Abstract
Introduction
Rituximab (RTX) has been shown to be effective in inducing immunological remission in patients with membranous nephropathy (MN). Some patients required more than one course of RTX to achieve immunological remission. Identifying patients who need more courses of RTX to achieve immunological remission is beneficial for better physician–patient communication, the assessment of treatment course, and the evaluation of medical costs. This study aims to establish a practical model to predict the probability of immunological remission after receiving one cycle of RTX.
Methods
This study enrolled 106 patients from the First Affiliated Hospital of Zhengzhou University in the modeling group and 30 patients from Henan Provincial Hospital of Traditional Chinese Medicine in the external validation group. Patients in the modeling group were divided into responders or nonresponders according to whether they achieved immunological remission or not after following up for 6 months. A nomogram was established based on the results of logistic regression analysis. The predictive performance of the nomogram was evaluated by the area under the receiver operating characteristic curve (AUC), calibration curves, and decision curve analysis (DCAs).
Results
In the modeling group, 75 (70.8%) patients achieved immunological remission within 6 months after receiving one cycle of RTX. Significant differences were observed between nonresponders and responders. Risk factors used in nomogram included PLA2R antibody, hemoglobin, and gender. The AUC value of nomogram was 0.797 (95% CI 0.701–0.894, P<0.001). The calibration curves demonstrated acceptable agreement between the predicted outcomes by the nomogram and the actual values. DCA curves showed good positive net benefits in the predictive model. The external validation also demonstrated the reliability of the prediction nomogram.
Conclusion
A predictive nomogram including PLA2R antibody, hemoglobin, and gender may provide a basis to predict the doses of RTX needed in MN patients.
Keywords: nomogram, external validation, immunological remission, rituximab, membranous nephropathy
Introduction
Membranous nephropathy has steadily increased in prevalence over the past few years and is now one of the most common pathological subtypes of primary nephrotic syndrome.1,2 Studies have found that most patients with membranous nephropathy have detectable autoimmune reactive antibodies.3–7 Phospholipase A2 receptor (PLA2R) antibody, as a major indicator for membranous nephropathy, can be used to assist the clinical diagnosis of membranous nephropathy and help judge the effectiveness of treatment.8,9 And because the change of PLA2R antibody titer can precede changes of proteinuria, PLA2R antibody could predict the remission and recurrence of membranous nephropathy in advance.3 To achieve complete remission, it would be necessary to reach immunological remission which is a major goal in the treatment of idiopathic membranous nephropathy.10
Rituximab (RTX), a drug targeting CD20 molecule on the surface of pre-B cells and mature B lymphocytes, induces the apoptosis of B-cell and inhibits the formation of autoantibody including PLA2R antibody.11,12 Many studies have confirmed that RTX can effectively induce immunological remission of membranous nephropathy, however we still have some questions in the clinical applicability of RTX. In clinical work, we found that different doses of RTX are required to achieve immunological remission in patients with membranous nephropathy. Some patients with membranous nephropathy required more than one course of RTX to achieve immunological remission. Identifying who needs more courses of RTX to achieve immunological remission before the use of RTX is beneficial for better physician–patient communication, the assessment of treatment course, and the evaluation of medical costs. It is necessary to distinguish patients who required multiple cycles of RTX from those who needed just one cycle. Unfortunately, until now clinicians lack a standard model to predict the immunological remission of membranous nephropathy patients treated by RTX. Therefore, we designed this retrospective study to develop a novel nomogram for clinical use. This may help clinicians choose the treatment strategy for patients with PLA2R antibody-associated membranous nephropathy to some extent.
Methods
Study Design and Patients
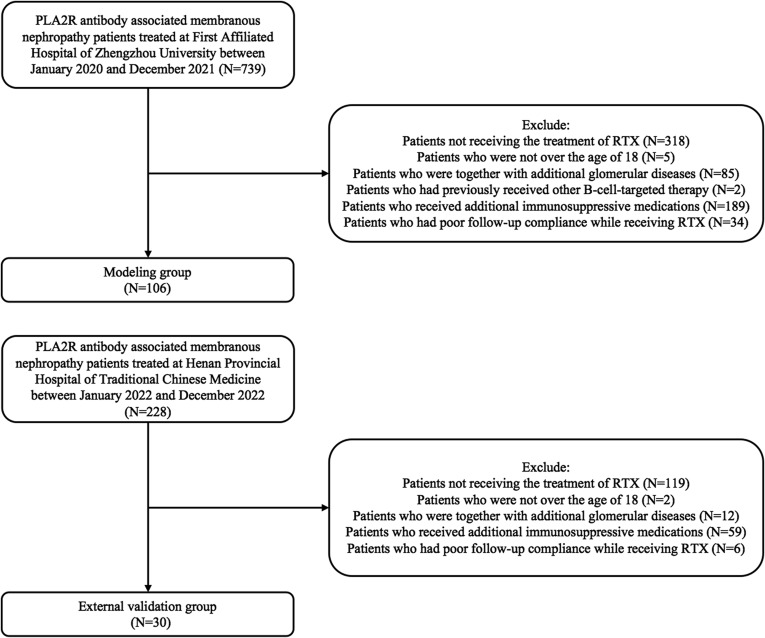
This is a retrospective study. This study comprised 106 PLA2R antibody associated membranous nephropathy patients treated at First Affiliated Hospital of Zhengzhou University between January 2020 and December 2021 and 30 PLA2R antibody associated membranous nephropathy patients treated at Henan Provincial Hospital of Traditional Chinese Medicine between January and December 2022. The following are the criteria: (1) Through glomerular PLA2R immunohistochemical staining or serum PLA2R antibody examination, all patients were diagnosed as PLA2R antibody associated membranous nephropathy; (2) All patients were over the age of 18; (3) Common causes of secondary membranous nephropathy were not identified; (4) All patients got one or more cycles of RTX, and after receiving one cycle of the medication, patients were monitored for a minimum of six months. The following are the requirements for exclusion: (1) Together with additional glomerular diseases; (2) Patients who received additional immunosuppressive medications or had poor follow-up compliance while receiving RTX; (3) Patients who had previously received other B-cell-targeted therapy. A study flow chart is shown in Figure 1.
Figure 1.
The flowchart of this study.
