Abstract
The growing demand for high food quality has been encouraging researchers in the food industry to apply biodegradable nanocomposites, which provide new opportunities and challenges for the advance of nanomaterials in the food industry. The objective of this study was to estimate the antibacterial activity and cytotoxicity effects of zinc oxide nanocomposite/zeolite (c/Zeo) with Aloe vera gel (AG) and its effect on the shelf life of chicken meat. The ZnONPs/Zeo was assessed using X‐ray fluorescence (XRF) and field emission scanning electron microscopy (FE‐SEM) analyses. The cytotoxicity effect of ZnONPs/Zeo was assessed by MTT assay. Then, the minimum inhibitory concentrations (MIC) and minimum bactericidal concentration (MBC) of ZnONPs/Zeo and ZnONPs/Zeo‐AG against Salmonella typhi and Salmonella para typhi A were investigated. Also, the preservative effect of nanocomposites on chicken fillets was evaluated. The results showed that these nanocomposites have the least cytotoxicity effect, resulting in good biocompatibility with the host. The MIC and MBC values of ZnONPs/Zeo‐AG were lower than the ZnONPs/Zeo against S. typhi and S. paratyphi A. Both ZnONPs/Zeo‐AG and ZnONPs/Zeo caused a significant decrease in the bacterial count of the chicken fillets. So, by spraying on meat, the number of bacteria presented a sharper decline as compared with the control group, resulting in an approximately 3.3 and 3‐log10 reduction over 48 h in the ZnONPs/Zeo‐AG and ZnONPs/Zeo treatment samples, respectively. In conclusion, antimicrobial packaging with ZnONPs containing A. vera is a beneficial solution for preserving and improving the quality, safety, and shelf life of fresh meat products.
Keywords: antibacterial activity, food packaging, food quality, nanocomposite, plant extracts, toxicology
The objective of this study was to estimate the antibacterial activity and cytotoxicity effects of zinc oxide nanocomposite/zeolite (ZnONC/Zeo) with Aloe vera gel (AG) and its effect on the shelf life of chicken meat.

1. INTRODUCTION
Appropriate food packaging prevents food degradation by microbial contamination. Hence, nanotechnology is used to meet the consumers' requirements to improve food quality by using antimicrobial agents to prolong the shelf life of foods under storage and delivery conditions (Youssef et al., 2016). Food industries have got to pay more attention to selecting which biodegradable packaging material is more appropriate for their food products. Also, with the increasing request for stability and environmental safety, a rising amount of studies have been concentrated on expanding food packaging materials that can rapidly degrade and entirely mineralize into rather safe products (Ahmadi et al., 2020).
Aloe vera has numerous pharmacological properties due to its various potentially bioactive components (Jangra et al., 2022). The main ingredients of aloe consist of anthraquinones, polysaccharides, lectin, alkylbenzenes, dehydroabietic acid, saponin, salicylic acid, and lignin (Moghaddam et al., 2018). Aloin is one of the most active anthraquinone glycosides of aloe latex. It shows an enhanced pharmacological effect because of its facile entrance into the target cells (Arbab et al., 2021). Moreover, A. vera displays many pharmacological activities, such as anti‐inflammatory, anti‐ulcer, antitumor, anti‐asthmatic, antidiabetic, and antimicrobial properties (Jangra et al., 2022).
Metal nanoparticles are used in the food industry due to their antimicrobial attributes, principally to cover food processing equipment or in food packaging to reduce foodborne diseases and food decay (Youssef et al., 2016). Zinc oxide NPs (ZnO NPs) is a metal nanoparticle which has attracted much attention due to their unique morphology, and antimicrobial activity against both Gram‐positive and Gram‐negative bacteria (Babapour et al., 2021). Besides, ZnO NPs have an eco‐friendly relationship with the environment and easy preparation. ZnO is approved by the Food and Drug Administration (FDA) as a safe food additive (Tamimi et al., 2021).
The antibacterial effect of ZnO NPs is related to interactions between them and cell membrane compounds, such as the bonding with amino acids, induction of reactive oxygen species (ROS) production, and membrane depolarization (Verma et al., 2018). Furthermore, nanofabrication has a challenge with aggregations, probable cytotoxicity, and expensive material (Razavi et al., 2018). Hereon, nanomaterials as a complex with zeolites will dominate the problems of translational research (Alswat et al., 2016). Even though the study of the biological use of zeolites is a novel field, it is gradually becoming an attractive area. Besides the harmless properties of zeolites, they can be widely used in the near future due to their reversible binding to minor molecules and their capacity of acting similarly to metalloenzymes and regulating immune response (Demirci et al., 2014). Also, nano zinc oxide‐doped zeolite has a major antibacterial potential (Alswat et al., 2016).
Microbial contamination is an important problem effective on food (exclusively meats) resulting in economic fatalities, quality decreases, and diminished product shelf life (Clarke et al., 2016). Salmonella spp. is the main common foodborne bacteria in animal‐source foods (ASF). Two species of Salmonella associated with ASF were recognized, including S. enterica and S. bongori. S. enterica is related to human salmonellosis (Rortana et al., 2021). Salmonella spp. may contaminate fresh meat during butchering or processing, transporting, storing, and selling at the markets (Rortana et al., 2021). These bacteria are generally distributed in the environment, mostly in livestock, such as chickens, and pigs, which colonization may be subclinical and difficult to diagnose before slaughter, then can contaminate carcasses and make humans sick through consumption (Koh et al., 2022). Among the different meats, chicken is broadly consumed because of its low‐fat, nourishing, and comparatively low value (Azlin‐Hasim et al., 2016). This meat is also susceptible to deterioration due to its protein ingredients, besides appropriate pH, permitting the growth of microbes (Takma & Korel, 2019).
