Abstract
Introduction:
Variability and prolongation of ventricular repolarization – measured by changes in QT interval and QT variability are independently associated with ventricular arrhythmias, sudden death, and mortality but such studies did not examine the role of sleep-disordered breathing. We aimed to determine whether sleep-disordered breathing moderated the association between measures of ventricular repolarization and overall mortality.
Methods:
Eight hundred participants were randomly selected from each of the following four groups in the Sleep Heart Health Study: mild, moderate, severe or no sleep disordered breathing (n = 200 each). Overnight electrocardiograms were analyzed for QTc duration and QT variability (standard deviation of QT intervals, normalized QT interval variance and the short-term interval beat-to-beat QT variability). Cox proportional hazards penalized regression modeling was used to identify predictors of mortality.
Results:
Eight hundred of 5600 participants were randomly selected. The participants (68 ± 10 years; 56.8% male) were followed for an average of 8.2 years during which time 222 (28.4%) died. QTc, SDQT, and QTVN were associated with the presence of SDB (p = 0.002, p = 0.014, and p = 0.024, respectively). After adjusting for covariates, the presence of sleep-disordered breathing did not moderate the association between QTc length, QT variability and mortality (p > 0.05).
Conclusion:
Sleep-disordered breathing was associated with some measures of ventricular repolarization. However, sleep-disordered breathing was not an effect modifier for the relationship between QTc and QT variability and mortality.
Keywords: Ventricular repolarization, QT interval, QT variability, Sleep disordered breathing, Sleep apnea, Mortality
1. Introduction
Sleep Disordered Breathing (SDB) is a prevalent condition affecting approximately 15–30% of males and 10–15% of females [1,2]. It consists primarily of obstructive sleep apnea (OSA), a chronic medical condition that is widely recognized as an independent risk factor for several clinical consequences including cardiac disease, stroke, hypertension, type 2 diabetes, motor vehicle accidents, arrhythmias, and sudden death [3–6]. Patients with untreated moderate to severe SDB have a two-to three-fold increased risk of all-cause mortality compared with individuals without SDB, independent of other risk factors such as obesity and cardiovascular disease [7–11].
Delayed ventricular repolarization as measured by QTc intervals in electrocardiograms (ECG) is associated with cardiac arrhythmias and sudden cardiac death [12]. The QTc interval has been shown to be prolonged at the onset of apnea compared to the active awake state [13]. There is further prolongation of the QTc interval during the apneic phase and abrupt shortening of the QTc interval during the post-apnea hyperventilation period [13,14]. This marker of ventricular repolarization has been shown to be abnormally prolonged in patients with SDB independent of known risk factors for cardiac arrhythmias [14–16].
Variability in QT interval has also been shown to be greater in patients with SDB than those without SDB [17]. Previous studies have demonstrated that QT variability is independently associated with ventricular arrhythmias, sudden cardiac death, and total mortality, but such studies did not examine the role of SDB [18]. In patients with SDB, prolonged QTc intervals and increased QT variability may be associated with ventricular arrhythmias, sudden cardiac death and overall mortality. The goal of this study to determine whether sleep-disordered breathing moderated the association between measures of ventricular repolarization and mortality.
2. Methods
2.1. Participants/study design
The study was granted an exemption from the University of Arizona Institutional Review Board. All participants were from the Sleep Heart Health Study (SHHS), details of which have been previously reported [19]. In summary, the SHHS is a prospective multicenter study cohort funded by the National Heart Lung and Blood Institute focusing on consequences of sleep disordered breathing. The SHHS consists of 6441 men and women over the age of 40 years evaluated between 1995 and 1998 (SHHS 1) and then again between 2001 and 2003 (SHHS 2) [20].
Our study focused on participants in SHHS 1 who had undergone unattended polysomnography with the CompuMedics P Series System monitor (Abbotsford, Australia) [21]. Signals available for review included oxyhemoglobin saturation, electroencephalogram (C3/A1 and C4/A2), bipolar electrocardiogram, bipolar electromyogram, bilateral electrooculogram, abdominal and thoracic inductance plethysmography, body position, ambient light, and oronasal thermocouple [22]. Additional details regarding the sleep study set-up and data quality are published [21].
Data was obtained from the National Sleep Research Resource (NSRR) which has 5600 polysomnograms in the database, but does not include participants from the Strong Heart Study cohort of the SHHS, and thus has few American Indians [23]. Participants with an apnea/hypopnea index (AHI) less or equal to 5/hour were considered not to have SDB and those with an AHI greater or equal to 5 per hour were considered to have SDB. Severity of SDB was classified as mild (AHI 5–14.99/hour), moderate (AHI 15–29.99/hour), and severe (AHI ≥30/hour) based on AHI [24]. We randomly selected two hundred participants from each of the mild, moderate, severe and no SDB groups in order to prevent selecting a cohort with predominantly no SDB and mild SDB participants given that approximately 83% of the SHHS participants were found to have no SDB or mild SDB [25]. We also chose to analyze a random sample of participants instead of all the participants in the SHHS 1 database given the rigor required for detailed ECG analysis as manual adjudication was performed for the signals to ensure validity and accuracy of the data. Sleep disordered breathing events were identified as apneas or hypopneas, the latter requiring a minimum 3% oxygen desaturation from baseline. Demographic, mortality, and co-morbid health conditions information were also obtained from the NSRR.