The 2021 KDIGO recommendations state that if an enzyme-linked immunosorbent test (ELISA) was used to assess PLA2R antibodies, a cut-off value of 2 RU/mL should be used to define complete immunologic remission.10 Therefore, definition of immunologic remission in this study is based on the 2021 KDIGO guidelines.13 Patients who achieved immunologic remission within 6 months after one cycle of RTX treatment were defined as responders. Patients were classified as nonresponders if they did not meet the aforementioned criteria after receiving RTX for 6 months. The study complies with the Declaration of Helsinki. The ethics committee of the First Affiliated Hospital of Zhengzhou University and the ethics commitment of Henan Provincial Hospital of Traditional Chinese Medicine authorized the study.
Clinical Data Collection
General clinical information, such as gender, age, blood pressure, body mass index, pathological information, and past treatment plans, were gathered before the administration of RTX. Clinical and laboratory parameters of patients including indicators associated with immune status, nephrotic syndrome, and renal function were collected before RTX infusion and repeated once a month after RTX administration for a total of 6 months. Follow-up ended when patients achieve immunological remission or the period of follow-up was greater than 6 months.
A commercial ELISA kit from EUROIMMUN AG, Lübeck, Germany, was used to identify PLA2R antibodies. According to age, race, and serum creatinine level, the estimated glomerular filtration rate (eGFR) was determined using the Chronic Kidney Disease Epidemiology Collaboration algorithm. The severity of proteinuria was assessed by urinary protein-to-creatinine ratio. Glomerular MN lesions were classified into four stages according to the Ehrenreich and Churg’s classification criteria.
Statistical Analysis
Data were expressed as mean ± standard deviation, median, interquartile range, or percentages. Independent sample t-test to compare respondents and nonresponders if the data follow a normal distribution. The Mann–Whitney U test is used to compare the two groups if the data are not distributed normally. The comparison between the two groups was performed by χ2 test or Fisher exact probability method if the type of data was categorical variables. Immunological remission predictions were estimated using logistic regression analysis. A nomogram was built based on the outcome of multivariate logistic regression analysis, which included PLA2R antibody, gender, and hemoglobulin level. We evaluate the PLA2R antibody, gender, hemoglobin, and nomogram by the area under the receiver operating characteristic curve (AUC). We also plotted calibration curves and decision curve analysis (DCAs). Lastly, goodness-of-fit model was evaluated using Hosmer–Lemeshow test. The SPSS 24.0 software program and R software (version 3.6.3, http://www.R-project.org) were for statistical analysis, and two-sided p-values were calculated.
Results
Baseline Characteristics at RTX Infusion
Between January 2020 and December 2021, we enrolled 106 patients with PLA2R antibody-associated membranous nephropathy whose follow-up time exceeded 6 months in the modeling group. The baseline characteristics are summarized in Table 1. In total, 82 (77.35%) patients in the modeling group were male, and the mean age was 50.2±12.9 years. Time from diagnosis to RTX initiation was 24.0 months (range, 6–40 months).
Table 1.
Baseline Characteristics of PLA2R Antibody-Associated MN Patients Treated with Rituximab
| Variables | Training (N=106) | Validation (N=30) | P-value |
|---|---|---|---|
| Gender (male/female) | 82/24 | 24/6 | 0.758 |
| Age (years) | 50.2±12.9 | 54.2±11.4 | 0.129 |
| Time since diagnosis to Rituximab (months) | 24.0 (6.0, 40.0) | 24.0 (5.3, 36.0) | 0.579 |
| BMI (kg/m2) | 25.4±4.1 | 25.1±3.7 | 0.796 |
| BP (mmHg) | |||
| Systolic | 131.8±14.4 | 131.7±13.8 | 0.783 |
| Diastolic | 82.1±9.5 | 82.6±8.2 | 0.981 |
| Pathologic stage of MN [n (%)] | |||
| Stage I | 22 (20.8) | 4 (13.3) | 0.799 |
| Stage I–II | 18 (17.0) | 4 (13.3) | |
| Stage II | 49 (46.2) | 17 (56.7) | |
| Stage II–III | 12 (11.3) | 3 (10.0) | |
| Stage III | 4 (3.8) | 1 (3.3) | |
| Stage IV | 1 (0.9) | 1 (3.3) | |
| Creatinine (µmol/L) | 91.0 (74.0, 132.0) | 86.0 (68.3, 131.0) | 0.836 |
| eGFR (mL/min/1.73m2) | 78.1 (53.1, 103.1) | 81.1 (51.4, 103.7) | 0.836 |
| eGFR >90 [n (%)] | 45 (42.5) | 13 (43.3) | 0.988 |
| eGFR 60–90 [n (%)] | 29 (27.4) | 8 (26.7) | |
| eGFR 30–60 [n (%)] | 23 (21.7) | 7 (23.3) | |
| eGFR 15–30 [n (%)] | 9 (8.5) | 2 (6.7) | |
| Protein-to-creatinine ratio (g/g) | 6.50 (3.98, 9.85) | 6.71 (4.73, 11.68) | 0.239 |
| Albumin (g/L) | 24.1 (19.4, 30.3) | 24.4 (19.1, 30.1) | 0.754 |
| PLA2R antibody titer (RU/mL) | 45.4 (14.8, 110.6) | 54.5 (23.6, 121.9) | 0.589 |
| Patients with immunological remission after previous therapies [n (%)] | 42 (52.5%) | 11 (52.4%) | 0.992 |
| Number (%) of patients with | |||
| Zero round of immunomeds | 26 (24.5) | 9 (30.0) | 0.779 |
| One round of immunomeds | 30 (28.3) | 6 (20.0) | |
| Two rounds of immunomeds | 23 (21.7) | 6 (20.0) | |
| Three rounds of immunomeds | 27 (25.5) | 9 (30.0) |
Note: Data presented as median (first-third interquartile range) or mean ± SD or number (percentage).
Abbreviations: BMI, body mass index; BP, blood pressure; MN, membranous nephropathy; eGFR, estimated glomerular filtration rate; PLA2R antibody, phospholipase A2 receptor antibody.
