Abstract
Mitochondrial genomes are expected to show adaptations for optimizing aerobic respiration in birds that make intense use of flight. However, there is limited empirical evidence of such a relationship. We here examine correlates of several mitochondrial genome characteristics and flight use across a diverse sample of 597 bird species. We developed an index of flight use intensity that ranged from 0 in flightless species to 9 in migratory hummingbirds and examined its association with nucleobase composition, amino acid class composition, and amino acid site allelic variation using phylogenetic comparative methods. We found no evidence of mitochondrial genome adaptations to flight intensity. Neither nucleotide composition nor amino acid properties showed consistent patterns related to flight use. While specific sites in mitochondrial genes exhibited variation associated with flight intensity, there was limited association between specific amino acid residues and flight intensity levels. Our findings suggest a complex genetic architecture for aerobic performance traits, where multiple genes in both mitochondria and the nucleus may contribute to overall performance. Other factors, such as gene expression regulation and anatomical adaptations, may play a more significant role in influencing flight performance than changes in mitochondrial genes. These findings highlight the need for comprehensive genomic analyses to unravel the intricate relationship between genetic variants and aerobic performance in birds.
Keywords: mtDNA, aerobic metabolism, avian flight, comparative analysis
Significance.
Despite the prominent role of mitochondria in aerobic metabolism, there is limited evidence of relationships between mitochondrial genetic variation and locomotor performance in animals. We analyzed the mitochondrial genomes of 597 bird species and found no association between several genomic characteristics and flight use intensity. Our findings suggest that the relationship between mitochondrial genomes and flight performance in birds may be highly complex.
Introduction
Flight is one of the most metabolically intense forms of animal locomotion, and birds display a suit of anatomical and physiological adaptations that make flight possible (Pennycuick 2008; Butler 2016). Being the organelles where aerobic respiration takes place, mitochondria should be a hotspot of adaptations for flight, and many of those adaptations could be encoded in its genome (Ballard and Melvin 2010; Shen et al. 2009, 2010). Those adaptations can be reflected in the mitochondrial genome in multiple ways. First, adaptations for high metabolic capacity can be coded in the DNA in regulatory or protein-coding sequences that change protein expression or function. Second, high metabolic intensity may affect the genome due to physicochemical effects on the DNA. Because the mitochondrial genome is immediately enclosed in the inner mitochondrial membrane, where the electron transport chain redox reactions take place, it is exposed to direct physicochemical side effects of cellular respiration. For example, high metabolic rates may produce oxidative stress or thermal stress that may exert selective pressures on the DNA molecules to prevent mutations and maintain stability. For example, it has been suggested that high guanine–cytosine (GC) content may provide higher thermal stability for the mitochondrial DNA (Musto et al. 2004; Yakovchuk et al. 2006), which may be relevant in light of the “hot mitochondria hypothesis” in which mitochondria may sustain higher temperatures than the surrounding cytoplasm due to the intensity of the physicochemical reactions taking place there (Chretien et al. 2018; Romshin et al. 2022). Alternatively, high metabolism may create mutation biases that affect the nucleobase composition of DNA sequence. For example, high adenine–thymine content in flying animals has been explained by a mutational bias due to high ATP production in the mitochondrial matrix (Pettigrew 1994).
Despite these direct links between aerobic activity and mitochondrial genomes, there are few studies showing empirical evidence of those links in nonhuman vertebrates (Garvin et al. 2015). Several studies focused on the detection of differences in rates of synonymous and nonsynonymous substitutions that can be attributed to purifying or positive selection consistent with a link between genetic variation and aerobic performance (Garvin et al. 2015). For example, differences in evolutionary rates have been associated with the degree of the use of flight in birds (Shen et al. 2009; Montoya et al. 2022), and bats showed signs of positive selection in their recent common ancestors, suggesting implications for the evolution of flight in this group (Shen et al. 2010). On the other hand, the analysis of amino acid convergence due to aerobically demanding behaviors in birds showed that convergence at the amino acid site may be rare if present at all (Burskaia et al. 2021). Even fewer studies have established a plausible mechanistic link between specific mitochondrial genetic variants and flight performance. For example, an amino acid substitution in the COX3 gene may be involved in adaptation for high altitude migration in the Bar-Headed Goose (Anser indicus, Scott 2010), and mitochondrial haplotypes with different aerobic performance are associated with different migratory routes in the Yellow-rumped Warbler (Setophaga coronata, Toews et al. 2014).
We here explored the relationship between the intensive use of flight and characteristics of the mitochondrial genome across a diverse sample of modern birds. To quantify flight use intensity, we developed an index based on flight mode, foraging flight, and seasonal movements (Table 1). We then correlated this index with several genomic characteristics that in theory could be affected by the intense metabolism required for flight.
Table 1.