2.2. Electrocardiogram signal analysis
All electrocardiographic signals were evaluated using the Comprehensive Analysis of Repolarization Signal (COMPAS) software developed at the University of Rochester Medical Center Rochester (NY). The software was applied to polysomnographic ECG signals for all available leads, providing the measurements of the RR and QT intervals measured from 10-beat median signals for the entire duration of each sleep study (mean total sleep duration/subject = 360 ± 65 min). Electrocardiograms were also manually adjudicated to ensure adequacy for automated analysis [26]. Furthermore, it was verified that all the participants were in a normal sinus rhythm. Bazett’s heart rate(QTc = QT/RR1/2) [27] correction was used to calculate QTc [27]. RR values used for QT interval correction for heart rate were the average heart rate across the 10 beats used to compute the median beat.
QT variability was measured based on guidelines established by the European Heart Rhythm Association and the European Society of Cardiology Working Group on Cardiac Cellular Electrophysiology [28]. The different QT variability measurements obtained included the standard deviation of QT intervals [28] (SDQT;), normalized QT interval variance [28] (QTVN; SDQT2 ) at = 5-min intervals and the short-term interval beat-to-beat QT variability [28] (STVQT; at 5-min intervals. In these equations N = number of beats evaluated, and n varies from 1 to N.
2.3. Statistical analysis
Univariate summary statistics for overall mortality as a binary (alive/dead) outcome include chi square statistics for categorical explanatory variables, and t-test statistics for continuous explanatory variables (Table 1). The primary analysis was a Cox proportional hazards model under the hypothesis of whether SDB moderates the relationship between mortality and QTc and QT variability, individually. This evaluation was performed for QTc and the QT variability through examination of the interaction between SDB and the QT variables. Selection for the optimal QT variability variable was chosen by a variable selection method, using regularized regression that optimally selects variables using a shrinkage estimate, between the QTVN, STVQT and SDQT as measures of QT variability (Table 3). Collinearity for all explanatory variables was evaluated using the variance inflation factor (VIF). The primary hypothesis has 0.80 power to detect a significant interaction between QTc and QT variability and SDB with a minimum of 128 deaths. The study was not powered to look at differences in QT variables based on SDB severity.
Table 1.
Demographic and QT variables stratified by mortality.
| Dead (n = 222) | Alive (n = 559) | P-value | |
|---|---|---|---|
| Age, years, mean (SD) | 74.9 (7.97) | 64.9 (9.19) | <0.001 |
| Age, 65 years and over, no. % | 202 (91.0%) | 299 (53.5%) | <0.001 |
| Men, no. % | 126 (56.8%) | 253 (45.3%) | <0.001 |
| Race, no. (%) | |||
| White | 202 (91.0%) | 512 (91.6%) | 0.976 |
| Black | 17 (7.7%) | 35 (6.3%) | |
| Other | 3 (1.4%) | 12 (2.1%) | |
| BMI, kg/m2, mean (SD) | 26.3 (4.34) | 27.4 (4.34) | <0.001 |
| Waist Circumference, cm, mean (SD) | 95.4 (12.6) | 95.0 (12.4) | <0.001 |
| Neck Circumference, mean (SD) | 37.6 (3.80) | 37.4 (3.87) | <0.001 |
| Smoking Status, no. (%) | |||
| Never | 94 (42.3%) | 282 (50.4%) | 0.256 |
| Current | 13 (5.9%) | 46 (8.2%) | |
| Former | 115 (51.8%) | 231 (41.3%) | |
| Hypertension, no. (%) | 135 (60.8%) | 216 (38.6%) | <0.001 |
| Diabetes, no. (%) | 30 (13.5%) | 28 (5.0%) | 0.594 |
| Cardiovascular Disease, no. (%) | 23 (10.4%) | 64 (11.4%) | 0.662 |
| Sleep Disordered Breathing, no. (%) | |||
| None | 45 (20.3%) | 120 (21.5%) | <0.001 |
| Mild | 49 (22.1%) | 194 (34.7%) | |
| Moderate | 61 (27.5%) | 136 (24.3%) | |
| Severe | 67 (30.2%) | 109 (19.5%) | |
| Heart Rate, mean (SD) | 65.6 (10.3) | 65.2 (8.82) | 0.191 |
| QTc interval, mean (SD)a | 422 (37.4) | 415 (28.3) | 0.002 |
| Standard deviation of the QT intervals (SDQT), mean (SD) | 11.9 (16.8) | 7.72 (8.89) | 0.014 |
| Normalized QT interval variance (QTVN), mean (SD) | 0.002 (0.006) | 0.001 (0.003) | 0.025 |
| The short-term interval beat-to-beat QT variability (STVQT), mean (SD) | 4.59 (4.95) | 3.33 (2.78) | 0.055 |
QTc = QT interval with Bazett’s heart rate correction.
Table 3.