Before RTX infusions, the serum PLA2R antibody titer of patients in the modeling group was 45.4 (14.8, 110.6) RU/mL, protein-to-creatinine ratio was 6.50 (3.98, 9.85) g/g, and the level of serum albumin was 24.1 (19.4, 30.3) g/L. As parameters of renal function, the serum creatinine level was 91.00 (74.0 to 132.0) µmol/L, and eGFR calculated from the CKD-EPI formula was 78.1mL/min/1.73m2 (IQR, 53.1 to 103.1).
In this study, 26 patients received RTX therapy as initial treatment. The remaining 80 patients underwent at least one course of immunosuppressive therapy prior to RTX therapy, including corticosteroids plus cyclophosphamide for 15 patients, corticosteroids plus cyclosporine for 25, tacrolimus for 74, and mycophenolate mofetil for 1. Forty-two of those 80 patients who had previously undergone treatment experienced immunological remission after prior immunosuppressive therapy.
This study also enrolled 30 PLA2R antibody-associated membranous nephropathy patients treated at Henan Provincial Hospital of Traditional Chinese Medicine between January and December 2022 as the external validation group. The baseline characteristics at RTX infusion showed no significant difference between the modeling group and external validation group (Table 1).
Immunological Outcomes
For a total of 6 months, every patient was followed up once a month. There were 75/106 (70.8%) patients who achieved immunological remission within 6 months in the modeling group. Seventeen of those patients experienced immunological remission in less than a month. At 2, 3, 4, 5, and 6 months after receiving RTX treatment, 24, 5, 4, 4, and 21 patients achieved immunological remission (Table 2).
Table 2.
Number of PLA2R Antibody-Associated MN Patients Achieving Immunological Remission Within 6 Months After Treated with Rituximab for One Cycle
| Time (Months) | Number of Patients Achieving Immunological Remission [n (%)] |
|---|---|
| 0 | 0 (0.0) |
| 1 | 17 (16.0) |
| 2 | 41 (38.7) |
| 3 | 46 (43.4) |
| 4 | 50 (47.2) |
| 5 | 54 (50.9) |
| 6 | 75 (70.8) |
Note: Data presented as number (percentage).
Abbreviations: MN, membranous nephropathy; PLA2R antibody, phospholipase A2 receptor antibody.
Comparison Between Responders and Nonresponders at RTX Initiation
Between responders and nonresponders in the modeling group, there were no significant differences in terms of age, body mass index, time since diagnosis to RTX, or pathologic phase of membranous nephropathy. We also observed an interesting phenomenon that the proportion of female patients in responders was higher (P=0.010). As an essential indicator of idiopathic membranous nephropathy, PLA2R antibody demonstrated a substantial difference between responders and nonresponders. Compared with nonresponders, the baseline level of PLA2R antibody titers was lower in responders [27.3 (10.5, 65.6) vs 98.5 (50.3, 278.8) RU/mL, P<0.001]. As nephrotic syndrome parameters, there was no significant difference in the baseline level of protein-to-creatinine ratio [3.97 (1.82, 7.56) vs 4.88 (3.55, 8.83) g/g, P=0.090]. Compared with nonresponders, serum albumin level was significantly higher in responders [23.5 (21.9, 32.0) vs 22.8 (16.0, 33.9) g/L, P=0.002].
In terms of indicators of renal function, responders had a better renal function than that of nonresponders, which was demonstrated by the lower level of serum creatinine [82.5 (67.5, 114.0) vs 114.0 (85.5, 139.5) µmol/L, P=0.006], higher eGFR [94.4 (59.3, 109.3) vs 61.6 (50.5, 86.7) mL/min/1.73m2, P=0.007], and the lower level of β2-MG [2.07 (1.81, 3.01) vs 3.56 (2.30, 6.19) mg/L, P=0.010]. In this study, we also observed that the level of hemoglobin was statistically different. Compared with nonresponders, the level of hemoglobin was higher [(121.8±20.2) vs (108.7±24.1) g/L, P=0.005] in responders (Table 3).
Table 3.
Comparison of Clinical Characteristics Between Responders and Nonresponders
| Variables | Responders (N=75) | Nonresponders (N=31) | P-value |
|---|---|---|---|
| Gender (male/female) | 53/22 | 29/2 | 0.010* |
| Age (years) | 49.0±12.5 | 52.9±13.7 | 0.172 |
| Time since diagnosis to rituximab (months) | 24.0 (5.0, 37.0) | 24.0 (13.5, 45.0) | 0.718 |
| BMI (kg/m2) | 25.3±4.3 | 25.6±3.6 | 0.742 |
| Pathologic phase of MN [n (%)] | |||
| Phase I | 14 (18.7) | 8 (25.8) | 0.517 |
| Phase I–II | 15 (20.0) | 3 (9.7) | |
| Phase II | 36 (48.0) | 13 (41.9) | |
| Phase II–III | 7 (9.3) | 5 (16.1) | |
| Phase III | 2 (2.7) | 2 (6.5) | |
| Phase IV | 1 (1.3) | 0 (0.0) | |
| PLA2R-antibody titer (RU/mL) | 27.3 (10.5, 65.6) | 98.5 (50.3, 278.8) | <0.001* |
| Protein-to-creatinine ratio (g/g) | 3.97 (1.82, 7.56) | 4.88 (3.55, 8.83) | 0.090 |
| Albumin (g/L) | 23.5 (21.9, 32.0) | 22.8 (16.0, 33.9) | 0.002* |
| Hemoglobin (g/L) | 121.8±20.2 | 108.7±24.1 | 0.005* |
| Creatinine (µmol/L) | 82.5 (67.5, 114.0) | 114.0 (85.5, 139.5) | 0.006* |
| eGFR (mL/min/1.73m2) | 94.4 (59.3, 109.3) | 61.6 (50.5, 86.7) | 0.007* |
| eGFR >90 [n (%)] | 38 (50.7) | 7 (22.6) | 0.015* |
| eGFR 60–90 [n (%)] | 18 (25.3) | 10 (32.3) | |
| eGFR 30–60 [n (%)] | 11 (14.7) | 12 (38.7) | |
| eGFR 15–30 [n (%)] | 7 (9.3) | 2 (6.5) | |
| UA (µmol/L) | 315.8±93.1 | 320.1±83.7 | 0.823 |
| β2-MG (mg/L) | 2.07 (1.81, 3.01) | 3.56 (2.30, 6.19) | 0.010* |
| Cystatin C (mg/L) | 1.10 (0.91, 1.62) | 1.90 (1.41, 2.17) | <0.001* |
| CD19 (per mm) | 249.0 (203.0, 261.0) | 141.0 (67.0, 239.5) | 0.023* |
| CD4 (per mm) | 915.0 (698.0, 1058.5) | 671.0 (316.0, 779.5) | 0.009* |
| CD8 (per mm) | 465.0 (343.5, 700.5) | 423.0 (278.0, 474.5) | 0.036* |
Notes: Data presented as median (first-third interquartile range) or mean ± SD or number (percentage). *Stands for p < 0.05.