Description of the Behavioral Characteristics Used for Generating an Index of Flight Use Intensity
| Category | Score | Description |
|---|---|---|
| Flight mode | 0 | Flightless. |
| 1 | Flight capable but limited to short bursts, mainly for takeoff. | |
| 2 | Both flapping and gliding/soaring used frequently. | |
| 3 | Continuous flapping during flight. | |
| 4 | Continuous flapping and regular hovering (hummingbirds). | |
| Foraging flight | 0 | No flights during foraging. |
| 1 | Short flights performed regularly during foraging (flycatching or flying from branch to branch). Short flights regularly needed for accessing new foraging substrates, moving from tree to tree, or from roosting or nesting sites to foraging sites. | |
| 2 | Long-duration flights performed regularly during foraging, typically for commuting or for searching for food or foraging substrates (gulls, soaring birds). | |
| 3 | Foraging performed entirely on the wings, including search, capture, and ingestion (hummingbirds, swifts, swallows, frigatebirds). | |
| Seasonal flight | 0 | Year-round sedentary. |
| 1 | Seasonal short-distance movements (e.g., altitudinal migration), nomadism, or a mix of sedentary and migratory populations. | |
| 2 | Long-distance migratory movements. |
Results
We assembled an alignment of mitochondrial coding sequences (10,947 bases in length) for 597 bird species, including all protein-coding sequences except for the ND6 gene (supplementary Table S1, Supplementary Material online). The index of flight intensity varied from 0 to 9 among the species in the alignment (Table 1, supplementary Table S1, Supplementary Material online). Species scoring 0 were flightless birds including ratites (Palaeognathae), flightless rails (Gruiformes), flightless pigeons (e.g., the Dodo, Columbiformes), penguins (Sphenisciformes), the Great Auk (Charadriiformes), the Kagu (Eurypygiformes), and the Kakapo (Psitaciformes), representing the species with the lowest aerobic demands. Terrestrial species that mostly use flight only to escape predators, such as most Galliformes, received a score of 1. Similar species that can sustain flight for longer for local displacements or seasonal movements received scores between 1 and 3. Most passerine birds, pigeons, raptors, and some aquatic birds such as rails occupied the middle range (4–5). Most aquatic and oceanic birds and mobile landbirds birds such as migratory passerines and open-land raptors scored 6. Highly active aerial specialists that spend most of their daily life on the wings, including swifts, terns, and hummingbirds occupied the upper range, with scores from 7 to 9.
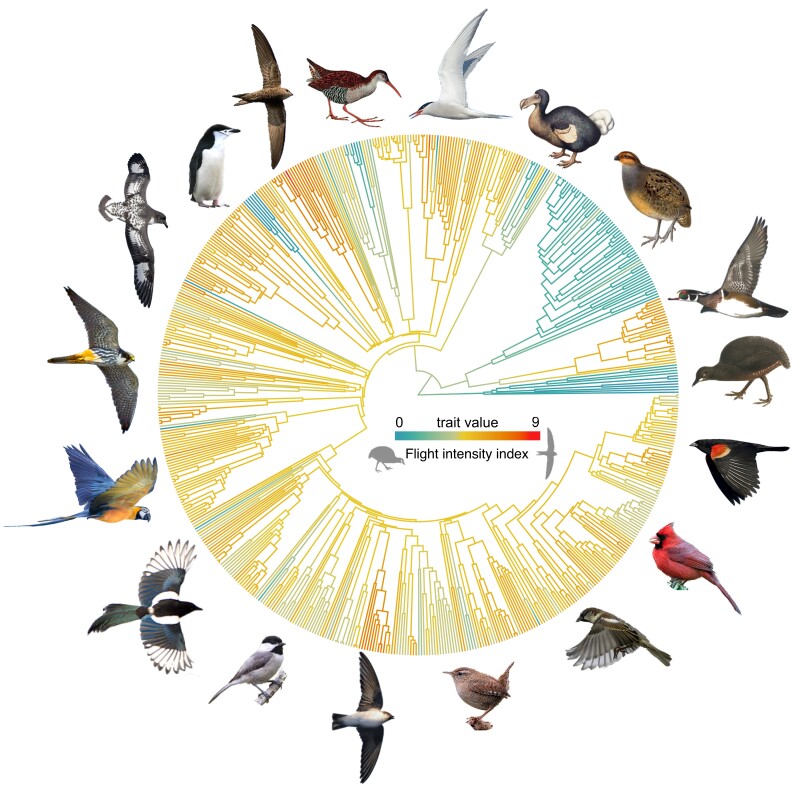
Mapping of the flight intensity index on a phylogenetic tree derived from the mtDNA alignment shows the repeated evolution of clades with high and low flight intensity across the radiation of modern birds (fig. 1), providing the necessary variation for comparative analyses, but also showing some degree of conservatism within clades. Overall, the index showed high phylogenetic inertia (Pagel's lambda = 0.94).
Fig. 1.