Regularized regression (Cox proportional hazards) with coefficients after applying shrinkage parameter and estimates from Cox proportional hazards without shrinkage estimation.
| Regularized Regression (Cox Model w/Shrinkage) | Cox Proportional Hazards (No Shrinkage) | ||
|---|---|---|---|
| Coefficient | Coefficient | HR (95% Cl) | |
| Agea | 0.1183 | 0.14043 | 1.1321 (1.1094, 1.1552) |
| BMIa | −0.0720 | −0.0908 | 0.9132 (0.8614, 0.9681) |
| Waist Circumference (cm)a | 0.0158 | 0.0212 | 1.0214 (1.0030, 1.0401) |
| Neck Circumference (cm) | 0.023 | 0.0349 | 1.0355 (0.9749, 1.0999) |
| Gender (Female) | −0.2953 | −0.2483 | 0.7801 (0.5144, 1.1830) |
| Race (Black) | −0.0674 | −0.1135 | 0.8927 (0.5282, 1.5086) |
| Never Smoker (vs Current) | −0.1928 | −0.2435 | 0.8927 (0.5282, 1.5086 |
| Former Smoker (vs Current) | N/Ab | −0.0257 | 0.9747 (0.5362, 1.7716) |
| Hypertension (Yes)a | 0.3630 | 0.4131 | 1.5115 (1.1380, 2.0075) |
| Diabetes (Yes)a | 0.4576 | 0.4903 | 1.6328 (1.1027, 2.4175 |
| Cardiovascular Disease (Yes)a | 0.5838 | 0.6716 | 1.9573 (1.2363, 3.0990) |
| QTca | 0.0043 | 0.0049 | 1.0049 (1.0003, 1.0096) |
| Log(SDQT)a | 0.3391 | 0.4546 | 1.5755 (1.1851,2.0946) |
| Log(STVQT) | −0.1398 | −0.2936 | 0.7455 (0.5230, 1.0628) |
| Heart Rate | 0.0103 | 0.0114 | 1.0115 (0.9968, 1.0264) |
| SDB | −0.1943 | −0.2720 | 0.7619 (0.5408, 1.0732) |
SDB = Sleep disordered breathing, QTc = QT with Bazett’s heart rate correction, SDQT = standard deviation of the QT intervals at 5-min intervals.
Statistically significant using Cox proportional hazards models.
Estimate after shrinkage set to zero for linear predictor calculation.
Additional statistical analyses included univariate data analyses, multivariable modeling, and the development of a prognostic classifier for mortality that included variable selection, and assessment of model performance evaluation (AUC) to predict mortality. Creation of the prognostic classifier was based on Cox proportional hazards modeling generalized from methods advocated by Harrell [29], and Moons [30] and implemented using a regularized regression approach described by Tibshirani [31] and Zou and Hastie. [32] Regularized regression methods are used to both select important predictors as well as develop a weighted linear “score”. The score is adjusted by a shrinkage factor that ameliorates the over-estimation of predictive performance that can occur with prediction and small effective sample sizes.
Lasso methods are a special case of these model-based approaches although we used an algorithm called the ‘elastic net’ as it is more robust to collinearity. There is no need for multiple testing adjustments, as these models are not selected based on hypothesis testing (e.g., p-values) and the adjustment for over-performance is already incorporated into the model selection process.
Coefficients of explanatory (or predictor) variables were multiplied by the values of the variables (Table 3) at the participant level and the sum of this product was the “linear predictor” or linear score. This linear predictor was therefore a weighted sum, with the coefficients describing the direction of the association (those that are associated with higher or lower risk of mortality) as well as relative strength (e.g., largely positive or negative contribute more to the score than lower ones). This linear predictor served as an individual score that was then evaluated using the area under the receiver operating characteristic (ROC) curve (AUC); typically, AUCs greater than 0.70 have “moderate” predictive ability. Since the response was mortality, the ROC curves, and subsequent AUC values, were evaluated over time periods to visually note any time dependencies (i.e., difference in performance was dependent on the length of follow-up time to CVD event). We used censored models for time-dependent covariates using the Nearest Neighbor Estimate (NNE) method as described by Heagerty, et. A1 [33].
3. Results
Complete data was available for seven hundred and eighty-one SHHS 1 participants. Age, male gender, body mass index (BMI), waist circumference, neck circumference, hypertension, sleep disordered breathing, QTc, SDQT and QTVN were associated with higher mortality (Table 1). Male gender, BMI, waist circumference, neck circumference, hypertension, mortality, QTc, SDQT, and QTVN were associated with the presence of SDB (Table 2). The primary hypothesis of whether SDB was an effect modifier for the relationship between QTc and QT variability and mortality was rejected for both QTc and SDQT, as the interaction terms were not statistically significant (p = 0.99 and p = 0.86, respectively). Both QTc and SDQT were statistically significantly associated with mortality after adjusting for SDB (p = 0.0011 and p < 0.0001, respectively); and SDB was statistically associated with mortality after adjusting for QTc and SDQT (p = 0.0020 and p = 0.0001, respectively).
Table 2.