Abbreviations: BMI, body mass index; BP, blood pressure; MN, membranous nephropathy; β2-MG, beta 2 microglobulin; CD, cluster of differentiation; UA, uric acid; eGFR, estimated glomerular filtration rate; PLA2R antibody, phospholipase A2 receptor antibody.
Effect of PLA2R Antibody, Gender, Hemoglobin on Prediction of Immunological Remission in PLA2R Antibody-Associated Membranous Nephropathy Patients Treated with RTX
In the modeling group, according to univariate logistic regression analysis and multivariable analysis, we found three strong predictors of immunological remission of patients: PLA2R antibody level (PLA2R-Ab titer ≤66.3 RU/mL/PLA2R-Ab titer >66.3RU/mL) (OR 15.564, 95% CI: 2.002, 121.002, P=0.009), gender (female/male) (OR 6.019, 95% CI: 1.321, 27.428, P=0.008), hemoglobin level (OR 1.092, 95% CI: 1.023, 1.165, P=0.008) (Table 4 and 5).
Table 4.
Predictive Factors Related to Immunological Remission in 108 PLA2R Antibody-Associated MN Patients Receiving One Cycle of Rituximab Within 6 Months: Univariate Analysis
| Variable | OR (95% CI) | P-value |
|---|---|---|
| Gender (female/male) | 6.019 (1.321, 27.428) | 0.020* |
| PLA2R-Ab titer >66.3RU/mL | 0.997 (0.995, 0.999) | 0.008* |
| PLA2R-Ab titer ≤66.3RU/mL | 1 | |
| Hemoglobin (g/L) | 1.030 (1.008, 1.053) | 0.008* |
| Albumin (g/L) | 1.116 (1.038, 1.199) | 0.003* |
| Creatinine (µmol/L) | 0.994 (0.987, 1.002) | 0.148 |
| eGFR (mL/min/1.73m2) | 1.018 (1.003, 1.034) | 0.018* |
| β2-MG (mg/L) | 0.685 (0.504, 0.932) | 0.016* |
| Cystatin C (mg/L) | 1.021 (0.916, 1.136) | 0.711 |
| CD19 (per mm) | 1.002 (0.999, 1.005) | 0.122 |
| CD4 (per mm) | 1.002 (1.000, 1.003) | 0.061 |
| CD8 (per mm) | 1.002 (1.000, 1.004) | 0.072 |
Note: *Stands for p < 0.05.
Abbreviations: MN, membranous nephropathy; β2-MG, beta 2 microglobulin; CD, cluster of differentiation; eGFR, estimated glomerular filtration rate; PLA2R antibody, phospholipase A2 receptor antibody.
Table 5.
Predictive Factors Related to Immunological Remission in 108 PLA2R Antibody-Associated MN Patients Receiving One Cycle of Rituximab Within 6 Months: Multivariate Logistic Regression Analysis (Forward, LR, α= 0.05)
| Variable | Partial Regression Coefficient | Standard Error | Wald χ2 | OR (95% CI) | P-value |
|---|---|---|---|---|---|
| Gender (female/male) | 4.888 | 1.853 | 6.955 | 6.019 (1.321, 27.428) | 0.008* |
| PLA2R-Ab titer ≤66.3RU/mL/PLA2R-Ab titer >66.3RU/mL | 2.745 | 1.046 | 6.882 | 15.564 (2.002, 121.002) | 0.009* |
| Hemoglobin (g/L) | 0.088 | 0.033 | 6.987 | 1.092 (1.023, 1.165) | 0.008* |
| Albumin (g/L) | −0.111 | 0.073 | 2.273 | 0.895 (0.775, 1.034) | 0.132 |
| eGFR (mL/min/ 1.73m2) | 0.037 | 0.026 | 1.936 | 1.037 (0.985, 1.092) | 0.164 |
| β2-MG (mg/L) | 0.089 | 0.253 | 0.123 | 1.093 (0.665, 1.796) | 0.726 |
Note: *Stands for p < 0.05.
Abbreviations: β2-MG, beta 2 microglobulin; eGFR, estimated glomerular filtration rate; MN, membranous nephropathy; PLA2R-Ab, phospholipase A2 receptor antibody.