Phylogenetic time-tree of 597 species of birds based on their mitochondrial genomes, with branch colors representing an index of flight use intensity that varies from 0 (flightless sedentary birds) to 9 (migratory aerial specialists). Illustrations show representative species spanning most of the spectrum of flight use intensity (not at scale, from extreme right counterclockwise): Crypturellus berlepschi, Aix sponsa, Rhynchortyx cinctus, Raphus cucullatus, Sterna paradisaea, Rallus caerulescens, Apus pallidus, Pygoscelis antarcticus, Daption capense, Falco subbuteo, Ara ararauna, Pica pica, Poecile carolinensis, Ptyonoprogne rupestris, Troglodytes troglodytes, Passer domesticus, Cardinalis cardinalis, and Agelaius phoneiceus (see supplementary Table S2, Supplementary Material online for image attribution and license).
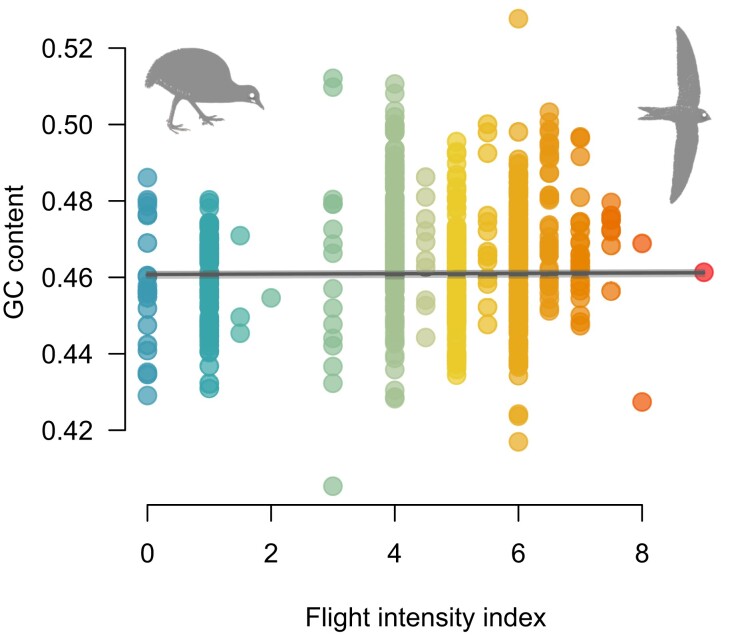
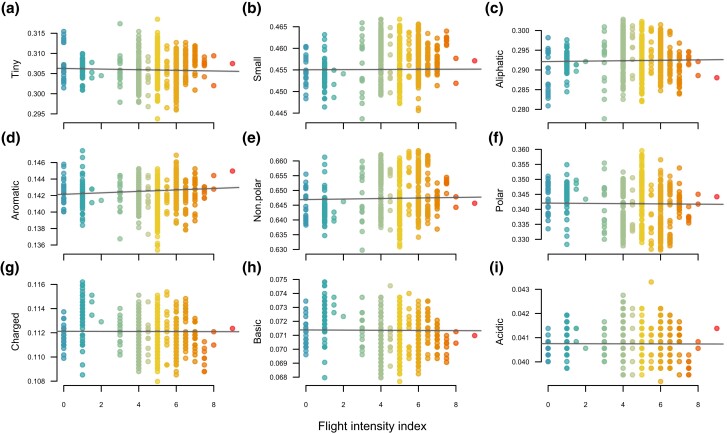
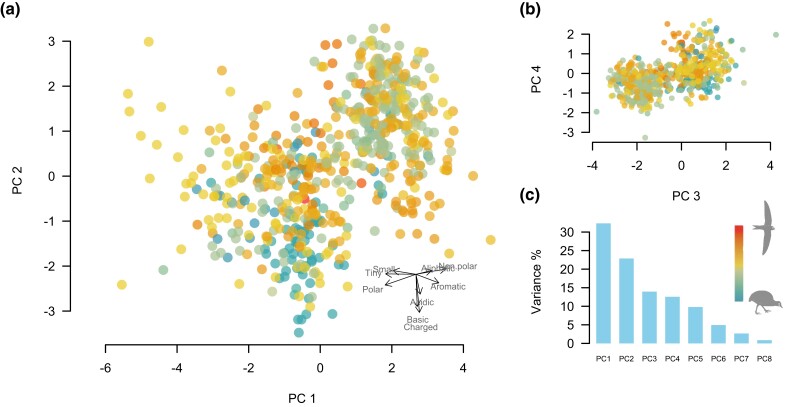
We examined variation in GC content across species and found no significant association between the index of flight intensity and GC content (fig. 2). We also translated the protein-coding sequences to amino acids, classified each amino acid into physicochemical classes following Bjellqvist et al. (1993), and calculated the proportions of each physicochemical category in each species. We examined the correlations between physicochemical category proportions and the flight intensity index using PGLS but found no significant association (fig. 3). We also looked for patterns in the multivariate space of amino acid physicochemical composition by using a Principal Component Analysis (PCA) to visualize trends. The first four principal components cumulative explained 82% of the total variance in amino acid category composition across species. However, there is a complete overlap of bird species with low, medium, and high flight intensity in the multivariate space defined by these four components (fig. 4).
Fig. 2.