Green packaging development can reduce adverse environmental influences by using biodegradable materials, plant extracts, and nanomaterials (Han et al., 2018). Many types of research have been reported on nanomaterials applications in food packaging, but the utmost materials are in the phase of possibility. The use of these materials in food packaging should be approved for their safety because there are public concerns about the possible adverse effects of these materials migrating into food environments (Cwiek‐Ludwicka & Ludwicki, 2017). So, the toxicity assessment of nanomaterials in the food intake of humans is a significant study emphasis.
The present work is a stage toward using bionanocomposites as food packaging supplies. We aimed to investigate the cytotoxicity effect of zinc oxide nanocomposite with A. vera gel (AG) on the caco‐2 cell line and to determine their effects on the shelf life of chicken meat.
2. MATERIALS AND METHODS
2.1. Materials
Zn(CH3CO2)2·2H2O and zeolite powder were purchased from Sigma Chemical Co. Caco‐2 cells were purchased from the National Cell Bank of Iran (Pasteur Institute of Iran, Tehran). Microbial strains, including Salmonella typhi and Salmonella paratyphi A were provided from our previous study (Nadi et al., 2020). All other chemicals and culture media used in the present study were of analytical grade and purchased from Merck Co.
2.2. Preparation of nano zinc oxide/zeolite
ZnONPs/Zeo has been prepared by a method formerly described by Partoazar et al. (2019) with slight modifications. Briefly, zeolite powder was mixed in deionized water for 1 h, and then the wastewater was filtered by a cellulose paper filter. The wash step was done three times and the following drying was performed at 80°C and was kept away from moisture. To get the Zn2+ exchanged zeolites, Zn(CH3CO2)2·2H2O (7 g) and zeolite powder (10 g) were added in 100 mL DW and with continuous stirring at 60°C for 1 h. Next, to create nanoparticles, NaOH 1 M solution was added to the suspension till pH = 12 was reached. After 2 h, the solution was washed extensively with DW by a cellulose filter to remove the residual zinc acetate. The nanoparticles were dried overnight at 80°C and then were calcined for 2 h at 400°C. Moreover, the above procedure measured entirely for ZnO/Ze composite construction without the addition of NaOH solution. Zeolite elementals were evaluated to characterize ZnO percentage by X‐ray fluorescence (XRF, PW2404; Philips) system. The morphology of ZnO nanomaterials was analyzed by a field emission scanning electron microscopy (FE‐SEM, MIRA3 TESCAN) system.
2.3. Extraction of the Aloe vera gel
Aloe vera gel has been extracted using a method previously described by Arsene et al. (2022) with a small modification. A. vera leaves were collected and cleaned with distilled water. Thirty grams (30 g) of A. vera leaf was weighed and added to sterile distilled water (270 mL) in a flask, and incubated at 25°C in a shaker incubator at 300 rpm for 3 h. Then, the mixture was filtered by using a cellulose paper filter. The A. vera extract was collected and placed in Petri dishes formerly weighed and then incubated open at 40°C until relative evaporation of aqueous solutions should be done. The extract was collected when the volume was small enough to store at 4°C.
2.4. Determining the cytotoxicity effects of ZnONPs/Zeo and ZnONPs/Zeo‐AG
To assess the cytotoxic effects of ZnONPs/Zeo and ZnONPs/Zeo‐AG on Caco‐2 cells, the MTT assay was performed based on Karimaei et al. (2022) study. Briefly, 5000 cells were seeded to each well of 96‐well plates comprising 200 μL of 10% FBS DMEM. After 24 h incubation and cell attachment, the cells were exposed to various concentrations of ZnONPs/Zeo and ZnONPs/Zeo‐AG, including (0.5, 1, 2, 4, 8, and 16 mg/mL) and the plates were incubated for 24, 48, and 72 h at 37°C. After incubation, the medium of each well was exchanged with 100 μL fresh DMEM without FBS. Then, 10 μL of 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT; 5 mg/mL; Sigma‐Aldrich) was added to each well for 4 h. Next, the medium was removed and 100 μL dimethyl sulfoxide (Sigma‐Aldrich) was added for 10 min. The optical density of each well was noted at 570 nm by an Eliza reader (Biorad). Finally, a cell survival of more than 75% was considered non‐cytotoxicity (Jung et al., 2019).
2.5. Determination of antibacterial activity
The antibacterial activity of A. vera extract, zeolite, ZnONPs/Zeo, and ZnONPs/Zeo‐AG was considered against two Gram‐negative pathogenic bacteria (S. typhi and S. paratyphi A). These bacteria were provided from our previous study (Nadi et al., 2020). The minimal inhibitory concentration (MIC) was estimated by the microdilution method according to the Clinical and Laboratory Standards Institute (CLSI) (Wayne, 2012). First, any of the above compounds were serially diluted in Mueller‐Hinton broth (MHB), at a volume of 100 μL. Then, the overnight bacterial cultures were diluted to reach a bacterial density of 0.5 McFarland standard. Next, 100 μL of bacterial suspension was added to each well, so resulting in a final volume of 200 μL, with different concentrations of compounds (0.25, 0.5, 1, 2, 4, and 8 mg/mL). The control contained only bacterial suspension. After incubation at 37°C for 24 h, MIC was determined as the lowest concentration at which no visible growth of bacteria compared with the control.
