Abstract
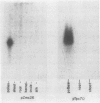
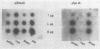
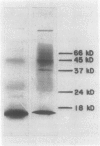
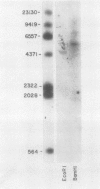
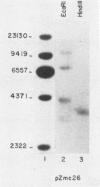
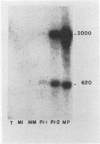
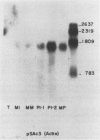
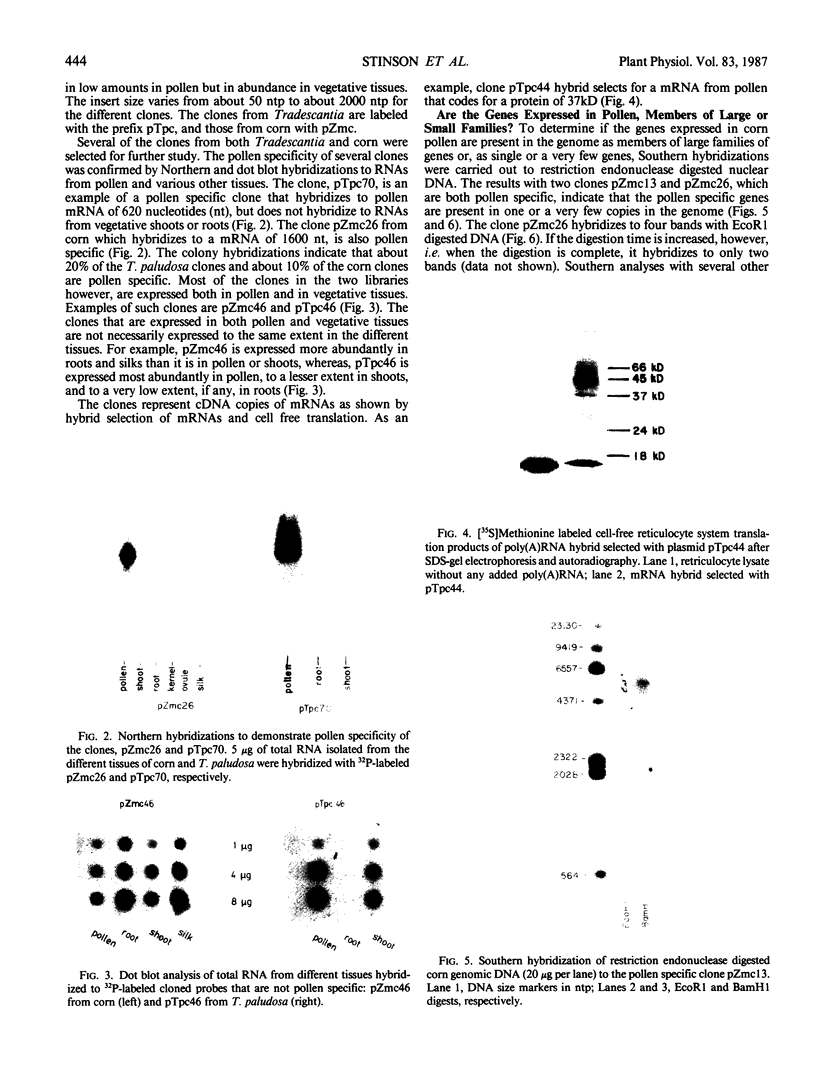
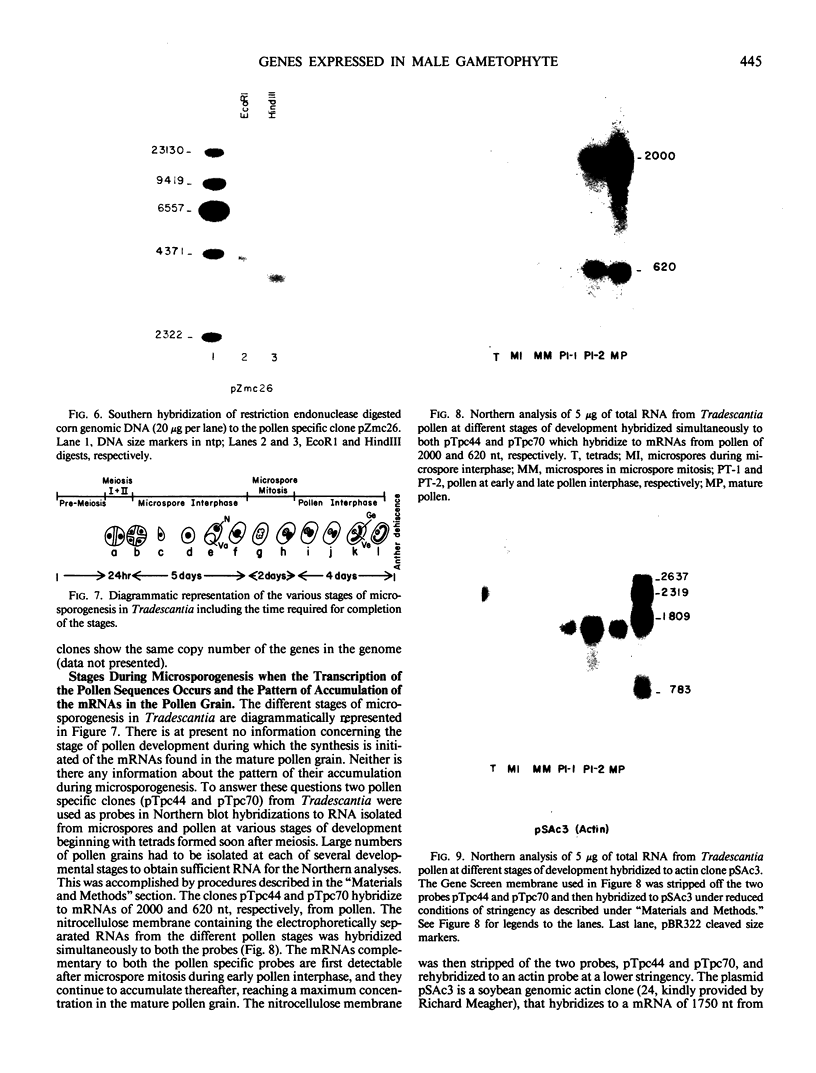
Recombinant cDNA libraries to poly(A)RNA isolated from mature pollen of Zea mays and Tradescantia paludosa have been constructed. Northern blot analyses indicate that several of the clones are unique to pollen and are not expressed in vegetative tissues. The majority, however, are expressed both in pollen and vegetative tissues. Southern hybridizations show that the pollen specific sequences in corn are present in one or a very few copies in the genome. By using several of the clones as probes, it was found that there are at least two different groups of mRNAs with respect to their synthesis. The mRNAs of the first group represented by the pollen specific clones are synthesized after microspore mitosis and increase in concentration up to maturity. The second group, exemplified by actin mRNA, begins to accumulate soon after meiosis, reaches its maximum by late pollen interphase, and decreases thereafter. Although the actin mRNA and the pollen specific mRNAs studied show very different patterns of initiation of synthesis and accumulation during pollen development, the rates of decline of these mRNAs during the first 60 minutes of germination and pollen tube growth in Tradescantia are similar and reflect the previously observed declines in rates of protein synthesis during this period.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander D. C., McKnight T. D., Williams B. G. A simplified and efficient vector-primer cDNA cloning system. Gene. 1984 Nov;31(1-3):79–89. doi: 10.1016/0378-1119(84)90197-5. [DOI] [PubMed] [Google Scholar]
- Bingham P. M., Judd B. H. A copy of the copia transposable element is very tightly linked to the Wa allele at the white locus of D. melanogaster. Cell. 1981 Sep;25(3):705–711. doi: 10.1016/0092-8674(81)90177-x. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheley S., Anderson R. A reproducible microanalytical method for the detection of specific RNA sequences by dot-blot hybridization. Anal Biochem. 1984 Feb;137(1):15–19. doi: 10.1016/0003-2697(84)90339-7. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Effect of growth conditions on the formation of the relaxation complex of supercoiled ColE1 deoxyribonucleic acid and protein in Escherichia coli. J Bacteriol. 1972 Jun;110(3):1135–1146. doi: 10.1128/jb.110.3.1135-1146.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danna K. J., Sack G. H., Jr, Nathans D. Studies of simian virus 40 DNA. VII. A cleavage map of the SV40 genome. J Mol Biol. 1973 Aug 5;78(2):363–376. doi: 10.1016/0022-2836(73)90122-8. [DOI] [PubMed] [Google Scholar]