Demographics and QT variables Stratified by Presence of Sleep Disordered Breathing.
| No SDB (n = 165) | SDB(n = 616) | P-value | |
|---|---|---|---|
| Age, years, mean (SD) | 67.1 (9.93) | 67.8 (9.9) | 0.795 |
| Men, no. % | 47 (28.5) | 332 (53.9) | <0.001 |
| Race, no. (%) | |||
| White | 151 (91.5) | 563 (91.4) | 0.976 |
| Black | 11 (6.7) | 41 (6.7) | |
| Other | 3 (1.8) | 12 (1.9) | |
| BM1, kg/m2, mean (SD) | 26.2 (3.57) | 27.3 (4.5) | 0.013 |
| Waist Circumference, cm, mean (SD) | 91.0 (12.8) | 96.2 (12.1) | 0.014 |
| Neck Circumference, mean (SD) | 35.7 (3.39) | 37.9 (3.8) | <0.001 |
| Smoking Status, no. (%) | |||
| Never | 90 (54.5) | 286 (46.4) | 0.256 |
| Current | 10 (6.1) | 49 (8.0) | |
| Former | 65 (39.4) | 281 (45.6) | |
| Hypertension, no. (%) | 66 (40.0) | 285 (46.3) | <0.001 |
| Diabetes, no. (%) | 12 (7.3) | 46 (7.5) | 0.594 |
| Cardiovascular Disease, no. (%) | 20 (12.1) | 67 (10.9) | 0.662 |
| Dead, no. (%) | 45 (27.3) | 177 (28.7) | <0.001 |
| QTc intervala | 422 (29.3) | 415 (31.7) | 0.002 |
| Standard deviation of QT interval (SDQT), mean (SD) | 6.84 (9.31) | 9.45 (12.4) | 0.014 |
| Normalized QT interval variance (QTVN), mean (SD) | 0.0010 (0.004) | 0.0014 (0.005) | 0.024 |
| The short-term interval beat-to-beat QT variability (STVQT), mean (SD) | 3.11 (2.04) | 3.84 (3.9) | 0.055 |
QTc = QT interval with Bazett’s heart rate correction.
Table 3 shows the estimated coefficients, after application of the shrinkage parameter, for explanatory variables selected by the regularized regression to predict mortality. The coefficients under typical Cox proportional hazards modeling are also shown, along with the hazard ratio’s and 95% confidence intervals. It is important to note some variables that were predictive were not statistically significant as the selection criteria for regularized regression method is based on change in model fit and not p-values. Age, BMI, waist circumference, presence of hypertension, diabetes, cardiovascular disease, prolonged QTc and increased SDQT were all significant contributors to mortality after adjusting for the other variables. Since QTVN is a function of SDQT, only one out of the two measures were chosen for the linear score.
Fig. 1 used the selected predictors shown in Table 3 to create a score describing predictive capacity of mortality for those with SDB. While the linear predictor has slightly better predictive capacity in year 2, there is little difference over time. This is likely due to the small number of deaths (n = 26 respectively) during the first two years, with an increasing number over time (16,15, and 158 in years 3,4 and 5, respectively). The results show that the linear score that is formed using the variables from Table 3 is moderately able to predict mortality in participants with SDB, and while we don’t see obvious time-dependency in this relationship, it is strongest at year 2 (AUC = 0.83).
Fig. 1.
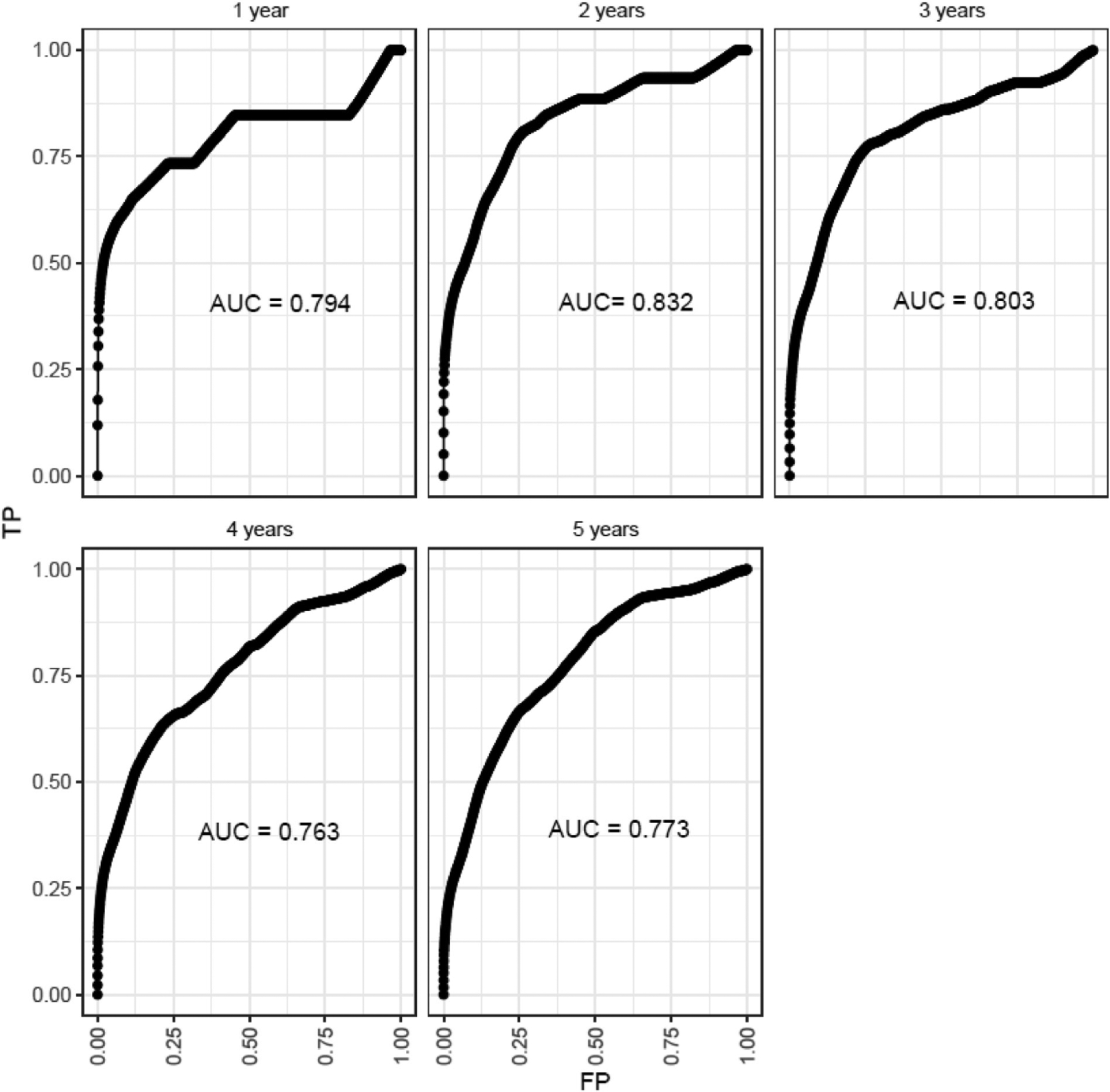
Auc estimates and ROC curves over Yearly time intervals from initial evaluation using nearest neighbor estimation.
Fig. 1. The predictive capacity of mortality for those with sleep disordered breathing utilizing the selected predictors in Table 3 is shown.
4. Discussion
Changes in QTc, SDQT, and QTVN were associated with the presence of SDB when compared to the absence of SDB. However, SDB was not found to be an effect modifier for the relationship between QTc, QT variability and overall mortality. Several variables including age, BMI, waist circumference, hypertension, diabetes, cardiovascular disease, QTc and SDQT were predictive of mortality in patients with OSA.
