined to be culled and disposed of, once logistics allow their further processing.
Vaccination of poultry in case of a change in the risk of HPAIV infection to prevent disease introduction and spread/to maintain freedom from disease/to prevent economic losses: this would lead to implement emergency protective vaccination , defined as (i) vaccination of poultry at risk of infection kept in affected countries/zones thereof but in establishments where the disease has not been confirmed nor is suspected or (ii) vaccination of poultry at risk of infection in non‐affected countries/zones in response to a change in the risk of HPAIV introduction. For instance, this vaccination strategy would be implemented in the case of rapid and uncontrolled spread of HPAIV infection in poultry or in wild birds in a neighbouring country/zone which implies a real change in the risk of infection.
Vaccination of poultry in the absence of a change in the risk of HPAIV infection to prevent disease introduction and spread/to maintain freedom from disease/to prevent economic losses: this would lead to implement a preventive vaccination, which is defined as vaccination of poultry in non‐affected countries/zones for preventive purposes other than the cases covered by emergency protective vaccination. Based on these definitions, a HPAI vaccination strategy can only be defined as ‘preventive’ if there is no change in the risk of HPAIV introduction in a non‐affected country/zone. For instance, this vaccination strategy would be implemented in the case of restocking of farms with vaccinated birds after HPAI eradication in a previously affected country/zone, or vaccination of highly susceptible poultry species during the summer to reduce the risk of HPAIV introduction from infected wild birds in the following migratory season, or preventive protection of poultry with high‐genetic value.
General considerations and examples of which vaccines could be potentially employed given according to the vaccination strategy (emergency protective or preventive) can be made according to the poultry species involved and the age.
In case of emergency protective vaccination, the following aspect of vaccines/vaccination should be taken into consideration:
vaccination could be target to poultry of all ages and all species/poultry types present in the vaccination zone;
use of vaccines with rapid onset of immunity, inactivated vaccines result in protection 2–3 weeks after vaccination;
inactivated vaccines can be used in all poultry species, but these vaccines should have a short antigenic distance to the circulating strain;
RNA (H5‐SRV vaccine®) and subunit vaccine (Volvac B.E.S.T. AI+ND®) tested by Grasland et al. are effective in mule ducks against currently circulating virus, although the onset of immunity is not exactly clear from the study design;
HVT vectored vaccines cannot be used in emergency vaccination programmes as the immunity against the vector is present in most gallinaceous poultry and it is not effective in Anseriformes poultry; ND vectored vaccines cannot be used in MS with ongoing vaccination programmes against ND because the pre‐existing immunity against the vector would interfere with vaccination efficacy;
due to lack of pre‐existing immunity against AIV, a single dose of an effective vaccine will in general be sufficient to curtail the transmission between farms;
no vaccine for mass vaccination is available under the current conditions in most of the MSs, thus each dose needs to be administered individually, requiring a vaccination capacity proportional to the target poultry population;
to enable quick administration, vaccine needs to be stockpiled, there should be a quick decision to implement vaccination and sufficient capacity to administer the vaccination should be in place;
if exposure to the virus (e.g. from wild birds) continues, repeated vaccinations may be needed to increase the length of the immunity and protection, but it is assumed that, in that situation, the emergency vaccination is followed by preventive vaccination.
In case of preventive vaccination, the following aspect of vaccines/vaccination should be taken into consideration:
vaccination could be targeted at those species/poultry types most susceptible and/or infectious in the region where vaccination is considered to be applied;
if vaccination is implemented in regular vaccination schemes (e.g. vaccination around hatch and in the rearing period), it takes a long time before the level of protection in the population reaches 70%;
inactivated vaccines can be used in all poultry species, but these vaccines should have a short antigenic distance to the circulating strain, vaccine fit to circulating field virus should be tested regularly;
RNA (H5‐SRV vaccine®) and subunit vaccine (Volvac B.E.S.T. AI+ND®) tested by Grasland et al. are effective in mule ducks against the currently circulating virus;
HVT vectored vaccines are suitable for vaccinating in ovo and day‐old gallinaceous poultry but are not suitable for Anseriformes poultry; ND vectored vaccines are not suitable in MS with vaccination programmes against ND because of pre‐existing (maternal) immunity against the vector;
Vaccines can be incorporated in regular vaccination programmes, but programmes might need adjustments (e.g. if HVT H5N1 is used HVT cannot be used against other diseases);
Repeated vaccination according to the specific species and age will be needed to ensure continued protection: from the evidence available, it is not clear whether a prime booster vaccination of young animals (e.g. vaccination around hatch and booster during the rearing period) is sufficient to protect long lived poultry (laying hens, breeding stock).
3.4.2. Specific vaccination scenarios for selected countries
3.4.2.1. Transmission‐risk maps
Figures 10, 11–12 show the high‐risk areas in the countries studied, that were determined based on the between‐farm reproduction numbers Rh quantified using their corresponding kernels. Areas with a low risk of between‐farm transmission (where an infected farm would mostly result in single or few outbreaks when the compulsory control measures are applied i.e. culling infected farms and implementing a 3 and 10 km zone for surveillance and movement control) were characterised by farms with estimated Rh < 1. However, for this assessment, a more conservative approach was taken by considering a threshold Rh <0.8 to discriminate between low‐risk and high‐risk areas. By taking this conservative approach we account for variation in the mean Rh estimates (the Rh from a farm whose estimated mean Rh is < 1 could still be 1 or higher due to the uncertainty associated with the estimate) increasing therefore the certainty of not missing high‐risk areas.
Figure 10.
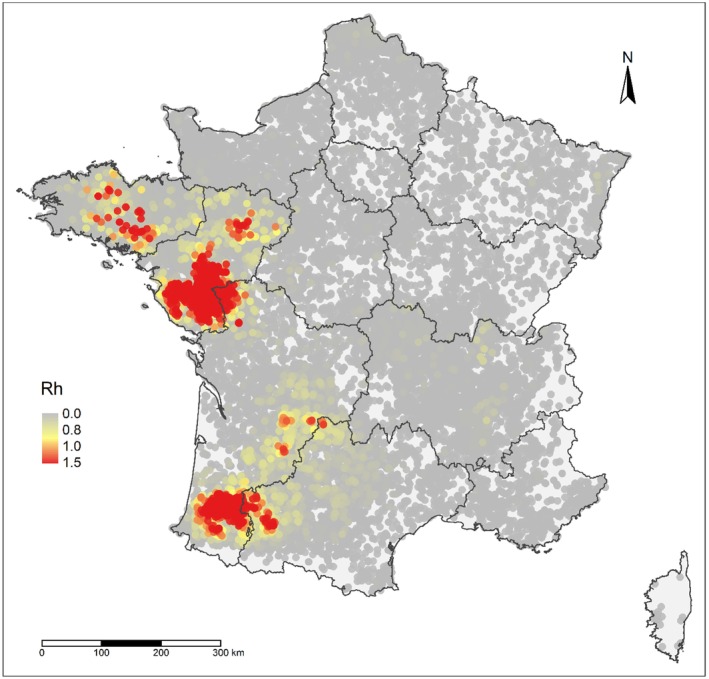
Between‐farm transmission risk map for HPAIV in France
- Rh are the between‐farm reproduction numbers quantified using the transmission kernel. Areas where Rh > 0.8 (farm density > 0.54 farm/km2) are considered high‐risk areas for transmission.
Figure 11.
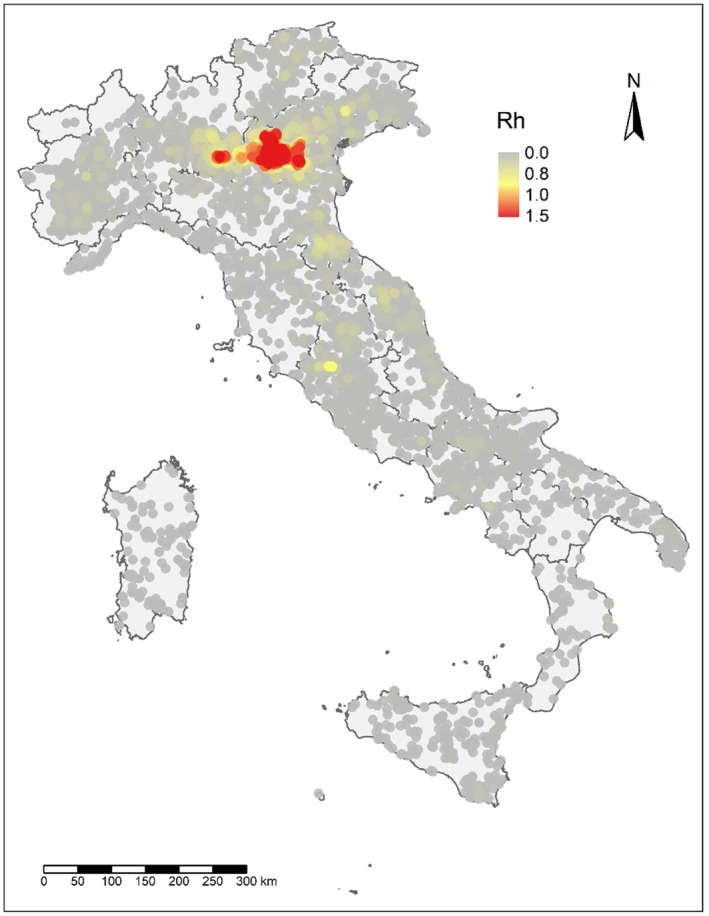
Between‐farm transmission risk map for HPAIV in Italy
- Rh are the between‐farm reproduction numbers quantified using the kernel. Areas where Rh > 0.8 (farm density > 0.52 farm/km2) are considered high‐risk areas for transmission.
Figure 12.
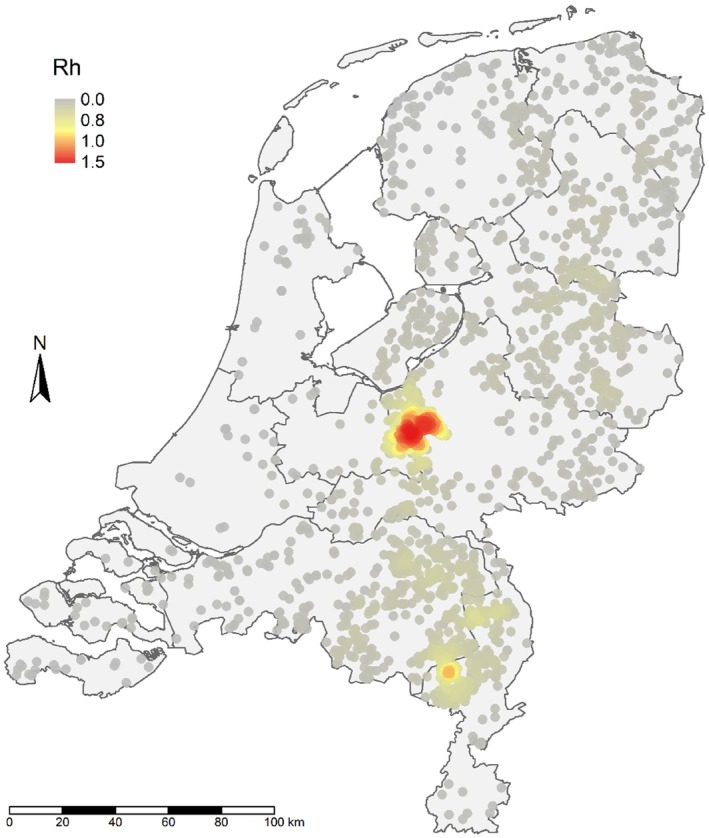
Between‐farm transmission risk map for HPAIV in The Netherlands
- Rh are the between‐farm reproduction numbers quantified using the kernel. Areas where Rh >0.8 (farm density > 0.84 farm/km2) are considered high‐risk areas for transmission.