- Eisenberg A. J., Holmes D. S. A note on the use of CsCl centrifugation to purify bacterial plasmids prepared by the rapid boiling method. Anal Biochem. 1982 Dec;127(2):434–434. doi: 10.1016/0003-2697(82)90199-3. [DOI] [PubMed] [Google Scholar]
- Goldberg D. A. Isolation and partial characterization of the Drosophila alcohol dehydrogenase gene. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5794–5798. doi: 10.1073/pnas.77.10.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- MOSES M. J., TAYLOR J. H. Desoxypentose nucleic acid synthesis during microsporogenesis in Tradescantia. Exp Cell Res. 1955 Dec;9(3):474–488. doi: 10.1016/0014-4827(55)90077-x. [DOI] [PubMed] [Google Scholar]
- Mascarenhas J. P., Bell E. Protein synthesis during germination of pollen: studies on polyribosome formation. Biochim Biophys Acta. 1969 Mar 18;179(1):199–203. doi: 10.1016/0005-2787(69)90136-1. [DOI] [PubMed] [Google Scholar]
- Nelson O. E. Intracistron Recombination in the Wx/wx Region in Maize. Science. 1959 Sep 25;130(3378):794–795. doi: 10.1126/science.130.3378.794. [DOI] [PubMed] [Google Scholar]
- Paterson B. M., Roberts B. E. Structural gene identification utilizing eukaryotic cell-free translational systems. Gene Amplif Anal. 1981;2:417–437. [PubMed] [Google Scholar]
- Schwartz D. Genetic control of alcohol dehydrogenase--a competition model for regulation of gene action. Genetics. 1971 Mar;67(3):411–425. doi: 10.1093/genetics/67.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah D. M., Hightower R. C., Meagher R. B. Complete nucleotide sequence of a soybean actin gene. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1022–1026. doi: 10.1073/pnas.79.4.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M. B., O'neill P. M., Knox R. B. Initiation of Postmeiotic beta-Galactosidase Synthesis during Microsporogenesis in Oilseed Rape. Plant Physiol. 1985 Jan;77(1):225–228. doi: 10.1104/pp.77.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson J., Mascarenhas J. P. Onset of Alcohol Dehydrogenase Synthesis during Microsporogenesis in Maize. Plant Physiol. 1985 Jan;77(1):222–224. doi: 10.1104/pp.77.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanksley S. D., Zamir D., Rick C. M. Evidence for Extensive Overlap of Sporophytic and Gametophytic Gene Expression in Lycopersicon esculentum. Science. 1981 Jul 24;213(4506):453–455. doi: 10.1126/science.213.4506.453. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing R. P., Mascarenhas J. P. Analysis of the Complexity and Diversity of mRNAs from Pollen and Shoots of Tradescantia. Plant Physiol. 1984 Jul;75(3):865–868. doi: 10.1104/pp.75.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]