To estimate MBC values, 10 μL of inoculums were taken in sterile conditions from negative wells that display the absence of observable turbidity and transferred onto Trypticase Soy Agar (TSA). After incubation at 37°C for 24 h, the lowest concentration caused the elimination of the tested bacteria, which was considered as MBC.
2.6. Chicken samples treatment
The chicken fillet meat was collected from different stores in Tehran. All samples were kept in a cooling box for refrigeration while being transported to the laboratory for testing. Determination of organoleptic properties and kinds of microbial tests containing (mold, yeast, and, all microorganisms), match instructions with National standards (Institute of Standards and Industrial Research of Iran [ISIRI], 1997). Treatment of the chicken fillet was performed at refrigeration temperature by spreading the ZnONPs/Zeo and ZnONPs/Zeo‐AG on all the chicken meat samples in two ways (spray on the meat and spray on the packaging). According to our results obtained from investigating the cytotoxic effects of ZnONPs/Zeo and ZnONPs/Zeo‐AG on Caco‐2 cells, the concentration of 8 mg/mL of these compounds was selected and tested on chicken fillets. The chicken fillet sample by adding distilled water was considered as a control. Storage was done at 4°C for 4 days; microbial features were assessed primarily and then at time points: 2, 4, 24, 48, 72, and 96 h.
2.7. Microbial evaluation of chicken fillet after treatment
To perform the microbial analysis, 5 g of chicken fillet samples were sterilely cut from different parts and mixed with 45 mL sterile normal saline, and allowed to soak for 20 min. Then, serial dilution was prepared (10−1 to 10−8) and 1 mL of the last three dilutions (10−6, 10−7, 10−8) was added to the previously sterilized and cooled TSB medium (15 mL). After mixing the medium with the inoculum, it was incubated at 37°C. Plates containing the culture medium without inoculation samples were considered as a negative control. Plates comprising colonies were counted, and the CFU/g was determined (Ahmadi et al., 2020).
2.8. Statistical analysis
Data from independently performed experiments are shown as mean ± standard deviation (SD). Statistical comparisons between individual groups were performed by one‐way analysis of variance (ANOVA) with Tukey's post hoc test using GraphPad Prism8 (GraphPad Software, Inc).
3. RESULTS
3.1. Composite investigation
In this study, the XRF technique was used for the evaluation of ZnO percentage in the experimental compounds. The result displays that the sample of NC has 19.34% ZnO among other elements although its quantities were 7.12% and 0.19% for ZnO/Zeo and raw Zeo, respectively (Table 1). Moreover, as shown in Figure 1, high magnification imaging by FE‐SEM showed that crystalline ZnO nanoparticles were formed on the zeolite surfaces.
TABLE 1.
Elemental analysis for ZnO percentage of the current compounds using the XRF system.
| Structure | XRF analysis/(wt. percentage) | ||||||
|---|---|---|---|---|---|---|---|
| ZnO | SiO2 | Al2O3 | CaO | MgO | Fe2O3 | P2O5 | |
| ZnONPs/Zeo | 19.34 | 54.23 | 8.12 | 3.67 | 0.75 | 0.89 | 0.08 |
| ZnO/Zeo | 7.12 | 63.91 | 9.83 | 4.01 | 0.99 | 2.29 | 0.11 |
| Zeo | 0.19 | 67.12 | 13.1 | 4.92 | 1.7 | 2.33 | 0.56 |
FIGURE 1.

Representative SEM imaging of ZnONPs/Zeo materials. The crystalline forms of Zinc Oxide nanoparticles doped on the surface of the zeolite structure are determined by yellow arrows.
3.2. The cytotoxicity effects of ZnONPs/Zeo and ZnONPs/Zeo‐AG on Caco‐2 cells
The cytotoxicity properties of ZnONPs/Zeo and ZnONPs/Zeo‐AG on Caco‐2 cells (human colorectal adenocarcinoma cells) were evaluated by MTT assay. As shown in Figure 2, the following concentrations: 0.5–8 mg/mL of ZnONPs/Zeo and ZnONPs/Zeo‐AG caused a minor cytotoxic effect on Caco‐2 cells for 72 h in comparison with the control (100%) (p < .05). However, the survival rate was significantly diminished at a concentration of 16 mg/mL in comparison to the control group (untreated cells). These results suggest that these nanocomposites have the least cytotoxicity effect and good biocompatibility with the host. In addition, in the combination of A. vera gel with ZnONPs/Zeo, the cell viability of Caco‐2 cells was significantly higher in all concentrations compared to ZnONPs/Zeo.
FIGURE 2.