Within the identified high‐risk areas (Rh > 0.8) for France, Italy and The Netherlands the median (range) estimated Rh were 1.58 (0.8–5.95), 1.52 (0.81–2.61) and 1.05 (0.81–1.40) respectively. The different median Rh among these countries could be attributed to their corresponding poultry production systems but also to other factors, such as the time between infection and detection of HPAI outbreaks (which depends on various factors including the type of affected species and timing of onset of clinical signs in those species). Significant differences in susceptibility and/or infectiousness between the different poultry species were identified (Table B.1 in Appendix B) which had an influence in the probability of between‐farm transmission. For all countries it was found that broiler farms were 2.2–7.5 fold less susceptible than commercial chicken layers and breeders (reference population for comparisons), whilst turkey farms were 1.3 to 3 times more susceptible than layers and breeders. Particularly in France the infectiousness of ducks and geese farms was significantly higher (19 times) than that of chicken layers and breeders whilst in Italy, the infectiousness of turkeys was 5.3 fold higher than chicken layers and breeders (Table B.1 in Appendix B).
Areas with Rh > 0.8 were considered areas with high risk for onward transmission. Farm densities in areas with Rh > 0.8 were > 0.54 farms/km2, > 0.52 farms/km2 and > 0.84 farms/km2 for France, Italy and The Netherlands, respectively. This means that within a 5‐km radius (using this radius to calculate density resulted in the highest correlation between farm density and Rh), a high‐risk area would have, on average, 43, 41 and 66 farms 9 , 9 in France, Italy and The Netherlands, respectively. We explored the correlation between farm densities and Rh as an alternative measure to identify areas where a high risk of transmission could be expected. These density measures could be used as reference for other MS.
3.4.2.2. Assessment of vaccination scenarios
Results for France
In France, several HPAIV subtype H5Nx epidemic waves occurred since the emergence of the currently circulating 2.3.4.4b clade following its introduction by migratory wild birds in 2014. The first major wave occurred in winter 2016–2017, during which France was the most heavily affected European country, with close to 500 poultry outbreaks (Guinat et al., 2018). Subsequently, France was affected by two other major waves in winters 2020–2021 and 2021–2022, with subtypes H5N8 and H5N1, respectively. The number of poultry outbreaks during these waves exceeded those caused by the 2016–2017 wave. Since the first major wave in 2016–2017, domestic ducks have been particularly affected, especially in the fattening duck production systems. Two high‐risk zones in south‐western and north‐western parts of France characterised by high‐poultry farm densities were incorporated into the French legislations from September 2021 (Guinat et al., 2019; Lambert et al., 2022). Due to the severity and frequency of outbreaks in the duck production systems, France was used as a case study to model different vaccination scenarios in which ducks would be targeted. According to the kernel estimation results, ducks and geese are characterised by 19‐fold higher infectiousness than layers and breeders in France (Table B.1 in Appendix B).
Figure 13 provides the results from the model simulations for each theoretical scenario in France (full details available in Table B.3 in Appendix B).
Figure 13.
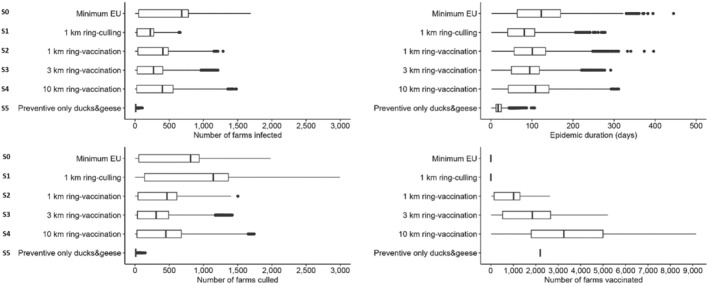
Results from the model simulations for each scenario in France
- The box extends from the first to the third quantile, the horizontal line extends from the 5th to the 95th percentile. The vertical line represents the median value and the black dots represent the outliers.
Table B.3.
Results from the model simulations for each scenario in France
| Scenario | Duration of epidemic (days) | Number of infected farms (a) | Number of culled farms (a) | Number of vaccinated farms (a) , (b) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | 5% | 95% | Median | 5% | 95% | Median | 5% | 95% | Median | 5% | 95% | |
| S0: Minimum EU | 122 | 12 | 241 | 681 | 5 | 1,452 | 812 | 5 | 1,727 | – | – | – |
| S1: 1‐km ring culling | 81 | 12 | 156 | 221 | 5 | 404 | 1,145 | 16 | 2,057 | – | – | – |
| S2: 1‐km ring vaccination | 101 | 12 | 193 | 407 | 5 | 897 | 467 | 5 | 1,073 | 1,005 | 7 | 2,243 |
| S3: 3‐km ring vaccination | 94 | 12 | 170 | 270 | 5 | 722 | 306 | 5 | 860 | 1,845 | 35 | 4,462 |
| S4: 10‐km ring vaccination | 108 | 11 | 199 | 397 | 4 | 1,074 | 451 | 5 | 1,276 | 3,248 | 238 | 7,756 |
| S5: Preventive vaccination ducks | 17 | 6 | 42 | 8 | 2 | 30 | 10 | 2 | 36 | 2,192 | ||
In this analysis a farm is considered to be characterised by unique location (XY coordinates), owner and poultry species. When a farm is detected infected, this and all the other farms in the same locations but with different poultry species or owner would be culled. This is the reason for the differences between the number of infected and culled.
This is the number of active farms (epidemiological units) being vaccinated under each scenario and do not indicate the number of times that poultry in a farm get vaccinated. For emergency vaccination one vaccination per farm is assumed. For preventive vaccination, poultry within a farm may receive more than one vaccination or the same farm might be subjected to more than one vaccine application when a new production cycle starts.
The minimum EU scenario (S0) shows the highest median number of infected farms and the longest epidemic duration of all scenarios tested. Furthermore, the upper bound (95%) of both the number of infected farms and the duration of the epidemic exceed those of the other scenarios.
The 1‐km ring culling scenario (S1) reduces the number of infected farms and the epidemic duration compared with the minimum EU scenario (S0). However, the median number of culled farms is clearly higher than in the minimum EU scenario (S0). Compared with all other scenarios the median and upper 95% of the number of culled farms is highest in this scenario.
Among the emergency vaccination scenarios (S2, S3 and S4), the 3‐km ring vaccination scenario (S3) performs best in terms of median number of infected and culled farms, and epidemic duration. In S2 with a 1‐km vaccination radius the virus has a high probability of having infected a farm outside the 1‐km area, making it very difficult for a farm to have protective immunity before exposure. For S4, the 10‐km vaccination radius, the limited vaccination capacity (20 farms per day) gives constraints when the virus jumps out of the 10‐km area, because the number of farms to be vaccinated will increase considerably. In addition, when an outbreak is detected outside the initial 10‐km area the allocation of vaccination in the model decreases the vaccination rate in the area first affected, because the probability for a farm to be vaccinated is the same across regions. S2 and S4 also show higher extremes (95%) of infected and culled farms compared with S3. The 3 km ring vaccination scenario (S3) shows an intermediate number of vaccinated farms compared with 1‐km and 10‐km ring vaccination scenarios. The ring vaccination scenarios (S2, S3 and S4) show a higher median number of infected farms and a longer epidemic duration than the ring‐culling scenario (S1) but a much lower median number of culled farms.
The preventive vaccination scenario (S5) results in the lowest number of infected and culled farms, and the shortest epidemic duration. This is likely due to the fact that ducks, that are the most susceptible and infectious upon infection, represent the main species in the high‐risk transmission area, and 70% of the farms are assumed to be fully protected before the virus is introduced; whereas in the emergency vaccination scenarios, a vaccinated farm has a probability of 70% to be fully protected 3 weeks after vaccination, creating a 3‐week window for virus spread. The 10‐km ring vaccination scenario (S4) shows a higher median number of vaccinated farms compared with the preventive vaccination (S5), likely due to the spread of the virus outside the high‐risk transmission area and the fact that in S4 all poultry types except broilers are vaccinated and in S5 only ducks.
Results for Italy
In the past three decades, Italy has experienced severe and persistent infections with both LPAI and HPAI viruses in poultry, with most cases concentrated in a HDPA stretching along the Po River valley in the north‐eastern part of the country. This area is characterised by the presence of high densities of poultry farms of highly susceptible species, especially layers and turkeys, near wetlands that harbour a remarkable avian biodiversity and abundance, representing an important crossroads of bird migration routes. In 2017–2018 and 2021–2022, Italy recorded major epidemic waves of HPAIV H5N8 and H5N1, respectively. Fattening turkeys, the second most common type of poultry production in the HDPA after broilers, were always severely affected during the HPAI epidemics, likely because of a higher probability of infection associated with their longer production cycle, species susceptibility and rearing practices (lamellae stable systems allowing for optimised temperature regulation but practically opening the stable interior to the outside). Italy was therefore used as a case study to model vaccination scenarios in which fattening turkeys will be primarily targeted.
Of note, Italy has faced multiple epidemics of H7 AI in the past. To combat these outbreaks, the country implemented compulsory vaccination programmes under official control, which ultimately resulted in eradication of infection. Initially, vaccination was applied as an emergency measure to contain outbreaks. However, between 2004 and 2006, vaccination was applied as a preventive measure, due to the high risk of re‐introduction from wild bird reservoirs (Capua and Marangon, 2000; Marangon and Capua, 2006).
Figure 14 provides the results from the model simulations for each theoretical scenario in Italy (full details available in Table B.4 in Appendix B).
Figure 14.
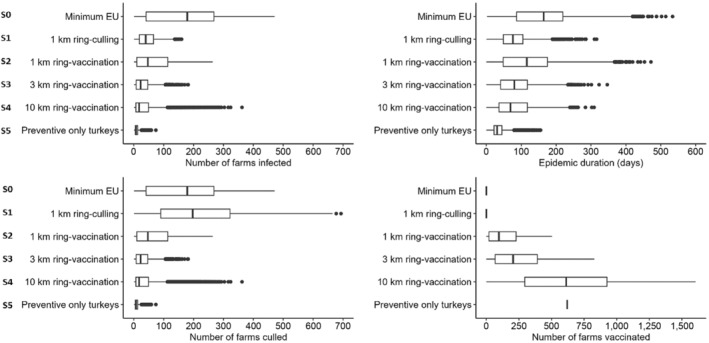
Results from the model simulations for each scenario in Italy
- The box extends from the first to the third quantile, the horizontal line extends from the 5th to the 95th percentile. The vertical line represents the median value and the black dots represent the outliers.
Table B.4.
Results from the model simulations for each scenario in Italy
| Scenario | Duration (days) (a) | Number of infected farms | Number of culled farms | Number of vaccinated farms (a) , (b) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | 5% | 95% | Median | 5% | 95% | Median | 5% | 95% | Median | 5% | 95% | |
| S0: Minimum EU | 164 | 28 | 303 | 179 | 8 | 343 | 179 | 8 | 343 | – | – | – |
| S1: 1‐km ring culling | 76 | 23 | 152 | 40 | 7 | 100 | 197 | 27 | 465 | – | – | – |
| S2: 1‐km ring vaccination | 111 | 6 | 252 | 47 | 2 | 175 | 47 | 2 | 175 | 96 | 1 | 327 |
| S3: 3‐km ring vaccination | 77 | 5 | 167 | 23 | 2 | 80 | 23 | 2 | 80 | 205 | 7 | 580 |
| S4: 10‐km ring vaccination | 66 | 4 | 177 | 18 | 2 | 148 | 18 | 2 | 148 | 612 | 68 | 1182 |
| S5: Preventive vaccination turkeys | 30 | 10 | 71 | 8 | 2 | 23 | 8 | 2 | 23 | 620 | ||
In this analysis a farm is considered to be characterised by unique location (XY coordinates), owner and poultry species. When a farm is detected infected, this and all the other farms in the same locations but with different poultry species or owner would be culled. This is the reason for the differences between the number of infected and culled.
