Abstract
Purpose:
This paper reviews the history of the radium dial workers in the United States, summarizes the scientific progress made since the last evaluation in the early 1990s, and discusses current progress in updating the epidemiologic cohort and applying new dosimetric models for radiation risk assessment.
Background:
The discoveries of radiation and radioactivity led quickly to medical and commercial applications at the turn of the 20th century, including the development of radioluminescent paint, made by combining radium with phosphorescent material and adhesive. Workers involved with the painting of dials and instruments included painters, handlers, ancillary workers, and chemists who fabricated the paint. Dial painters were primarily women and, prior to the mid to late 1920s, would use their lips to give the brush a fine point, resulting in high intakes of radium. The tragic experience of the dial painters had a significant impact on industrial safety standards, including protection measures taken during the Manhattan Project. The dial workers study has formed the basis for radiation protection standards for intakes of radionuclides by workers and the public.
Epidemiologic approach:
The mortality experience of 3,276 radium dial painters and handlers employed between 1913 and 1949 is being determined through 2019. The last epidemiologic follow-up was 30 years ago when most of these workers were still alive. Nearly 65% were born before 1920, 37.5% were teenagers when first hired, and nearly 50% were hired before 1930 when the habit of placing brushes in mouths essentially stopped. Comprehensive dose reconstruction techniques are being applied to estimate organ doses for each worker related to the intake of 226Ra, 228Ra, and associated photon exposures. Time dependent dose-response analyses will estimate lifetime risks for specific causes of death.
Discussion:
The study of radium dial workers is part of the Million Person Study of low-dose health effects that is designed to evaluate radiation risks among healthy American workers and veterans. Despite being one of the most important and influential radiation effects studies ever conducted, shifting programmatic responsibilities and declining funding led to the termination of the radium program of studies in the early 1990s. Renewed interest and opportunity have arisen. With scientific progress made in dosimetric methodology and models, the ability to perform a study over the entire life span, and the potential applicability to other scenarios such as medicine, environmental contamination and space exploration, the radium dial workers have once again come to the forefront.
Keywords: Radium, mesothorium, dial painter, Million Person Study
Introduction
The epidemiologic investigation of United States radium dial workers is one of the most important and influential radiation effects studies ever conducted (Rowland 1994; Fry 1998). The last epidemiological follow-up was conducted more than 30 years ago, at which time most of the radium dial workers were still alive (Stebbings et al. 1984; Rowland 1994; Department of Energy (DOE) 2021). Ongoing extended follow-up will provide new information on the lifetime risk of cancer and other adverse effects of ionizing radiation among women following intakes of radium. The study of radium dial workers is part of the Million Person Study (MPS) of low-dose health effects that is designed to evaluate radiation risks among healthy American workers and veterans (Boice et al. 2019). This paper briefly reviews the history of the radium dial workers in the United States, summarizes the scientific progress made since the last epidemiologic analysis of this cohort (Rowland 1994), and discusses current progress in expanding and applying updated models to the original cohort.
Historical context
There are numerous excellent reviews and books that discuss the history of the dial painters (e.g. Martland 1929; Sharpe 1978; Rowland 1994; Fry 1998; Gunderman and Gonda 2015), including Kate Moore’s novel The Radium Girls (2017) that focuses on the women’s experiences. From this tragedy, information was learned about radiation-induced osteosarcomas as well as mastoid and paranasal sinus carcinomas. Here we provide a summary to provide context for the discussion that follows.
Marie and Pierre Curie discovered radium in 1898 and soon after radium was being marketed as a medicinal cure-all (Cothern and Smith 1987). The early 1900s also brought the recognition that radium could be combined with phosphorescent material (e.g. zinc sulfide) to make self-luminous paint; several proprietary formulas were eventually developed. Dr. Sabin von Sochocky, who studied both medicine and atomic physics (the latter under Dr. Ernest Rutherford), is credited with inventing a radioluminous paint used widely in the United States that was cheaper than that developed in Europe (NYT 1928; Sharpe 1978). In 1913, seven years after immigrating to the United States (US), he began selling radioluminescent watches commercially and two years later became an original founder of what would become the US Radium Corporation (USRC); he left the company in 1922. Dial-painting enterprises prospered early on due to the wartime demand for radioluminescent dials (Rowland 1994).
226Ra (t1/2 = 1600 y), an alpha-emitter, was used in dial paint through the summer of 1919, at which point some facilities, notably USRC in New Jersey, also began to use 228Ra (t1/2 = 5:75 y), a beta-emitting decay product of 232Th commonly referred to as mesothorium (Keane et al. 1994). Figure 1 contains decay schemes for these two radium isotopes. Despite being chemically identical, 228Ra was cheaper than 226Ra. The processing of thorium ore results in the production of thorium nitrate, which was used at the time in the manufacture of incandescent gas mantles. 228Ra was a byproduct of this process that could be obtained locally, used after a year or two following extraction to allow in-growth of alpha-emitting progeny, necessary to achieve reasonable luminescence. Additionally, with a higher specific activity, 228Ra also had a ‘greater practical luminosity’ than 226Ra and was thus used in some locations to supplement 226Ra in dial paint (MLR 1926; Stewart 1929; Sharpe 1978). Retrospective analysis of USRC dial paint determined an average ratio of 228Ra to 226Ra of about 8.4 for paints used between 1919 and 1925, with other years’ paint likely 226Ra only (Keane et al. 1994).
Figure 1.
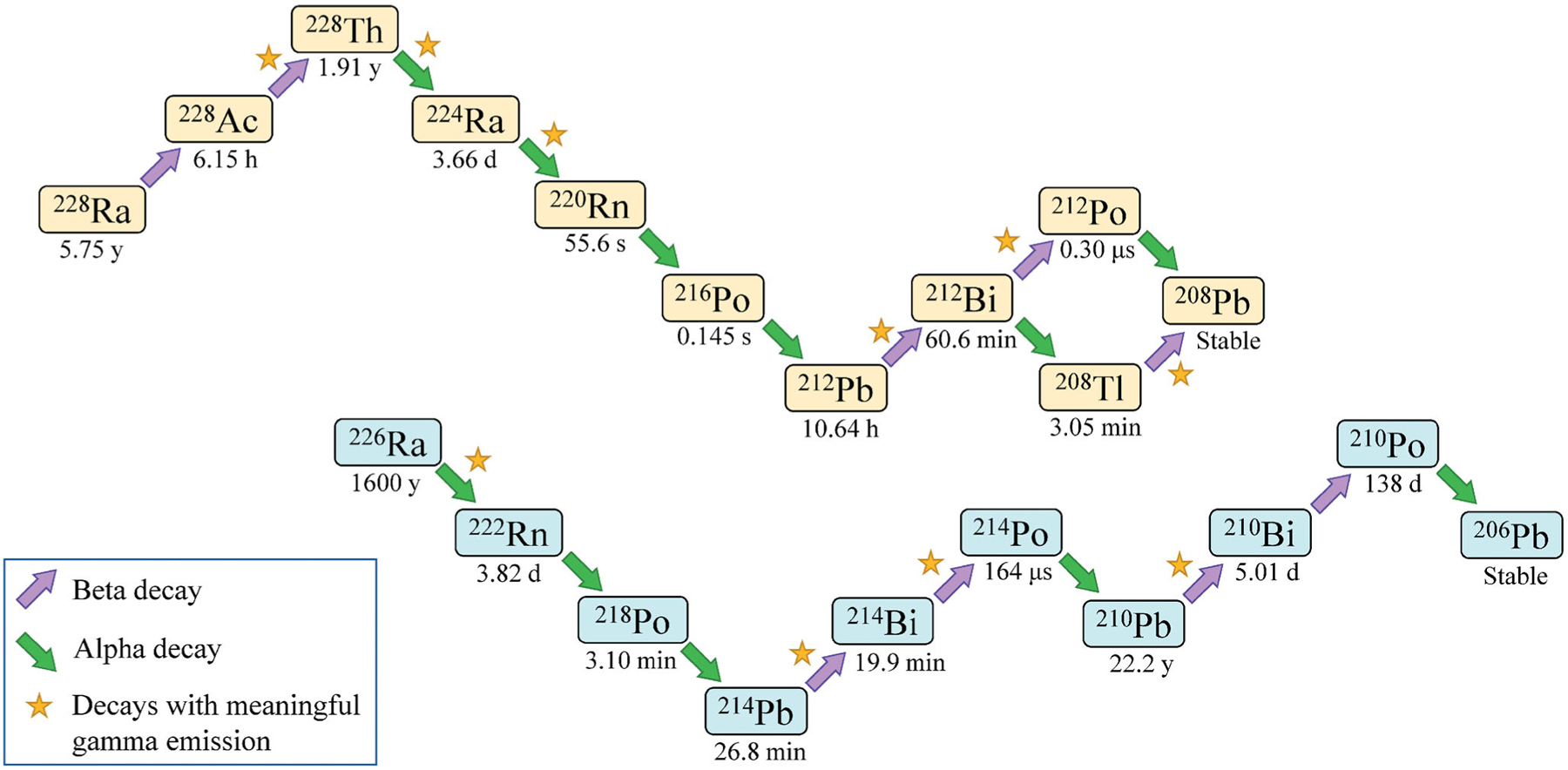
Decay chains for 228Ra (top) and 226Ra (bottom). Arrows pointing up and to the right indicate beta decay. Arrows pointing down and to the right indicate alpha decay. Stars indicate decays that are associated with meaningful gamma emission. Radiological half-lives are listed below each nuclide (International Commission on Radiological Protection (ICRP) 2008).
Workers in dial painting facilities included dial painters, dial handlers, chemists, and other ancillary workers. Making up the largest percentage (90.2%) of the current epidemiological cohort are the dial painters. Thousands of workers in the current study, mainly women (96.4%), painted dials and instruments with radium paint, using their lips to finely point their camel hair paint brushes to paint delicate pieces quickly and precisely. Being a dial painter was considered glamorous and patriotic, and early on little if any concern was expressed as to worker health and safety; many individuals believed radium exposure was ‘good for you.’ Interestingly, Moore reports an anecdote from 1918, where a New Jersey dial painter remembers von Sochocky, as he quickly passed through the room, telling her not to put the brush in her mouth because she would get sick (Moore 2017). In 1919, cloths were provided to help the painters shape the brushes but were removed due to their ‘waste’ of paint (Moore 2017). Despite this passing concern, it appears supervisors and managers remained either unconvinced or unconcerned that radium had negative health consequences, and most dial painters continued to moisten and/or point the brush with their mouth for several years following.
It wasn’t until 1923 that Dr. Theodor Blum, a dentist in Orange, New Jersey, first publicly reported that osteomyelitis of the mandible and maxilla had occurred in a young painter of luminous dials, a condition termed ‘radium jaw’ (Blum 1924; Fry 1998), although Dr. Martin Szamatolski, consulting chemist for New Jersey Department of Labor, is often credited with the earliest written suspicion (January 1923) that radium was the source of this occupational disease (Stewart 1929; Sharpe 1978). Research on health effects of internal radium contamination began in earnest in the mid-1920s when unusual occurrences of bone and other conditions began appearing among New Jersey radium workers (Martland 1929; Martland and Humphries 1929; Aub et al. 1952; Rowland 1994; Stebbings 2001). A report by Harvard Medical School health and safety experts Drs. W.B. Castle, Katherine Drinker, and Cecil Drinker was written for USRC in June 1924 and published in 1925 despite objections from the company (Castle et al. 1925; Rowland 1994). This report was one of the first studies to link exposure to radium with blood changes and jaw necrosis observed in dial painters (Castle et al. 1925; Rowland 1994). Dr. Frederick Hoffman, a statistician by education, published independent observations the same year, concluding that detrimental effects observed in dial painters were most likely due to direct contact with radium in the paint via lip pointing, although effects were attributed to 228Ra (Hoffman 1925; Sharpe 1978). This assumption was seemingly based on the fact that, at the time, affected women were from USRC and believed to have painted with only 228Ra-containing paint, contrasted with other facilities using 226Ra paint. Ultimately this resulted in 228Ra no longer being used in dial paint (Stewart 1929), which is supported by the retrospective paint analysis mentioned above (Keane et al. 1994). Interestingly, one difference between facilities that had an impact on radium intake was the type of adhesive used. Compared to an oil-varnish adhesive, paint applied with a water-based adhesive usually resulted in more frequent lip-pointing as water tends to separate brush hairs and was also less objectionable to put in the mouth. Also, paint was easier to apply with a stylus of some kind (e.g. glass rod or metal pen) with oil-varnish adhesive, which is likely why European dial painters did not exhibit the same effects as early American dial painters (Stewart 1929, Sharpe 1978).