The association of demographic-related variables such as BMI, waist circumference and gender with mortality and the presence of SDB are consistent with existing literature [34–37] which shows a clear association between mortality and SDB with these variables. Similarly, the association of hypertension with mortality and SDB is consistent with the literature [38]. Contrary to existing literature [39,40], our study did not find an association between mortality and SDB among those with diabetes or cardiovascular disease likely due to the small number of participants with these conditions in our limited SHHS-1 cohort.
While we had hypothesized that QTc would be increased in participants with SDB when compared to participants without SDB, this was not the case. The QTc of participants without SDB group was 422(±29.3) mm while the QTC of participants with SDB was 415 (±31.7) mm, p = 0.002. This finding is not inconsistent with existing literature as both an increase in QTc with SDB [16,41–51] and a decrease in QTc with SDB [52–54] when compared to controls have been reported, with a majority of the studies showing an increase in QTc with SDB. Additional studies closely evaluating the relationship between QTc and SDB are needed to further clarify the relationship.
Also consistent with existing literature, is the association of QT variability with SDB [17,48,50,55,56]. The measures of QT variability we examined included SDQT, QTVN and STVQT, as recommended by the European Heart Rhythm Association and the European Society of Cardiology Working Group on Cardiac Cellular Electrophysiology [28]. In our sample SDQT and QTVN were associated with mortality and SDB however, STVQT was not. This may be due to the differences in computing QT variability using SDQT and QTVN. The latter incorporates the SDQT in its calculation whereas the STVQT does not. Due to subtle differences in these QT variability parameters, it is usually helpful to use more than one measurement of QT variability [28].
The predictive model for mortality included age, BMI, waist circumference, hypertension, diabetes, cardiovascular disease, and SDQT as significant predictors. This information while not surprising, is novel and can be further validated and strengthened for use in future clinical-decision support tools.
There are multiple strengths to the study, including a well identified SDB cohort based on current American Academy of Sleep Medicine (AASM) guidelines, detailed evaluations of QT-related parameters, and likely generalizability of the results to patients with SDB. Despite these strengths, there are some limitations to this study including the small sample size as well as the retrospective nature of the analysis. An additional possible limitation is the use of 10-beat median measurements for RR and QT intervals instead of beat-to-beat analysis. We do not believe this materially alters our findings because of evidence demonstrating that use of the 10-beat median approach does not lead to significant differences when compared to beat-to-beat analysis [57]. Finally, QTc and QT variability were averaged for the entire night and were not analyzed per specific apnea/hypopnea event due to the nature of how the sleep studies were tagged for respiratory events in the SHHS. Therefore, any QT effect that was apnea/hypopnea specific as has been previously reported [13,14] may have been diluted. Nevertheless, to our knowledge, most of the studies associating the changes in markers of ventricular repolarization with sleep apnea are small and none to our knowledge have also included clinically relevant outcomes such as mortality. Additional research is needed to evaluate the changes in QTc and QT variability with every apnea/hypopnea event in real-time and to clarify the relationship between the QT-related parameters, SDB and patient outcomes including arrhythmias and mortality.