This is the number of active farms (epidemiological units) being vaccinated under each scenario and do not indicate the number of times that poultry in a farm get vaccinated. For emergency vaccination one vaccination per farm is assumed. For preventive vaccination, poultry within a farm may receive more than one vaccination or the same farm might be subjected to more than one vaccine application when a new production cycle starts.
The minimum EU scenario (S0) shows the highest median number of infected farms and the longest epidemic duration. Furthermore, the 95% of both the number of infected farms and the duration of the epidemic clearly exceed those of the other scenarios.
The 1‐km ring culling scenario (S1) reduces the number of infected farms and the epidemic duration compared with the minimum EU scenario (S0). However, the median number of culled farms is slightly above the one estimated in the minimum EU scenario (S0). The median and the upper bound (95%) of the number of culled farms are highest in this scenario of all scenarios tested.
Among the emergency vaccination scenarios (S2, S3 and S4), the 1‐km ring vaccination scenario (S2) results in the highest number of infected and culled farms, and epidemic duration. The likely reason is that in S2 the virus has a high probability of having infected a farm outside the 1‐km vaccination radius and, consequently, a farm has a high probability of exposure whilst being unvaccinated. The 10‐km ring vaccination scenario (S4) appears to be the most effective in optimising the median number of infected and culled farms, and the epidemic duration, although the difference with the 3‐km scenario is small. In addition, the 10‐km ring vaccination (S4) has higher extremes (95%) of infected and culled farms compared with the 3‐km radius, likely due to the limited vaccination capacity of 20 farms per day, which may create a problem in the runs where the virus spreads outside the 10‐km radius. In addition, S4 involves the highest number of vaccinated farms when compared with 1‐km and 3‐km ring vaccination scenarios. The 3‐km and 10‐km ring vaccination scenarios (S3 and S4) show a similar median number of infected farms than the ring culling scenario (S1) but a much lower number of culled farms.
The preventive vaccination scenario (S5) results in the lowest number of infected and culled farms, and the shortest epidemic duration. This is likely due to the fact that turkey farms are the most susceptible and infectious species in the high‐risk transmission area. The number of vaccinated farms in the preventive scenario is comparable to the median in S4 but higher than the median in S3. However, the upper bound (95%) of the number of vaccinated farms in S4 is the highest among all vaccination scenarios tested likely due to the fact that in S4, all poultry except broilers are vaccinated and farms outside the high‐risk area can be vaccinated if the vaccination rings expand outside the high‐risk area.
Results for The Netherlands
The Netherlands suffered from a large epidemic of HPAI H7N7 in 2003. During this epidemic the most affected poultry production systems were commercial chicken layers (Stegeman et al., 2004) and between‐farm transmission was mostly concentrated in two areas where the farm density was higher than in the rest of the country (HDPA): the Gelderland Valley and the south‐east of the country. In these areas, the risk of sustained between‐farm transmission whilst taking the minimum EU measures, quantified in terms of the between farm reproduction ratio (Rh) was estimated to be high (Rh ≥ 1; Boender et al., 2007). Since 2014, The Netherlands have been experiencing mostly primary introductions of HPAIV H5Nx, with the highest number of outbreaks recorded between 2020 and 2022. During these years, sporadic infections in farms located in HDPA were observed, and in some cases there was limited between‐farm transmission. Commercial layers and broilers as well as chicken breeder farms constitute more than 90% of the poultry production sector in the country.
Figure 15 provides the results from the model simulations for each theoretical scenario in The Netherlands (full details available in Table B.5 in Appendix B).
Figure 15.
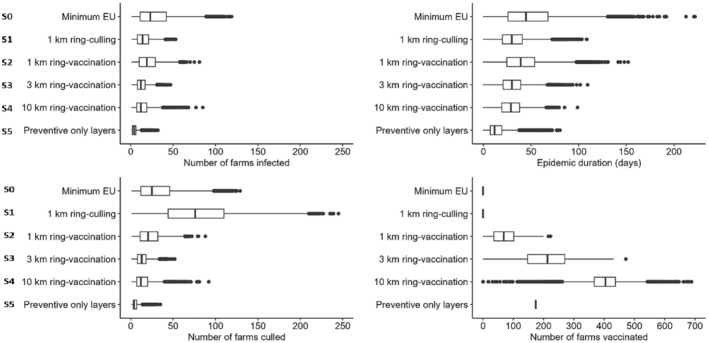
Results from the model simulations for each scenario in The Netherlands
- The box extends from the first to the third quantile, the horizontal line extends from the 5th to the 95th percentile. The vertical line represents the median value and the black dots represent the outliers.
Table B.5.
Results from the model simulations for each scenario in The Netherlands
| Scenario | Duration (days) | Number of infected farms (a) | Number of culled farms (a) | Number of vaccinated farms (a) , (b) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | 5% | 95% | Median | 5% | 95% | Median | 5% | 95% | Median | 5% | 95% | |
| S0: Minimum EU | 45 | 10 | 104 | 23 | 4 | 70 | 25 | 4 | 76 | – | – | – |
| S1: 1‐km ring culling | 30 | 9 | 60 | 14 | 3 | 31 | 76 | 14 | 157 | – | – | – |
| S2: 1‐km ring vaccination | 39 | 10 | 78 | 19 | 4 | 44 | 20 | 4 | 48 | 69 | 10 | 146 |
| S3: 3‐km ring vaccination | 30 | 8 | 55 | 12 | 3 | 24 | 13 | 3 | 26 | 213 | 54 | 333 |
| S4: 10‐km ring vaccination | 29 | 8 | 52 | 12 | 3 | 33 | 12 | 3 | 36 | 404 | 261 | 494 |
| S5: Preventive vaccination layers | 12 | 0 | 33 | 4 | 1 | 11 | 4 | 1 | 13 | 174 | ||
In this analysis a farm is considered to be characterised by unique location (XY coordinates), owner and poultry species. When a farm is detected infected, this and all the other farms in the same locations but with different poultry species or owner would be culled. This is the reason for the differences between the number of infected and culled.
This is the number of active farms (epidemiological units) being vaccinated under each scenario and do not indicate the number of times that poultry in a farm get vaccinated. For emergency vaccination one vaccination per farm is assumed. For preventive vaccination, poultry within a farm may receive more than one vaccination or the same farm might be subjected to more than one vaccine application when a new production cycle starts.
The minimum EU scenario (S0) shows the highest median number of infected farms and the longest epidemic duration. Furthermore, the predicted extreme outcomes (95%) of both the number of infected farms and the duration of the epidemic clearly exceed those of the other scenarios.
The 1‐km ring‐culling scenario (S1) reduces the median number of infected farms and the epidemic duration compared with the minimum EU scenario (S0). However, the median number of culled farms (and extreme values) is higher than the one estimated in the minimum EU scenario (S0).
Among the emergency vaccination scenarios (S2, S3 and S4), the 1‐km ring vaccination scenario (S2) shows the highest number of infected and culled farms, and epidemic duration. The likely reason is that, based on the transmission kernel, the virus has a high probability of infecting a farm outside the 1‐km radius vaccinated area, resulting in a higher likelihood of virus exposure in unvaccinated farms than S3 and S4. The 3‐km and 10‐km ring vaccination scenarios (S3 and S4) appear to be the more effective in optimising the number of infected and culled farms, and the epidemic duration. The 10‐km ring vaccination (S4) has slightly higher extremes (95%) of infected and culled farms compared with the 3‐km radius, likely due to the limited vaccination capacity of 20 farms per day, which may become a problem in the runs where the virus spreads outside the 10 km radius. The 3‐km and 10‐km ring vaccination scenarios (S3 and S4) show a quite similar median number of infected farms than the ring culling scenario (S1) but a much lower median number of culled farms.
The preventive vaccination scenario (S5) results in the lowest number of infected and culled farms, and the shortest epidemic duration. This is likely due to the fact that layer farms are the dominant farm type in the high‐risk transmission area. The number of vaccinated farms is intermediate to those estimated for the 1‐km and 10‐km ring vaccination scenarios (S2, S4), similar to the median of the 3‐km ring vaccination scenario (S3).
Results for the three countries
Overall, in all three countries, the minimum EU scenario (S0) results in the highest number of infected farms and the longest duration of the epidemic of the scenario's simulated here. In addition, preventive vaccination of the most susceptible target species (S5) in the high‐risk transmission areas consistently resulted in the lowest number of infected farms and the shortest duration of the epidemic. Among the emergency vaccination scenarios (S2, S3 and S4), the 3‐km ring vaccination scenario (S3) performs best in all three countries in terms of median number of infected, culled and vaccinated farms, and epidemic duration. S3 and S4 showed quite comparable numbers of infected farms and epidemic duration to the 1‐km ring‐culling scenario (S1), but with fewer culled farms. In France, S1 performed slightly better than in the other two countries, but that is likely the result of the higher culling capacity in France (six farms/day) compared with Italy (one farm/day) and The Netherlands (two farms/day). However, the 10‐km ring vaccination (S4) performed poorer in France than in Italy and The Netherlands. This is likely due to the combination of the high infectiousness of ducks and the limited vaccination capacity (20 farms per day). The latter increases the likelihood of the virus jumping out of the vaccinated area and if that happens the vaccination capacity will be overwhelmed. This was confirmed by running a simulation with a vaccination capacity of 60 farms per day where the median number of infected farms dropped to 80 (from 397) and the median duration to 70 days (from 108).
3.5. General considerations and practical aspects on vaccination scenarios and sources of uncertainty (ToR 2)
It is important to note that the results of the scenarios rely on the accuracy of the model's assumptions and parameter values. The parameters are based on data provided by the studied countries, including information on poultry population data, HPAI outbreaks and pre‐emptive culling. However, the model is a simplification of real‐world complexities, including the spectrum of situations found in reality (e.g. diversity of contexts, MSs, geography and livestock systems). The quality and precision of the parameters and assumptions play a significant role in the reliability and validity of the model outcomes.
Backyard poultry were not considered in the scenarios due to data limitations. Also, previous studies in The Netherlands and France have shown that captive bird and backyard holdings likely played a limited role on disease spread (Bavinck et al., 2009; Souvestre et al., 2019). However, it is important to note that Regulation (EU) No 2023/361, regulates the use of vaccination against HPAI also in captive bird and backyard holdings.
The different characteristics of vaccine type and products described in Section 3.1 were not explicitly integrated into the scenarios since, as already mentioned in Section 3.3. there is no single option/solution for a given vaccination scenario. However, general considerations and examples of which vaccines could be potentially employed were given according to the vaccination strategy (emergency protective or preventive), the poultry species involved and the age (Section 3.4.1.2).
It should be noted that the results of the number of infected, vaccinated and culled farms should be carefully interpreted in the view of the definition that was used in this analysis of a ‘farm’ (i.e. considered to be characterised by unique location (XY coordinates), owner and poultry species). Also, in cases an infected farm was identified, the assumption was made that not only the affected farm but also all other farms located at the same geographical location would be culled. This explains the differences observed between the number of infected and culled farms in the same scenario. In relation to the results of farms being vaccinated under each scenario, this does not include if poultry within a farm may receive more than one vaccination, or the same farm might be subjected to more than one vaccine application when a new production cycle starts (e.g. as expected in case of preventive vaccination).
In the emergency protective vaccination, the assumption that a vaccinated farm is protected against transmission 3 weeks after detecting the index case is crucial for the scenario's outcomes. This implies the immediate start of vaccination, sufficient vaccine availability and prepared vaccination teams/farmers.