The top panel of Figure 2 shows yearly averages in previously computed initial systemic intakes of 226Ra (left) and 228Ra (right) for individuals in the current cohort; non-zero intakes have been reported for dial workers through 1929 for 228Ra and 1949 for 226Ra (Department of Energy (DOE) 2021; Rowland 1994). Error bars represent the standard error of the mean and do not include consideration of measurement or modeling uncertainty. Estimates of initial systemic intake are available for 1,558 individuals in the current cohort of 3,276 radium dial workers (see the following section). Initial systemic intake, or the amount of radium that entered systemic circulation during an individual’s exposure period, was calculated based on measurements of body burden, or residual radium content in the body (Rowland 1994). It was, however, sometimes necessary to estimate an individual’s 228Ra intake from colleagues’ results or from measurement of exposure materials (Department of Energy (DOE) 2021). Initial systemic intake was found to be a useful metric for developing dose-response type of relationships as it was time-invariant and involved no assumptions as to the critical tissues at risk (Rowland and Lucas 1982).
Figure 2.
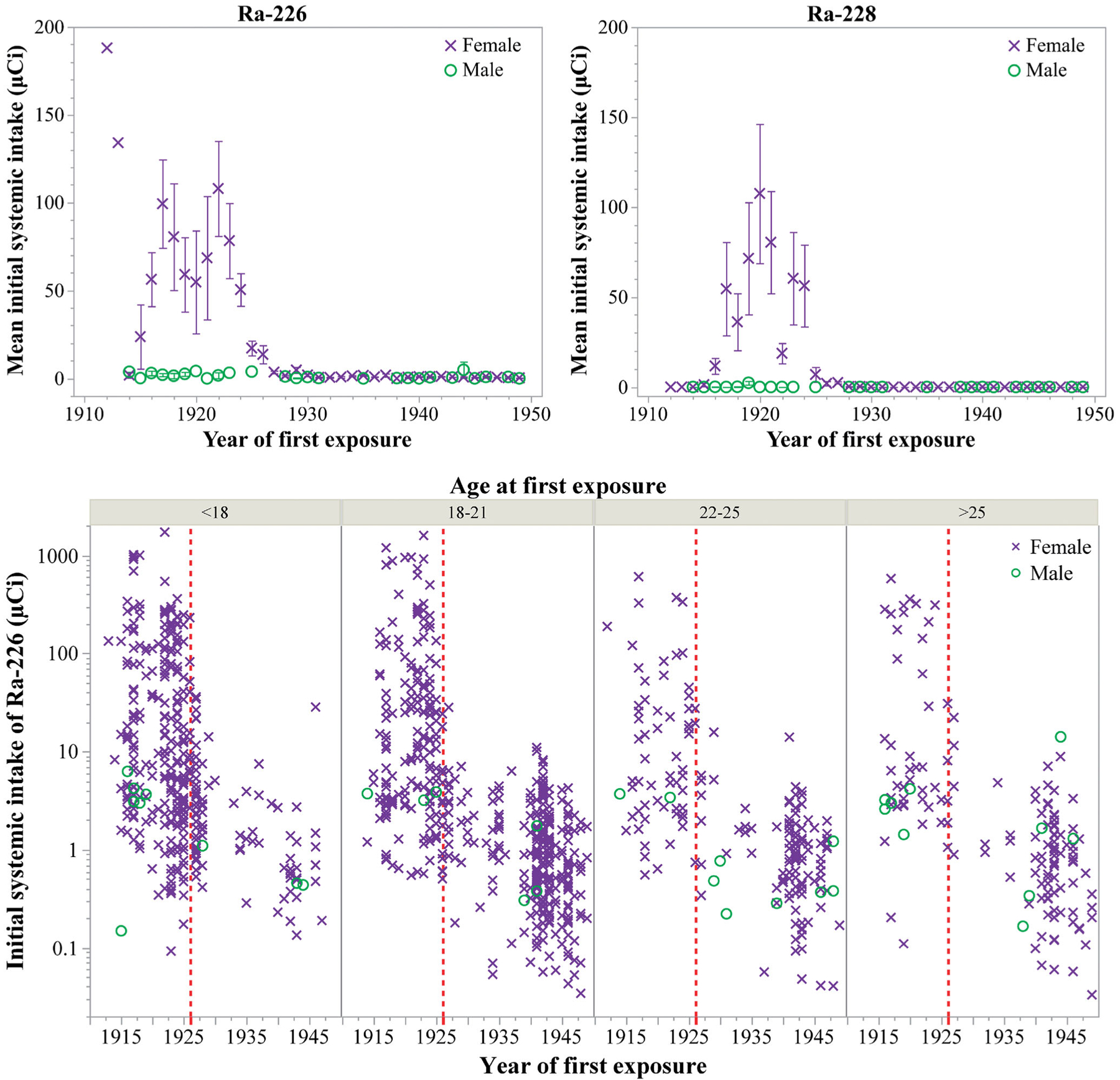
Available initial systemic intakes of radium (μCi) for individuals in the current cohort by year of first exposure, generally taken to be the year an individual was first hired. (×) represent females and (o) represent males. Data were drawn from the Comprehensive Epidemiologic Data Resource (DOE 2021). (Top panel) Mean initial systemic intakes (linear scale) of 226Ra (left) and 228Ra (right). Error bars represent the standard error of the mean in reported intakes and do not include consideration of measurement or modeling uncertainty. (Bottom panel) Individual initial systemic intakes (log scale) of 226Ra grouped by age at first exposure. Vertical dashed lines represent the year 1926.
Many dial-painting facilities reported prohibiting lip-pointing around 1925 (Martland 1929), although some seem to have delayed implementing the rule (Stewart 1929) and reports from painters themselves describe continuing to lip-point for a year or two more (Rowland and Lucas 1982; Moore 2017). Despite some uncertainty in when and to what extent lip-pointing was discontinued, calculated intakes of radium by dial workers were much lower in the years following 1926 (Figure 2).
The bottom panel of Figure 2 contains individual initial systemic intakes of 226Ra for those in the current cohort for whom estimates are available (Department of Energy (DOE) 2021; Rowland 1994). Widespread publicity of the hazards of dial-painting and tragic consequences to early dial workers is attributed to the reduction in number of employees following 1925, with renewed interest in the 1940s associated with the wartime demand for luminous dials (Rowland and Lucas 1982). Figure 2 also highlights the overlap of age and year of first exposure. Prior to the 1926 benchmark, a large number of dial workers were under 18 years old. Looking forward to the 1940s, there are comparatively fewer teenage workers. This may be in part due to the Fair Labor Standards Act, first passed in 1938 (29 USC Chapter 8; Department of Labor (DOL) 2011).
Perhaps the most extensive of the early research into the effects observed in dial workers was the body of work led by Dr. Harrison Martland, a pathologist at Newark City Hospital who became the medical examiner for Essex County, New Jersey in 1925 (Martland et al. 1925; Sharpe 1978; ; Rowland 1994; Fry 1998). He reports in a series of papers detailed exposure history, symptoms, pathology, and prognosis of the radium-induced diseases seen in dial workers (e.g. jaw necrosis, aplastic anemia, and osteosarcomas) along with corresponding radiological measurements (Martland et al. 1925; Martland 1926, 1929, 1931; Martland and Humphries 1929; Aub et al. 1952). Martland and colleagues, including von Sochocky, were among the first to develop and use techniques for in vivo measurement of radioactivity (Martland et al. 1925; Martland 1929). Martland was an advocate for the dial workers, and his papers frequently included sociolegal aspects of the occupational circumstances (e.g. Martland 1929; Fry 1998).
The early health studies were revived by the Atomic Energy Commission following World War II, partly because of the importance of radium studies in predicting the health effects of plutonium, a new bone-seeking alpha-emitting radionuclide. In 1969, the three major human studies of radium were centralized at Argonne National Laboratory, following an initial proposal made by Dr. Robley D. Evans as to the need for a National Center of Human Radiobiology. Evans was a physicist at the Massachusetts Institute of Technology who made substantial contributions to the radium studies starting in the early 1930s, fresh out of graduate school, through his retirement in 1972. The Argonne program was terminated in the early 1990s and materials were transferred to Washington State University and stored at the National Human Radiobiology Tissue Repository (NHRTR) in Richland WA (Rowland 1994). The United States Transuranium and Uranium Registries (USTUR) research program is a federal-grant-funded human tissue research program providing long-term study of actinide biokinetics in former nuclear workers with accidental internal depositions of these elements. The USTUR conducts autopsies and performs radiochemical analyses of voluntarily donated tissue samples (Kathren and Tolmachev 2019; Tolmachev et al. 2019). NHRTR holds all tissues donated to the USTUR, along with specimens acquired from the US Radium Worker Studies (Rowland 1994). The USTUR/NHRTR is a unique resource for retrospective analyses and distribution studies of plutonium, uranium, americium, radium, and barium in the human whole body, as well as in specific tissues and organs. In fact, the USTUR repository contains 1000s of specimens from the radium dial workers and has been accessed to help inform dosimetric models, e.g. radiochemical determination of radium in brain tissue of a painter (Leggett et al. 2018; Kathren and Tolmachev 2019; Tolmachev et al. 2019)
Cohort definition
Over the years, there have been several epidemiologic studies and analyses of the radium dial workers (Polednak et al. 1978; Polednak 1978a, 1978b; Rowland, Stehney, Brues, et al. 1978; Rowland, Stehney, Lucas 1978; Rowland and Lucas 1982, 1984; Rowland et al. 1983, 1989; Stebbings et al. 1984; Rowland 1993, 1994). The current population is composed of the dial painters studied by Polednak et al. 1978 (900 dial painters first hired prior to 1930) and Stebbings et al. 1984 (approximately 2,600 dial painters hired prior to 1950) supplemented with workers available from the comprehensive dataset from Argonne National Laboratory described by Rowland (1994) and digitally archived at the US Department of Energy (DOE) Comprehensive Epidemiologic Data Resource (CEDR). This dataset contains information on about 6,000 individuals with radium exposure, including not only dial workers, but also radium chemists, patients treated with radium, individuals who were known to have ingested radium water (e.g. Radithor), and other miscellaneous exposures (Department of Energy (DOE) 2021; Rowland 1994). While the information available in CEDR and in Rowland (1994) helps define the study population, a key source of information is the 100,000 s of pages of individual microfilm and microfiche records that were converted to optical character read format. Detailed clinical data, dosimetry data, and follow-up data for individuals provides a treasure trove of information to supplement and enhance the epidemiologic data. These data were made available through the DOE USTUR.
The population selection and the incremental cost for data abstraction and tracing were balanced against a small benefit from including a substantially expanded set of radium dial workers who had very low or minimal radium intakes (e.g. those first employed after about 1925, and certainly after 1950). The final defined population includes all dial painters (DP) and dial handlers (DH) employed prior to 1950. The final population size consists of 3,276 workers, including a small number (n = 119) of male painters and handlers.
Vital status and cause of death determination
Vital status, date and cause of death as of 31 December 2019 (aka vital status tracing) are being sought from link-ages with the National Death Index (NDI); state mortality files; the Social Security Administration (SSA) Death Master File; the SSA Service to Epidemiological Researchers (which confirms alive status); and credit reporting agencies using the methods outlined in Mumma et al. (2018). The Centers for Disease Control and Prevention LinkPlus program, which incorporates a probabilistic scoring system that does not require exact matches on all variables, was used for in-house matches (Campbell 2008). Online ancestry providers (Ancestry.com) and credit record providers (Transunion) are important sources to help complete and correct key demographic and linking data, such as Social Security Number, last names (which often changed since employment), and dates of birth and death. Vital status (VS) tracing (Figure 3) continues, and preliminary results are presented in Table 1. End-of-follow-up (EOFU) in Table 1 refers to the date a person is no longer considered at-risk for analytic purposes. It is their date of death, 95th birthday or December 31 of the calendar year. We anticipate that most of the study participants still being traced will be deceased as of December 2019.
Figure 3.