Relationship between the flight intensity index and guanine–cytosine (GC) content of the mitochondrial genomes of 597 species of birds. Each dot represents a species and the values of the flight intensity index vary from 0 (flightless sedentary birds) to 9 (migratory hummingbirds). Regression line from a phylogenetic least squares model (F = 0.04, P = 0.85, R2 < 0.01), including 50 replicates across bootstrap (nearly identical fit).
Fig. 3.
Relationship between an index of flight use intensity and the proportions of amino acid physicochemical classes across mitochondrial protein-coding genes in 597 species of birds. Each dot represents a species, and the values of the flight intensity index vary from 0 (flightless sedentary birds) to 9 (migratory hummingbirds). Regression line from phylogenetic least squares models (not significantly different from zero in all cases), including 50 replicates across bootstrap (nearly identical fit).
Fig. 4.
Principal component analysis of variation in amino acid physicochemical class composition of mitochondrial protein-coding genes across 597 species of birds. (a) Principal components 1 and 2 and inset showing variable loading (physicochemical classes). (b) Principal components 3 and 2. (c) Proportion of variance explained by each component (“scree plot”), and reference color bar representing the values of the index of flight intensity from blue (0, flightless sedentary birds) to red (9, migratory hummingbirds).
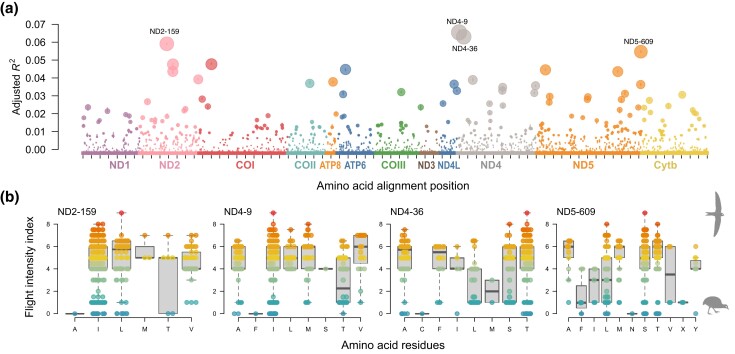
Finally, we evaluated associations of particular amino acid sites with high or low values of the flight intensity index fitting an ANOVA-style PGLS model to each amino acid site in the alignment (alleles as discrete predictors of the index of flight use intensity). The two sites with the greatest effect were in the ND4 gene and explained about 6.5% of the variation in flight intensity (positions 9 and 36, fig. 5a). Two other sites, one in the ND2 gene (position 159) and the other in the ND5 gene (position 609), had also similar levels of explanatory power (adjusted R2 > 5%). However, the distribution of amino acid alleles at these sites does not suggest any clear predictive power of particular amino acids regarding flight use intensity (fig. 5b). For example, for the ND4–9 site, other than the flightless Porphyrio hochstetteri having an Alanine in that position, amino acids are distributed across a wide range of flight intensity scores (fig. 5b). A similar lack of pattern is shown by the other three sites (fig. 5b).
Fig. 5.
Phylogenetic factorial models in which each amino acid site is used to predict flight use intensity in 597 species of birds. (a) Adjusted coefficient of determination (adjusted R2) of the 3,649 amino acid site models (dot size is proportional to adjusted R2, vertical bar within bubble span max–min estimates from a sample of 50 bootstrap trees). (b) Profile of the four sites that showed adjusted R2 greater than 0.05, showing the distribution of the flight intensity index across the alternative amino acids at the site; the index of flight intensity varies from 0 in flightless sedentary birds, to 9 in migratory hummingbirds.
Discussion
We explored the effects of the intensive use of flight on the mitochondrial genomes of 597 species of birds. Because the mitochondrial genomes encode important enzymes of the oxidative phosphorylation reactions, it should be a hotspot for adaptations for cellular respiration that can affect the aerobic metabolism of birds (Ballard and Melvin 2010). Moreover, because the mitochondrial genome is adjacent to where the oxidative phosphorylation reactions take place, it may also endure selective pressures for functioning in the challenging physicochemical environment that surrounds it. However, we did not find any signal of adaptation of the mitochondrial genomes to these two types of selective pressures. Overall, our findings suggest that the relationship between the mitochondrial genome and flight intensity in birds is complex and the differences in the use of flight across species do not have a strong impact on the mitochondrial genome composition and sequence.
We did not find any pattern in the DNA base composition across a wide range of flight use intensities. The GC content was not higher in species that show intensive and frequent flies, suggesting that the mitochondrial genome of birds is not much influenced by requirements for thermal stability (see also Hurst and Merchant 2001). This may be explained by the relative uniform body temperature across birds that increases little during high aerobic activity (Prinzinger et al. 1991), likely due to efficient heat dissipation mechanisms. Also, the heat produced by the oxidative phosphorylation reactions diffuses very rapidly through the cell, minimizing temperature gradients between the mitochondrion and its surroundings (Fahimi and Matta 2021). Conversely, adenine–thymine content was not higher for these species either, giving no support for the mutational bias hypothesis explaining nucleotide compositional biases in volant animals (Pettigrew 1994).