In all vaccination scenarios, it was assumed that only 70% of the vaccinated farms would be effectively protected. This percentage, estimated by expert knowledge, takes into account different aspects that could limit reaching 100% protection (e.g. concurrent infections, flaws in the vaccination process, etc.). Moreover, particularly when considering preventive vaccination, immune waning over time may result in the loss of protection in some farms. Increasing the percentage of effectively protected farms is expected to enhance the performance of vaccination beyond what is shown in this analysis (Busani et al., 2009). A lower value of level of protection of 50% was also tested, and did not lead to changes in the order of magnitude of the outcomes in the different scenarios (data not shown), hence leading to the same conclusions. Variations in the percentage of vaccinated birds within farms was not assessed in this study, although this would need further investigation.
According to the assessment of VE (ToR1), it is assumed in the model that 3 weeks after vaccination a far is protected (R < 1), so virus introduction will not lead to an outbreak. Identified vaccines capable of reaching this level of protection (ToR 1) all require individual injection of a bird, either in ovo or day‐old or later in life. This implies that emergency protective vaccination using vaccines that are efficacious (according to this assessment) requires individual injection of birds in the species targeted for vaccination. This can be difficult because of the husbandry systems (e.g. aviary) or because of the size of the flock or animals (e.g. male turkeys late in the productive cycle), reducing the number of farms that can be vaccinated per day.
Vaccination of broiler farms is not considered effective giving their short life span compared with other poultry production systems, resulting in a no or a too short period to build sufficient immunity. This was corroborated by emergency vaccination scenarios simulations where broilers were included (data not shown) resulting in outcomes similar to those presented here. However, this may reduce the effectiveness of emergency vaccination scenarios in regions where broilers farms represent the major poultry type. Nevertheless, the low susceptibility of broiler farms compared with the other poultry types and the control measures implemented in affected areas, such as ban of restocking, will likely mitigate the risk of infection of this poultry production type.
Although different susceptibility and infectiousness levels were considered in the model based on the different species, the effect of housing systems, such as free‐range housing, on vaccination outcomes remains unclear and would need further investigation in future models.
In some cases, farms with poultry of high‐genetic value or rare poultry breeds can also be targeted for vaccination to ensure their protection. Besides, vaccinating poultry farms in an area along the border of an infected country (barrier vaccination) can be an option to be considered in order to prevent virus spreading into or from a neighbouring country.
Because the model draws a 1‐, 3‐ or 10‐km circle around each infected farm, a number of farms located outside the high‐risk areas may be vaccinated as part of these emergency ring vaccination scenarios. As all farms within the ring area have the same chance of being vaccinated, and given the limited vaccination capacity, in the emergency vaccination scenarios, restricting vaccination to the high‐risk area would likely increase the effectiveness of the 10‐km scenario in particular.
Vaccination teams may also enhance transmission in a region in case of emergency vaccination. To minimise transmission by such vaccination teams, vaccination could be applied outside – inwards, starting with the outside of the ring and moving towards the centre. In particular, in the 10‐km scenario this may increase the number of infections in the centre, but decrease the likelihood of virus escape from the vaccination zone.
Vaccination in low density regions was not modelled, because the compulsory EU measures are, by themselves, sufficient to stop between farm transmission in those regions. Nevertheless, farms in such regions may be at risk for HPAIV outbreaks if they are located in regions with circulation of the virus among wild water birds. Consequently, preventive vaccination could be implemented in such regions to prevent outbreaks caused by primary introductions from wild water birds. If vaccination is implemented in such regions and farms are protected by vaccination before virus exposure, the expected reduction of the number of outbreaks in each poultry species will be inversely proportional to the number of vaccinated farms (assuming random probabilities of each farm to be immunised and infected).
A quantitative sensitivity analysis of the model could not be performed due to time constraints. However, some parameters are expected to influence the duration of the epidemic and the number of infected, culled and vaccinated farms, including a lower poultry density or a shorter infectious period. As an example, reducing the time between infection and detection of an outbreak (i.e. increasing early detection capacity) will decrease the infectious period, and increasing vaccination capacity or increasing culling capacity will decrease the number of infected, culled, vaccinated farms, as well as the length of the epidemic. 10
Logistic constraints that might affect the vaccination effectiveness should be considered in real scenarios, such as the vaccine characteristics (administration route, number and timing of vaccinations, storage conditions), the number of vaccine doses available and the number of poultry farms that can be vaccinated per day. This also applies to control measures that will be implemented in addition to the vaccination programme, such as the culling capacity, the number of poultry farms that can be culled per day. Therefore, the outcome of the kernel, particularly in case of emergency vaccination, might be negatively impacted by the time needed to implement vaccination or control measures.
The simulations were based on kernels that were derived from epidemics where prevention and control measures detailed by EU regulations were implemented. This implies that the results consider that additional surveillance, enhanced biosecurity and movement control measures are always implemented, alongside the vaccination programme as complementary measure, to prevent or early detect future outbreaks (to be assessed in ToRs 3 and 4).
The results obtained with the scenarios were robust across the three studied countries, which suggest that both emergency ring‐vaccination (particularly a 3‐Km radius) and preventive vaccination could be considered as complementary measures, to the minimum measures required by the EU, to effectively control or prevent epidemics. Caution should be done when extrapolating these findings to other countries. Additional research, taking into account the specific characteristics of each country, in terms of poultry population demographics, disease dynamics and other factors, is necessary to validate the effectiveness of these measures in different contexts.
4. Conclusions and Recommendations
4.1. ToR 1: Update on the available vaccines against HPAI for poultry
4.1.1. Available vaccine types and technologies
Conclusions
A large array of vaccine types and technologies has been developed against AI. However, only a small proportion has been produced commercially and used in the field outside of scientific studies. Among them, classical oil‐adjuvanted inactivated whole virus vaccines remain the most widely used group of vaccines as this vaccine type is not bound to poultry species‐specific limitations, allows for easy manufacturing and offers potential versatility in strain adjustment.
Whole virus vaccines are less fit for serological DIVA strategies compared with recombinant ones. Serosurveillance concerning recombinant vaccines, which lack expression of internal viral proteins, can rely on already commercially available and consolidated serological assays.
Although there is no specific experience with AI vectored vaccines in the EU, the same vector backbone technology (e.g. recombinant HVT) is widely used for prevention of other diseases (e.g, IBD, NDV, ILT). Therefore, adequately trained personnel that is a very important requirement to ensure effectiveness of this type of vaccine in the field is already available in chicken hatcheries in the EU.
Nucleic acid‐based vaccines hold promise for the poultry sector particularly for their characteristic to allow for a smooth adaptation to the circulating strains compared with whole virus vaccines, further increasing the array of potentially suitable vaccines.
Recommendations
Continuing research and development of AIV vaccines is required to further improve efficacy, application versatility and widen surveillance options.
Data collection, analyses and network discussions of experiences with the deployment of AIV vaccine candidates and licensed AIV vaccines in real world scenarios should be fostered.
4.1.2. Characteristics of available vaccines
Conclusions
Currently there is only a single authorised vaccine against AI in the EU (Nobilis AI H5N2); however, this vaccine has a wide antigenic distance from the strain currently circulating in EU and was not efficacious to stop its transmission in experimental settings. Outside the EU, several different commercialised vaccines are used in the field for vaccination against HPAI, for either H5 or H7 or both.
The review of available literature sources revealed a significant lack of usable and harmonised data regarding the characteristics of available monovalent and bivalent H5/H7 vaccines that hampers a detailed description and comparison of these vaccines. As an example, a harmonised testing protocol to define onset and duration of protective immunity, as well as recommending a vaccination schedule, are often lacking or experimental conditions and assessment parameters are not harmonised among the different studies that were retrieved.
Most available poultry vaccines are designed for and evaluated in chickens, with few vaccines having been tested in other poultry species.
Off‐label use of vaccines in species other than chicken is possible, but effectiveness can vary due to differences in formulations, adjuvants and inherent limitations that restrict their use to specific poultry species (e.g. inefficient replication of HVT in ducks does not allow the use of HVT‐vectored vaccines in this species).
Most of the available poultry vaccines are administered through injection, requiring time‐ and labour‐consuming manipulation of individual birds that can cause stress and impact production. This approach is particularly challenging for heavier birds and large populations, and might increase the risk of virus spread since vaccination teams enter several farms consecutively.
Vaccines suitable for mass‐application methods outside hatcheries, such as via drinking water or spray/aerosolisation, are currently not available, except for one vaccine based on a live NDV strain as vector for the HA gene (Vaxigen NewH5).
For some of the identified vectored vaccines, pre‐existing immunity against the vector can interfere with vaccination efficacy (e.g. NDV, Marek virus).
Administration of different vaccines based on the same type of vector has been demonstrated to affect replication and consequently immunity elicited by each vaccine.
Vaccines have varying minimum age recommendations for the first administration, ranging from 1 day to 6 weeks of age. Some live vectored vaccines can be administered in‐ovo/in the hatchery (e.g. Vaxigen Ad‐H5 and Vectormune HVT‐AIV).
Knowledge of the time required for vaccination to confer protection and of the duration of the immunity induced is often lacking but is key to planning and implementation of effective vaccination campaigns.
The influence of maternal immunity on vaccination schedule and the number of vaccine doses administered has been studied for few available vaccines.
Certain live vectored vaccines, such as HVT‐vectored vaccines, are less affected by maternal immunity than NDV‐vectored vaccines and can be given early even in the presence of maternally derived antibodies.
Humoral immunity has been measured from 10 to 14 days following primary vaccination, however more time or even successive vaccine doses may be required to obtain full protective immunity.
There is evidence for HVT‐vectored vaccines of a slower onset of immunity, which is reached approximately from 4 weeks onwards after vaccination in chickens.
Benefits of vaccination are extremely limited for short‐lived poultry such as broiler chickens.
Recommendations
Efforts should be made to set standards to generate suitable and harmonised data by conducting standardised trials with available vaccines for H5, H7 and H5 + H7 subtypes, aiming to provide comprehensive and consistent information for a detailed description and to allow comparison of these vaccines.
The development of mass applicable AI vaccines to minimise the labour intensive and time consuming individual manipulations of birds, as well as to reduce stress to the animals, should be encouraged.
To better understand and compare vaccine effectiveness in the field and to enable efficient vaccination campaign planning, future studies should be designed in a harmonised way to provide information on: (i) the onset and duration of immunity following vaccination, particularly for long living poultry types, (ii) the impact of maternal immunity and its sequelae on vaccination schedules, (iii) the indications of vaccines for poultry species other than chickens and considering different poultry production types.
The development of vaccines suitable for diverse poultry species and production types or their extension of indication to species other than chicken should be encouraged. Considering all the characteristics of the available vaccines and technologies, vaccination programmes might require the (subsequent) use of different vaccines, alone or in combination (e.g. heterologous booster), to overcome immunity waning over time.
In the planning of vaccination strategies, possible interference with existing vaccination schemes against other diseases should be carefully considered to avoid interference with maternal and vector‐related immunity.
4.1.3. Antigenic distance
Conclusions
Previous experiences of vaccination conducted outside the EU highlighted the importance of minimising antigenic distance, between vaccine seed strains and field viruses. A low‐antigenic distance of HA related epitopes by cross HI assays is useful to increase the likelihood of selection of the most effective vaccine antigens although final proof will only come from vaccination‐challenge experiments in the target species.
Evidence on the association between antigenic distance and protection is mostly based on inactivated vaccines and the association is less investigated for other types of vaccines.
Most vaccines are found to have high‐antigenic distance from the consensus sequence of recent circulating European gs/GD HPAIV H5N1 of clade 2.3.4.4b: Only four vaccines (Mefluvac H5N8, Vaxigen Flu and Duck H5‐SRV vaccine®), all belonging to the 2.3.4.4b clade, have close antigenic distances (i.e. < 2.4 AU; Table 3).
Low protection due to wide antigenic distance of vaccine strains from circulating viruses might be overcome by broadening immune responses using effective adjuvants and high potency vaccines (e.g. vaccines with high content of immunogenically relevant antigens) or the combination of vaccines stimulating predominantly the humoral immunity (e.g. inactivated or subunit vaccines) with vaccines inducing cellular immunity against conserved epitopes (e.g. live vectored vaccines).