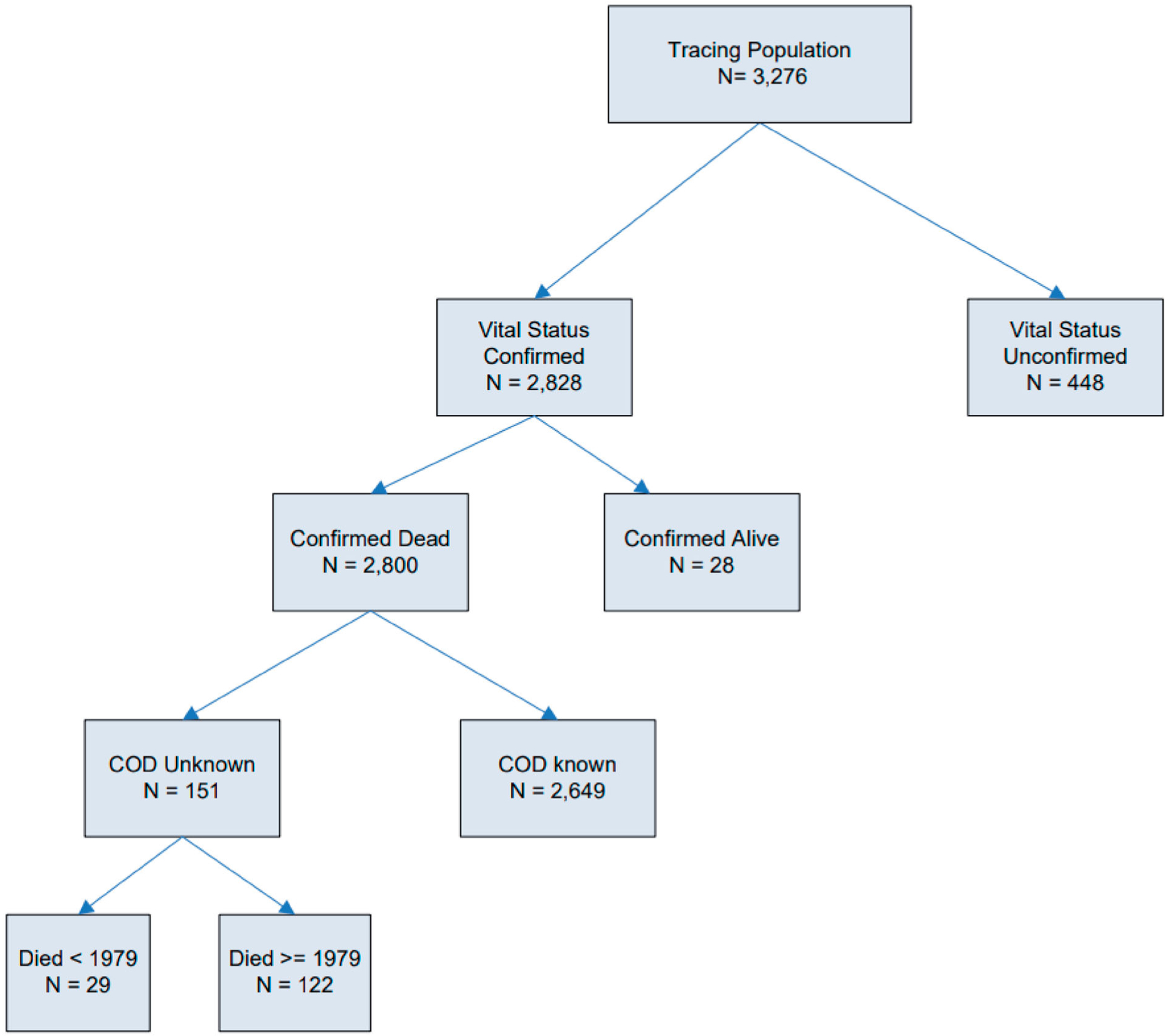
Schematic of the selection and vital status tracing results as of 31 December 2019 for the study population of 3,276 radium dial painters and radium dial handlers employed prior to 1950. COD indicates Cause of Death.
Table 1.
Descriptive characteristics of the 3,276 radium dial painters and handlers first employed 1910–1949 that comprise the epidemiologic cohort being studied.
| Characteristic | N | % |
|---|---|---|
| Primary employer | ||
| Bendix | 266 | 8.1 |
| Elgin National Watch Company | 52 | 1.6 |
| Ingraham Clock Company | 78 | 2.4 |
| Luminous Engineering/Processes | 978 | 29.9 |
| Radium Dial Company | 396 | 12.1 |
| Standard Chemical | 83 | 2.5 |
| US Radium Company | 594 | 18.1 |
| Waltham Watch Company | 88 | 2.7 |
| Waterbury Clock Company | 457 | 13.9 |
| Other | 284 | 8.7 |
| Job category | ||
| Dial painter | 2,955 | 90.2 |
| Dial handler | 321 | 9.8 |
| Sex | ||
| Male | 119 | 3.6 |
| Female | 3,157 | 96.4 |
| Year of birth | ||
| 1856–1899 | 473 | 14.4 |
| 1900–1919 | 1,637 | 50.0 |
| 1920–1932 | 711 | 21.7 |
| Unknown | 455 | 13.9 |
| Year of first hire | ||
| 1912–1919 | 529 | 16.1 |
| 1920–1924 | 546 | 16.7 |
| 1925–1929 | 445 | 13.6 |
| 1930–1939 | 172 | 5.3 |
| 1940–1949 | 1,481 | 45.2 |
| Unknown | 103 | 3.1 |
| Age at first hire | ||
| 10–15 years | 183 | 5.6 |
| 16–19 years | 1,044 | 31.9 |
| 20–24 years | 822 | 25.1 |
| 25–29 years | 334 | 10.2 |
| 30–39 years | 263 | 8.0 |
| 40 years or older | 121 | 3.7 |
| Unknown | 509 | 15.5 |
| Vital status as of 31 December 2019 | ||
| Confirmed dead | 2,800 | 85.5 |
| Confirmed alive | 28 | 0.9 |
| Currently being traced | 448 | 13.7 |
| Age at death or EOFU (Dec. 31, 2019)-VS confirmed | ||
| <40 years | 155 | 5.5 |
| 40–49 years | 89 | 3.1 |
| 50–59 years | 171 | 6.0 |
| 60–69 years | 348 | 12.3 |
| 70–79 years | 571 | 20.2 |
| 80–89 years | 829 | 29.3 |
| 90 year and older | 539 | 19.1 |
| Unknown | 126 | 4.5 |
EOFU: end of follow up; VS: vital status.
Career doses
Dosimetry records documenting radiation exposure received after employment at dial painting facilities will be sought from additional sources: the DOE Radiation Exposure Monitoring System (REMS), including historic DOE radiation exposure data not included in REMS; the Nuclear Regulatory Commission (NRC) Radiation Exposure Information and Reporting System (REIRS) and Landauer, Inc. dosimetry records (Department of Energy (DOE) 2018; National Council on Radiation Protection and Measurements (NCRP) 2018; Nuclear Regulatory Commission (NRC) 2019; Yoder et al. 2021). Based on the age structure of the population, we do not anticipate many additional external dose records. All organ/tissue-specific doses from each source will be added together to obtain the total organ/tissue-specific external and internal dose received by each worker for each calendar year, following the procedures outlined in Boice et al. (2006) and Ellis et al. (2018).
Integration of radiation biology, dosimetry, and epidemiology for the radium dial worker cohort
Epidemiology and health outcomes
As above, the mortality experience of a new cohort of 3,276 radium dial painters and handlers employed between 1913 and 1949 is being examined through 2019. Nearly 65% were born prior to 1920, 37.5% were teenagers when first hired, 96.4% were female, 90.2% were dial painters, and nearly 50% were hired before 1930 when the habit of placing brushes in mouths essentially stopped (Table 1). The large number of dial workers first employed 1940–1949 (46.7%), represents a relatively large low-dose group for comparison. The cohort was assembled over the years from over 10 different companies located primarily in New Jersey, Connecticut, and Illinois (Table 1) (Polednak et al. 1978; Rowland 1994). A comprehensive approach to ascertaining vital status (Mumma et al. 2018) has already confirmed to date that 86% have died; 99% of those with known vital status have died. Comprehensive dose reconstruction techniques are being applied to estimate organ doses for each worker related to the intake of 226Ra, 228Ra, and associated photon exposures. Time dependent dose-response analyses will estimate lifetime risks for specific causes of death, with a particular focus on osteosarcoma, mastoid and paranasal sinus carcinoma; leukemia; cancers of the lung, breast, and brain; ischemic heart disease; and dementia, Alzheimer’s, Parkinson’s, and motor neuron disease.
A comprehensive review of the published epidemiological studies of radium workers can be found in Rowland (1994); Dr. Robert E. Rowland, a biophysicist, was the first director of the Center for Radiation Biology at Argonne National Laboratory and heavily involved in the radium studies. Updates and informative reviews also are available in the Biological Effects of Ionizing Radiations (BEIR) IV Report (National Academies/National Research Council (NA/NRC) 1988) and articles by Fry (1998) and Stebbings (2001). Over the years, different epidemiologic cohorts were defined, e.g. women first employed before 1930 (Polednak et al. 1978), women first employed before 1950 (Stebbings et al. 1984) and women and men first employed before 1950 as radium workers (Rowland and Lucas 1984). Further, some publications focused primarily on dose-response relationships and included only women with measured intakes of radium (Rowland, Stehney, and Lucas 1978), and some included other types of radium workers in addition to dial painters (Rowland 1994). As mentioned above, the current cohort under study includes radium dial painters and a few associated workers, e.g. dial handlers, first employed before 1950 and previously studied in large part by Stebbings et al. (1984) and supplemented as described in Rowland (1994).
Briefly, osteosarcomas and head carcinomas (mastoid and paranasal sinus carcinomas) have been convincingly associated with internal radium exposure. The distribution of these cancers in radium-exposed persons as of 1990 is shown in Table 2; note that five individuals were diagnosed with both osteosarcomas and head carcinomas. About 1.5% (64 individuals) of dial workers were diagnosed with osteosarcomas, a cancer which was found to be more effectively induced by 228Ra compared with 226Ra (Rowland, Stehney, and Lucas 1978). About 0.6% (24 individuals) of dial workers were diagnosed with head carcinomas, attributed to the accumulation of 222Rn in the sinus cavities (National Academies/National Research Council (NA/NRC) 1988; Rowland 1994). All these cancers were observed in female dial painters, attributed primarily to their higher levels of intake compared to dial handlers or male dial painters; few men painted dials (Rowland et al. 1983) and those who did generally had measured intake levels of 226Ra that were much lower than those among women (Rowland 1994; see also Figure 2).
Table 2.
Distribution of bone sarcomas and head carcinomas* in radium-exposed persons as of 1990 by exposure circumstance (radium dial worker or other**), measurement status*** and sex, modified from Rowland (1994)
| Number of persons | Osteosarcomas (%) | Head carcinomas (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Radium-Exposure Circumstance | Female | Male | All | Female | Male | All | Female | Male | All |
| Persons measured | |||||||||
| Dial worker | 1747 | 161 | 1908 | 46 (2.6%) | 0 (0.0%) | 46 (2.4%) | 19 (1.1%) | 0 (0.0%) | 19 (1.0%) |
| Other | 156 | 319 | 475 | 15 (9.6%) | 3 (1.4%) | 18 (3.8%) | 8 (5.1%) | 5 (1.6%) | 13 (2.7%) |
| Total | 1903 | 480 | 2383 | 61 (3.2%) | 3 (0.6%) | 64 (2.7%) | 27 (1.4%) | 5 (1.0%) | 32 (1.3%) |
| Persons not measured | |||||||||
| Dial worker | 1910 | 315 | 2225 | 18 (0.9%) | 0 (0.0%) | 18 (0.8%) | 5 (0.3%) | 0 (0.0%) | 5 (0.2%) |
| Other | 871 | 1196 | 2067 | 1 (0.1%) | 2 (0.2%) | 3 (0.1%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Total | 2781 | 1511 | 4292 | 19 (0.7%) | 2 (0.1%) | 21 (0.5%) | 5 (0.2%) | 0 (0.0%) | 5 (0.1%) |
| All persons | |||||||||
| Dial worker | 3657 | 476 | 4133 | 64 (1.8%) | 0 (0.0%) | 64 (1.5%) | 24 (0.7%) | 0 (0.0%) | 24 (0.6%) |
| Other | 1027 | 1515 | 2542 | 16 (1.6%) | 5 (0.3%) | 21 (0.8%) | 8 (0.8%) | 5 (0.3%) | 13 (0.5%) |
| Total | 4684 | 1991 | 6675 | 80 (1.7%) | 5 (0.3%) | 85 (1.3%) | 32 (0.7%) | 5 (0.3%) | 37 (0.6%) |
Carcinomas originating in the paranasal sinuses or mastoids.