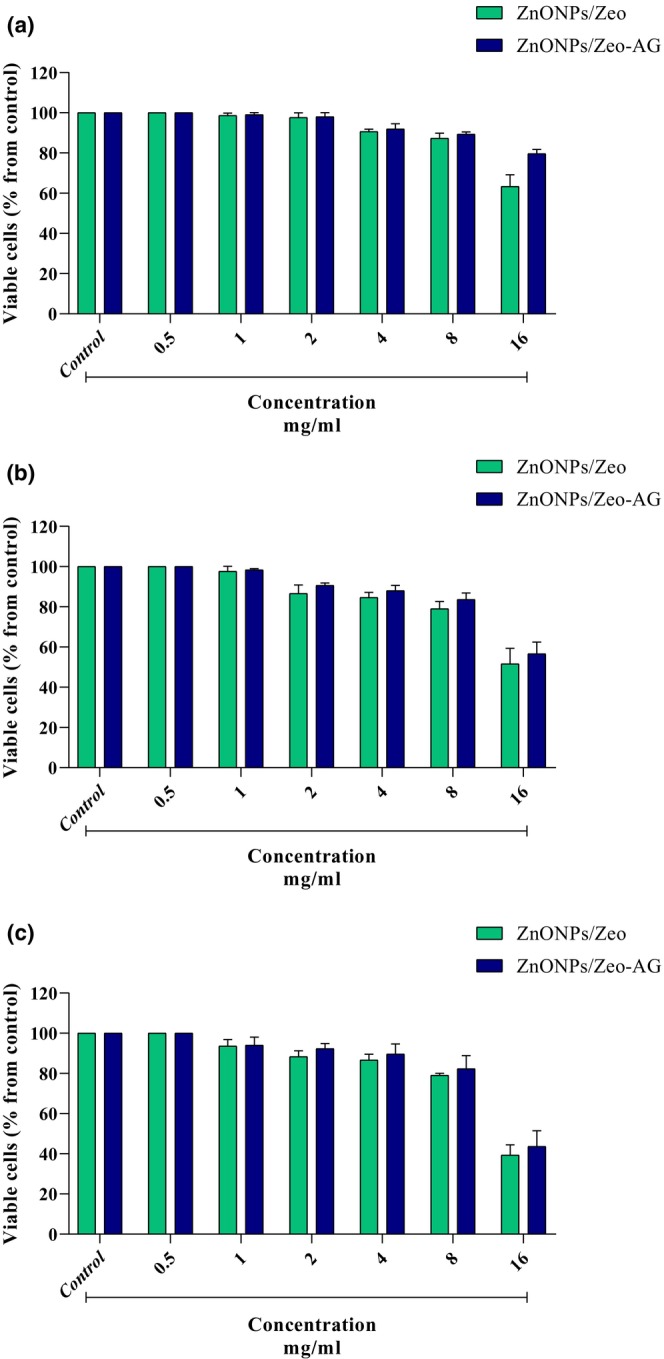
Effect of different concentrations of ZnONPs/Zeo and ZnONPs/Zeo‐AG on the viability of Caco‐2 cells at 24 h (a), 48 h (b), and 72 h (c).
3.3. Antibacterial effects of ZnONPs/Zeo and ZnONPs/Zeo‐AG
The susceptibility of two bacteria (S. typhi and S. paratyphi A) to zeolite, A. vera gel, ZnONPs/Zeo, and ZnONPs/Zeo‐AG was assessed using the microdilution method. Results showed the MIC of 8 and 4 mg/mL for ZnONPs/Zeo, in S. typhi and S. paratyphi A, respectively. On the other hand, the combination of ZnONPs/Zeo with A. vera gel inhibited the growth of S. typhi and S. paratyphi A at a lower concentration (MIC = 4 and 2 mg/mL, respectively). Also, none of the zeolite and A. vera compounds separately could inhibit the growth of the mentioned bacteria. The MBC test gave results equal to the MIC. That means, the MBC amount was 8 and 4 mg/mL for ZnONPs/Zeo in S. typhi and S. paratyphi A, respectively, and was 4 and 2 mg/mL for ZnONPs/Zeo‐AG in S. typhi and S. paratyphi A, respectively.
3.4. The preservative effect of nanocomposites on chicken fillets
The number of viable microorganisms such as bacteria and fungi that chicken fillets contain at different time intervals (0, 2, 4, 24, 48, 72, and 96 h) and in the five examined groups was investigated. The findings showed that the number of bacterial colonies changes with time, so the highest shelf life of the samples was up to 48 h, and after that, an increase in the number of bacterial colonies was observed. It should be noted that in the method of spraying on chicken meat, the number of counted colonies was less compared with the spraying on the packaging. Also, the ZnONPs/Zeo‐AG caused a significant reduction in the number of bacterial colonies in both methods and was more effective in increasing the shelf life of chicken meat than the ZnONPs/Zeo (Figure 3).
FIGURE 3.