Recommendations
When relying on vaccines that primarily induce strain‐specific humoral immunity, antigenic distance should be given strong consideration.
Standardised continued in silico antigenic distance calculations and HI assay‐based antigenic cartography of relevant HPAIV strains and variants circulating in Europe should be carried out.
Recommendations on the most relevant strains to be included in AIV vaccines in Europe should be issued from harmonised vaccination‐challenge experiments which are based on strains selected from antigenic distance evaluations.
Reference strains and sequences of recommended vaccine viruses should be made readily available to vaccine producers upon request.
It is recommended that the authorised vaccines can be rapidly updated should this be required based on the match with the circulating AIV strains. For this purpose, continuous surveillance efforts to monitor evolution of circulating AIV are needed.
4.1.4. Vaccine efficacy
Conclusions
In experimental settings, vaccination can stop transmission within flocks as evidenced by decreasing basic reproduction number, R0, significantly below 1. This has been demonstrated for inactivated (chickens and ducks), HVT (chickens) and DNA/RNA vaccines (mule ducks).
In experiments studying the effect of vaccination on transmission, the reduction of susceptibility (infection upon challenge of donor birds) and shedding was associated with reduction of transmission.
Vaccines that have been identified to reduce R0 below 1 achieved that level of protection between 14 and 21 days after single administration in experimental settings.
Whilst vaccination‐challenge experiments, particularly transmission experiments, remain the most valuable methods to assess vaccine efficacy, they are time consuming, costly and require animal experiments.
Maternal immunity against AIV or against vaccine vector backbones could interfere with VE as it may hamper the development of protective immunity.
Recommendations
Harmonised data should be generated and collected on VE to reduce R0 below 1 under experimental condition, including standardised information on the vaccine virus strain and challenge strain assessed.
It is recommended to assess the effectiveness of vaccination to stop virus transmission (R0 < 1) also in field trials, taking into account regional differences, e.g. prevalence of certain immunosuppressive diseases, specific logistic constraints, level of training of poultry operators or local resource availability.
To better understand and compare vaccine effectiveness in the field and to enable efficient vaccination campaign planning, future studies should be designed in a way to provide information on: (i) the onset and duration of immunity following vaccination in birds, particularly for long‐lived poultry types, (ii) the maternal immunity and its impact on vaccination schedules and the number of doses required, (iii) the indications of vaccines considering different poultry production types and species, (iv) the effectiveness of vaccination to stop virus transmission.
Authorised vaccines should be rapidly updated to match the circulating strains, thus maintaining their effectiveness over time.
For long‐lived poultry types, the duration of immunity to reduce transmission and immune waning should be studied also in the advanced stages of the production cycle.
The development of alternative methods replacing animal experiments in VE studies should be encouraged.
4.1.5. Correlates of protections
Conclusions
The most readily accessible parameter of vaccine‐induced protective immunity is the amount of specific neutralising antibodies against the target virus. The HI assay is a widely accepted surrogate for the neutralisation assay.
There is a positive association between HI titre and protection against transmission in chickens, although no uniform threshold could be defined.
Routine measurement of cell mediated immunity and in vitro evaluation of its impact on protection is not currently feasible.
Current routine in vitro assays, including those targeting humoral effectors, remain a proxy and do not allow assessment of protective immunity in all its aspects.
Recommendations
Development of a methodology to assess the level of protection in vaccinated birds in the field should be encouraged.
Development of methods accessible to routine laboratories to measure cell‐mediated immunity upon vaccination should be encouraged.
4.2. ToR 2: Vaccination strategies
When planning a vaccination programme, the most adequate vaccine type and vaccination scheme should be selected considering the epidemiological situation, antigenic distance from the circulating strain, population‐specific parameters (poultry species, age, production type, other vaccination programmes), supervision capacities and the vaccination strategy, in particular:
in case of emergency protective vaccination, inactivated vaccines that can be administered to all poultry species/production types/age, leading to rapid onset of immunity and short antigenic distance should be used, while vectored vaccines, in some cases, cannot be used due to the pre‐existing immunity against the vector; also, in principle, a single dose of an effective vaccine would be sufficient to curtail the between farms virus transmission;
in case of preventive vaccination, this could target those species/poultry types most susceptible and/or infectious in the area at the highest risk of introduction; in addition to inactivated vaccines, vectored vaccines are suitable for vaccinating in ovo and day‐old but are not suitable for all poultry species and/or inMSs with vaccination programmes that include the same vector for vaccination against other diseases of poultry; in addition, repeated vaccination according to each poultry species and length of the production cycle will be needed to ensure continued protection.
The results of the model simulations provided insights into the effectiveness of different vaccination scenarios in controlling HPAI in poultry. While the assessed vaccination scenarios serve as illustrative examples of possible vaccination options, it is important to note that they do not cover all possible scenarios. Also, the choice and tailoring of those vaccination scenarios that are hypothetical can vary based on the specific situation in each MS. However, the results obtained with the scenarios were similar across France, Italy and The Netherlands, therefore consistent conclusions and recommendations can be drawn.
Conclusions
Duck and turkey farms are significantly more infectious than chicken (layers and breeders) farms; turkey farms were also more susceptible, whereas broiler farms resulted the least susceptible poultry production species in the three studied countries.
The control measures currently detailed by EU legislation applied to the scenarios simulating virus introduction to a densely populated area, with high‐risk of transmission, showed limitations in limiting the disease spread in the three countries considered. In fact, the minimum EU scenario (culling of all infected poultry farms) resulted in the highest median number of infected farms and longest epidemic durations compared to all other scenarios.
The 1‐km ring‐culling scenario performed better or similarly in terms of number of infected farms and duration of the epidemic compared with the emergency protective ring vaccination scenarios but resulted in the highest number of culled farms.
Among the emergency protective vaccination scenarios, the 1‐km ring vaccination resulted in a higher number of infected farms than the 3‐km and the 10‐km ring vaccination scenarios, which resulted in a lower median number of infected farms and duration of epidemic in The Netherlands and Italy. In France, the 3‐km ring vaccination scenario performed better than the 10‐km vaccination scenario due to the limited vaccination capacity assumed in the simulations. Overall, 3‐km ring vaccination resulted in similar efficacy to that observed with 10‐km ring vaccination whilst a lower number of farms (around 50% less) need to be vaccinated.
If the goal is to minimise the number of outbreaks and duration of epidemic, then the model predicts preventive vaccination of the most susceptible and/or infectious target species in the high‐risk transmission areas as optimal vaccination strategy.
For areas with high risk of introduction from wild birds and low‐farm density, preventive vaccination could be considered to reduce the number of outbreaks resulting from primary introductions.
Recommendations
To minimise the number of infected and culled farms and epidemic duration, preventive vaccination of the most susceptible and/or infectious poultry species is recommended in high‐risk transmission areas. Depending on the region, these species are ducks, geese, turkeys and layers chickens.
In case of an outbreak in a high‐risk transmission area, emergency protective vaccination in a 3‐km radius is recommended, as it showed to be the most effective strategy among the three emergency vaccination scenarios tested.
Monitoring of vaccine efficacy over time should be planned under the implementation of every vaccination strategy, due to possible changes in the antigenicity of circulating HPAI viruses, changes that can also be accelerated by the selection pressure exerted by vaccine‐induced immunity.
Vaccines fit for the purpose for either emergency protective or preventive vaccination should be carefully selected. For the emergency vaccination strategy, vaccines requiring multiple administrations cannot be used. However, for preventive vaccination strategy, booster vaccinations could further enhance the achieved protection.
It is a crucial prerequisite that vaccination should not replace other preventive and control measures such as infection monitoring in wild birds, early detection and biosecurity, but complement them to reinforce their impact, so to adopt an integrated disease prevention and control approach.
Abbreviations
- AI
avian influenza
- AIV
avian influenza virus
- CVMP
Committee for Veterinary Medicinal Products
- DIVA
differentiating infected from vaccinated animals
- EMA
European Medicines Agency
- FPV
Fowlpox virus
- GMO
genetically modified organism
- HDPA
high‐density poultry areas
- HI
haemagglutination inhibition
- HP
highly pathogenic
- HPAI
highly pathogenic avian influenza
- HPAIV
highly pathogenic avian influenza virus
- LP
low pathogenic
- LPAI
low pathogenic avian influenza
- LPAIV
low pathogenic avian influenza virus
- NDV
Newcastle Disease Virus
- SAN
specific antibody negative
- SPF
specific pathogen free
- TRL
technology readiness level
- USDA
United States Department of Agriculture
- VE
vaccine efficacy
- WOAH
World Organisation for Animal Health
Appendix A – Protocol: translation of ToR into assessment questions and sub‐questions (using APRIO)
A.1. Step 1: Formulate the problem
A.1.1. Translate mandate into assessment question
Following the Technical report on Problem Formulation (EFSA, 2022), the APRIO approach was used to formulate the assessment questions and subsequent sub‐questions to be answered in this Scientific Opinion. The process for questions formulation can be found in Table A.1, where each ToR was formulated in a single assessment question.
Table A.1.
APRIO elements for formulating the assessment question and sub‐questions
| Number as appeared in the mandate | Mandate element | Agent | Pathway | Receptor | Intervention | Output | Lower or higher order sub‐questions |
|---|---|---|---|---|---|---|---|
| ToR1 | Update on the available vaccines against HPAI for poultry | HPAIV | All forms of transmission (direct and indirect) | Poultry | Vaccination | Identification and description of vaccines against HPAI, including aspects that could jeopardise a swift eradication | AQ1: Which are the HPAI available vaccines against HPAIV for poultry and their characteristics, including those aspects that could jeopardise a swift eradication? |
| 1.1 | Identification and description of vaccines against HPAIV in poultry | HPAIV | All forms of transmission (direct and indirect) | Poultry | Vaccination | Identification and description of current HPAI vaccine technologies and their characteristics |
SQ1.1: Which are the available vaccine technologies and what are the main characteristics of such technologies? SQ1.2: Which are the available vaccines against HPAIV and what are the characteristics of those vaccines (e.g. target poultry species, possibility to use during lay, efficacy, pharmaceutical form, stability, administration route, age of vaccination, number of doses required to induce protection, onset of immunity and duration of immunity, antigenic match with currently circulating strains, possibility to adapt the vaccine strain with that technology) |
| 1.2 | Aspects related to different vaccines/type that could jeopardise a swift eradication | HPAIV | All forms of transmission (direct and indirect) | Poultry | Vaccination | Description and evaluation of practical aspects related to the use of each vaccine type | SQ1.3: Which are the drawbacks of vaccination in the control and eradication programmes according to the different type of vaccine? |
| ToR 2 | Vaccination strategies | HPAIV | All forms of transmission (direct and indirect) | Poultry | Vaccination | Definition and assessment of possible vaccination scenarios | AQ2: Which are the efficacy and recommended parameters for each vaccination scenario, considering its objectives and factors triggering them? |
| 2.1 | Definition of possible vaccination strategies | HPAIV | All forms of transmission (direct and indirect) | Poultry | Vaccination | Definition of the possible vaccination scenarios and corresponding vaccination strategies based on their objective and reasons for implementation | SQ2.1: What are the different vaccination scenarios, the corresponding vaccination strategies and factors that trigger them? |
| 2.2 | Effectiveness assessment and recommendations for the implementation of the different vaccination strategies, within the identified scenarios | HPAIV | All forms of transmission (direct and indirect) | Poultry | Vaccination | Effectiveness evaluation of the vaccination strategies and recommended parameters for their successful implementation | SQ2.2: What are the recommended parameters for the different vaccination strategies and the efficacy of each of them? |
| ToR3 | Surveillance in vaccinated zone/establishments | HPAIV | All forms of transmission (direct and indirect) | Poultry | Vaccination and surveillance | AQ3: Which are the possible surveillance approaches and their efficacy in the different vaccination scenarios? | |
| 3.1 | Definition of possible surveillance approaches | HPAIV | All forms of transmission (direct and indirect) | Poultry | Vaccination and surveillance | Definition of the possible surveillance approaches for each vaccination scenario | SQ3.1: Which are the possible surveillance approaches for each vaccination scenario? |
| 3.2 | Assessment of efficacy of the different surveillance approaches and the recommended details for each of them | HPAIV | All forms of transmission (direct and indirect) | Poultry | Vaccination and surveillance | Efficacy evaluation of the surveillance approaches for each vaccination scenario for their successful implementation | SQ3.2: How efficient are the different surveillance approaches and in which conditions should they be implemented to ensure their maximum efficacy for each vaccination scenario? |
| ToR 4 | Restrictions and risk mitigation measures | HPAIV | All forms of transmission (direct and indirect) | Poultry | Vaccination and risk mitigation measures | AQ4: Which are the suitable restriction and risk mitigation measures suitable in vaccinated zones? |
A.1.2. Define the sub‐questions of each assessment question and their relationship (conceptual model)
Following the assessment elements mentioned in the ToRs, the assessment questions were broken down into four higher order sub‐questions (AQ) and lower order sub‐questions (SQ).