Other includes chemists, physicists, laboratory technicians, nurses, offspring born to female subjects who had been exposed to radium, persons and patients injected with radium, and persons who drank Radithor (a form of radium available to the public in the 1920s which included bottled drinking water spiked with radium and sold over the counter or by mail).
Whether or not measurements were made to determine radium body burden. The highest measurements were found among workers in the dial industry, patients treated with radium and from self-administered radium, e.g. drinking Radithor.
Other previously studied health outcomes included leukemia (Spiers et al. 1983), breast cancer (Adams and Brues 1980; Stebbings 2001), fertility (Polednak 1980; Schieve et al. 1997), multiple myeloma (Stebbings et al. 1984; Stebbings 2001), thyroid tumors (Polednak 1986) and cataracts (Adams et al. 1983). Although increased risks were reported for some of these conditions, the associations were either not statistically meaningful and/or were not convincingly related to estimated intakes of radium.
The current work will expand upon these previous observations and will provide a complete assessment of lifetime risks related to radium ingestion. The health outcomes considered will include the previously studied cancer and non-cancer outcomes and have been broadened to include cognitive deficits possibly related to radiation exposure to brain tissue (Marazziti et al. 2012; Parihar et al. 2016, 2020; Azizova et al. 2020; Pasqual et al. 2021). Interestingly, NASA is concerned about possible behavioral and cognitive impairments from high energy heavy ions in space [galactic cosmic radiation (GCR)] that might jeopardize long missions, and, possibly, lead to Alzheimer’s, Parkinson’s or dementia later in life (Boice 2019; National Council on Radiation Protection and Measurements (NCRP) 2019). There are no human exposure circumstances similar to GCR in space that can provide direct information on cognition or neurological diseases following such high-LET exposure to brain tissue. The intake of radium can result in meaningful exposure to brain tissue from alpha particles, and the medical records of the dial painters are substantial, going back as early as the 1920s. Although an imperfect analog, the study will be able to address the likelihood that high-LET exposures to brain might cause cognitive impairment and provide some guidance as to the seriousness of this threat for space exploration.
Uncertainties associated with this work are those typically encountered in epidemiological studies evaluating radiation-induced health effects, although the current study has the unique benefit of documented radiological measurements for about half of the population, the continued monitoring and clinical visits of individuals in the population, and the current availability of thousands of tissue samples, and bones, of the dial painters available at USTUR. Dose reconstructions and analysis will follow current best practices with respect to evaluation of uncertainty (e.g. National Council on Radiation Protection and Measurements (NCRP) 2009, 2018), including consideration of the new approaches to address uncertainty in worker studies following intakes of plutonium, another bone-seeker (Stram et al. 2021).
Cellular dosimetry
Peripheral blood slides have been reviewed from a cohort of 166 radium dial painters and ancillary workers (Goans et al. 2019). The blood slides were prepared in 1960–1975 during medical follow-up and were made available in collaboration with the USTUR. The cohort contained 107 dial painters, 22 dial handlers, 19 radium chemists, and other personnel dealing with radium. Members of the cohort had ingestion of 226Ra and 228Ra at an early age (average age 20.6 ± 5.4 y; range 13–40 y) during the years 1914–1955. Exposure duration ranged from 1 to 1,820 weeks with red marrow dose 1.5–6,750 mGy.
The cell of interest in the peripheral smear is the pseudo-Pelger Huët cell (PH). PH is characterized in neutrophils by a bi-lobed nucleus whose lobes are joined by a thin chromatin bridge. PH in this case is caused by a radiation-induced decrease in the amount of the Lamin B receptor (LBR). The gene that encodes the LBR is known to be located on the long arm of chromosome 1, 1q42.12.
PH has been described as a novel, permanent, radiation-induced biomarker in circulating neutrophils (Goans et al. 2015, 2017). In studies involving a group of workers from the Y-12 criticality accident (1958) and from controlled primate studies at the Armed Forces Radiobiology Research Institute (AFRRI), PH has shown a linear dose response for mixed dose in red marrow from 1–10 Gy. In the radium dial painter cohort, PH expressed as a percentage of total neutrophils has been shown to rise in a sigmoidal fashion over five decades of red marrow dose, best fit with a sigmoid function and suggestive of a threshold effect (Goans et al. 2019). These results are consistent with health outcome findings (discussed above) in that no bone sarcomas were observed in the radium dial cohort at total systemic intakes below 100 μCi (Rowland 1994). Thus, PH percentages from peripheral blood tracks alpha dose to bone marrow and have the potential to be a useful metric for supporting dose estimates. Figure 4 shows a plot of PH percent (mean % ± standard error of the mean) from this cohort versus date of entry into the workforce. A decrease toward control values is seen for entry into the workplace after 1930.
Figure 4.
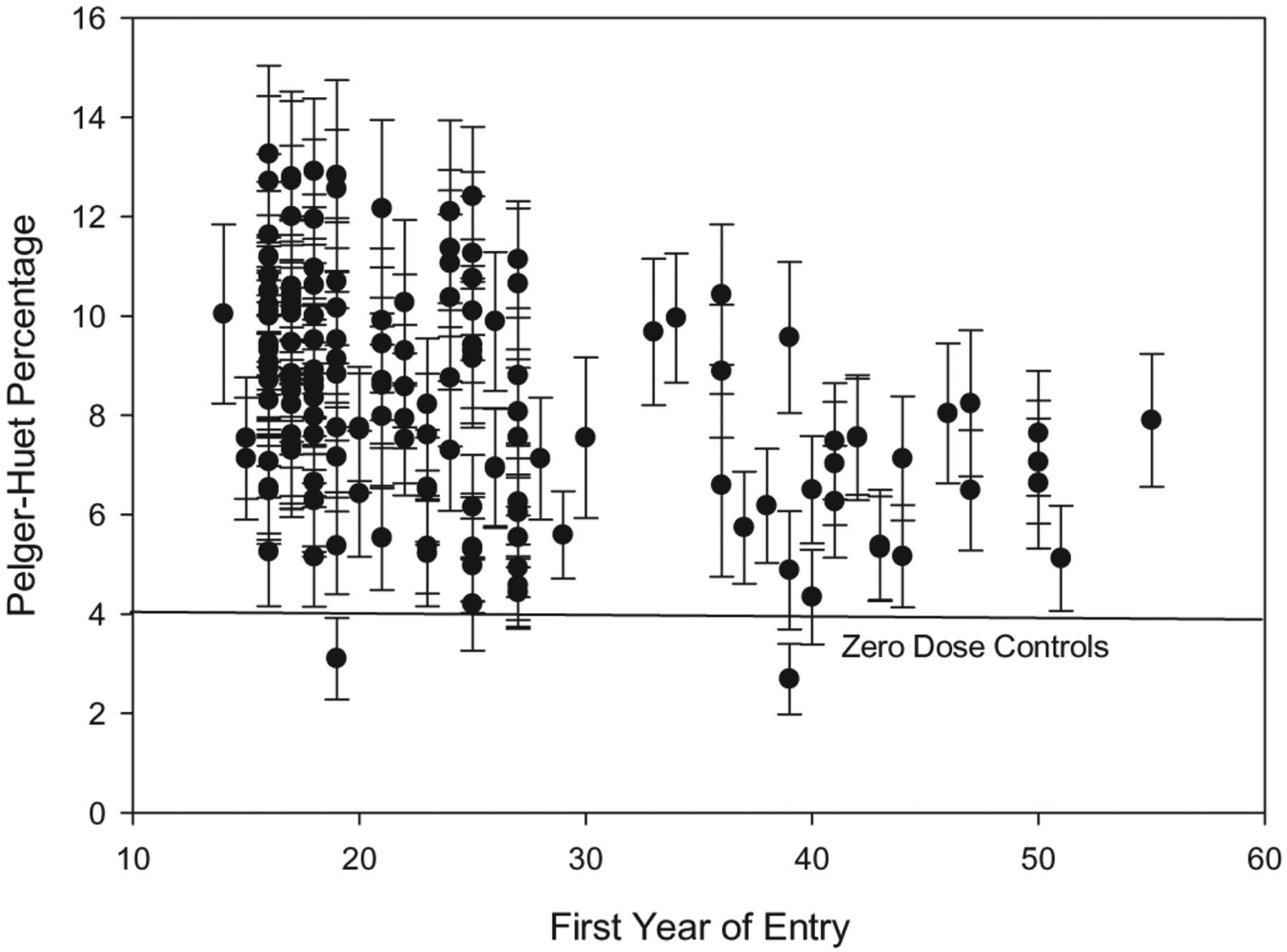
Pelger Huët percent (mean % ± SEM) observed in a cohort of 166 radium dial painters and ancillary workers versus date of entry into the workforce.
Radium biokinetics and associated models
Overview of radium kinetics in the body
Radium belongs to the alkaline earth family (Group 2) of the periodic table and is a physiological analogue of the lighter alkaline earth metals calcium, strontium, and barium. The rates of uptake and removal by tissues differs from one alkaline earth to another due to discrimination by biological membranes and hydroxyapatite crystals of bone (Leggett, 1992; International Commission on Radiological Protection (ICRP) 1993). The biokinetics of radium in the human body resembles that of barium more closely than that of calcium or strontium.
Biokinetic data for radium in adult humans were reviewed in International Commission on Radiological Protection (ICRP) Publication 137 (International Commission on Radiological Protection (ICRP) 2017). Briefly, the biokinetics of the individual alkaline earth elements and the comparative behaviors of different pairs of these elements have been studied extensively in human subjects and laboratory animals. Based on controlled studies on adult human subjects it is estimated that about a third of radium atoms leaving blood deposit in excretion pathways, predominantly in the colon. Soft tissues initially accumulate a substantial portion of retained systemic radium but lose most of the deposited activity within a few days. Bone soon becomes the primary systemic repository of radium after its acute uptake to blood. Radium and other alkaline earths entering bone initially deposit on bone surfaces, from which they are removed over a period of hours or days back to blood and to a lesser extent to a bone volume pool referred to as exchangeable bone volume. The rate of loss of alkaline earth elements from exchangeable bone over the first few months after uptake to blood increases in the order radium > barium > strontium > calcium. A portion of radium, barium, strontium, or calcium entering exchangeable bone volume returns to blood over a period of months and a smaller portion becomes firmly fixed in bone crystals and is retained there until removed by bone restructuring processes. Calcium, strontium, barium, and radium are all about equally likely to transfer from bone surface to exchangeable bone volume, but the likelihood of becoming firmly fixed in bone crystal decreases in the order calcium > strontium > barium > radium. The rate at which the non-exchangeable (firmly fixed) alkaline earth elements are removed from bone volume to blood appears to depend completely on the rate of turnover of the bone type (trabecular or cortical bone) and thus is independent of the element. The portion of acutely injected radium in bone of a mature adult human typically is about 20–40% after 1 day, 6–12% after 1 month and 2–4% after 1 year.
Information is available on the systemic behavior of radium in immature humans (International Commission on Radiological Protection (ICRP) 1973; Parks et al., 1978; Muth and Glöbel, 1983; Parks and Keane, 1983; Keane and Schlenker, 1987). More detailed data on the age-specific behavior of systemic radium are available for laboratory animals, particularly dogs (Lloyd, Mays, and Atherton 1976; Lloyd, Mays, Atherton, et al. 1976; Lloyd et al. 1982; Bruenger et al. 1983; Lloyd, Bruenger, Jones et al. 1983; Lloyd, Bruenger, Mays et al. 1983; Lloyd, Jones, et al. 1983; Lloyd, Taylor, et al. 1983; Bruenger and Lloyd 1989). Differences with age in the systemic behavior of radium are consistent with findings for the other alkaline earth elements. That is, retention of radium is greater in growing bone than in mature bone; changes with age in uptake of radium by the skeleton are roughly proportional to the age-specific rate of calcium addition to bone from bone growth plus bone remodeling; at times remote from exposure, skeletal burdens acquired during periods of growth tend to remain higher than those acquired by mature skeletons except for skeletal burdens acquired during or soon after infancy; and both deposition and removal of radium appear to be greater in areas of bone undergoing rapid remodeling than in areas of relatively slow remodeling.