5. Conclusions
Although QTc and markers of QT variability (SDQT and QTVN) were associated with the presence of SDB, SDB was not found to be moderating the relationship between these parameters and mortality. Risk prediction (using regularized regression) selected age, BMI, waist circumference, presence of hypertension, diabetes, cardiovascular disease, prolonged QTc and increased SDQT as significant contributors to mortality after adjusting for the other variables as optimal predictors of future mortality. Time-dependent ROC analysis showed that these predictors were optimal for classifying those with a death within 2 years of the study (AUC = 0.832) though all AUC were greater than 0.75. Additional research is needed to clarify the relationship between QTc, QT variability, and outcomes including arrhythmias and mortality in patients with SDB.
Funding
During the writing of this manuscript, Dr. S. Patel was supported by grants from the American Academy of Sleep Medicine Foundation (AASMF; 203-JF-18), National Institutes of Health (HL126140), a University of Arizona Health Sciences Career Development Award and Faculty Seed Grant Award. Dr. Combs was supported by an American Heart Association Career Development Award (19CDA34740005), National Institutes of Health (HL151254, 2L30HL154400-023) and a University of Arizona Health Sciences Career Development Award. Dr. I. Patel was supported by National Institutes of Health (HL126140). Dr. Parthasarathy was supported by National Institutes of Health (NIH HL126140, AG065346; HL140144; AG059202, OD028307, HL151254, HL138377; OT2-HL156912); PCORI (DI-2018C2-13161, CER-2018C2-13262; EADI-16493; PCS-1504-30430) and American Academy of Sleep Medicine Foundation (AASMF; 169-SR-17). Dr. Woosley was supported by grants from the Agency for Healthcare Research and Quality (1R18HS02666621) and the Flinn Foundation. Dr. Quan was supported by HL151637 and HL53938.
Abbreviations
- AASM
American Academy of Sleep Medicine
- AHI
Apnea/Hypopnea index
- AUC
Area Under the receiver operating characteristic Curve
- COMPAS
Comprehensive Analysis of Repolarization Signal
- ECG
Electrocardiograms
- NSRR
National Sleep Research Resource
- OSA
Obstructive Sleep Apnea
- QTVN
Normalized QT Interval Variance
- ROC
Receiver Operating Characteristic
- SDB
Sleep Disordered Breathing
- SDQT
Standard Deviation of QT Intervals
- SHHS
Sleep Heart Health Study
- STVQT
Short-term Interval Beat-to-Beat QT Variability
- VIF
Variance Inflation Factor
Footnotes
CRediT authorship contribution statement
Salma I. Patel: Conceptualization, Methodology, Software, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Writing – review & editing, review and editing, Visualization, Supervision, Project administration, Funding acquisition. Wojciech Zareba: Conceptualization, Methodology, Resources, Writing – review & editing, Supervision, (mentorship), Funding acquisition. Bonnie LaFleur: Conceptualization, Methodology, Software, Validation, Formal analysis, Resources, Data curation, Supervision, Writing - review & editing, Writing – review & editing, (mentorship), review and editing, Visualization, Funding acquisition. Jean-Phillipe Couderc: Conceptualization, Methodology, Software, Validation, Formal analysis, Resources, Data curation, Writing - review & editing, Funding acquisition. Xiaojuan Xia: Conceptualization, Methodology, Software, Validation, Formal analysis, Resources, Data curation, Writing - review & editing, Funding acquisition. Raymond Woosley: Conceptualization, Methodology, Writing – review & editing, Supervision, (mentorship), Funding acquisition. Imran Y. Patel: Conceptualization, Writing - review & editing. Daniel Combs: Writing – review & editing. Saif Mashaqi: Writing - review & editing. Stuart F. Quan: Conceptualization, Supervision, Writing - review & editing, (mentorship), review and editing. Sairam Parthasarathy: Conceptualization, Methodology, Writing – review & editing, Supervision, (mentorship), Funding acquisition.
Declaration of competing interest
There are no conflicts of interest to report.