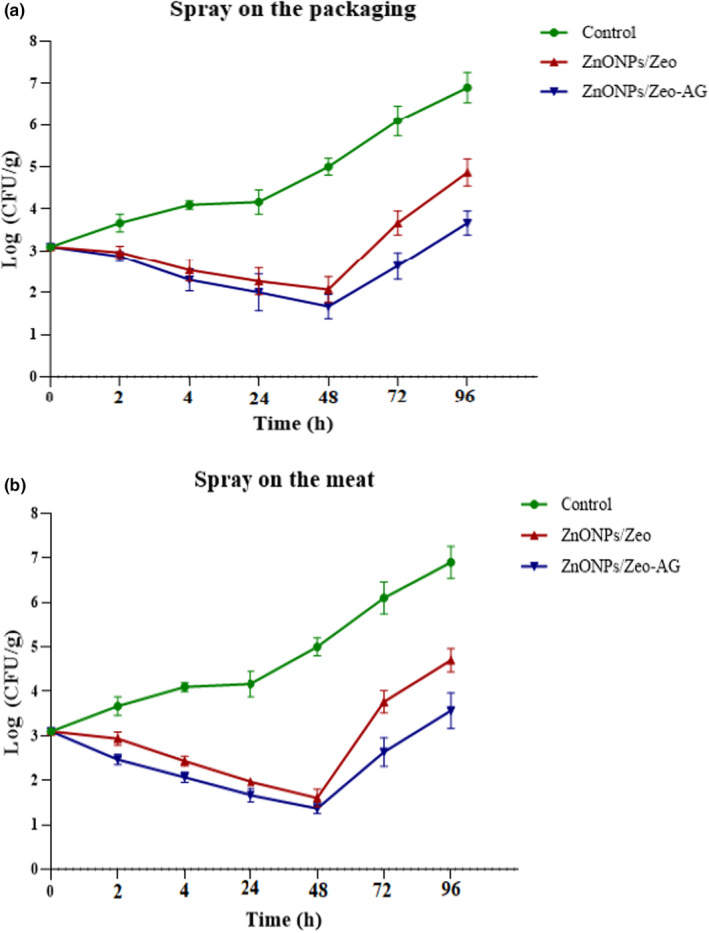
Colony counts of bacteria in chicken fillet samples were treated with the ZnONPs/Zeo and ZnONPs/Zeo‐AG by two methods: (a) spray on the packaging and (b) spray on the meat. Data are presented as the mean (−SD) of three independent biological replicates.
4. DISCUSSION
Packaging is a key stage in the food industry; though its penetrable nature is the main defect in conventional packaging materials (Sharma et al., 2017). The researchers seek novel, cost‐effective, eco‐friendly, and biodegradable food packaging systems to preserve the quality of foods. It is an important factor driving the innovation of food packaging materials to be continuously evaluated (Sharma et al., 2017). The subsequent two types of materials are in attention: (1) inorganic and metal nanoparticles and (2) plant extract mixtures incorporated in biopolymers (Youssef et al., 2016; Yu et al., 2016).
Nanomaterials have unique properties compared with their bulk counterparts which have increased research on the synthesis, characterization, applications, and evaluation of these materials and have led to promoting the scientific progression and development of the agrifood area (Huang et al., 2018). Various reports have focused on the potential usage of nanomaterials as contributors to guarantee food quality, and rectify packaging problems (Huang et al., 2018).
The reason for gaps in understanding the toxicology of nanomaterials, and the expansion of their applications is associated with safety concerns. In the case of food, the primary phases of consumers' exposure are the movements of nanoparticles from packaging to food products (Störmer et al., 2017). Therefore, it is important to investigate the cytotoxic effects of these substances on human cells. In the present study, the cytotoxic effects of ZnONPs/Zeo and ZnONPs/Zeo‐AG on Caco‐2 cells were done using the MTT assay. As shown in Figure 2, in concentrations 0.5–8 mg/mL, ZnONPs/Zeo and ZnONPs/Zeo‐AG led to a slight cytotoxic effect on Caco‐2 cells for 72 h in comparison with the control (100%) (p < .05). Moreover, a significant cytotoxic effect was seen at the concentrations of 16 mg/mL of both substances in comparison with the negative control (100%) (p < .05). These results suggest that these nanocomposites have the least cytotoxicity effect and good biocompatibility with the host. Also, the cell viability of Caco‐2 cells was significantly higher in all ZnONPs/Zeo‐AG concentrations compared with ZnONPs/Zeo.
For assessing the susceptibility of pathogenic strains (S. typhi and S. paratyphi A), the microdilution method was used. The result showed that as the amount of both ZnONPs/Zeo and ZnONPs/Zeo‐AG increased, bacteria growth inhibition was observed. It is clear from growth inhibition that both of these materials have effective bactericidal activity. The higher impact was seen against S. paratyphi A, for which the MIC was 4 and 2 mg/mL for ZnONPs/Zeo, and ZnONPs/Zeo‐AG, respectively. Overall, the combination of ZnONPs/Zeo and A. vera gel had a superior inhibitory effect than the ZnONPs/Zeo alone. Moreover, experiments previously showed that ZnONPs/Zeo has significant antibiofilm activity against Klebsiella pneumoniae and Staphylococcus aureus strains in their sublethal concentrations (Partoazar et al., 2020, 2021).
Zeolite suspensions display no inhibitory effect, while the ZnONPs/Zeo suspensions for both tested bacteria presented antibacterial activity. These results are consistent with those found by Alswat et al. (2016). These antibacterial activities are because of different mechanisms. The first one is related to the ZnO nanoparticles size, which is the antibacterial activity enhancement with a reduction in the nanoparticle size (Alswat et al., 2016). The other mechanism is a result of the release of Zn 2+ ions to the medium comprising bacteria (Alswat et al., 2016).
Although antibacterial effects were reported for A. vera extract in previous studies (Arbab et al., 2021; Arsene et al., 2022), in our study, these materials separately did not have any antibacterial activity on the studied bacteria. This observation proposes that additional examination at concentrations higher than our tested concentrations should be done to measure their antimicrobial activity against S. typhi and S. paratyphi A. A. vera extract in combination with ZnONPs/Zeo displayed significant antibacterial activity against both tested bacterial strains. In the previous study by Ali et al. (2016), ZnONP‐AG showed differential antibacterial activity against Gram‐positive and Gram‐negative bacteria.