Biokinetic models for radium previously applied to dial workers
Mainly on the basis of follow-up data for Elgin State Hospital patients administered known amounts of 226Ra via intravenous injection, Norris et al. (1955) proposed that fractional retention of absorbed radium as a function of time t (days) could be described by the power function R(t) = 0.54t−0.52. This retention function was used for years to estimate intake of 226Ra by radium dial workers, based on long-term retention of 226Ra as judged from external measurement or 222Rn exhalation.
ICRP Publication 20 (1973) introduced a relatively complex model of retention R of the alkaline earths calcium, strontium, barium, and radium as a function of time, t (days), based on an extensive review of data on the behavior of these elements in human subjects:
| (1) |
Some of the parameters in this retention function are element dependent and others represent physiological or unknown, element-independent processes:
λ is the rate of resorption of compact bone,
σ is the ratio of turnover rates of trabecular and compact bone,
β is the fraction of bone volume activity deposited in compact bone,
m is a rate constant representing an early exponential process,
p is the fraction of retention not in the early exponential process,
ε (element-specific) is related to fast turnover of an initial pool,
b (element-specific) is related to diffusion of exchangeable activity from bone,
r (element specific) is related to redeposition of activity at bone-forming sites.
In ICRP Publication 20, retention in soft tissues was calculated as the difference between total-body retention R(t) and components of the model interpreted as representing activity in bone and blood.
Schlenker et al. (1982) concluded from a review of the distribution and retention of radium in soft tissues that the model of ICRP Publication 20 did not accurately depict the time-dependent distribution of radium between bone and soft tissue. They modified selected parameter values for radium to obtain a better fit to their collected data.
As described by Rowland (1993, 1994), measurements of retention of 226Ra in the dial painters made 30–60 years after exposure indicated faster loss of radium from the body than predicted by the model of ICRP Publication 20. To address this issue, Rowland (1993) modified Schlenker’s revision of the ICRP model to incorporate a higher bone turnover rate. It was later observed that case-specific modifications of the Schlenker model may sometimes be needed because the rate of bone resorption may be greatly reduced in cases of extremely high intake of 226Ra due to radiation damage to bone (Rowland 1994).
Biokinetic model for systemic radium applied in the present study
The latest version of the ICRP’s biokinetic model for systemic radium will be used as the starting place for reconstructing intake of 226Ra and 228Ra by radium dial painters. As described below, it is expected that parameter values of the ICRP model will be modified where feasible to improve estimates of the intake and subsequent behavior of radium in individual dial painters or subgroups of dial painters.
The ICRP’s latest biokinetic model for a reference adult is described in Publication 137 (2017), which is Part 3 of an ICRP series of reports on occupational intake of radionuclides (OIR). An age-specific version of that model has been developed and will be described and applied in an upcoming series of ICRP reports on environmental intake of radionuclides (EIR) by members of the public. The structure of the model is shown in Figure 5. Reference age-specific transfer coefficients are listed in Table 3. The following modifications of the radium model described in Figure 5 and Table 3 are planned for application to the radium dial painters:
Figure 5.
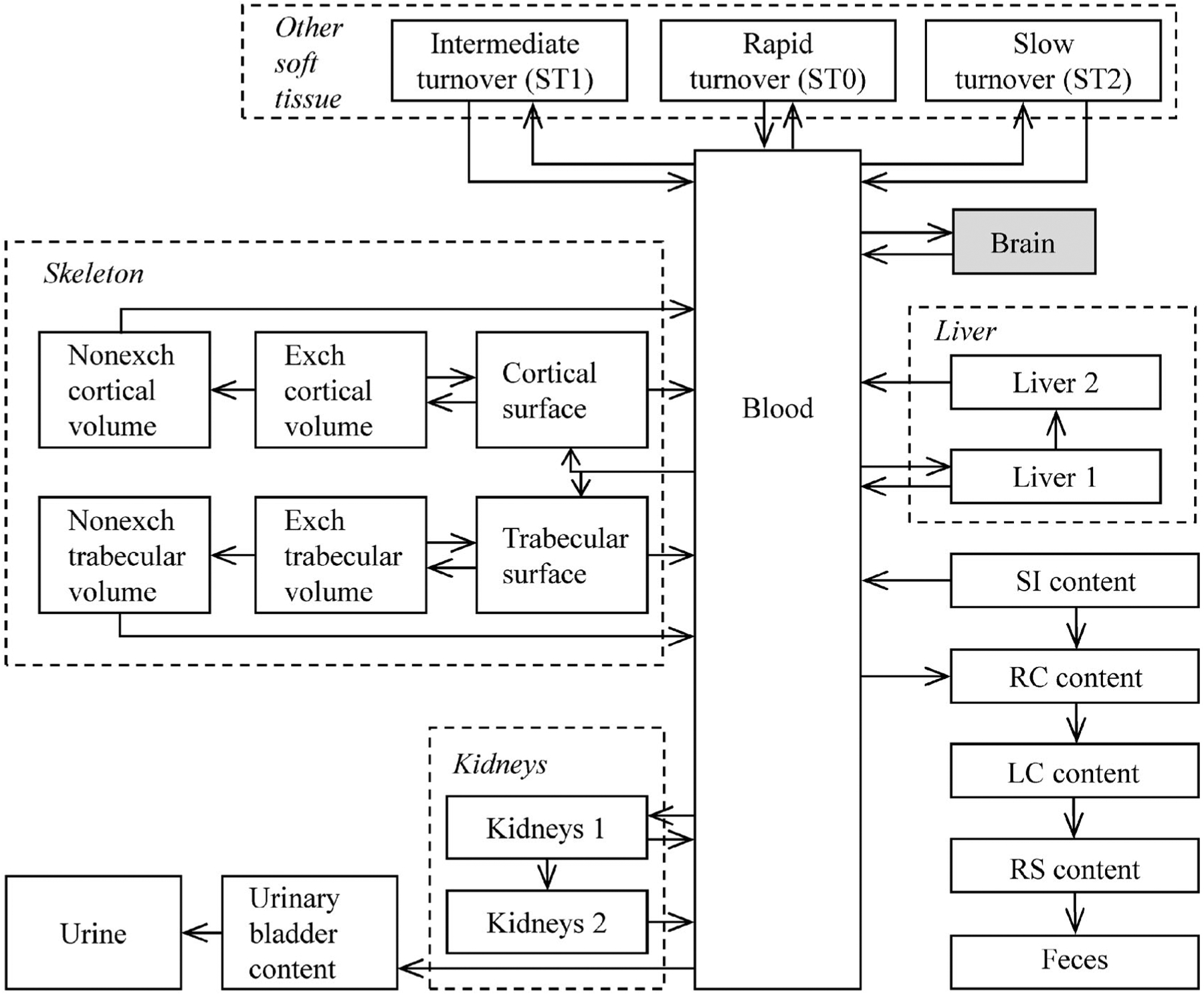
Structure of the ICRP’s current biokinetic model for systemic radium (International Commission on Radiological Protection (ICRP) 2017) with the addition of the brain (shaded).
Table 3.
ICRP’s age-specific transfer coefficients for radium (adopted for use and to be published in the environmental intake of radionuclide [EIR] series).
| Transfer coefficient (d−1) | ||||||
|---|---|---|---|---|---|---|
| Patha | 100 d | 1 y | 5 y | 10 y | 15 y | Adult |
| Blood to urinary bladder content | 2.02E-01 | 4.44E-01 | 4.85E-01 | 3.56E-01 | 2.10E-01 | 6.06E-01 |
| Blood to right colon content | 7.26E+00 | 1.60E+01 | 1.74E+01 | 1.28E+01 | 7.55E+00 | 2.18E+01 |
| Blood to Trab bone surf | 1.05E+01 | 6.30E+00 | 6.23E+00 | 9.87E+00 | 1.44E+01 | 9.72E+00 |
| Blood to Cort bone surf | 4.20E+01 | 2.52E+01 | 2.18E+01 | 2.93E+01 | 3.74E+01 | 7.78E+00 |
| Blood to other 3 | 6.98E+00 | 1.53E+01 | 1.67E+01 | 1.23E+01 | 7.26E+00 | 2.09E+01 |
| Blood to other 4 | 1.17E+00 | 2.57E+00 | 2.80E+00 | 2.05E+00 | 1.21E+00 | 3.50E+00 |
| Blood to other 5 | 2.33E-02 | 5.13E-02 | 5.60E-02 | 4.11E-02 | 2.43E-02 | 7.00E-02 |
| Blood to liver 1 | 1.40E+00 | 3.08E+00 | 3.36E+00 | 2.46E+00 | 1.46E+00 | 4.20E+00 |
| Blood to kidneys 1 | 4.67E-01 | 1.03E+00 | 1.12E+00 | 8.21E-01 | 4.85E-01 | 1.40E+00 |
| Trab bone surf to blood | 5.78E-01 | 5.78E-01 | 5.78E-01 | 5.78E-01 | 5.78E-01 | 5.78E-01 |
| Trab bone surf to Exch trab bone vol | 1.16E-01 | 1.16E-01 | 1.16E-01 | 1.16E-01 | 1.16E-01 | 1.16E-01 |
| Cort bone surf to Blood | 5.78E-01 | 5.78E-01 | 5.78E-01 | 5.78E-01 | 5.78E-01 | 5.78E-01 |
| Cort bone surf to Exch trab bone vol | 1.16E-01 | 1.16E-01 | 1.16E-01 | 1.16E-01 | 1.16E-01 | 1.16E-01 |
| Other 3 to blood | 6.98E+00 | 6.98E+00 | 6.98E+00 | 6.98E+00 | 6.98E+00 | 6.98E+00 |
| Other 4 to blood | 6.93E-01 | 6.93E-01 | 6.93E-01 | 6.93E-01 | 6.93E-01 | 6.93E-01 |
| Other 5 to blood | 3.80E-04 | 3.80E-04 | 3.80E-04 | 3.80E-04 | 3.80E-04 | 3.80E-04 |
| Liver 1 to blood | 6.91E-01 | 6.91E-01 | 6.91E-01 | 6.91E-01 | 6.91E-01 | 6.91E-01 |
| Liver 1 to liver 2 | 2.08E-03 | 2.08E-03 | 2.08E-03 | 2.08E-03 | 2.08E-03 | 2.08E-03 |
| Liver 2 to blood | 1.90E-03 | 1.90E-03 | 1.90E-03 | 1.90E-03 | 1.90E-03 | 1.90E-03 |
| Kidneys 1 to blood | 2.07E+00 | 2.07E+00 | 2.07E+00 | 2.07E+00 | 2.07E+00 | 2.07E+00 |
| Kidneys 1 to kidneys 2 | 6.24E-03 | 6.24E-03 | 6.24E-03 | 6.24E-03 | 6.24E-03 | 6.24E-03 |
| Kidneys 2 to blood | 1.90E-03 | 1.90E-03 | 1.90E-03 | 1.90E-03 | 1.90E-03 | 1.90E-03 |
| Exch trab bone vol to Trab bone surf | 1.85E-02 | 1.85E-02 | 1.85E-02 | 1.85E-02 | 1.85E-02 | 1.85E-02 |
| Exch to Nonexch trab bone vol | 4.60E-03 | 4.60E-03 | 4.60E-03 | 4.60E-03 | 4.60E-03 | 4.60E-03 |
| Exch cort bone vol to Cort bone surf | 1.85E-02 | 1.85E-02 | 1.85E-02 | 1.85E-02 | 1.85E-02 | 1.85E-02 |
| Exch to Nonexch cort bone vol | 4.60E-03 | 4.60E-03 | 4.60E-03 | 4.60E-03 | 4.60E-03 | 4.60E-03 |
| Nonexch cort bone vol to Blood | 8.22E-03 | 2.88E-03 | 1.53E-03 | 9.04E-04 | 5.21E-04 | 8.21E-05 |
| Nonexch trab bone vol to Blood | 8.22E-03 | 2.88E-03 | 1.81E-03 | 1.32E-03 | 9.59E-04 | 4.93E-04 |
Trab: trabecular; Cort: cortical; surf: surface; vol: volume; Exch: exchangeable; Nonexch: nonexchangeable.
