Abstract
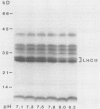
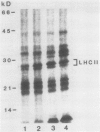
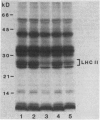
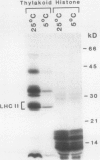
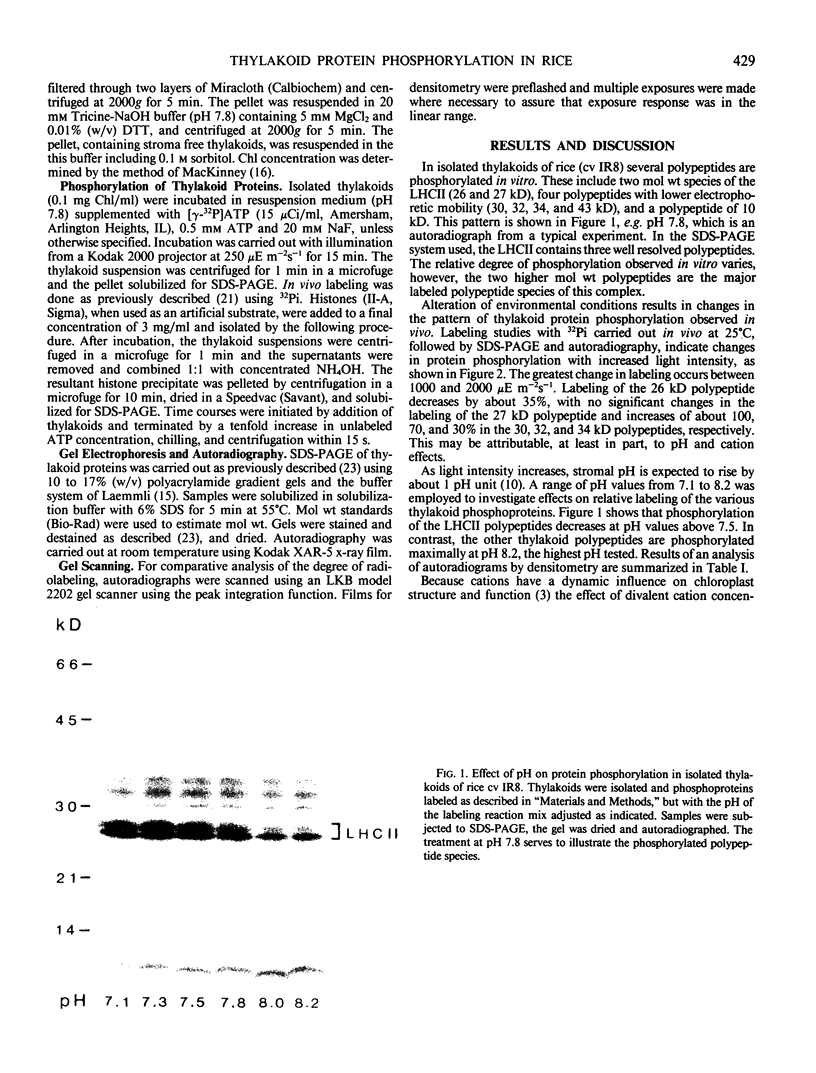
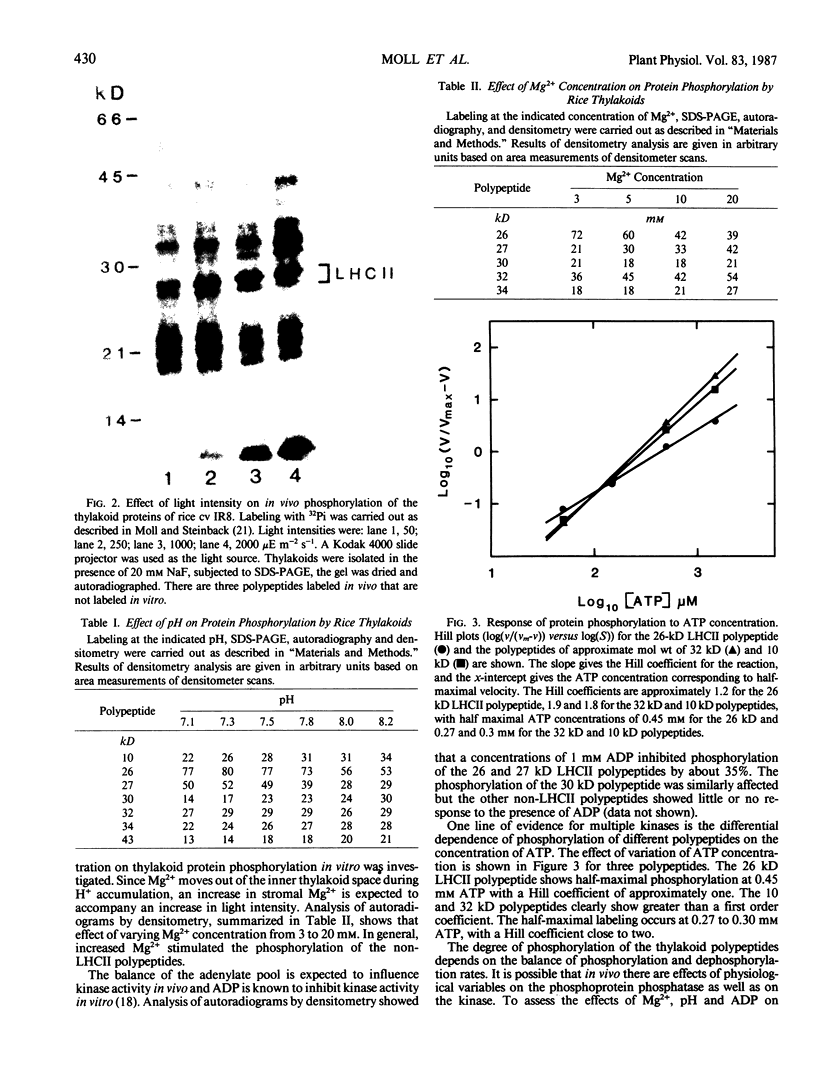
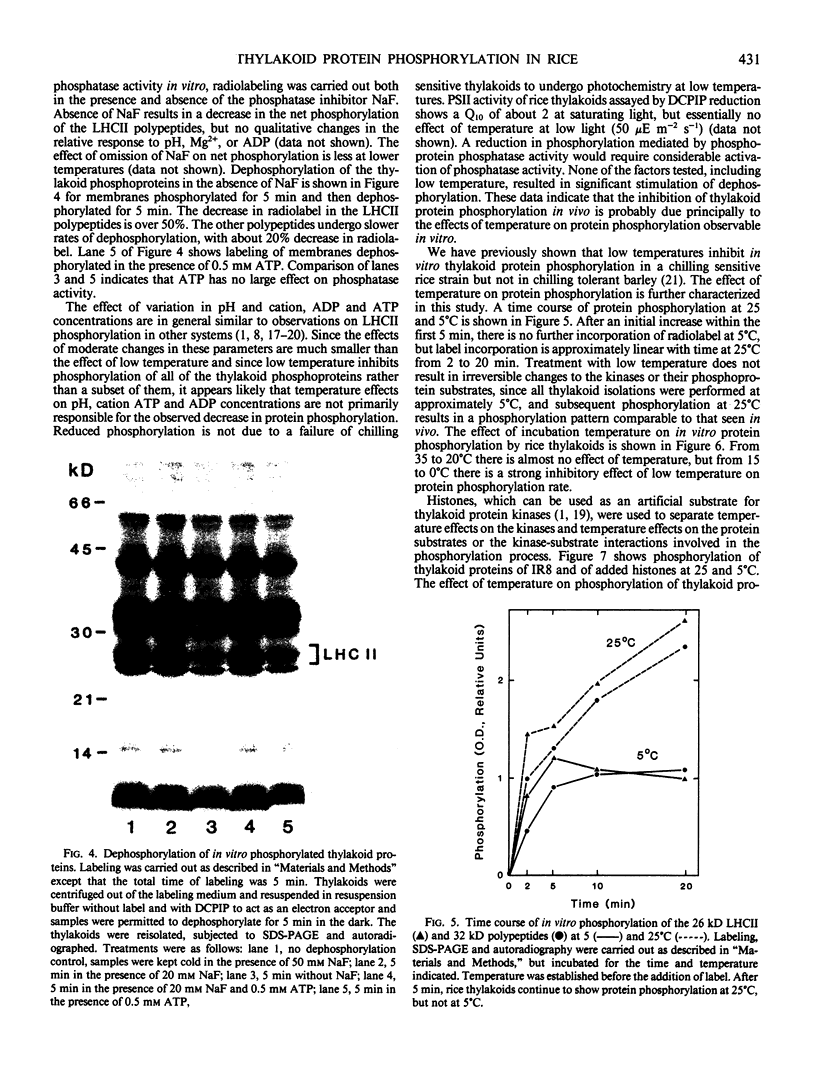
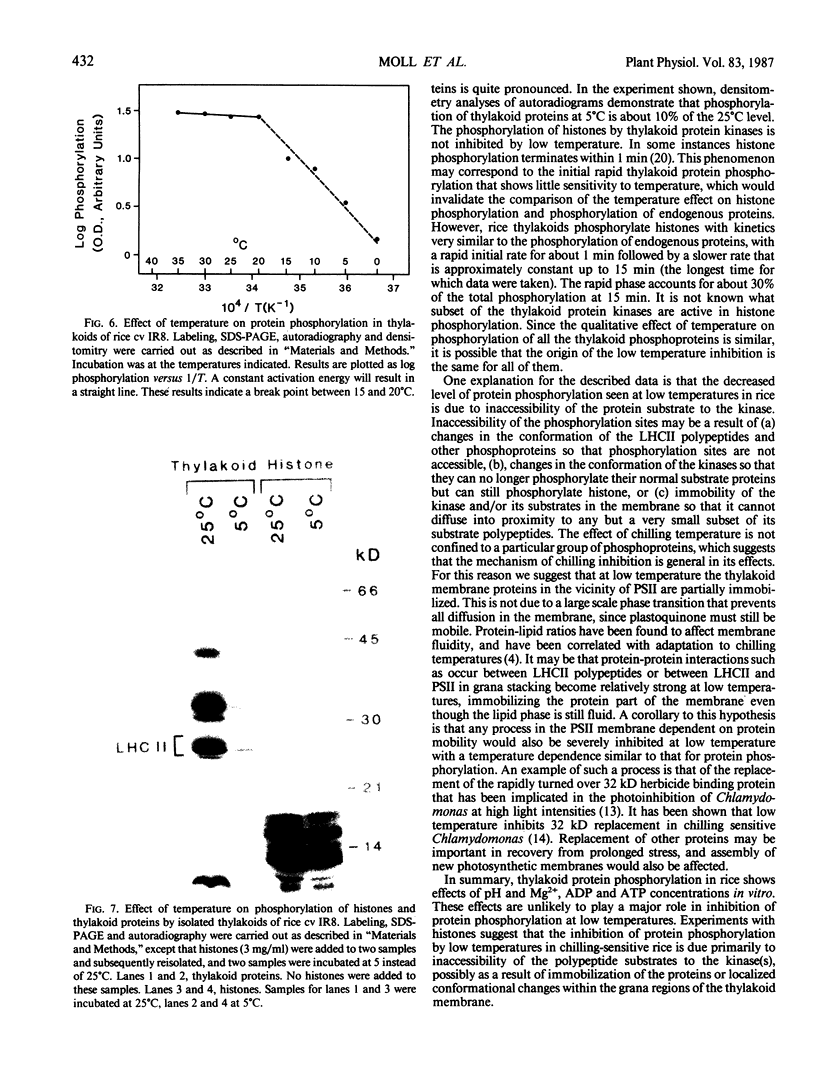
The phosphorylation of thylakoid proteins of rice (Oryza sativa L.) was studied in vitro using [γ-32P]ATP. Several thylakoid proteins are labeled, including the light-harvesting complex of photosystem II. Protein phosphorylation is sensitive to temperature, pH, and ADP, ATP, and divalent cation concentrations. In the range pH 7 to 8.2, phosphorylation of the light-harvesting polypeptides declines above pH 7.5, whereas labeling of several other thylakoid polypeptides increases. Increasing divalent cation concentration from 3 to 20 millimolar results in a decrease in phosphorylation of the 26 kilodalton light-harvesting complex polypeptide and increased phosphorylation of several other polypeptides. ADP has an inhibitory effect on the phosphorylation