References
- [1].Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177: 1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Young T, Palta M, Dempsey J, Peppard PE, Nieto FJ, Hla KM. Burden of sleep apnea: rationale, design, and major findings of the Wisconsin Sleep Cohort study. Wis Med J 2009;108:246–9. [PMC free article] [PubMed] [Google Scholar]
- [3].Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation 2010;122:352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med 2015;3:310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep 2008;31:1079–85. [PMC free article] [PubMed] [Google Scholar]
- [6].Sassani A, Findley LJ, Kryger M, Goldlust E, George C, Davidson TM. Reducing motor-vehicle collisions, costs, and fatalities by treating obstructive sleep apnea syndrome. Sleep 2004;27:453–8. [DOI] [PubMed] [Google Scholar]
- [7].Fu Y, Xia Y, Yi H, Xu H, Guan J, Yin S. Meta-analysis of all-cause and cardiovascular mortality in obstructive sleep apnea with or without continuous positive airway pressure treatment. Sleep Breath 2017;21:181–9. [DOI] [PubMed] [Google Scholar]
- [8].Ludka O, Konecny T, Somers V. Sleep apnea, cardiac arrhythmias, and sudden death. Tex Heart Inst J 2011;38:340–3. [PMC free article] [PubMed] [Google Scholar]
- [9].Molnar MZ, Mucsi I, Novak M, et al. Association of incident obstructive sleep apnoea with outcomes in a large cohort of US veterans. Thorax 2015;70: 888–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005;353:2034–41. [DOI] [PubMed] [Google Scholar]
- [11].Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 2008;31: 1071–8. [PMC free article] [PubMed] [Google Scholar]
- [12].Moss AJ. The QT interval and torsade de pointes. Drug Saf 1999;21(1):5–10.; discussion 81–7. [DOI] [PubMed] [Google Scholar]
- [13].Gillis AM, Stoohs R, Guilleminault C. Changes in the QT interval during obstructive sleep apnea. Sleep 1991;14:346–50. [DOI] [PubMed] [Google Scholar]
- [14].Sokmen E, Ozbek SC, Celik M, Sivri S, Metin M, Avcu M. Changes in the parameters of ventricular repolarization during preapnea, apnea, and postapnea periods in patients with obstructive sleep apnea. Pacing Clin Electrophysiol 2018. [DOI] [PubMed] [Google Scholar]
- [15].Bilal N, Dikmen N, Bozkus F, et al. Obstructive sleep apnea is associated with increased QT corrected interval dispersion: the effects of continuous positive airway pressure. Braz J Otorhinolaryngol 2018;84:298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Schmidleitner C, Arzt M, Tafelmeier M, et al. Sleep-disordered breathing is associated with disturbed cardiac repolarization in patients with a coronary artery bypass graft surgery. Sleep Med 2018;42:13–20. [DOI] [PubMed] [Google Scholar]
- [17].Viigimae M, Karai D, Pirn P, Pilt K, Meigas K, Kaik J. QT interval variability index and QT interval duration in different sleep stages: analysis of polysomnographic recordings in nonapneic male patients. BioMed Res Int 2015:2015:963028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Niemeijer MN, van den Berg ME, Eijgelsheim M, et al. Short-term QT variability markers for the prediction of ventricular arrhythmias and sudden cardiac death: a systematic review. Heart 2014;100:1831–6. [DOI] [PubMed] [Google Scholar]
- [19].Quan SF, Howard BV, Iber C, et al. The sleep heart health study: design, rationale, and methods. Sleep 1997;20:1077–85. [PubMed] [Google Scholar]
- [20].Sleep heart health study. 2020. 2020, at, https://sleepdata.org/datasets/shhs. [Accessed 6 February 2020],
- [21].Redline S, Sanders MH, Lind BK, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep 1998;21:759–67. [PubMed] [Google Scholar]
- [22].SHHS operations manual. 1997. 2020, at, https://sleepdata.org/datasets/shhs/files/m/browser/documentation/SHHSl_Manual_of_Operations.pdf?inline=l. [Accessed 6 February 2020],
- [23].Dean DA 2nd, Goldberger AL, Mueller R, et al. Scaling up scientific discovery in sleep medicine: the national sleep research Resource. Sleep 2016;39: 1151–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Medicine AAoS. International classification of sleep disorders. 3 ed. Darien, 1L: American Academy of Sleep Medicine; 2014. [Google Scholar]
- [25].Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 2009;6:el000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Couderc JP, Garnett C, Li M, et al. Highly automated QT measurement techniques in 7 thorough QT studies implemented under 1CH El4 guidelines. Ann Noninvasive Electrocardiol 2011;16:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bazett HC. An analysis of the time-relations of the electrocardiograms. Heart 1920;7:353–70. [Google Scholar]
- [28].Baumert M, Porta A, Vos MA, et al. QT interval variability in body surface ECG: measurement, physiological basis, and clinical value: position statement and consensus guidance endorsed by the European Heart Rhythm Association jointly with the ESC Working Group on Cardiac Cellular Electrophysiology. Europace 2016;18:925–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361–87. [DOI] [PubMed] [Google Scholar]
- [30].Moons KG, Kengne AP, Woodward M, et al. Risk prediction models: 1. Development, internal validation, and assessing the incremental value of a new (bio)marker. Heart 2012;98:683–90. [DOI] [PubMed] [Google Scholar]
- [31].Tibshirani R The lasso method for variable selection in the Cox model. Stat Med 1997;16:385–95. [DOI] [PubMed] [Google Scholar]