We also did not observe any discernible pattern in the predicted amino acid properties across a wide range of flight use intensities. For example, a high proportion of polar amino acids have been associated with increased mobility for the enzymes that are embedded in the inner membrane, which may provide stability and enhanced function under high metabolic rates (Kitazoe and Tanaka 2014). However, our findings reveal no consistent trend in the proportion of polar amino acids among birds, with both flightless and highly aerial species exhibiting completely overlapping profiles in terms of their amino acid properties (figs. 3 and 4).
Examining specific sites using phylogenetic linear models, we detected a number of sites associated with variation in flight intensity use. In particular, it is interesting that the sites with the greatest estimated effects are in NADH dehydrogenase genes, and in particular ND2, ND4, and ND5, three genes that have been implied in adaptations for high aerobic performance in animals (da Fonseca et al. 2008; Shen et al. 2010; Gronek et al. 2013; Stager et al. 2014; Garvin et al. 2015). However, the distribution of amino acid alleles at these sites shows that there is a limited association of particular amino acid residues with flight intensity; most residues are present both in sedentary species and in highly volant species (fig. 5).
Most of the evidence of an association between mitochondrial genomes and aerobic activity comes from the study of changes in substitution rates. For example, there is some evidence of a relationship between locomotion speed and substitution rates in birds and mammals in which slower species experience more nonsynonymous substitutions, attributed to a relaxation of selective pressures on the mitochondrial machinery (Shen et al. 2009). ND2, ND5, and Cytb genes in Tachycineta swallows (Hirundinidae) experienced positive selection that may be attributed to a faster pace of life (Stager et al. 2014), and small ovenbirds (Furnariidae) showed a positive relationship between substitution rates and flight use proxies (Montoya et al. 2022). However, there are cases where positive selection has been observed in species exhibiting low aerobic performance (Zhang and Broughton 2015). Finally, the relationship between substitution rates and aerobic activity may be modified by factors including environmental mutagens such as temperature and UV radiation controlling mutation rates, as well as population sizes, which can affect substitution rates (Montoya et al. 2022).
Overall, our results align with those of Burskaia et al. (2021), who did not find general patterns of convergence between amino acid changes associated with activities demanding high aerobic performance in birds. We posit that these results can be reconciled with previous studies detecting selection in mitochondrial genes if we consider the complex genetic architecture of aerobic performance traits—the phenotype–genotype map (Alberch 1991; Pigliucci 2010). Genetic changes that affect aerobic metabolism are likely distributed across multiple genes in both mitochondria and the nucleus and a similar level of aerobic performance may be attained by different combinations of alleles across sites. Individual changes in such a system may have small and nonlinear effects on aerobic performance, and those changes may interact with variants in multiple genes and potentially coevolve. This complex phenotype–genotype map may result in the lack of general macroevolutionary patterns in the encoding of genetic variants associated with aerobic performance in diverse groups such as birds.
Finally, other elements of the avian anatomy and metabolism may exert a greater influence on performance than changes in mitochondrial genes. For example, increased aerobic requirements for locomotor performance can be attained by modifying levels of gene expression, changing the number and distribution of mitochondria in muscle cells, and optimizing oxygen intake and transport at the tissue, organ, and organismal levels (Storz et al. 2010; Scott 2011; Butler 2016; Condell et al. 2022). In addition, other metabolic pathways such as the transport of fatty acids across cells and membranes may be the rate-limiting factor for sustained flight muscle activity in birds (Butler 2016). These alternative ways of increasing aerobic performance may diminish and even mute selective pressures on the mitochondrial genome. Mapping these other changes to the genotype would require the analysis of entire genomes with methods that can take into account high levels of causal complexity in the phenotype–genotype map.
Methods
Mitochondrial Genomes and Phylogeny
We downloaded from GenBank 597 raw bird mitochondrial genome sequences that were available as of July 2017 (supplementary Table S1, Supplementary Material online). The rRNAs, tRNAs, control region, and protein-coding genes within each mitochondrial genome were identified and extracted, and the program MAFFT v7 (Katoh and Standley 2013) was used to align the sequences within each gene using the program's default settings. We kept all protein-coding genes except for ND6 for downstream analyses (ATPase6, ATPase8, COI, COII, COIII, Cytb, ND1, ND2, ND3, ND4, ND4L, and ND5). ND6 was excluded as it is encoded on the opposite strand and has been partially duplicated in some bird genomes. The final aligned dataset is available as an annotated nexus file as Supplementary material.