A.1.3. Select the approach to be followed
The first lower order sub‐questions (SQ) defined for ToR 1 will be addressed by qualitative assessment of the existing evidence on the available HPAI vaccines and technologies in poultry. However, for the remaining ToRs, a quantitative approach will be followed, that will be complemented with an initial scenario setting phase and discussion of the quantitative results.
A.2. Step 2. Plan the methods for conducting the assessment
| Mandate stages | Sub‐question | 2.1 Evidence needs | Data collection | 2.2. Assessment methods to be used |
|---|---|---|---|---|
| 1.1 Identification and description of vaccines against HPAIV in poultry | SQ1.1: Which are the available vaccine technologies and what are the main characteristics of such technologies? | Information on HPAI poultry vaccine technologies | Literature search, request of information to EMA and EURL | Descriptive analysis of the information collected (summary table and descriptive text) |
| SQ1.2: Which are the available vaccines against HPAIV and what are the characteristics of those vaccines (e.g. target poultry species, possibility to use during lay, protection capacity, pharmaceutical form, stability, administration route, age of vaccination, number of doses required to induce protection, onset of immunity and duration of immunity, antigenic match with currently circulating strain, possibility to adapt the vaccine strain with that technology) | Information on the available vaccines against HPAIV (e.g. technology, poultry species, number of doses, administration route, antigenic match with current strain) | Literature search, request of information to EMA, EURL and WOAH network | Descriptive analysis of the information collected (summary table and descriptive text) | |
| 1.2 Aspects related to different vaccines/technologies that could jeopardise a swift eradication | SQ1.3: Which are the drawbacks of vaccination in the control and eradication programmes | Information on practical aspects that could jeopardise a swift eradication of the disease according to the different type of vaccine/technologies (e.g. condition for silent circulation of HPAIV due to poor antigenic match, inefficient adjuvant, low thermostability, vaccination in presence of maternal antibodies) | Literature search, request of information to EMA and EURL | Descriptive analysis of the information collected (summary table and descriptive text) |
| 2.1 Definition of possible vaccination strategies | SQ2.1: Which are the different vaccination strategy scenarios based on their objectives and factors that trigger them? | Information on vaccination objectives and factors triggering them from existing European Regulations, International standard and expert opinions | Literature search and WG discussion | Summary table and description of the different possible vaccine strategy scenarios based on the combination of objectives and the factors triggering each of them |
| 2.2 Efficacy assessment and recommendations for the implementation of the different vaccination strategies | SQ2.2: Which are the recommended parameters for the different vaccination scenarios and the efficacy of each of them? | Infectious parameters of HPAI spread in different poultry species Vaccination data | Model (transmission kernel) parameters obtained by fitting the model to HPAI epidemics (e.g. species, production category, location, date of suspicion) and poultry population data (e.g. species, production category, location) and literature |
A kernel disease spread model will be used to assess the different vaccination strategy scenarios and define the recommended parameters for each scenario (e.g. type of vaccine to be used depending on population characteristics, the frequency of vaccination and minimum coverage) Vaccination strategies will be assessed according to different poultry production characteristics in a country or zone mainly: (1) chickens farms are mostly present, (2) turkey farms are mostly present or (3) ducks farms are mostly present |
| 3.1 Definition of possible surveillance approaches | SQ3.1: Which are the suitable surveillance approaches for each vaccination strategy? | Information on surveillance strategies and objectives from existing European Regulations, International standard Regulation and expert opinions | Literature search and WG discussion | Description of the possible surveillance approaches for each vaccination strategy, including the reinforced surveillance set out in the Delegated Regulation (EU) No 2023/361, the WOAH standards and alternatives to those two |
| 3.2 Assessment of efficacy of the different surveillance approaches and the recommended details for each of them | SQ3.2: How efficient are the different surveillance approaches and in which conditions should they be implemented to ensure their maximum efficacy for each vaccination scenario? |
Infectious parameters of HPAI spread in different poultry species Vaccination data Surveillance and testing details |
Model parameters from literature search, data model from HPAI epidemics (e.g. species, production category, location, date of suspicion) and poultry population data (e.g. species, production category, location) |
Suitable models to each surveillance strategy will be implemented; for example, a compartmental model could be used to assess surveillance approaches for early detection during emergency response or a scenario tree model could be used to assess freedom once an epidemic has been controlled. Based on the results of the used models, specific surveillance parameters will be recommended for each scenario (i.e. sampling schemes, testing procedures, duration of the surveillance) |
| 4. Restrictions and risk mitigation measures | SQ4: Which are the suitable restriction and risk mitigation measures in vaccinated zones? |
Parameters of HPAI spread in different poultry species Vaccination data Restriction measures Efficacy details |
Model (transmission kernel) parameters obtained by fitting the model to HPAI epidemics (e.g. species, production category, location, date of suspicion) and poultry population data (e.g. species, production category, location) and/or literature | A kernel disease spread model will be used to assess the efficacy of the different restriction measures for the different vaccination scenarios, including the measures set out in the Delegated Regulation and alternatives proposed by the WG |
Appendix B – Estimation of between‐farm transmission kernel parameters and results of model simulations
1.
The results of the between‐farm transmission kernel parameters that have been estimated by fitting the model to data of HPAI epidemics in the Netherlands, Italy and France are presented in Table 9. These kernel parameters for each of the assessed countries were used to simulate the spread of HPAIV and assess the effect of different vaccination scenarios on the control of HPAI in poultry.
Using the estimated transmission kernel parameters, between‐farm reproduction numbers were quantified for each farm in each country and then used to generate transmission risk maps (Figures 10, 11–12). Furthermore, the relationship between and farm density was explored (data not shown) to express the transmission risk in terms of farm density.
From the epidemic data the median length of the infectious period was derived for each country (Table B.2) and this data was later used for the simulations to assess the effectiveness of different vaccination scenarios.
The detailed results of the kernel model simulations conducted on the data from France, Italy and the Netherlands are reported in Tables B.3–B.5.
Annex A – Supplementary information on ToR 1 data
1.
The Annex to this Scientific Opinion is available on the EFSA Knowledge Junction community on Zenodo at: https://doi.org/10.5281/zenodo.8354898
Suggested citation: EFSA AHAW Panel (EFSA Panel on Animal Health and Animal Welfare), European Union Reference Laboratory for Avian Influenza , Nielsen, S. S ., Alvarez, J ., Bicout, D. J ., Calistri, P ., Canali, E ., Drewe, J. A ., Garin‐Bastuji, B ., Gonzales Rojas, J. L ., Gortázar, C ., Herskin, M ., Michel, V ., Miranda Chueca, M. A ., Padalino, B ., Roberts, H. C ., Spoolder, H ., Stahl, K ., Velarde, A ., … Viltrop, A . 2023. Vaccination of poultry against highly pathogenic avian influenza – part 1. Available vaccines and vaccination strategies. EFSA Journal, 21(10), 1–87. 10.2903/j.efsa.2023.8271
Requestor European Commission
Question number EFSA‐Q‐2022‐00550
Panel members Søren Saxmose Nielsen, Julio Alvarez, Dominique Joseph Bicout, Paolo Calistri, Elisabetta Canali, Julian Ashley Drewe, Bruno Garin‐Bastuji, Jose Luis Gonzales Rojas, Christian Gortázar, Mette Herskin, Virginie Michel, Miguel Ángel Miranda Chueca, Barbara Padalino, Helen Clare Roberts, Hans Spoolder, Karl Stahl, Antonio Velarde, Christoph Winckler and Arvo Viltrop.
Map disclaimer The designations employed and the presentation of material on any maps included in this scientific output do not imply the expression of any opinion whatsoever on the part of the European Food Safety Authority concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries.
Declarations of interest If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
Acknowledgement EFSA and EURL wish to thank the following Member States and their representatives who provided data on HPAI vaccines, poultry population and HPAI outbreaks: Karen Bucher, Bianca Quercetti and Andrea Jimenez Pellicer for France; Andrea Maroni Ponti and Luigi Ruocco for Italy; Borbála Bende and Gábor Wyszoczky for Hungary; Annemarie Bouma and Wim Pelgrim for The Netherlands. As well as thanking pharmaceutical industries whose information was available on their website, EFSA and EURL also thank Avimex salud animal, Boehringer‐Ingelheim Animal Health, CEVA, Huvepharma, Kemin Biologics, MSD, Yebio and Zoetis who provided data on vaccine products for this scientific output. EFSA and EURL wish to acknowledge Beatrice Grasland as hearing expert from ANSES, the European Medicines Agency (EMA), Christine Miras as hearing expert from the Committee for Veterinary Medicinal Products (CVMP) at the EMA, Alexandre Fediaevsky as hearing expert from the World Organisation for Animal Health (WOAH), Francesca Scolamacchia as Individual Scientific Advisor (contract EOI/EFSA/SCIENCE/2020/01), Barbara Lanfranchi from Randstad (contract CP/IHUF2060/374), Linnea Kero Lindgren (contract OC/EFSA/DATA/2021/01), Stefano Marangon, and Gina Ciocata from EFSA for the support provided to this scientific output; the WBVR for the work done on quantification of between‐farm transmission and simulation of vaccination scenarios under contract OC/EFSA/ALPHA/2020/01, and for the statistical analysis of vaccine efficacy (project KB‐37‐003‐047‐WBVR); the CoVetLab consortium for support with the systematic literature review (project DACRAH‐4, OC/EFSA/ALPHA/2020/01); the authors, originating and submitting laboratories of the sequences from GISAID's EpiFlu™ Database.
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
Adopted: 13 September 2023
Note: A plain language summary of this scientific report is available at https://www.efsa.europa.eu/en/plain-language-summary/vaccination-poultry-against-highly-pathogenic-avian-influenza-available
Note: A plain language summary of this scientific report is available at https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2023.p211001
Notes
In this analysis, a farm was considered to be characterised by a unique location (XY coordinates), owner and poultry species.
Regulation (EU) 2016/429 of the European Parliament and of the Council of 9 March 2016 on transmissible animal diseases and amending and repealing certain acts in the area of animal health (‘Animal Health Law’). OJ L 84, 31.3.2016, p. 1.
Commission Delegated Regulation (EU) 2020/687 of 17 December 2019 supplementing Regulation (EU) 2016/429 of the European Parliament and the Council, as regards rules for the prevention and control of certain listed diseases. OJ L 174, 3.6.2020, p. 64–13.
Commission Delegated Regulation (EU) 2023/361 of 28 November 2022 supplementing Regulation (EU) 2016/429 of the European Parliament and the Council as regards rules for the use of certain veterinary medicinal products for the purpose of prevention and control of certain listed diseases. OJ L 52, 20.2.2023, p. 1–42.