- [32].Zou H, Hastie T. Regularization and variable selection via the elastic net. J Roy Stat Soc B 2005;67:301–20. [Google Scholar]
- [33].Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics 2000;56:337–44. [DOI] [PubMed] [Google Scholar]
- [34].Kang HH, Kang JY, Ha JH, et al. The associations between anthropometric indices and obstructive sleep apnea in a Korean population. PLoS One 2014;9: el 14463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chen X, Pensuksan WC, Lohsoonthorn V, Lertmaharit S, Gelaye B, Williams MA. Obstructive sleep apnea and multiple anthropometric indices of general obesity and abdominal obesity among young adults. Int J Soc Sci Stud 2014;2:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Newman AB, Foster G, Givelber R, Nieto FJ, Redline S, Young T. Progression and regression of sleep-disordered breathing with changes in weight: the Sleep Heart Health Study. Arch Intern Med 2005;165:2408–13. [DOI] [PubMed] [Google Scholar]
- [37].Redline S, Schluchter MD, Larkin EK, Tishler PV. Predictors of longitudinal change in sleep-disordered breathing in a nonclinic population. Sleep 2003;26:703–9. [DOI] [PubMed] [Google Scholar]
- [38].Phillips CL, O’Driscoll DM. Hypertension and obstructive sleep apnea. Nat Sci Sleep 2013;5:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tasali E, Mokhlesi B, Van Cauter E. Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest 2008;133:496–506. [DOI] [PubMed] [Google Scholar]
- [40].Drager LF, McEvoy RD, Barbe F, Lorenzi-Filho G, Redline S, Initiative I. Sleep apnea and cardiovascular disease: lessons from recent trials and need for team science. Circulation 2017;136:1840–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fisser C, Marcinek A, Hetzenecker A, et al. Association of sleep-disordered breathing and disturbed cardiac repolarization in patients with ST-segment elevation myocardial infarction. Sleep Med 2017;33:61–7. [DOI] [PubMed] [Google Scholar]
- [42].Karacop E, Karacop HB. Correlation between apnea-hypopnea index and Tp-Te interval, Tp-Te/QT, and Tp-Te/QTc ratios in obstructive sleep apnea. Ann Noninvasive Electrocardiol 2020:el2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kilicaslan F, Tokatli A, Ozdag F, et al. Tp-e interval, Tp-e/QT ratio, and Tp-e/QTc ratio are prolonged in patients with moderate and severe obstructive sleep apnea. Pacing Clin Electrophysiol 2012;35:966–72. [DOI] [PubMed] [Google Scholar]
- [44].Luo YQ, Yang Y, Yu SF. [Influence of obstructive sleep apnea-hypopnea syndrome on the QTc interval], Zhong Nan Da Xue Xue Bao Yi Xue Ban 2004;29: 97–8. [PubMed] [Google Scholar]
- [45].Lyshova OV, Borodin NV, Kostenko II. [Predictors of electrical myocardial instability in men with arterial hypertension and obstructive sleep apnea-hypopnea syndrome]. Kardiologiia 2017;57:23–9. [PubMed] [Google Scholar]
- [46].Rusu A, Nita C, Bala C, Hancu N. Effect of sleep apnea syndrome on QT dispersion and QT corrected interval in patients with type 2 diabetes. Romanian J Diabetes Nut Metabolic Dis 2011; 18. [Google Scholar]
- [47].Sayin MR, Altuntas M, Aktop Z, et al. Presence of fragmented QRS complexes in patients with obstructive sleep apnea syndrome. Chin Med J (Engl) 2015;128:2141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Schmidt M, Baumert M, Penzel T, Malberg H, Zaunseder S. Nocturnal ventricular repolarization lability predicts cardiovascular mortality in the Sleep Heart Health Study. Am J Physiol Heart Circ Physiol 2019;316:H495–505. [DOI] [PubMed] [Google Scholar]
- [49].Shamsuzzaman A, Amin RS, van der Walt C, et al. Daytime cardiac repolarization in patients with obstructive sleep apnea. Sleep Breath 2015;19: 1135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Viigimae M, Karai D, Pilt K, et al. Influence of gender on the QT interval variability and duration in different wake-sleep stages in non-sleep apneic individuals: analysis of polysomnographic recordings. J Electrocardiol 2017;50:444–9. [DOI] [PubMed] [Google Scholar]
- [51].Walker M, Blackwell JN, Stafford P, et al. Daytime QT by routine 12-lead ECG is prolonged in patients with severe obstructive sleep apnea. Sleep Disord 2020;2020:3029836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Roche F, Barthelemy JC, Garet M, Duverney D, Pichot V, Sforza E. Continuous positive airway pressure treatment improves the QT rate dependence adaptation of obstructive sleep apnea patients. Pacing Clin Electrophysiol 2005;28: 819–25. [DOI] [PubMed] [Google Scholar]
- [53].Roche F, Gaspoz JM, Court-Fortune I, et al. Alteration of QT rate dependence reflects cardiac autonomic imbalance in patients with obstructive sleep apnea syndrome. Pacing Clin Electrophysiol 2003;26:1446–53. [DOI] [PubMed] [Google Scholar]
- [54].Nunes JP, Oliveira NP. Cardiac structure and apnea/hypopnea index in patients with arterial hypertension and excessive weight. Kidney Blood Press Res 2006;29:159–64. [DOI] [PubMed] [Google Scholar]
- [55].Viigimae M, Karai D, Pilt K, et al. QT interval variability index and QT interval duration during different sleep stages in patients with obstructive sleep apnea. Sleep Med 2017;37:160–7. [DOI] [PubMed] [Google Scholar]
- [56].Baumert M, Smith J, Catcheside P, et al. Variability of QT interval duration in obstructive sleep apnea: an indicator of disease severity. Sleep 2008;31: 959–66. [PMC free article] [PubMed] [Google Scholar]
- [57].Postema PG, Wilde AA. The measurement of the QT interval. Curr Cardiol Rev 2014;10:287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]