Age- and sex-specific biokinetics of radium and progeny throughout life. The ICRP’s biokinetic model for radium addresses differences with age in radium kinetics only through age 25 y and does not address gender differences at any age. For application to the radium dial painters, the ICRP model will be modified to address age and gender differences in radium biokinetics throughout life. For example, the model will address age and gender differences in bone remodeling rates throughout life and in rates of net bone loss starting in the fifth decade of life. The ICRP model will also be modified to address differences with gender in the rates of bone maturation during adolescence.
The relatively simple model applied by the ICRP to radon produced in vivo by decay of radium will be replaced with the more sophisticated ICRP model for radon as a parent.
The structure of the ICRP’s current biokinetic model for systemic radium (Figure 5) will be modified, insofar as allowed by available biokinetic data, to improve dose estimates for tissues of interest that are not explicitly depicted in the ICRP model. For example, a compartment representing the brain will be added. Parameter values describing uptake and removal of radium by the brain will be taken from an earlier paper (Leggett et al. 2018).
As in the ICRP’s OIR and EIR series, the assumption of independent kinetics of radioactive progeny will be applied in dose reconstructions for radium dial painters. That is, radioactive progeny produced in the body after intake of 226Ra or 228Ra will be assumed to follow the characteristic biological behaviors of those elements (as opposed to following the biokinetics of radium) following production in soft tissues or on bone surface. Radioactive progeny other than radon produced in bone volume will be assumed to be removed from bone volume only through bone remodeling or net bone loss.
Radium distribution in brain
Analysis of brain tissue of a female individual occupationally exposed to radium was conducted at USTUR recently to study the distribution of 226Ra. The concentrations of 226Ra were measured with inductively coupled plasma mass spectrometry (ICP-MS) at the University of Missouri in the corpus callosum, the white and gray matter of the cerebrum lobes, the cerebellum, and brainstem segments of the brain. Preliminary results indicate that 226Ra concentration in the white matter (18.3 ± 3.0 Bq kg−1) was about 3.5 times higher than the average of all other brain segments (ranged 4.9–5.7 Bq kg−1). With only one case studied, current preliminary results suggest non-uniformly distribution of radium in the human brain. In the future, this finding might have an impact on biokinetic modeling of internally deposited radionuclides in the brain as well as on the assessment of radiation doses to the brain. Current systemic biokinetic models, recommended by the ICRP assume a uniform distribution in the brain for any specific element because at present, it is not feasible to characterize with much confidence (1) the distribution of most individual elements among different regions of the brain or (2) element-specific biokinetics in individual regions of the brain. Thus, the assumption still would be that the element is uniformly distributed in the entire brain. We recognize, however, the importance of different distributions of radium within different brain regions and would address such issues moving forward as new information becomes available (Boice et al. 2021; NCRP 2021).
Overview of methodology for internal dose calculation
The starting point for internal dose calculation will be measurement-based radium body burden estimates performed in the past. The timing, number, and type of radiological measurement(s) made to estimate body burdens in dial workers varied by individual and were dependent on the available instrumentation and technology. For example, thallium-activated sodium iodide (NaI(Tl)) crystal was reported to be an effective scintillation material in 1948 (Hine 1977), but neither useful crystals, nor appropriate electronic circuitry to collect and interpret the light signal from a NaI(Tl) crystal, were readily available at the time. This limited the practical application of the NaI(Tl) detector for the next few years. Common types of measurements made, though, included radon breath measurements, whole-body gamma-ray measurements, and autopsy or other posthumous measurements (e.g. autoradiography of bones) (Rowland 1994).
Body burden estimates provide the activity of 226Ra and/or 228Ra in the body at the time of measurement, which can then be coupled with biokinetic models and the time of first intake from an individual’s work history to develop a time series of relevant activity levels in the body. In other words, the latest biokinetic models described above will use previously determined body burdens to develop estimates of activity versus time in each pertinent source region in the body for radium and its progeny. The temporal activity data will then be customized to each dial worker based on their age and duration of employment. This work will also treat the intake of radium as chronic over their employment. Other factors, such as whether a dial painter’s work bridged a transition from mouth tipping of brushes to cessation of this practice (somewhere around 1926) can be folded into the chronic intake model.
The absorbed dose rate, , to a particular target region, T, is computed using Equation (2) where AS is the activity at a given time in a source region, S, and S(rT ← rS) is the S-coefficient, defined as the absorbed dose to a target region, rT, per nuclear transformation taking place in a source region, rS.
| (2) |
The S-coefficient is computed as shown in Equation (3) and depends on the energy, ER,i, and yield, YR,i, of emission, i, of radiation type, R, from a given radionuclide. The last term in Equation (3), Φ(rT ← rS, ER,i), is the specific absorbed fraction which is defined as the fraction of emitted energy from a source region absorbed in a target region per mass of the target. The specific absorbed fraction depends on the energy, radiation type, and specific source-target geometry. Before adulthood, the S-coefficient also varies with respect to age.
| (3) |
The absorbed dose rates as functions of time will be integrated to provide annualized absorbed doses over life. Similarly, committed absorbed dose over life will be computed for the sake of comparison to past committed absorbed dose calculations.
Dosimetric targets of interest include the bone endosteum, red (active) marrow, breast, brain, liver, lung, heart wall, and others. While the bone endosteum is considered the current target of interest for radiogenic bone cancer (Gossner et al. 2000, Gossner 2003, Bolch et al. 2007), dose to the entire bone volume will also be computed since it has been used in past studies on this cohort.
The absorbed dose to the red (active) marrow resulting from alpha particles emitted in the bone surface will not be uniform across the marrow cavity due to the short range of the alpha particles. The current definition of the bone endosteum target is the first 50 mm of marrow space adjacent to the bone surface. This region contains red marrow and, for the case of radiations emitted from the bone, the endosteum dose is equivalent to the shallow red marrow dose. The difference between the shallow marrow dose and the marrow dose averaged over the entire cavity may provide some insight into deterministic and stochastic hematopoietic response, or the lack thereof as seen in previous reports of radium dial painters (Spiers et al. 1983; Priest 1989).
Use of the latest energy absorption data
Energies and yields of the various radionuclide emissions will be taken from ICRP Publication 107 (International Commission on Radiological Protection (ICRP) 2008). In 2016, the ICRP published new specific absorbed fractions for reference adults (International Commission on Radiological Protection (ICRP) 2016). These specific absorbed fractions were computed using the latest whole-body voxel phantoms and detailed models of the skeleton, alimentary, and respiratory regions. Similar data for reference children were recently published by the ICRP (Schwarz et al. 2021a, 2021b) using an age-dependent set of reference phantoms (International Commission on Radiological Protection (ICRP) 2020a) and models.
The energy deposition of alpha particles emitted from the skeleton is of particular importance to the ingestion of radium given its high uptake to the bone. Figure 6 contains plots of the absorbed fractions provided in Publication 133 compared to values in Publication 30 (International Commission on Radiological Protection (ICRP) 1979). The differences are due to new definitions of source and target regions and an improved capability to perform radiation transport calculations in complex geometries such as the skeleton.
Figure 6.
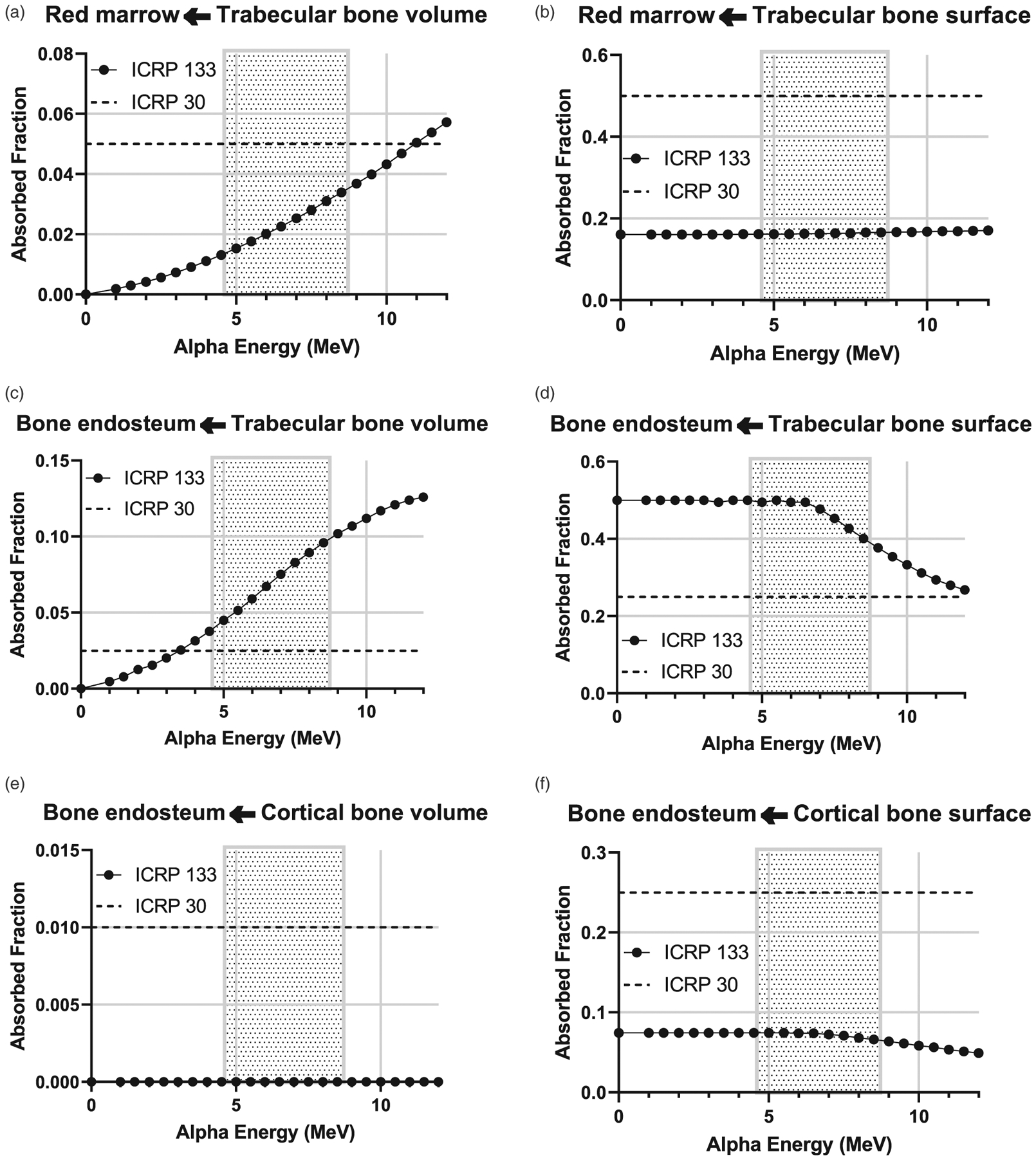
Plots of the fraction of the energy absorbed in a target tissue per alpha energy emitted from a skeletal source region. Data points (circles) are from ICRP Publication 133 (2016) and dashed lines are the values in ICRP Publication 30 (1979). The shaded region represents the range of alpha particle energies emitted in the 226Ra and 228Ra decay chains.
Age and sex dependency of dosimetric calculations
As referenced earlier, the updated dosimetry calculations will include consideration of the age and sex of the workers at the time of their ingestion of radium. This will impact both the biokinetic, or activity term in Equation (2) and the energy absorption term.
Figure 7 is a histogram of the ages of dial workers when they began their work. Most dial workers were teenagers at the time of first employment (see also Figure 2) and therefore at the time they were ingesting radium. Figure 8 gives the absorbed dose rates for selected target tissues due to ingestion of 1 Bq of 226Ra as a function of time after intake. Due to significant differences in skeletal growth at age 15 compared to age 25 (adult), intake of radium as a teenager will result in extended retention of radium and its progeny in the skeleton.
Figure 7.