of the light-harvesting complex polypeptides. Phosphorylation of the 26 kilodalton light-harvesting polypeptide requires 0.45 millimolar ATP for half-maximal phosphorylation, compared to 0.3 millimolar for the 32 kilodalton phosphoprotein. Low temperature inhibits the phosphorylation of thylakoid proteins in chilling-sensitive rice. However, phosphorylation of histones by thylakoid-bound kinase(s) is independent of temperature in the range of 25 to 5°C, suggesting that the effect of low temperature is on accessibility of the substrate, rather than on the activity of the kinase.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfonzo R., Nelson N., Racker E. A Light-dependent Protein Kinase Activity of Chloroplasts. Plant Physiol. 1980 Apr;65(4):730–734. doi: 10.1104/pp.65.4.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. Chloroplast phosphoproteins. The protein kinase of thylakoid membranes is light-dependent. FEBS Lett. 1979 Jul 15;103(2):342–344. doi: 10.1016/0014-5793(79)81358-7. [DOI] [PubMed] [Google Scholar]
- Bennett J., Steinback K. E., Arntzen C. J. Chloroplast phosphoproteins: regulation of excitation energy transfer by phosphorylation of thylakoid membrane polypeptides. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5253–5257. doi: 10.1073/pnas.77.9.5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt W. H., Werdan K., Milovancev M., Geller G. Alkalization of the chloroplast stroma caused by light-dependent proton flux into the thylakoid space. Biochim Biophys Acta. 1973 Aug 31;314(2):224–241. doi: 10.1016/0005-2728(73)90137-0. [DOI] [PubMed] [Google Scholar]
- Kyle D. J., Ohad I., Arntzen C. J. Membrane protein damage and repair: Selective loss of a quinone-protein function in chloroplast membranes. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4070–4074. doi: 10.1073/pnas.81.13.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markwell J. P., Baker N. R., Bradbury M., Thornber J. P. Use of zinc ions to study thylakoid protein phosphorylation and the state 1-state 2 transition in vitro. Plant Physiol. 1984 Feb;74(2):348–354. doi: 10.1104/pp.74.2.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millner P. A., Widger W. R., Abbott M. S., Cramer W. A., Dilley R. A. The effect of adenine nucleotides on inhibition of the thylakoid protein kinase by sulfhydryl-directed reagents. J Biol Chem. 1982 Feb 25;257(4):1736–1742. [PubMed] [Google Scholar]
- Moll B. A., Steinback K. E. Chilling Sensitivity in Oryza sativa: The Role of Protein Phosphorylation in Protection against Photoinhibition. Plant Physiol. 1986 Feb;80(2):420–423. doi: 10.1104/pp.80.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinback K. E., Bose S., Kyle D. J. Phosphorylation of the light-harvesting chlorophyll-protein regulates excitation energy distribution between photosystem II and photosystem I. Arch Biochem Biophys. 1982 Jun;216(1):356–361. doi: 10.1016/0003-9861(82)90221-1. [DOI] [PubMed] [Google Scholar]
- Steinback K. E., Burke J. J., Arntzen C. J. Evidence for the role of surface-exposed segments of the light-harvesting complex in cation-mediated control of chloroplast structure and function. Arch Biochem Biophys. 1979 Jul;195(2):546–557. doi: 10.1016/0003-9861(79)90381-3. [DOI] [PubMed] [Google Scholar]