COUNCIL DIRECTIVE 2005/94/EC of 20 December 2005 on Community measures for the control of avian influenza and repealing Directive 92/40/EEC. OJ L 10, 14.1.2006, p. 16–65.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, p. 1–24.
Regulation (EU) 2019/6 of the European Parliament and of the Council of 11 December 2018 on veterinary medicinal products and repealing Directive 2001/82/EC OJ L 4, 7.1.2019, p. 43–167.
In this analysis, a farm was considered to be characterised by a unique location (XY coordinates), owner and poultry species.
Simulation done in the Italian scenario with reduced infectious period to 12 days showed a reduction of ~ 25% in transmission (data not shown).
References
- Backer JA, van Roermund HJ, Fischer EA, van Asseldonk MA and Bergevoet RH, 2015. Controlling highly pathogenic avian influenza outbreaks: an epidemiological and economic model analysis. Preventive Veterinary Medicine, 121, 142–150. 10.1016/j.prevetmed.2015.06.006 [DOI] [PubMed] [Google Scholar]
- Bauzile B, Durand B, Lambert S, Rautureau S, Fourtune L, Guinat C, Andronico A, Cauchemez S, Paul MC and Vergne T, 2023. Impact of palmiped farm density on the resilience of the poultry sector to highly pathogenic avian influenza H5N8 in France. Veterinary Research, 54, 56. 10.1186/s13567-023-01183-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavinck V, Bouma A, van Boven M, Bos ME, Stassen E and Stegeman JA, 2009. The role of backyard poultry flocks in the epidemic of highly pathogenic avian influenza virus (H7N7) in The Netherlands in 2003. Preventive Veterinary Medicine, 88, 247–254. 10.1016/j.prevetmed.2008.10.007 [DOI] [PubMed] [Google Scholar]
- Beato MS, Toffan A, De Nardi R, Cristalli A, Terregino C, Cattoli G and Capua I, 2007. A conventional, inactivated oil emulsion vaccine suppresses shedding and prevents viral meat colonisation in commercial (Pekin) ducks challenged with HPAI H5N1. Vaccine, 25, 4064–4072. 10.1016/j.vaccine.2007.02.042 [DOI] [PubMed] [Google Scholar]
- Bertran K, Clark A and Swayne DE, 2018. Mitigation strategies to reduce the generation and transmission of airborne highly pathogenic avian influenza virus particles during processing of infected poultry. International Journal of Hygiene and Environmental Health, 221, 893–900. 10.1016/j.ijheh.2018.05.013 [DOI] [PubMed] [Google Scholar]
- Boender GJ, Hagenaars TJ, Bouma A, Nodelijk G, Elbers ARW, de Jong MCM and van Boven M, 2007. Risk maps for the spread of highly pathogenic avian influenza in poultry. PLOS Computational Biology, 3, e71. 10.1371/journal.pcbi.0030071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos MEH, Nielen M, Koch G, Stegeman A and De Jong MCM, 2008. Effect of H7N1 vaccination on highly pathogenic avian influenza H7N7 virus transmission in turkeys. Vaccine, 26, 6322–6328. 10.1016/j.vaccine.2008.09.022 [DOI] [PubMed] [Google Scholar]
- Busani L, Toson M, Stegeman A, Pozza MD, Comin A, Mulatti P, Cecchinato M and Marangon S, 2009. Vaccination reduced the incidence of outbreaks of low pathogenicity avian influenza in northern Italy. Vaccine, 27, 3655–3661. 10.1016/j.vaccine.2009.03.033 [DOI] [PubMed] [Google Scholar]
- Cagle C, To TL, Nguyen T, Wasilenko J, Adams SC, Cardona CJ, Spackman E, Suarez DL and Pantin‐Jackwood MJ, 2011. Pekin and Muscovy ducks respond differently to vaccination with a H5N1 highly pathogenic avian influenza (HPAI) commercial inactivated vaccine. Vaccine, 29, 6549–6557. 10.1016/j.vaccine.2011.07.004 [DOI] [PubMed] [Google Scholar]
- Capua I and Marangon S, 2000. The avian influenza epidemic in Italy, 1999—2000: a review. Avian Pathology, 29, 289–294. 10.1080/03079450050118403 [DOI] [PubMed] [Google Scholar]
- Cattoli G, Fusaro A, Monne I, Coven F, Joannis T, El‐Hamid HS, Hussein AA, Cornelius C, Amarin NM, Mancin M, Holmes EC and Capua I, 2011a. Evidence for differing evolutionary dynamics of A/H5N1 viruses among countries applying or not applying avian influenza vaccination in poultry. Vaccine, 29, 9368–9375. 10.1016/j.vaccine.2011.09.127 [DOI] [PubMed] [Google Scholar]
- Cattoli G, Milani A, Temperton N, Zecchin B, Buratin A, Molesti E, Aly MM, Arafa A and Capua I, 2011b. Antigenic drift in H5N1 avian influenza virus in poultry is driven by mutations in major antigenic sites of the hemagglutinin molecule analogous to those for human influenza virus. Journal of Virology, 85, 8718–8724. 10.1128/jvi.02403-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H and Bu Z, 2009. Development and application of Avian Influenza vaccines in China. In: Compans RW and Orenstein WA (eds). Vaccines for Pandemic Influenza. Berlin, Heidelberg, Springer, Berlin, Heidelberg. pp. 153–162. [DOI] [PubMed] [Google Scholar]
- Chen T, Tan Y, Song Y, Wei G, Li Z, Wang X, Yang J, Millman AJ, Chen M, Liu D, Huang T, Jiao M, He W, Zhao X, Greene CM, Kile JC, Zhou S, Zhang R, Zeng X, Guo Q and Wang D, 2023. Enhanced environmental surveillance for avian influenza A/H5, H7 and H9 viruses in Guangxi, China, 2017–2019. Biosafety and Health, 5, 30–36. 10.1016/j.bsheal.2022.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, 2023. BIRD SHOTS As an avian influenza virus devastates the poultry industry, the United States considers an unprecedented step: vaccinating its flocks. Science, 24–27. [Google Scholar]
- Delpont M, Guinat C, Guérin J‐L, Le leu E, Vaillancourt J‐P and Paul MC, 2021. Biosecurity measures in French poultry farms are associated with farm type and location. Preventive Veterinary Medicine, 195, 105466. 10.1016/j.prevetmed.2021.105466 [DOI] [PubMed] [Google Scholar]
- Dorigatti I, Mulatti P, Rosà R, Pugliese A and Busani L, 2010. Modelling the spatial spread of H7N1 avian influenza virus among poultry farms in Italy. Epidemics, 2, 29–35. 10.1016/j.epidem.2010.01.002 [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , online. Highly pathogenic avian influenza Geographical distribution. Available online: https://animal-diseases.efsa.europa.eu/HPAI/
- EFSA (European Food Safety Authority) , 2022. Problem Formulation for EFSA Scientific Assessments. EFSA Supporting Publications 2022;19(7):7349E, 74 pp. 10.2903/sp.efsa.2022.EN-7349 [DOI] [Google Scholar]
- EFSA Scientific Committee , Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Younes M, Craig P, Hart A, Von Goetz N, Koutsoumanis K, Mortensen A, Ossendorp B, Martino L, Merten C, Mosbach‐Schulz O and Hardy A, 2018. Guidance on Uncertainty Analysis in Scientific Assessments. EFSA Journal 2018;16(1):5123, 39 pp. 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsobky Y, El Afandi G, Abdalla E, Byomi A and Reddy G, 2020. Possible ramifications of climate variability on HPAI‐H5N1 outbreak occurrence: Case study from the Menoufia, Egypt. PLoS One, 15, e0240442. 10.1371/journal.pone.0240442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMA (European Medicines Agency) , online. CVMP: Agendas, minutes and reports. Available online: https://www.ema.europa.eu/en/committees/cvmp/cvmp-agendas-minutes-reports
- EMBL‐EBI , online. EMBOSS Cons Multiple Sequence Alignment. Available online: https://www.ebi.ac.uk/Tools/msa/emboss_cons/
- European Union , online. Veterinary Medicines. Available online: https://medicines.health.europa.eu/veterinary/en
- Gass JD, Hill NJ, Damodaran L, Naumova EN, Nutter FB and Runstadler JA, 2023. Ecogeographic drivers of the spatial spread of highly pathogenic avian influenza outbreaks in Europe and the United States, 2016–Early 2022. International Journal of Environmental Research and Public Health, 20, 6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germeraad EA, Sanders P, Hagenaars TJ, de Jong MCM, Beerens N and Gonzales JL, 2019. Virus Shedding of Avian Influenza in Poultry: A Systematic Review and Meta‐Analysis. Viruses, 11, 812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germeraad EA, Velkers FC, de Jong MCM, Gonzales JL, de Wit JJ, Stegeman JA, Beerens N and (Wageningen Bioveterinary Research) , 2023. Transmissiestudie met vier vaccins tegen H5N1 hoogpathogeen vogelgriepvirus (clade 2.3.4.4b) BO‐43‐111‐074 Available online: https://edepot.wur.nl/584306
- Gilbert M, Cinardi G, Da Re D, Wint WGR, Wisser D and Robinson TP, 2022. Global chickens distribution in 2015 (5 minutes of arc). V1 Edition. Place Harvard Dataverse, Harvard Dataverse.