Microbial contamination is the most common disadvantage in the food industry, particularly in meat products, due to weakening quality and decreased shelf life of them. For this reason, in the current study, the effect of ZnONPs/Zeo‐AG on the shelf life of chicken was investigated. According to the results, for spraying on meat, 48 h after chicken fillet samples exposure to ZnONPs/Zeo‐AG and ZnONPs/Zeo, the number of bacteria reached 4.8, 1.5, and 1.8 log CFU/g in the control and ZnONPs/Zeo‐AG and ZnONPs/Zeo treatment samples, respectively (Figure 3). This number increased significantly after 48 h during the storage time, though the control had a quicker rate of increase in this parameter. This is due to bacterial resistance to the surrounding environment caused by the membrane and cell wall, with their components providing numerous penetrability pathways for NPs in bacteria (Lesniak et al., 2013). In Gram‐negatives, the cell wall's outer membrane consists of lipopolysaccharide (LPS), lipoproteins, and phospholipids. This causes resistance to the passage of substances. These structural attributes of the outer membrane prevent lipid peroxidation by ROS formed via ZnO NPs and decrease their susceptibility to ZnO (Kumar et al., 2017). At the end of the storage period, the number of bacteria was 7.2 log CFU/g in the control sample. The results also presented that after 48 h, a meaningfully higher count was seen in the control samples relative to the treatment sample sprayed with the ZnONPs/Zeo‐AG and ZnONPs/Zeo. Mostly, the nanocomposite containing ZnO NPs had a significant antimicrobial effect against the bacteria. A previous study suggests that NPs have superior antibacterial activity, similar to our study (Amjadi et al., 2019). Also, Esmaeili et al. (2021) previously reported that A. vera packaging can decrease decay by suppressing bacteria growth. This result approved the antibacterial activity of nanocomposites, particularly ZnONC/Zeo‐AG, and their potential for increasing the shelf life of chicken meat.
5. CONCLUSION
In the current research, an eco‐friendly, active nanocomposite containing ZnO NPs and A. vera was introduced. Considering the results of the microdilution test and bacterial count, the ZnO NPs displayed antibacterial activity against S. typhi and S. paratyphi A and it had no cytotoxic effect on Caco‐2 cells. In conclusion, antimicrobial active packaging comprising ZnO NPs and A. vera is a beneficial solution for preserving and improving the quality, safety, and shelf life of fresh meat products. It should be noted that it is better to conduct studies on migration assays and risk assessments of nanocomposite materials. Therefore, it allows the use of nanomaterials in the food packaging field.
AUTHOR CONTRIBUTIONS
Mohammad Mehdi Soltan Dallal: Conceptualization (equal); project administration (equal); writing – review and editing (equal). Samira Karimaei: Formal analysis (equal); writing – original draft (equal); writing – review and editing (equal). Maryam Hajighasem: Investigation (equal). Seyed Jamal Hashemi: Formal analysis (equal); software (equal). Abbas Rahimi Foroushani: Formal analysis (equal). Mahmoud Ghazi‐Khansari: Formal analysis (equal); software (equal). Alireza Partoazar: Conceptualization (equal); supervision (equal).
FUNDING INFORMATION
This study was part of a research project approved by the Food Microbiology Research Center, Tehran University of Medical Sciences, Tehran, Iran (Contract No. 46801), and has the ethics code IR.TUMS.VCR.REC.1399.209. We are grateful to the Vice‐Chancellor of Research at Tehran University of Medical Sciences who sponsored this research project.
CONFLICT OF INTEREST STATEMENT
No competing interests to declare.
Acknowledgements
None.
Soltan Dallal, M. M. , Karimaei, S. , Hajighasem, M. , Hashemi, S. J. , Rahimi Foroushani, A. , Ghazi‐Khansari, M. , & Partoazar, A. (2023). Evaluation of zinc oxide nanocomposite with Aloe vera gel for packaging of chicken fillet against Salmonella typhi and Salmonella para typhi A. Food Science & Nutrition, 11, 5882–5889. 10.1002/fsn3.3528
DATA AVAILABILITY STATEMENT
All data that support the findings of this study are available in the manuscript.