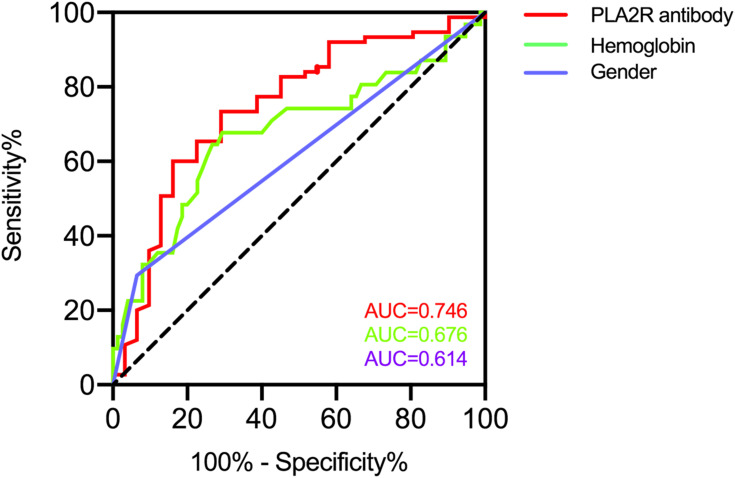
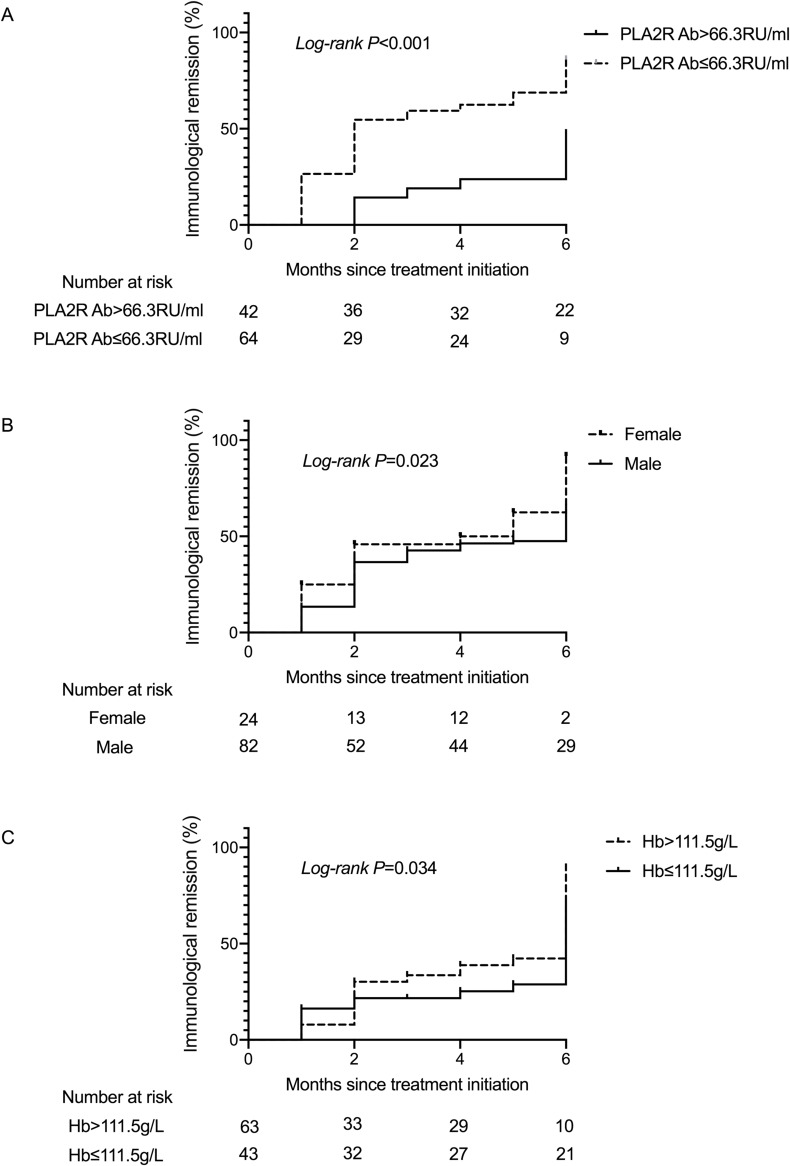
PLA2R antibody has been widely used in the diagnosis, assessment of the severity, and evaluation of remission rates in PLA2R antibody associated membranous nephropathy. We calculated the AUC to explore the predictive value of PLA2R antibody in the immunological remission of membranous nephropathy patients. According to ROC studies, the PLA2R antibody had an AUC of 0.746 (95% CI: 0.630–0.852, P<0.001). The optimal cut-off point for the PLA2R antibody to determine immunological remission was 66.3RU/mL (73.3% sensitivity; 71.0% specificity) (Figure 2). In order to investigate the relationship between PLA2R antibody and the probability of immunological remission of patients, we plotted a Kaplan–Meier curve for patients according to PLA2R antibody levels; results indicated that the cumulative immunological remission rate of patients with PLA2R antibody ≤66.3 RU/mL was higher than with PLA2R antibody >66.3 RU/mL (P<0.001) (Figure 3A).
Figure 2.
ROC curves for PLA2R antibody, hemoglobin, and gender to predict immunological remission in patients with membranous nephropathy.
Abbreviations: ROC, receiver operating characteristic; PLA2R, phospholipase A2 receptor.
Figure 3.
Kaplan–Meier curve for the the cumulative immunological remission rate of patients stratified by PLA2R antibody (A), gender (B) and hemoglobin (C) is shown for immunological remission.
Abbreviation: PLA2R, phospholipase A2 receptor.
The other factor hemoglobin had an AUC of 0.676 (95% CI: 0.553–0.799, P=0.045), and the optimal cut-off point for the hemoglobin level to determine immunological remission was 111.5g/L (67.8% sensitivity; 70.7% specificity) (Figure 2). Gender had an AUC of 0.614 (95% CI: 0.504–0.725, P=0.048). Kaplan–Meier curve also showed that the cumulative immunological remission rate of patients with hemoglobin >111.5g/L and female gender was higher (Figure 3B and C).