- GISAID , online. GISAID online database. Available online: https://gisaid.org/
- Grasland B, Schmitz A, Niqueux É, Busson R, Morin N, Guillemoto C, Orosco A, Souchaud F, Bougeard S, Fx B, Martenot C, Cherbonnel M, Massin P, Rose N, Louboutin K, Pierre I, Amelot M, Delpont M, Pouvelle L, Soubies S, Keita A, Guerin J‐L, Eterradossi N and (ANSES ENVT) , 2023. Expérimentation de vaccination des canards mulards en élevage contre un virus influenza aviaire hautement pathogène A(H5N1) clade 2.3.4.4b Rapport intermédiaire: "Evaluation expérimentale de la protection clinique et de l'excrétion virale". Available online: https://hal.inrae.fr/hal-04158032
- Guinat C, Nicolas G, Vergne T, Bronner A, Durand B, Courcoul A, Gilbert M, Guérin J‐L and Paul MC, 2018. Spatio‐temporal patterns of highly pathogenic avian influenza virus subtype H5N8 spread, France, 2016 to 2017. Eurosurveillance, 23, 1700791. 10.2807/1560-7917.ES.2018.23.26.1700791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinat C, Artois J, Bronner A, Guérin JL, Gilbert M and Paul MC, 2019. Duck production systems and highly pathogenic avian influenza H5N8 in France, 2016–2017. Scientific Reports, 9, 6177. 10.1038/s41598-019-42607-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinat C, Comin A, Kratzer G, Durand B, Delesalle L, Delpont M, Guérin J‐L and Paul MC, 2020a. Biosecurity risk factors for highly pathogenic avian influenza (H5N8) virus infection in duck farms, France. Transboundary and Emerging Diseases, 67, 2961–2970. 10.1111/tbed.13672 [DOI] [PubMed] [Google Scholar]
- Guinat C, Durand B, Vergne T, Corre T, Rautureau S, Scoizec A, Lebouquin‐Leneveu S, Guérin JL and Paul MC, 2020b. Role of live‐duck movement networks in transmission of avian influenza, France, 2016–2017. Emerging Infection Disease, 26, 472–480. 10.3201/eid2603.190412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenaars TJ, Boender GJ, Elbers ARW, Gonzales JL and Hobbelen P, 2023. Preventief ruimen bij vogelgriep in pluimveedichte gebieden en mogelijkheden voor aanvullende bemonstering. Wageningen Bioveterinary Research, 2321589. [Google Scholar]
- Henning J, Hesterberg UW, Zenal F, Schoonman L, Brum E and McGrane J, 2019. Risk factors for H5 avian influenza virus prevalence on urban live bird markets in Jakarta, Indonesia—Evaluation of long‐term environmental surveillance data. PLoS One, 14, e0216984. 10.1371/journal.pone.0216984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbelen PHF, Elbers ARW, Werkman M, Koch G, Velkers FC, Stegeman A and Hagenaars TJ, 2020. Estimating the introduction time of highly pathogenic avian influenza into poultry flocks. Scientific Reports, 10, 12388. 10.1038/s41598-020-68623-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoda N, Twabela AT, Bazarragchaa E, Ogasawara K, Hayashi H, Wang ZJ, Kobayashi D, Watanabe Y, Saito K, Kida H and Sakoda Y, 2020. Re‐invasion of H5N8 high pathogenicity avian influenza virus clade 2.3.4.4b in Hokkaido, Japan, 2020. Viruses, 12, 12. 10.3390/v12121439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K and Standley DM, 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution, 30, 772–780. 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH and Samal SK, 2019. Innovation in newcastle disease virus vectored avian influenza vaccines. Viruses, 11. 10.3390/v11030300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J‐K, Kayali G, Walker D, Forrest HL, Ellebedy AH, Griffin YS, Rubrum A, Bahgat MM, Kutkat MA, Ali MAA, Aldridge JR, Negovetich NJ, Krauss S, Webby RJ and Webster RG, 2010. Puzzling inefficiency of H5N1 influenza vaccines in Egyptian poultry. Proceedings of the National Academy of Sciences, 107, 11044–11049. 10.1073/pnas.1006419107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert S, Durand B, Andraud M, Delacourt R, Scoizec A, Le Bouquin S, Rautureau S, Bauzile B, Guinat C, Fourtune L, Guérin J‐L, Paul MC and Vergne T, 2022. Two major epidemics of highly pathogenic avian influenza virus H5N8 and H5N1 in domestic poultry in France, 2020–2022. Transboundary and Emerging Diseases, 69, 3160–3166. 10.1111/tbed.14722 [DOI] [PubMed] [Google Scholar]
- Lambert S, Hobbelen PHF, Gonzales JL, Elbers ARW and Vergne T, 2023. Supporting policy by estimating the transmission dynamics of highly pathogenic avian influenza H5 infections in poultry flocks from France and The Netherlands. Proceedings SVEPM. [Google Scholar]
- Lardinois A, Vandersleyen O, Steensels M, Desloges N, Mast J, van den Berg T and Lambrecht B, 2016. Stronger interference of avian influenza virus‐specific than newcastle disease virus‐specific maternally derived antibodies with a recombinant NDV‐H5 vaccine. Avian Disease, 60, 191–201. 10.1637/11133-050815-Reg [DOI] [PubMed] [Google Scholar]
- Marangon S and Capua I, 2006. Control of avian influenza in Italy: from stamping out to emergency and prophylactic vaccination. Developmental Biology (Basel), 124, 109–115. [PubMed] [Google Scholar]
- Palya V, Tatar‐Kis T, Walkone Kovacs E, Kiss I, Homonnay Z, Gardin Y, Kertesz K and Dan A, 2018. Efficacy of a recombinant Turkey Herpesvirus AI (H5) vaccine in preventing transmission of heterologous highly pathogenic H5N8 Clade 2.3.4.4b challenge virus in commercial broilers and layer pullets. Journal of Immunology Research, 2018, 3143189. 10.1155/2018/3143189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantin‐Jackwood MJ and Suarez DL, 2013. Vaccination of domestic ducks against H5N1 HPAI: a review. Virus Research, 178, 21–34. 10.1016/j.virusres.2013.07.012 [DOI] [PubMed] [Google Scholar]
- Peeters B, Reemers S, Dortmans J, de Vries E, de Jong M, van de Zande S, Rottier PJM and de Haan CAM, 2017. Genetic versus antigenic differences among highly pathogenic H5N1 avian influenza A viruses: consequences for vaccine strain selection. Virology, 503, 83–93. 10.1016/j.virol.2017.01.012 [DOI] [PubMed] [Google Scholar]
- Poetri ON, Van Boven M, Claassen I, Koch G, Wibawan IW, Stegeman A, Van den Broek J and Bouma A, 2014. Silent spread of highly pathogenic Avian Influenza H5N1 virus amongst vaccinated commercial layers. Research Veterinary Science, 97, 637–641. 10.1016/j.rvsc.2014.09.013 [DOI] [PubMed] [Google Scholar]
- Qiao C, Tian G, Jiang Y, Li Y, Shi J, Yu K and Chen H, 2006. Vaccines developed for H5 highly pathogenic avian influenza in China. Annals of the New York Academy of Sciences, 1081, 182–192. 10.1196/annals.1373.022 [DOI] [PubMed] [Google Scholar]
- Rudolf M, Poppel M, Frohlich A, Breithaupt A, Teifke J, Blohm U, Mettenleiter T, Beer M and Harder T, 2010. Longitudinal 2 years field study of conventional vaccination against highly pathogenic avian influenza H5N1 in layer hens. Vaccine, 28, 6832–6840. 10.1016/j.vaccine.2010.08.038 [DOI] [PubMed] [Google Scholar]
- Santos JJS, Obadan AO, Garcia SC, Carnaccini S, Kapczynski DR, Pantin‐Jackwood M, Suarez DL and Perez DR, 2017. Short‐ and long‐term protective efficacy against clade 2.3.4.4 H5N2 highly pathogenic avian influenza virus following prime‐boost vaccination in turkeys. Vaccine, 35, 5637–5643. 10.1016/j.vaccine.2017.08.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreuder J, de Knegt HJ, Velkers FC, Elbers ARW, Stahl J, Slaterus R, Stegeman JA and de Boer WF, 2022. Wild bird densities and landscape variables predict spatial patterns in HPAI outbreak risk across The Netherlands. Pathogens, 11, 549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolamacchia F, Mulatti P, Mazzucato M, Barbujani M, Harvey WT, Fusaro A, Monne I and Marangon S, 2021. Different environmental gradients associated to the spatiotemporal and genetic pattern of the H5N8 highly pathogenic avian influenza outbreaks in poultry in Italy. Transboundary and Emerging Diseases, 68, 152–167. 10.1111/tbed.13661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Deng G, Ma S, Zeng X, Yin X, Li M, Zhang B, Cui P, Chen Y, Yang H, Wan X, Liu L, Chen P, Jiang Y, Guan Y, Liu J, Gu W, Han S, Song Y, Liang L, Qu Z, Hou Y, Wang X, Bao H, Tian G, Li Y, Jiang L, Li C and Chen H, 2018. Rapid evolution of H7N9 highly pathogenic viruses that emerged in China in 2017. Cell Host and Microbe, 24, 558–568.e557. 10.1016/j.chom.2018.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si Y, Wang T, Skidmore AK, de Boer WF, Li L and Prins HHT, 2010. Environmental factors influencing the spread of the highly pathogenic avian influenza H5N1 virus in wild birds in Europe. Ecology and Society, 15. [Google Scholar]
- Sims LD and Peiris M, 2013. One health: the Hong Kong experience with avian influenza. Current Topics in Microbiology and Immunology, 365, 281–298. 10.1007/82_2012_254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaras I, 2017. On the role of vaccine dose and antigenic distance in the transmission dynamics of Highly Pathogenic Avian Influenza (HPAI) H5N1 virus and its selected mutants in vaccinated animals. Wageningen University. Available online: https://edepot.wur.nl/424464 [Google Scholar]
- Sitaras I, 2020. Antigenic cartography: overview and current developments. Methods in Molecular Biology, 2123, 61–68. 10.1007/978-1-0716-0346-8_5 [DOI] [PubMed] [Google Scholar]
- Sitaras I, Rousou X, Peeters B and de Jong MCM, 2016. Mutations in the haemagglutinin protein and their effect in transmission of highly pathogenic avian influenza (HPAI) H5N1 virus in sub‐optimally vaccinated chickens. Vaccine, 34, 5512–5518. 10.1016/j.vaccine.2016.10.002 [DOI] [PubMed] [Google Scholar]
- Smith DJ, Lapedes AS, de Jong JC, Bestebroer TM, Rimmelzwaan GF, Osterhaus AD and Fouchier RA, 2004. Mapping the antigenic and genetic evolution of influenza virus. Science, 305, 371–376. 10.1126/science.1097211 [DOI] [PubMed] [Google Scholar]
- Souvestre M, Guinat C, Niqueux E, Robertet L, Croville G, Paul M, Schmitz A, Bronner A, Eterradossi N and Guérin JL, 2019. Role of backyard flocks in transmission dynamics of highly pathogenic avian influenza A(H5N8) Clade 2.3.4.4, France, 2016–2017. Emerging Infectious Disease, 25, 551–554. 10.3201/eid2503.181040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegeman A, Bouma A, Elbers AR, de Jong MC, Nodelijk G, de Klerk F, Koch G and van Boven M, 2004. Avian influenza A virus (H7N7) epidemic in The Netherlands in 2003: course of the epidemic and effectiveness of control measures. Journal of Infection Disease, 190, 2088–2095. 10.1086/425583 [DOI] [PubMed] [Google Scholar]
- Stokstad E, 2022. Wrestling with bird flu, Europe considers once‐taboo vaccines. Science, 376, 682–683. [DOI] [PubMed] [Google Scholar]
- Tatar‐Kis T, Dan A, Felfoldi B, Balint A, Ronai Z, Dauphin G, Penzes Z, El‐Attrache J, Gardin Y and Palya V, 2019. Virus‐like particle based vaccine provides high level of protection against homologous H5N8 HPAIV challenge in mule and pekin duck, including prevention of transmission. Avian Diseases, 63, 193–202. 10.1637/11882-042718-Reg.1 [DOI] [PubMed] [Google Scholar]
- Textor J, van der Zander B, Gilthorpe MS, Liskiewicz M and Ellison GT, 2016. Robust causal inference using directed acyclic graphs: the R package ‘dagitty’. International Journal of Epidemiology, 45, 1887–1894. 10.1093/ije/dyw341 [DOI] [PubMed] [Google Scholar]
- van der Goot JA, Koch G, de Jong MCM and van Boven M, 2005. Quantification of the effect of vaccination on transmission of avian influenza (H7N7) in chickens. Proceedings of the National Academy of Sciences, 102, 18141–18146. 10.1073/pnas.0505098102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Goot JA, van Boven M, Stegeman A, van de Water SG, de Jong MC and Koch G, 2008. Transmission of highly pathogenic avian influenza H5N1 virus in Pekin ducks issignificantly reduced by a genetically distant H5N2 vaccine. Virology, 382, 91–97. 10.1016/j.virol.2008.08.037 [DOI] [PubMed] [Google Scholar]
- Wu J, Ke C, Lau EHY, Song Y, Cheng KL, Zou L, Kang M, Yen H‐L and Peiris M, 2019. Influenza H5/H7 virus vaccination in poultry and reduction of zoonotic infections, guangdong province, China, 2017–2018. Emerging Infectious Disease Journal, 25, 116–118. 10.3201/eid2501.181259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Liu K, Luo J, Tan S, Quan C, Zhang S, Chai Y, Qi J, Li Y, Bi Y, Xiao H, Wong G, Zhou J, Jiang T, Liu W, Yu H, Yan J, Liu Y, Shu Y, Wu G, Wu A, Gao GF and Liu WJ, 2018. Heterosubtypic protections against human‐infecting avian influenza viruses correlate to biased cross‐T‐cell responses. mBio, 9. 10.1128/mBio.01408-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Chen P, Liu L, Deng G, Li Y, Shi J, Kong H, Feng H, Bai J, Li X, Shi W, Tian G and Chen H, 2016. Protective efficacy of an H5N1 inactivated vaccine against challenge with lethal H5N1, H5N2, H5N6, and H5N8 influenza viruses in chickens. Avian Diseases, 60, 253–255. [DOI] [PubMed] [Google Scholar]