REFERENCES
- Ahmadi, A. , Ahmadi, P. , & Ehsani, A. (2020). Development of an active packaging system containing zinc oxide nanoparticles for the extension of chicken fillet shelf life. Food Science & Nutrition, 8(10), 5461–5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali, K. , Dwivedi, S. , Azam, A. , Saquib, Q. , Al‐Said, M. S. , Alkhedhairy, A. A. , & Musarrat, J. (2016). Aloe vera extract functionalized zinc oxide nanoparticles as nanoantibiotics against multi‐drug resistant clinical bacterial isolates. Journal of Colloid and Interface Science, 472, 145–156. [DOI] [PubMed] [Google Scholar]
- Alswat, A. A. , Ahmad, M. B. , Saleh, T. A. , Hussein, M. Z. B. , & Ibrahim, N. A. (2016). Effect of zinc oxide amounts on the properties and antibacterial activities of zeolite/zinc oxide nanocomposite. Materials Science & Engineering: C, Materials for Biological Applications, 68, 505–511. [DOI] [PubMed] [Google Scholar]
- Amjadi, S. , Emaminia, S. , Nazari, M. , Davudian, S. H. , Roufegarinejad, L. , & Hamishehkar, H. (2019). Application of reinforced ZnO nanoparticle‐incorporated gelatin bionanocomposite film with chitosan nanofiber for packaging of chicken fillet and cheese as food models. Food and Bioprocess Technology, 12, 1205–1219. [Google Scholar]
- Arbab, S. , Ullah, H. , Weiwei, W. , Wei, X. , Ahmad, S. U. , Wu, L. , & Zhang, J. (2021). Comparative study of antimicrobial action of Aloe vera and antibiotics against different bacterial isolates from skin infection. Veterinary Medicine and Science, 7(5), 2061–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsene, M. M. , Viktorovna, P. I. , Sergei, G. V. , Hajjar, F. , Vyacheslavovna, Y. N. , Vladimirovna, Z. A. , Aleksandrovna, V. E. , Nikolayevich, S. A. , & Sachivkina, N. (2022). Phytochemical analysis, antibacterial and antibiofilm activities of Aloe vera aqueous extract against selected resistant Gram‐negative bacteria involved in urinary tract infections. Fermentation, 8(11), 626. [Google Scholar]
- Azlin‐Hasim, S. , Cruz‐Romero, M. C. , Morris, M. A. , Padmanabhan, S. C. , Cummins, E. , & Kerry, J. P. (2016). The potential application of antimicrobial silver polyvinyl chloride nanocomposite films to extend the shelf‐life of chicken breast fillets. Food and Bioprocess Technology, 9, 1661–1673. [Google Scholar]
- Babapour, H. , Jalali, H. , & Mohammadi Nafchi, A. (2021). The synergistic effects of zinc oxide nanoparticles and fennel essential oil on physicochemical, mechanical, and antibacterial properties of potato starch films. Food Science & Nutrition, 9(7), 3893–3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke, D. , Molinaro, S. , Tyuftin, A. , Bolton, D. , Fanning, S. , & Kerry, J. P. (2016). Incorporation of commercially‐derived antimicrobials into gelatin‐based films and assessment of their antimicrobial activity and impact on physical film properties. Food Control, 64, 202–211. [Google Scholar]
- Cwiek‐Ludwicka, K. , & Ludwicki, J. K. (2017). Nanomaterials in food contact materials; considerations for risk assessment. Roczniki Państwowego Zakładu Higieny, 68(4), 321–329. [PubMed] [Google Scholar]
- Demirci, S. , Ustaoğlu, Z. , Yılmazer, G. A. , Sahin, F. , & Baç, N. (2014). Antimicrobial properties of zeolite‐X and zeolite‐A ion‐exchanged with silver, copper, and zinc against a broad range of microorganisms. Applied Biochemistry and Biotechnology, 172(3), 1652–1662. [DOI] [PubMed] [Google Scholar]
- Esmaeili, Y. , Zamindar, N. , Paidari, S. , Ibrahim, S. A. , & Mohammadi Nafchi, A. (2021). The synergistic effects of Aloe vera gel and modified atmosphere packaging on the quality of strawberry fruit. Journal of Food Processing and Preservation, 45(12), e16003. [Google Scholar]
- Han, J. W. , Ruiz‐Garcia, L. , Qian, J. P. , & Yang, X. T. (2018). Food packaging: A comprehensive review and future trends. Comprehensive Reviews in Food Science and Food Safety, 17(4), 860–877. [DOI] [PubMed] [Google Scholar]
- Huang, Y. , Mei, L. , Chen, X. , & Wang, Q. (2018). Recent developments in food packaging based on nanomaterials. Nanomaterials, 8(10), 830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Standards and Industrial Research of Iran (ISIRI) . (1997). Standard methods for preparation of food samples and enumeration of microorganisms in food .