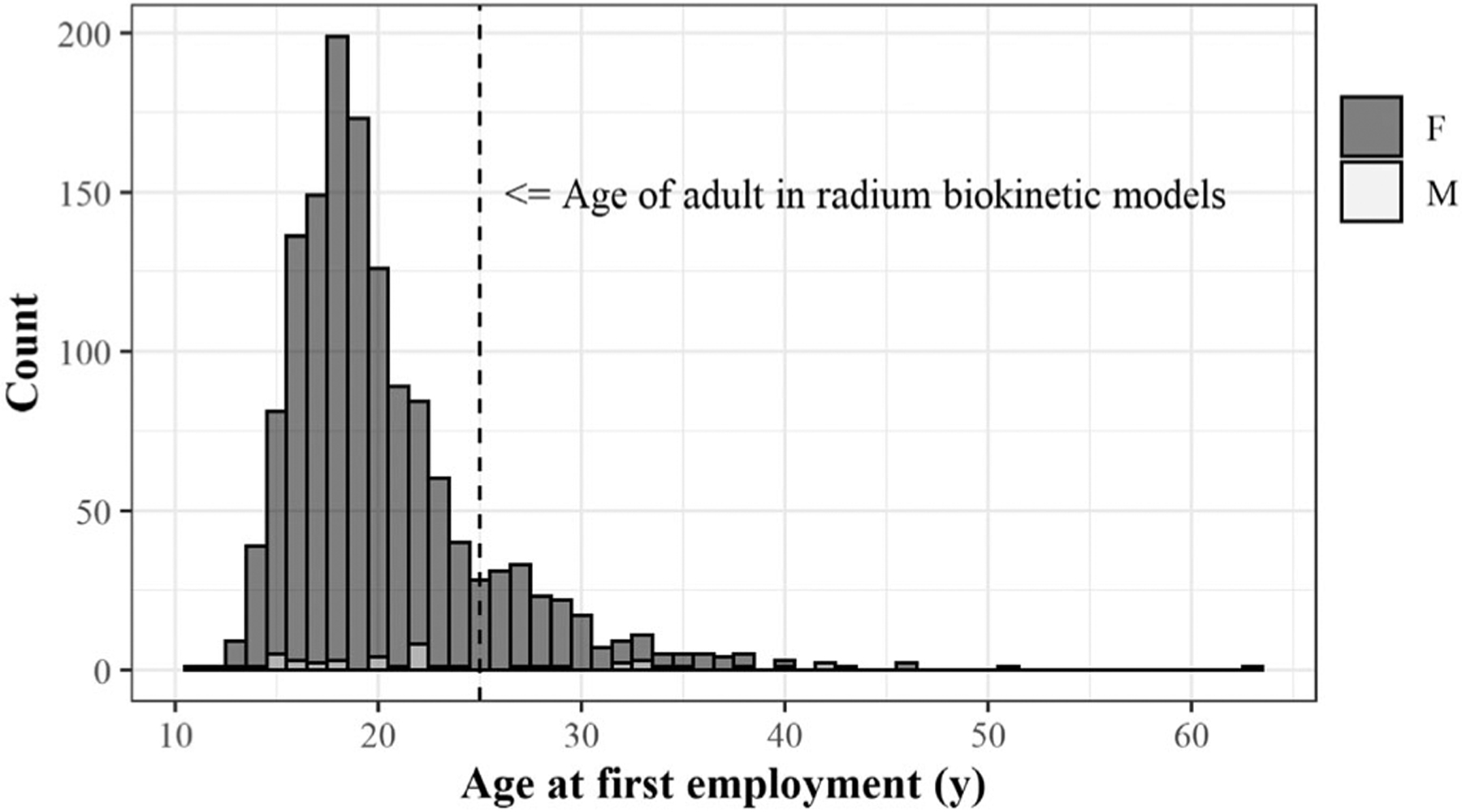
1,558 members of the radium dial painter cohort by sex and age at time of first employment. The vertical dashed line is at age 25 years and is the age of the adult in systemic biokinetic models for radium and its progeny.
Figure 8.
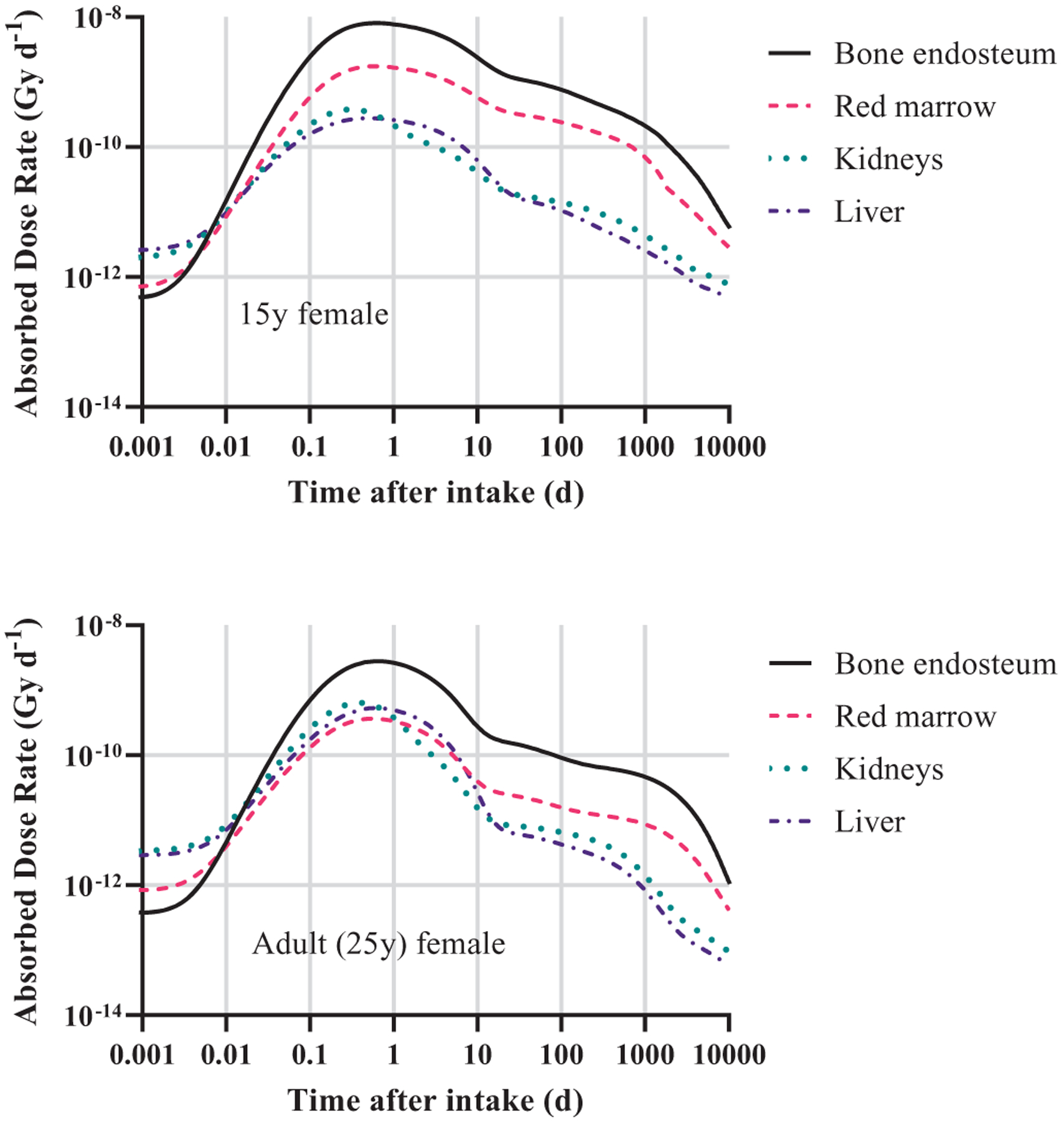
Absorbed dose rates to selected target regions for ingestion of 1 Bq of 226Ra in a reference 15-year-old female (top) and reference adult female (bottom). The plots include contributions from radium progeny created post-ingestion.
Also of note is that age and sex influence body weight. ICRP Publication 23 (International Commission on Radiological Protection (ICRP) 1975) lists weight of the reference total body as 70 kg for men and 58 kg for women. Current ICRP reference total body masses are 60 kg for adult females and 53 kg for 15-year-old females (International Commission on Radiological Protection (ICRP) 2009, 2020a). The United States did not start collecting detailed, comprehensive data on heights and weights of Americans until 1960 (Stoudt et al. 1960; Flegal 2009), although pockets of data were collected as early as 1858 for adults (Hathaway and Foard 1960) and 1877 for children and teenagers (Hathaway 1957). Interestingly, a 1923 dataset drawing from 12 schools in the North Eastern and North Central states (about 55,000 girls) determined a standard weight for 15-year-old females (depending on height) to be about 53 kg (Hathaway 1957), giving additional confidence in applying the current ICRP model to teenagers in our cohort.
Unique temporal aspects of dose resulting from 226Ra and 228Ra
The two radium decay chains (Figure 1) have several differences in their half-lives and elemental constituents. As mentioned previously, 226Ra has a significantly longer half-life than 228Ra and is itself an alpha emitter unlike the beta-emitting 228Ra. However, the comparatively short half-life of 228Ra allows for in-growth of the alpha-emitting progeny 228Th and 224Ra which are important dose contributors. Figure 9 contains plots of the absorbed dose rate to selected target tissues at times following the ingestion of 1 Bq of the two radium isotopes. Note that the dose rate due to 226Ra and progeny peaks at under 1-day following ingestion. Conversely, the absorbed dose rate due to 228Ra and progeny reaches has two peaks. The early peak at 1 day is due to dose from the beta-emitting 228Ra and removal of the non-absorbed radium via the alimentary tract. The late peak occurring just before 1000 days after ingestion is due to the in-growth of alpha-emitting progeny 228Th, 224Ra and others from the fraction of radium which was absorbed into the skeleton and systemic tissue. It takes time for the in-growth of important 228Ra progeny in the body.
Figure 9.
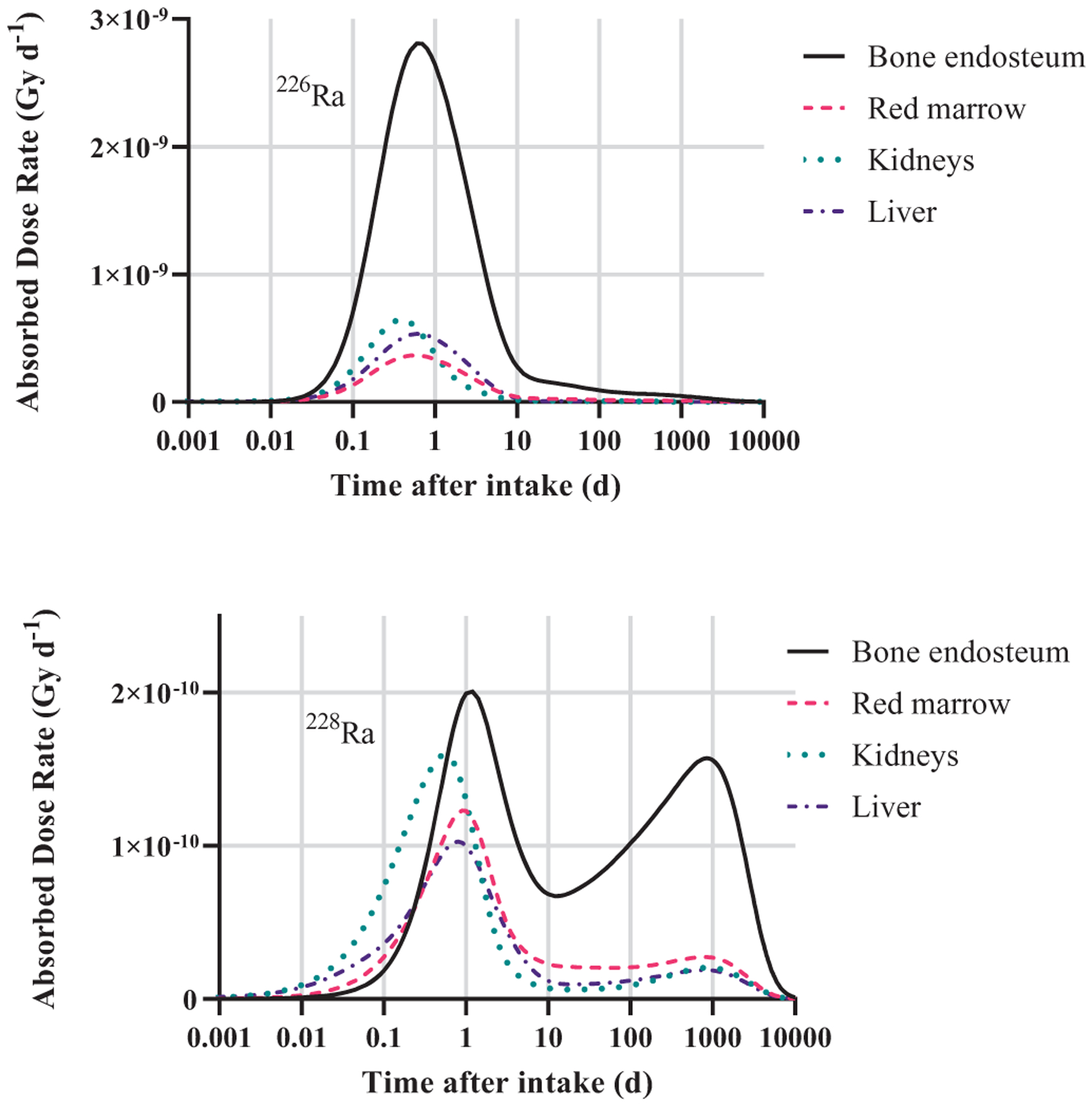
Absorbed dose rate to selected targets versus time following the ingestion of 1 Bq of 226Ra (top) and 228Ra (bottom) including contributions from radium progeny created post-ingestion.