With this alignment, we built a reference tree as follows. We first inferred a maximum likelihood tree using IQ-tree 2.2.2.6 for Mac OS (Minh et al. 2020) as follows. We conducted maximum likelihood optimizations using the automatic model selection option on partitioned data sets (Chernomor et al. 2016; Kalyaanamoorthy et al. 2017), restricting the models to the general time reversible family of models of nucleotide substitution combined with the gamma mixture model of rate heterogeneity (Posada and Crandall 2001). We evaluated two alternative partitioning schemes, by codon position and by gene, and compared them using the Akaike Information Criterion (AIC, Akaike 1974). Using the best partitioning scheme, we obtained a maximum likelihood tree and a bootstrapped sample of 1,000 trees using the ultrafast bootstrap algorithm (Hoang et al. 2018). We used topological constraints to enforce the monophyly of calibration clades (see below) and to ensure that basal relationships within Neoaves agree with current phylogenomic evidence (Braun et al. 2019; Kimball et al. 2019; Kuhl et al. 2021, constraint tree provides as supporting material). We then used the function midpoint (R package phangorn, Schliep 2011) to root the trees using the midpoint criterion, resulting in the accepted root of Neornithes at the Palaeognathae–Neognathae split.
Finally, we time-calibrated the trees using maximum likelihood methods and fossil-derived calibration information. We obtained calibration information for 18 clades from Claramunt and Cracraft (2015) (supplementary Table S3, Supplementary Material online), who used mathematical models of the bounds of truncated distributions to derive empirical calibration density functions from the fossil record (Marshall 2019; Claramunt 2022). We specified the minimum possible age of the clade as indicated by the minimum possible age of the oldest fossil in the clade. We obtained an upper bound for clade age from the estimated 95% quantile of the log-normal or exponential distributions specified by Claramunt and Cracraft (2015). We used these calibration bounds (supplementary Table S3, Supplementary Material online) in maximum likelihood divergence time estimation using a relaxed molecular clock method in the function chronos (R package ape, Paradis 2013; Paradis and Schliep 2019). We assumed a relaxed clock consisting of a mixture model of 10 discrete rate categories (Paradis 2013).
Index of Flight Intensity
To characterize the intensity of flying activities across the 597 species, we generated an index of flight use intensity by assigning points for the presence of behaviors that require high flight demands: flight mode, use of flight on a daily basis for foraging and commuting, and use of flight seasonally for long-distance movements (e.g., migration, Table 1). Behavioral information for each species was obtained from the Handbook of the Birds of the World and Birds of North America (del Hoyo et al. 1992–2013; Billerman et al. 2022). Species with ambiguous scores or fitting multiple categories were assigned averages of the corresponding category scores. The final score for each species was the sum of the individual scores for each category.
We mapped the index along the branches of the phylogenetic tree using maximum likelihood ancestral trait estimation and interpolation implemented in the function contMap (R package phytools, Revell 2012, 2013). Phylogenetic signal in the index was estimated with the function phylosig in the same package, using the lambda method (Pagel 1999).
Nucleotide Base Composition
We examined nucleobase composition biases across the mitochondrial genome of bird species and evaluated the association between the flight intensity index and GC content using phylogenetic generalized least squares regression (PGLS, Freckleton et al. 2002). The calibrated maximum likelihood tree was used to define a phylogenetic correlation structure using the lambda method for controlling levels of phylogenetic nonindependence (Freckleton et al. 2002). We fit models using the package phylolm, which implements a fast algorithm for fitting phylogenetic Gaussian models (Ho and Ané 2014). To evaluate the effect of phylogenetic uncertainty, we repeated the analysis using a sample 50 bootstrap trees.
Amino Acid Composition
We translated the alignment of coding sequences into the corresponding amino acids, classified each amino acid into physicochemical classes following Bjellqvist et al. (1993) and calculated the frequency of each category for each species using function AAstat in the seqinr 4.2-30 R package (Charif and Lobry 2007). Amino acids were classified into the following categories: tiny, small, aliphatic, aromatic, nonpolar, polar, charged, basic, and acidic. We then evaluated the correlation between the proportion of amino acid categories and flight use intensity using PGLS as before. Finally, we explored multivariate patterns in amino acid properties across species using a phylogenetic principal components analysis (Revell 2009) based on the correlation matrix using function phyl.pca in phytools 1.9 (Revell 2012).
Association Between Amino Acid Residues and Flight Intensity
To evaluate possible associations between particular amino acid residues and the index of flight use intensity, we used phylogenetic generalized least squares models in which we fit a model for each amino acid position in the alignment with alternative amino acids observed at each site considered levels of a factor predicting the value of the flight intensity index. We fit PGLS models with a lambda correlation structure (Freckleton et al. 2002) in function phylolm as before (Ho and Ané 2014), using the main calibrated tree and a sample of 50 bootstrap trees. For each site model, we collected fit statistics including the coefficient of determination R2, as an indicator of absolute model fit, and adjusted R2 and the AIC (Akaike 1974) as a way to compare models with different numbers of alleles.
Supplementary Material
Tables S1 to S3 are available at Genome Biology and Evolution online (http://www.gbe.oxfordjournals.org/).
Supplementary Material
Acknowledgments
We acknowledge support from the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant RGPIN-2018-06747 to S.C.