- Jangra, A. , Sharma, G. , Sihag, S. , & Chhokar, V. (2022). The dark side of miracle plant‐Aloe vera: A review. Molecular Biology Reports, 49(6), 5029–5040. [DOI] [PubMed] [Google Scholar]
- Jung, O. , Smeets, R. , Hartjen, P. , Schnettler, R. , Feyerabend, F. , Klein, M. , Wegner, N. , Walther, F. , Stangier, D. , Henningsen, A. , Rendenbach, C. , Heiland, M. , Barbeck, M. , & Kopp, A. (2019). Improved in vitro test procedure for full assessment of the cytocompatibility of degradable magnesium based on ISO 10993‐5/‐12. International Journal of Molecular Sciences, 20(2), 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimaei, S. , Shabestari, A. N. , Mirzaei, A. , & Fatahi, B. (2022). Apoptosis, cytotoxicity and expression of metastatic suppressor genes increased in human bladder and renal carcinoma cells by Nisin. Translational Research in Urology, 4(2), 83–88. [Google Scholar]
- Koh, Y. , Bae, Y. , Lee, Y. S. , Kang, D. H. , & Kim, S. H. (2022). Prevalence and characteristics of Salmonella spp. isolated from raw chicken meat in the Republic of Korea. Journal of Microbiology and Biotechnology, 32(10), 1307–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, R. , Umar, A. , Kumar, G. , & Nalwa, H. S. (2017). Antimicrobial properties of ZnO nanomaterials: A review. Ceramics International, 43(5), 3940–3961. [Google Scholar]
- Lesniak, A. , Salvati, A. , Santos‐Martinez, M. J. , Radomski, M. W. , Dawson, K. A. , & Åberg, C. (2013). Nanoparticle adhesion to the cell membrane and its effect on nanoparticle uptake efficiency. Journal of the American Chemical Society, 135(4), 1438–1444. [DOI] [PubMed] [Google Scholar]
- Moghaddam, A. B. , Shirvani, B. , Aroon, M. A. , & Nazari, T. (2018). Physico‐chemical properties of hybrid electrospun nanofibers containing polyvinylpyrrolidone (PVP), propolis and Aloe vera . Materials Research Express, 5(12), 125404. [Google Scholar]
- Nadi, Z. R. , Salehi, T. Z. , Tamai, I. A. , Foroushani, A. R. , Sillanpaa, M. , & Dallal, M. M. S. (2020). Evaluation of antibiotic resistance and prevalence of common Salmonella enterica serovars isolated from foodborne outbreaks. Microchemical Journal, 155, 104660. [Google Scholar]
- Partoazar, A. , Bideskan, F. R. , Partoazar, M. , Talaei, N. , & Dallal, M. M. S. (2020). Inhibition of biofilm formation of Staphylococcus aureus strains through ZnO/zeolite nanocomposite and its cytotoxicity evaluation. BioNanoScience, 10, 714–720. [Google Scholar]
- Partoazar, A. , Bideskan, F. R. , Takzaree, N. , & Dallal, M. M. S. (2021). Antibiofilm activity of ZnO/zeolite nanocomposite (ZnO/zeonc) against Klebsiella pneumoniae and its biocompatibility in an animal model. Anti‐Infective Agents, 19(2), 174–181. [Google Scholar]
- Partoazar, A. , Talaei, N. , Bahador, A. , Pourhajibagher, M. , Dehpour, S. , Sadati, M. , & Bakhtiarian, A. (2019). Antibiofilm activity of natural zeolite supported NanoZnO: Inhibition of Esp gene expression of Enterococcus faecalis . Nanomedicine (London, England), 14(6), 675–687. [DOI] [PubMed] [Google Scholar]
- Razavi, S. , Partoazar, A. , Takzaree, N. , Fasihi‐Ramandi, M. , Bahador, A. , & Darvishi, M. H. (2018). Silver sulfadiazine nanoethogel for burn healing: Characterization and investigation of its in vivo effects. Nanomedicine (London, England), 13(11), 1319–1331. [DOI] [PubMed] [Google Scholar]
- Rortana, C. , Nguyen‐Viet, H. , Tum, S. , Unger, F. , Boqvist, S. , Dang‐Xuan, S. , Koam, S. , Grace, D. , Osbjer, K. , Heng, T. , Sarim, S. , Phirum, O. , Sophia, R. , & Lindahl, J. F. (2021). Prevalence of Salmonella spp. and Staphylococcus aureus in chicken meat and pork from Cambodian markets. Pathogens, 10(5), 556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, C. , Dhiman, R. , Rokana, N. , & Panwar, H. (2017). Nanotechnology: An untapped resource for food packaging. Frontiers in Microbiology, 8, 1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Störmer, A. , Bott, J. , Kemmer, D. , & Franz, R. (2017). Critical review of the migration potential of nanoparticles in food contact plastics. Trends in Food Science & Technology, 63, 39–50. [Google Scholar]
- Takma, D. K. , & Korel, F. (2019). Active packaging films as a carrier of black cumin essential oil: Development and effect on quality and shelf‐life of chicken breast meat. Food Packaging and Shelf Life, 19, 210–217. [Google Scholar]
- Tamimi, N. , Mohammadi Nafchi, A. , Hashemi‐Moghaddam, H. , & Baghaie, H. (2021). The effects of nano‐zinc oxide morphology on functional and antibacterial properties of tapioca starch bionanocomposite. Food Science & Nutrition, 9(8), 4497–4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma, S. K. , Jha, E. , Panda, P. K. , Das, J. K. , Thirumurugan, A. , Suar, M. , & Parashar, S. K. S. (2018). Molecular aspects of core‐shell intrinsic defect induced enhanced antibacterial activity of ZnO nanocrystals. Nanomedicine (London, England), 13(1), 43–68. [DOI] [PubMed] [Google Scholar]
- Wayne, P. (2012). Clinical and Laboratory Standards Institute (CLSI), approved standard M07‐A9, methods for dilution antimicrobial susceptibility, tests for bacteria that grow aerobically. Clinical and Laboratory Standards Institute. [Google Scholar]
- Youssef, A. M. , el‐Sayed, S. M. , el‐Sayed, H. S. , Salama, H. H. , & Dufresne, A. (2016). Enhancement of Egyptian soft white cheese shelf life using a novel chitosan/carboxymethyl cellulose/zinc oxide bionanocomposite film. Carbohydrate Polymers, 151, 9–19. [DOI] [PubMed] [Google Scholar]
- Yu, H.‐Y. , Yang, X. Y. , Lu, F. F. , Chen, G. Y. , & Yao, J. M. (2016). Fabrication of multifunctional cellulose nanocrystals/poly (lactic acid) nanocomposites with silver nanoparticles by spraying method. Carbohydrate Polymers, 140, 209–219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data that support the findings of this study are available in the manuscript.