Nomogram Model Establishment and Validation
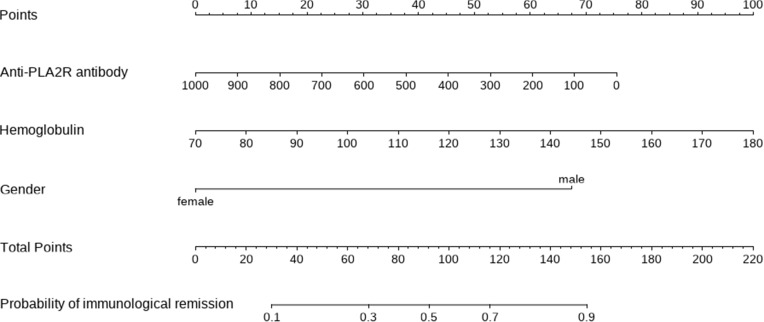
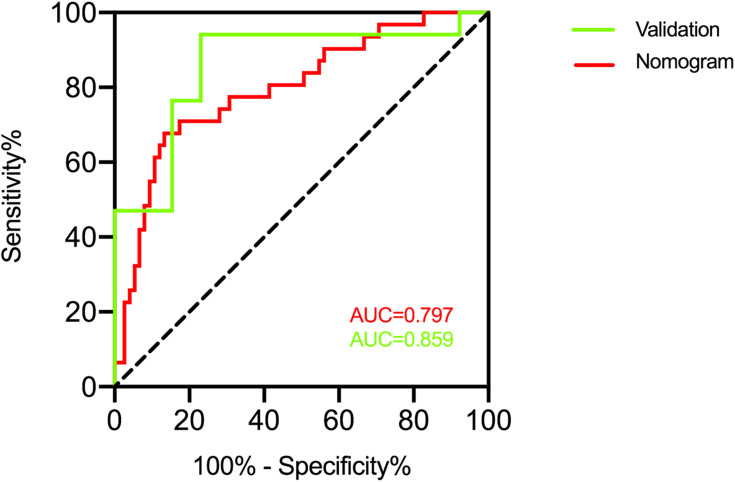
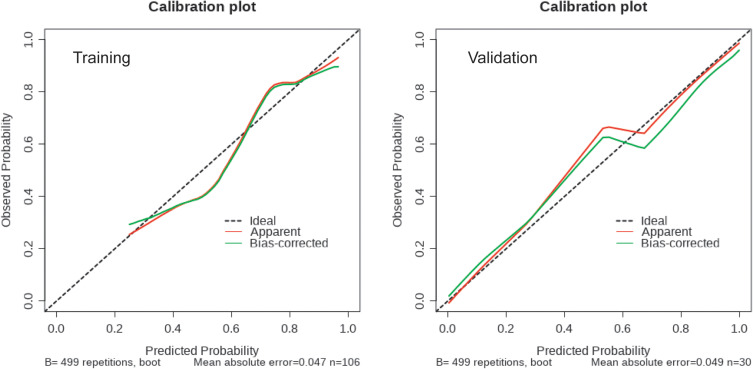
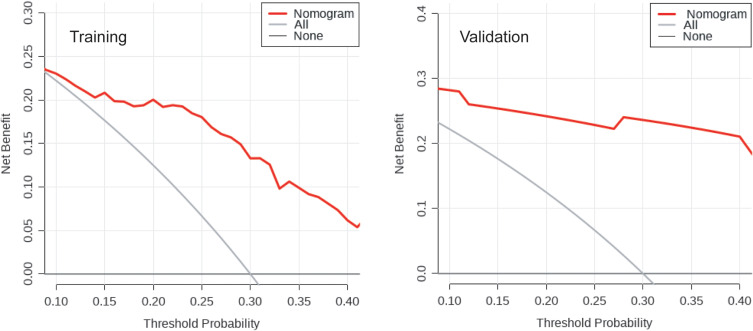
Based on the outcomes of multivariable logistic model, a nomogram was constructed with 3 factors (Figure 4). We evaluated the discrimination of the nomogram by the ROC curve and AUC value. The AUC value was 0.797 (95% CI 0.701–0.894), which is higher than that of factors including PLA2R antibody, gender, and hemoglobin (Figure 5). The calibration curves demonstrate that there is good consistency between the predicted values and the real outcomes (Figure 6). The results of Hosmer–Lemeshow test for goodness-of-fit of discriminative model showed that χ2 was 4.342, and p value was 0.825, indicating that the goodness-of-fit of the nomogram model was good. At last, the DCA plot indicated good positive net benefits in the predictive nomogram model among majority threshold probabilities (Figure 7).
Figure 4.
A novel nomogram predicting for the probability of immunological remission. To use the nomogram, the specific point for each variable of the patient lies on each variable axis. Draw a vertical line upward to determine the point at which each variable accepts. The sum of these points is located on the Total Points axis, and draw a vertical line down to horizontal axis to determine the probability of immunological remission.
Figure 5.
The ROC curve and the AUC value to evaluate the discrimination ability of the nomogram in the training and validation datasets.
Abbreviations: ROC, receiver operating characteristic; AUC, area under the receiver operating characteristic curve.
Figure 6.
Calibration curves of the nomogram in the training and validation datasets. The y-axis indicates the observed cumulative incidence for immunological remission, and the x-axis is the predicted probability of immunological remission based on the predictive model. The line adjacent to the ideal line represents the predictive accuracy.
Figure 7.
Decision curve analysis plot of the nomogram in the training and validation datasets. Red line represents the clinical net benefits according to the threshold probabilities; horizontal line assumes no cases will get immunological remission; grey line assumes all cases will get immunological remission.
To comprehensively validate and evaluate the prediction performance of the nomogram, we analyzed the ROC, DCA, and calibration curves in the external validation datasets. The results showed that the nomogram displayed overall satisfactory and stable predictive performance since the AUC (95% CI) achieved 0.859 (0.718–0.981) in validation datasets (Figure 5). The calibration curve achieved approximately ideal agreement between observed outcomes and predictions in the external validation datasets, which further confirmed the prediction accuracy and robustness of the nomogram (Figure 6). For the DCA curves, the results suggest that the nomogram can achieve a good clinical net benefit in validation datasets (Figure 7).
Discussion
The safety and efficacy of RTX in patients with membranous nephropathy have been widely reported.14–16 2021 KDIGO guidelines suggest that the discovery of PLA2R antibody has positioned immune remission as a main goal in the treatment of PLA2R antibody-associated membranous nephropathy.10 The guidelines also suggest that the level of PLA2R antibody should be evaluated after receiving RTX treatment for 6 months, and whether more courses of RTX treatment are needed should be determined according to the level of PLA2R antibody. In the clinical work, we have used RTX reasonably and extensively in the treatment of membranous nephropathy for more than 3 years. We note that some patients with membranous nephropathy could achieve immunological remission after receiving one cycle of RTX, while some patients still needed to receive more than one cycle of RTX. This was the first study to establish a practical model to predict the probability of immunological remission after receiving one cycle of RTX.
This study showed that PLA2R antibody is a major determinant of the doses of RTX required in patients with membranous nephropathy. Our study found that more than one cycle of RTX may be necessary for patients with PLA2R antibody >66.3RU/mL. Previous studies showed that PLA2R antibody disappearance was less likely in membranous nephropathy patients treated with RTX who had PLA2R antibody titers higher than 152 relative units/mL,12 which was different from our findings. There are several reasons for the differences between our research and previous experiments. Firstly, the discovery of PLA2R antibody defined in this previous study is less than 20RU/mL. The immunological remission in our study was that PLA2R antibody measured by ELISA was less than 2RU/mL, which was much lower than that in previous study. The difficulty of the goal may lead to a lower cut-off value of PLA2R antibody in our study. Secondly, this previous study did not get the cut-off value calculated by ROC curve, and its results showed that if the antibody level was lower than 152RU/mL, nearly 80% of the patients might obtain the disappearance of PLA2R antibody after 6 months of RTX treatment. In our study, if the PLA2R antibody is lower than 66.3RU/mL, the proportion of patients who have achieved immunological remission at 6 months will reach 85.9%, higher than that in the previous study. In general, because of the differences in the definition of immunological remission and research design, the cut off values of PLA2R antibody that affect whether immunological remission is achieved are different.