Contributor Information
Santiago Claramunt, Department of Ecology and Evolutionary Biology, University of Toronto, Toronto, Ontario, Canada; Department of Natural History, Royal Ontario Museum, Toronto, Ontario, Canada.
Oliver Haddrath, Department of Natural History, Royal Ontario Museum, Toronto, Ontario, Canada.
Data Availability
The mitochondrial genomes used in this study are publicly available in GenBank (see supplementary Table S1, Supplementary Material online for accession numbers). DNA sequence alignments, flight use data, and phylogenetic trees are provided as Supplementary material.
Literature Cited
- Akaike H. 1974. A new look at the statistical model identification. IEEE Trans Automa Control. 19:716–723. [Google Scholar]
- Alberch P. 1991. From genes to phenotype: dynamical systems and evolvability. Genetica 84:5–11. [DOI] [PubMed] [Google Scholar]
- Ballard JWO, Melvin RG. 2010. Linking the mitochondrial genotype to the organismal phenotype. Mol Ecol. 19:1523–1539. [DOI] [PubMed] [Google Scholar]
- Billerman SM, Keeney BK, Rodewald PG, Schulenberg TS. 2022. Birds of the world. Ithaca: Cornell Laboratory of Ornithology. [Google Scholar]
- Bjellqvist B, et al. 1993. The focusing positions of polypeptides in immobilized pH gradients can be predicted from their amino acid sequences. Electrophoresis 14:1023–1031. [DOI] [PubMed] [Google Scholar]
- Braun EL, Cracraft J, Houde P. 2019. Resolving the avian tree of life from top to bottom: the promise and potential boundaries of the phylogenomic era. In: Kraus RHS, editor. Avian genomics in ecology and evolution: from the lab into the wild. Cham, Switzerland: Springer. p. 151–210. [Google Scholar]
- Burskaia V, Artyushin I, Potapova NA, Konovalov K, Bazykin GA. 2021. Convergent adaptation in mitochondria of phylogenetically distant birds: does it exist? Genome Biol Evol. 13:evab113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PJ. 2016. The physiological basis of bird flight. Philos Trans R Soc Lond B Biol Sci. 371:20150384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charif D, Lobry J, Bastolla U, Porto M, Roman H. 2007. Seqinr 1.0-2: a contributed package to the R project for statistical computing devoted to biological sequences retrieval and analysis. In: Bastolla U, Porto M, Roman H, Vendruscolo M, editors. Structural approaches to sequence evolution: molecules, networks, populations. New York: Springer. p. 207–232. [Google Scholar]
- Chernomor O, von Haeseler A, Minh BQ. 2016. Terrace aware data structure for phylogenomic inference from supermatrices. Syst Biol. 65:997–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chretien D, et al. 2018. Mitochondria are physiologically maintained at close to 50 °C. PLoS Bol. 16:e2003992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claramunt S. 2022. Cladedate: calibration information generator for divergence time estimation. Methods Ecol Evol. 13(11):2331–2338. [Google Scholar]
- Claramunt S, Cracraft JL. 2015. A new time tree reveals Earth history's Imprint on the evolution of modern birds. Sci Adv. 1:e1501005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condell K, Robinson WD, Moore RP, Rourke B. 2022. Myoglobin as a conservation-relevant predictor of short-distance flight capacity in neotropical forest birds. Biotropica 54:327–333. [Google Scholar]
- da Fonseca RR, Johnson WE, O'Brien SJ, Ramos MJ, Antunes A. 2008. The adaptive evolution of the mammalian mitochondrial genome. BMC Genomics. 9:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Hoyo J, Elliott A, Sargatal J, Christie D. 1992–2013. Handbook of the birds of the world. Vols. 1 to 16. Barcelona: Lynx Edicions. [Google Scholar]
- Fahimi P, Matta CF. 2021. The hot mitochondrion paradox: reconciling theory and experiment. Trends Chem. 2:96–110. [Google Scholar]
- Freckleton RP, Harvey PH, Pagel M. 2002. Phylogenetic analysis and comparative data: a latest and review of evidence. Am Nat. 160:712–726. [DOI] [PubMed] [Google Scholar]
- Garvin MR, Bielawski JP, Sazanov LA, Gharrett AJ. 2015. Review and meta-analysis of natural selection in mitochondrial complex I in metazoans. J Zool Syst Evol Res. 53:1–17. [Google Scholar]
- Gronek P, Holdys J, Kryściak J, Stanisławski D. 2013. Maximal oxygen uptake is associated with the snp 13470 G>C polymorphism of the mitochondrial NADH dehydrogenase subunit 5 gene (mtND5) in Caucasians from Poland. Trends Sport Sci. 4:189–196. [Google Scholar]
- Ho LST, Ané C. 2014. A linear-time algorithm for Gaussian and non-Gaussian trait evolution models. Syst Biol. 63(3):397–408. [DOI] [PubMed] [Google Scholar]
- Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS. 2018. UFBoot2: improving the ultrafast bootstrap approximation. Mol Biol Evol. 35:518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst LD, Merchant AR. 2001. High guanine–cytosine content is not an adaptation to high temperature: a comparative analysis amongst prokaryotes. Proc Biol Sci. 268:493–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS. 2017. Modelfinder: fast model selection for accurate phylogenetic estimates. Nat Methods. 14:587–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30:772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball RT, et al. 2019. A phylogenomic supertree of birds. Diversity (Basel). 11(7):109. [Google Scholar]