In addition, we also found that patients with lower level of hemoglobin were less likely to obtain immunological remission when receiving RTX treatment. Comparison of the clinical data between the two groups and univariate logistic regression analysis also showed that the renal function of nonresponders was worse, which was manifested by higher serum creatinine level, lower eGFR and higher β2-MG level. Renal insufficiency also suggests that patients with membranous nephropathy may have tubulointerstitial injury. The lower hemoglobin level of nonresponders may be related to potential interstitial fibrosis and worse renal function, which may be induced by the ineffectiveness of previous therapies, persistent massive proteinuria, or previous use of calcineurin inhibitors. The treatment of membranous nephropathy with renal insufficiency is a difficult problem because those patients always have a poorer response to immunosuppressants than those with normal renal function.17 This might be the reason why the effectiveness of RTX in membranous nephropathy patients with renal insufficiency is worse.
In this study, data showed that gender was an important predictor of the response to RTX in PLA2R antibody associated membranous nephropathy patients. Of the 24 women, 22 (91.7%) achieved immunological remission at 6 months after RTX infusion, while only 64.6% males progressed to the same endpoint. A recent study also confirmed that female patients with membranous nephropathy are more likely to achieve partial or complete remission of nephrotic syndrome after receiving RTX treatment.18 Gender seems to play a specific role in the effect of MN treated with RTX. Why do gender had such a strong impact on the outcome upon exposure to RTX even after adjusting for the potential confounders, including serum albumin level, renal function, and PLA2R antibody titer? There is no clear explanation for this question. Sex hormones may be responsible for this phenomenon. For example, estrogen has effects on the renin-angiotensin and endothelin systems, thereby affecting glomerular hemodynamics. Other potential mechanisms include receptor-mediated effects of estrogen on TGF-β signaling,19 which in turn regulates glomerular cell proliferation, matrix accumulation, apoptosis, and antioxidant capacity.20–23 Interim studies are needed to investigate this interesting question further, and more direct evidences are needed to explain the influence of gender on this outcome.
One of the limitations of the article is the small sample size. Possible reasons are as follows: RTX failed to be widely used in the treatment of membranous nephropathy due to the expensive price, and RTX would be combined with other immunosuppressive agents such as calcineurin inhibitors, which resulted in a smaller population for inclusion in this study. Whether the difference of immunosuppressants will affect the rate of immunological remission and shorten the treatment time effectively and safely is still unknown. In addition, studies have confirmed that membranous nephropathy patients treated with RTX are less prone to relapse than calcineurin inhibitors including tacrolimus. However, it is indeed observed that some patients with membranous nephropathy develop immunological relapse during B-cell reconstitution. Whether patients prone to relapse can be found or not before the treatment of RTX is still unknown, which is also a problem we will try to solve in the future. While, several factors including serum RTX levels and the existence of anti-RT antibodies that have been demonstrated to influence the efficacy of RTX in the treatment of membranous nephropathy.24 Studies have confirmed that low RTX levels at month 3 correlate with high anti-PLA2R antibody titer at months 3, 6 and 12, and with proteinuria at months 3, 6 and 12.25,26 Close monitoring of serum RTX levels can improve the efficacy of RTX. Serum RTX levels are related to drug dose, and proteinuria.27 A large inter-individual variability among patients even treated with the same schedule of RTX may also correlate with serum RTX levels. RTX recycling depends on endothelial cells via FcRn, and the efficacy of recycling seems different among membranous nephropathy patients due to the polymorphism of FcRn.26 However, at present, serum RTX levels cannot be tested in our hospital. In the future, we hope to carry out this examination to identify patients who are likely to be resistant or less effective to RTX, and improve the efficacy of the treatment.
However, our findings show that the level of PLA2R antibody, hemoglobin, and gender might be indicators to judge whether patients with membranous nephropathy need more cycles of RTX treatment.
Conclusion
In conclusion, in the treatment of membranous nephropathy, RTX is an effective treatment to induce immunological remission. A predictive nomogram including PLA2R antibody, hemoglobin, and gender can be used as a predictor of the doses of RTX needed to achieve immunological remission in patients with membranous nephropathy.
Acknowledgment
Thanks to the support of Renal Pathology Laboratory, the First Affiliated Hospital of Zhengzhou University.
Funding Statement
This work was supported by the National Natural Science Foundation of China (grant no. 82200839).
Data Sharing Statement
Data could be obtained upon request to the corresponding author.
Ethics Approval
The study was approved by the ethics commitment of the First Affiliated Hospital of Zhengzhou University and the ethics commitment of Henan Provincial Hospital of Traditional Chinese Medicine.
Informed Consent
Informed consent was derived from the participants.
Disclosure
The authors declare that there is no conflict of interest in this work.
References
- 1.du Buf-Vereijken P, Branten A, Wetzels J. Idiopathic membranous nephropathy: outline and rationale of a treatment strategy. Am J Kidney Dis. 2005;46(6):1012–1029. doi: 10.1053/j.ajkd.2005.08.020 [DOI] [PubMed] [Google Scholar]
- 2.Francis J, Beck L, Salant D. Membranous nephropathy: a journey from bench to bedside. Am J Kidney Dis. 2016;68(1):138–147. doi: 10.1053/j.ajkd.2016.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ronco P, Beck L, Debiec H, et al. Membranous nephropathy. Nat Rev Dis Primers. 2021;7(1):69. doi: 10.1038/s41572-021-00303-z [DOI] [PubMed] [Google Scholar]
- 4.Tomas N, Beck L, Meyer-Schwesinger C, et al. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med. 2014;371(24):2277–2287. doi: 10.1056/NEJMoa1409354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reinhard L, Machalitza M, Wiech T, et al. Netrin G1 is a novel target antigen in primary membranous nephropathy. J Am Soc Nephrol. 2022;33(10):1823–1831. doi: 10.1681/ASN.2022050608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoxha E, Reinhard L, Stahl R. Membranous nephropathy: new pathogenic mechanisms and their clinical implications. Nat Rev Nephrol. 2022;18(7):466–478. doi: 10.1038/s41581-022-00564-1 [DOI] [PubMed] [Google Scholar]
- 7.Sethi S. New “Antigens” in membranous nephropathy. J Am Soc Nephrol. 2021;32(2):268–278. doi: 10.1681/ASN.2020071082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van de Logt A, Fresquet M, Wetzels J, Brenchley P. The anti-PLA2R antibody in membranous nephropathy: what we know and what remains a decade after its discovery. Kidney Int. 2019;96(6):1292–1302. doi: 10.1016/j.kint.2019.07.014 [DOI] [PubMed] [Google Scholar]
- 9.Burbelo P, Joshi M, Chaturvedi A, et al. Detection of PLA2R autoantibodies before the diagnosis of membranous nephropathy. J Am Soc Nephrol. 2020;31(1):208–217. doi: 10.1681/ASN.2019050538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rovin BH, Adler SG, Barratt J, et al. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100(4S):S1–S276. doi: 10.1016/j.kint.2021.05.021 [DOI] [PubMed] [Google Scholar]
- 11.Pescovitz M. Rituximab, an anti-cd20 monoclonal antibody: history and mechanism of action. Am J Transplant. 2006;6(5):859–866. doi: 10.1111/j.1600-6143.2006.01288.x [DOI] [PubMed] [Google Scholar]
- 12.van de Logt A, Dahan K, Rousseau A, et al. Immunological remission in PLA2R-antibody-associated membranous nephropathy: cyclophosphamide versus rituximab. Kidney Int. 2018;93(4):1016–1017. doi: 10.1016/j.kint.2017.12.019 [DOI] [PubMed] [Google Scholar]
- 13.Rovin B, Adler S, Barratt J, et al. Executive summary of the KDIGO 2021 guideline for the management of glomerular diseases. Kidney Int. 2021;100(4):753–779. doi: 10.1016/j.kint.2021.05.015 [DOI] [PubMed] [Google Scholar]
- 14.Fervenza F, Appel G, Barbour S, et al. Rituximab or cyclosporine in the treatment of membranous nephropathy. N Engl J Med. 2019;381(1):36–46. doi: 10.1056/NEJMoa1814427 [DOI] [PubMed] [Google Scholar]
- 15.Scolari F, Delbarba E, Santoro D, et al. Rituximab or cyclophosphamide in the treatment of membranous nephropathy: the RI-CYCLO randomized trial. J Am Soc Nephrol. 2021;32(4):972–982. doi: 10.1681/ASN.2020071091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dahan K, Debiec H, Plaisier E, et al. Rituximab for severe membranous nephropathy: a 6-month trial with extended follow-up. J Am Soc Nephrol. 2017;28(1):348–358. doi: 10.1681/ASN.2016040449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peponis V, Kyttaris V, Chalkiadakis S, Bonovas S, Sitaras N. Ocular side effects of anti-rheumatic medications: what a rheumatologist should know. Lupus. 2010;19(6):675–682. doi: 10.1177/0961203309360539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perna A, Ruggiero B, Podestà M, et al. Sexual dimorphic response to rituximab treatment: a longitudinal observational study in a large cohort of patients with primary membranous nephropathy and persistent nephrotic syndrome. Front Pharmacol. 2022;13:958136. doi: 10.3389/fphar.2022.958136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim D, Lee A, Jung Y, et al. Tamoxifen ameliorates renal tubulointerstitial fibrosis by modulation of estrogen receptor α-mediated transforming growth factor-β1/Smad signaling pathway. Nephrol Dial Transplant. 2014;29(11):2043–2053. doi: 10.1093/ndt/gfu240 [DOI] [PubMed] [Google Scholar]
- 20.Kim S, Lee K, Kim J, Choi H. Estrogenic compound attenuates angiotensin II-induced vascular smooth muscle cell proliferation through interaction between LKB1 and estrogen receptor α. J Pharmacol Sci. 2016;132(1):78–85. doi: 10.1016/j.jphs.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Ding X, Li H, Zhang Y, Bao J. Role of G protein-coupled estrogen receptor 1 in modulating transforming growth factor-β stimulated mesangial cell extracellular matrix synthesis and migration. Mol Cell Endocrinol. 2014;391(1–2):50–59. doi: 10.1016/j.mce.2014.04.014 [DOI] [PubMed] [Google Scholar]
- 22.Doublier S, Lupia E, Catanuto P, et al. Testosterone and 17β-estradiol have opposite effects on podocyte apoptosis that precedes glomerulosclerosis in female estrogen receptor knockout mice. Kidney Int. 2011;79(4):404–413. doi: 10.1038/ki.2010.398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matrai M, Hetthéssy J, Nadasy G, et al. Estrogen therapy may counterbalance eutrophic remodeling of coronary arteries and increase bradykinin relaxation in a rat model of menopausal hypertension. Menopause. 2016;23(7):778–783. doi: 10.1097/GME.0000000000000654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teisseyre M, Cremoni M, Boyer-Suavet S, et al. Advances in the management of primary membranous nephropathy and rituximab-refractory membranous nephropathy. Front Immunol. 2022;13:859419. doi: 10.3389/fimmu.2022.859419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seitz-Polski B, Dahan K, Debiec H, et al. High-dose rituximab and early remission in PLA2R1-related membranous nephropathy. Clin J Am Soc Nephrol. 2019;14(8):1173–1182. doi: 10.2215/CJN.11791018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyer-Suavet S, Andreani M, Cremoni M, et al. Rituximab bioavailability in primary membranous nephropathy. Nephrol Dial Transplant. 2019;34(8):1423–1425. doi: 10.1093/ndt/gfz041 [DOI] [PubMed] [Google Scholar]
- 27.Jacobs R, Langer-Jacobus T, Duong M, et al. Detection and quantification of rituximab in the human urine. J Immunol Methods. 2017;451:118–121. doi: 10.1016/j.jim.2017.09.001 [DOI] [PubMed] [Google Scholar]