- Kitazoe Y, Tanaka M. 2014. Evolution of mitochondrial power in vertebrate metazoans. PLoS One. 9:e98188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl H, et al. 2021. An unbiased molecular approach using 3′-UTRs resolves the avian family-level tree of life. Mol Biol Evol. 38(1):108–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CR. 2019. Using the fossil record to evaluate timetree timescales. Front Genet. 10:1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minh BQ, et al. 2020. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol Biol Evol. 37:1530–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya P, Cadena CD, Claramunt S, Duchêne DA. 2022. Environmental niche and flight intensity are associated with molecular evolutionary rates in a large avian radiation. BMC Ecol Evol. 22:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musto H, et al. 2004. Correlations between genomic GC levels and optimal growth temperatures in prokaryotes. FEBS Lett. 573:73–77. [DOI] [PubMed] [Google Scholar]
- Pagel M. 1999. Inferring the historical patterns of biological evolution. Nature 401:877–884. [DOI] [PubMed] [Google Scholar]
- Paradis E. 2013. Molecular dating of phylogenies by likelihood methods: a comparison of models and a new information criterion. Mol Phylogenet Evol. 67:436–444. [DOI] [PubMed] [Google Scholar]
- Paradis E, Schliep K. 2019. Ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35:526–528. [DOI] [PubMed] [Google Scholar]
- Pennycuick CJ. 2008. Modelling the flying bird. Burlington: Elsevier. [Google Scholar]
- Pettigrew JD. 1994. Flying DNA. Current Biol. 4:277–280. [DOI] [PubMed] [Google Scholar]
- Pigliucci M. 2010. Genotype–phenotype mapping and the end of the ‘genes as blueprint’ metaphor. Philos Trans R Soc Lond B Biol Sci. 365:557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada D, Crandall KA. 2001. Selecting the best-fit model of nucleotide substitution. Syst Biol. 50(4):580–601. [PubMed] [Google Scholar]
- Prinzinger R, Preßmar A, Schleucher E. 1991. Body temperature in birds. Comp Biochem Physiol A Physiol. 99:499–506. [Google Scholar]
- Revell LJ. 2009. Size-correction and principal components for interspecific comparative studies. Evolution 63:3258–3268. [DOI] [PubMed] [Google Scholar]
- Revell LJ. 2012. Phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol Evol. 3:217–223. [Google Scholar]
- Revell LJ. 2013. Two new graphical methods for mapping trait evolution on phylogenies. Methods Ecol Evol. 4:754–759. [Google Scholar]
- Romshin AM, et al. 2022. Heat release by isolated mouse brain mitochondria detected with diamond thermometer. Nanomaterials 13:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliep KP. 2011. Phangorn: phylogenetic analysis in R. Bioinformatics 27:592–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott GR. 2011. Elevated performance: the unique physiology of birds that fly at high altitudes. J Exp Biol. 214:2455–2462. [DOI] [PubMed] [Google Scholar]
- Shen YY, et al. 2010. Adaptive evolution of energy metabolism genes and the origin of flight in bats. Proc Natl Acad Sci USA. 107:8666–8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen YY, Shi P, Sun YB, Zhang YP. 2009. Relaxation of selective constraints on avian mitochondrial DNA following the degeneration of flight ability. Genome Res. 19:1760–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stager M, Cerasale DJ, Dor R, Winkler DW, Cheviron ZA. 2014. Signatures of natural selection in the mitochondrial genomes of Tachycineta swallows and their implications for latitudinal patterns of the ‘pace of life’. Gene 546:104–111. [DOI] [PubMed] [Google Scholar]
- Storz JF, Scott GR, Cheviron ZA. 2010. Phenotypic plasticity and genetic adaptation to high-altitude hypoxia in vertebrates. J Exp Biol. 213:4125–4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toews DPL, Mandic M, Richards JG, Irwin DE. 2014. Migration, mitochondria, and the yellow-rumped warbler. Evolution 68:241–255. [DOI] [PubMed] [Google Scholar]
- Yakovchuk P, Protozanova E, Frank-Kamenetskii MD. 2006. Base-stacking and base-pairing contributions into thermal stability of the DNA double helix. Nucleic Acids Res. 34:564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Broughton RE. 2015. Heterogeneous natural selection on oxidative phosphorylation genes among fishes with extreme high and low aerobic performance. BMC Evol Biol. 15:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The mitochondrial genomes used in this study are publicly available in GenBank (see supplementary Table S1, Supplementary Material online for accession numbers). DNA sequence alignments, flight use data, and phylogenetic trees are provided as Supplementary material.