By computing dose rate and annualized doses, the current study will allow for the important temporal contributions of the two radium isotopes and their progeny to be included in the dosimetry. This is particularly important since radiogenic disease occurred at varying times in members of the cohort.
Rowland, Stehney and Lucas (1978), among others, previously described various strategies for weighting 228Ra compared to 226Ra for the purpose of assessing dose-effect relationships, ultimately assigning different values for different biological effects. For example, Rowland, Stehney and Lucas (1978) describe three different ways to weight 228Ra with respect to the induction of osteosarcomas: comparative effective alpha energy per decay, comparative energy deposited over the average time to sarcoma appearance, and comparison of dose-response curves between two groups with intakes of predominantly one isotope. The average value of these approaches, 2.5, was taken for comparative effectiveness of 228Ra in the induction of osteosarcomas. Corresponding dose-response relationships (where ‘dose’ is taken to be systemic intake) were developed and analyzed for a total systemic intake of 226Ra activity plus 2.5 times 228Ra activity.
Comparatively, only 226Ra intake was considered in developing dose-response relationships for the induction of mastoid and paranasal sinus carcinomas. None of these types of cancers were observed in those whose intake was primarily 228Ra; this has been attributed to the importance of radon progeny accumulating in the sinus cavities, as touched on previously (Rowland 1994). 220Rn (t1/2 = 55.6 s), fifth progeny of 228Ra, does not have time to migrate to the sinus cavities, compared to 222Rn (t1/2 = 3.82 d), first progeny of 226Ra (Figure 1).
Missing body burden measurements
A job exposure matrix will be developed to provide body burden estimates, based on work history, for those members of the cohort without body burden measurements. This matrix will use the ingestion rate for other cohort members performing the same task in the same workplace during the same period of time. A model will be developed to provide a probability distribution of possible doses to these individuals.
Additional routes of exposure
Dial workers were exposed to gamma radiations emitted from the paint and inhalation of airborne activity. The magnitude of these exposures depends on the working conditions, e.g. number of coworkers, ventilation, and work practices. Bloomfield and Knowles (1933) surveyed several facilities and reported that painters were daily handling 50–500 μg (1.9–19 MBq) of radium. Measured external dose rates ranged from 0.7 to 46 cGy/y (0.8–5.3 μGy/h). Airborne activity of radium and radon were also observed. The average airborne activity concentration of radon was 0.051 μCi/m3 (1.8 kBq/m3) and that of radium was 260 pCi/m3 (9.6 Bq/m3). Estimates of the dose to the female breast and other tissues can be derived from these measurements and applying the dose rate coefficients of ICRP Publication 144 (International Commission on Radiological Protection (ICRP) 2020b). The dose contribution due to inhalation of airborne radium would largely follow the above methodology with the respiratory tract being the entrance into the body. The dose contribution of the airborne radon can be derived using the methods of ICRP Publication 137 (International Commission on Radiological Protection (ICRP) 2017) with due consideration to the workplace air ventilation and potential unattached radon short-lived progeny. Tissue dose coefficients can be derived from the measured external dose rates using the dose rate coefficients of ICRP Publication 144 (International Commission on Radiological Protection (ICRP) 2020b), which are based on the computational phantoms of ICRP Publication 110 (International Commission on Radiological Protection (ICRP) 2009), in the manner outlined in Appendix E of NCRP Report 178 (National Council on Radiation Protection and Measurements (NCRP) 2018).
These contributions are in addition to the potential contribution of internally deposited radium. Rowland et al. (1989) concluded that the elevated breast cancer risk among the dial painters cannot be attributed to the external dose and questioned the potential contribution of internally deposited radium. This conclusion is consistent with the studies in the United Kingdom (UK) where the habit of licking brushes was not seen, and any radiation exposure was primarily from external gamma radiation (mean absorbed breast dose 330 mGy) (Baverstock and Papworth 1989). The possibility that external radiation as well as radium intake among young women might be related to breast cancer (Stebbings 2001) will be reexamined, addressing personal characteristics, such as nulliparity, which are related to breast cancer risk (Adams and Brues 1980; Schieve et al. 1997).
Applicability to current exposure circumstances
The current work ultimately seeks to address female-specific health risks following intakes of radium; provide information on public and worker health risks relevant to environmental cleanups of former nuclear facilities and weapons testing; provide information on cognitive function following brain exposure relevant to flight crews at high altitude as well as astronaut crews on space missions; provide insights and information relevant to novel clinical therapeutic uses of radium and alpha emitting isotopes; and yield new scientific quantitative knowledge on the risks associated with radium- and radon progeny-induced cancers of the bone, bone marrow, mastoid and paranasal sinus cells, lung, breast, brain and heart.
As a specific example of broader relevance, in clinical oncology there is increasing interest in therapy or a combined imaging and therapy (i.e. theranostics) with alpha particle emitting radiopharmaceuticals (International Commission on Radiological Protection (ICRP) 2019; Nelson et al., 2020). Initially, treatment of diffuse skeletal or bone metastases were explored (Pandit-Taskar et al. 2004) and 223Ra-dichloride has been shown effective for castration-resistant prostate cancer bone metastases (Parker et al. 2013; Dauer et al. 2014; Pandit-Taskar et al. 2014). In addition, alpha particle emitters are increasingly being evaluated for radioimmunotherapies (Sgouros et al. 2010; Larson et al. 2015) that can deliver high LET, short range efficient tumor cell killing while sparing nearby healthy tissue (Jurcic and Rosenblat 2014). As these clinical applications are moving forward, there is interest in addressing uncertainties associated with actual local absorbed doses (especially in the bone) and the associated selection of the most appropriate radiation weighting factors for alpha dosimetry (Sgouros et al. 2010; Lassmann and Nosske, 2013). This updated study of the radium dial worker cohort and the associated improvements in the understanding of the dosimetry of alpha emitters (along with their progeny) in the body will be informative for the ongoing clinical development of effective and optimal treatment protocols using radium or other alpha particles.
Conclusion
In the 1967 report proposing a National Center of Human Radiobiology (Rowland 1994), Evans made arguments still relevant today for continuing the study of the dial workers, e.g.: the nuclear era necessitates valid radiation protection criteria; human protection criteria are best derived from human evidence and, despite tragic outcomes for many of the exposed and their families, the dial workers represent a unique scientific opportunity not likely to be repeated; and gaining information from this group will benefit current and future generations, with progressing results likely applicable to other exposure scenarios not yet envisioned (such as in medicine, environmental contamination and space exploration).
Acknowledgements
We are tremendously indebted to innumerable individuals, including the scientists, physicians, epidemiologists, statisticians, technicians, librarians, etc. who have studied these workers and who have published and otherwise preserved associated information since the 1920s. We also acknowledge that in many cases, radium dial workers suffered tremendously and died, and that this suffering and loss was also acutely felt by their loved ones and their communities.
Funding
The study of radium dial painters, a component of the Million Person Study, is supported in part by grants from the US Department of Energy [Grant No. DE-AU0000042 and DE-AU0000046] awarded to the National Council on Radiation Protection and Measurements, and a grant from the National Aeronautics and Space Administration [80NSSC17M0016]. Further, contract support was received by Oak Ridge National Laboratory from the Office of Radiation and Indoor Air, US Environmental Protection Agency, under Interagency Agreement DOE No. 1824 S581-A1, under contract No. DE-AC05-00OR22725 with UT-Battelle. The United States Transuranium and Uranium Registries is funded by US Department of Energy, Office of Domestic and International Health Studies (AU-13), under grant award DE-HS0000073 to the College of Pharmacy and Pharmaceutical Sciences at Washington State University.
Biographies

Nicole E. Martinez, Certified Health Physicist, is an Associate Professor at Clemson University in the Department of Environmental Engineering and Earth Sciences. She also holds a Joint Faculty Appointment at Oak Ridge National Laboratory (ORNL) within the Center for Radiation Protection Knowledge (CRPK). Her current research focuses on dosimetric modeling and the behavior and effects of radiological contaminants in the environment. She serves on ICRP Committee 4, Application of the Commission’s Recommendations.

Derek W. Jokisch is Professor of Physics and Chair of the Department of Physics and Engineering at Francis Marion University in Florence, South Carolina. He holds a Joint Faculty Appointment at ORNL within the CRPK and is a member of ICRP Committee 2 on Doses from Radiation Exposure and is a member of the US Scientific Review Group for the Department of Energy’s Russian Health Studies program.

Lawrence T. Dauer is Associate Attending Physicist specializing in radiation protection at Memorial Sloan Kettering Cancer Center in the Departments of Medical Physics and Radiology. He is a Council and Board member of the National Council on Radiation Protection and Measurements (NCRP) and served as a member of the ICRP Committee 3, Protection in Medicine.

Keith F. Eckerman retired in 2013 from ORNL and is an emeritus member of the NCRP and ICRP Committee 2. He served on ICRP Committee 2 for over 20 years and chaired their task group on dose calculations.

Ron Goans has worked in the field of nuclear physics and radiation effects since 1966. He received his PhD in radiation physics from the University of Tennessee in 1974, his MD from the George Washington University School of Medicine in 1983, and the MPH from the Tulane School of Public Health and Tropical Medicine in 2000. He is currently Senior Medical Advisor with MJW Corporation and Senior Medical/Scientific Advisor with the Radiation Emergency Assistance Center/Training Site (REAC/TS).

John D. Brockman is an Associate Professor in the Department of Chemistry at the University of Missouri. He researches problems in diverse fields that benefit from radio-analytical techniques. Currently, his research group is working on problems in pre-and post-detonation nuclear forensic analysis, trace element epidemiology, and nuclear engineering.

Sergey Y. Tolmachev is a Research Professor in the College of Pharmacy and Pharmaceutical Sciences, Washington State University, where he directs the United States Transuranium and Uranium Registries and the associated National Human Radiobiology Tissue Repository. He has over 20 years of experience in the development of analytical methods and in actinide analyses of environmental and biological samples. Dr. Tolmachev is currently a Council member of the NCRP and is a vice-chair of NCRP Scientific Committee 6–12 ‘Development of Models for Brain Dosimetry for Internally Deposited Radionuclides’.

Maia Avtandilashvili is an Assistant Research Professor at the U.S. Transuranium and Uranium Registries (USTUR), College of Pharmacy and Pharmaceutical Sciences, Washington State University. She has over 15 years of experience in internal radiation dosimetry and biokinetic modeling of actinides. Dr. Avtandilashvili serves on NCRP scientific committee (SC) 6–12 ‘Development of Models for Brain Dosimetry for Internally Deposited Radionuclides’ and is a member of the European Radiation Dosimetry Group (EURADOS) Working Group 7 on Internal Dosimetry.

Michael T. Mumma is the Director of Information Technology at the International Epidemiology Institute and the International Epidemiology Field Station for Vanderbilt University Medical Center. He has over 20 years of experience in data analysis and conducting epidemiologic investigations. He has published on methodological topics, including geocoding and comprehensive radiation exposure assessment, and is currently developing methods to determine socioeconomic status based on residential history.

John D. Boice is past President of the NCRP and Professor of Medicine at Vanderbilt University. He is an international authority on radiation effects and served on the Main Commission of the ICRP and on the United Nations Scientific Committee on the Effects of Atomic Radiation. He directs the Million Person Study of Low-Dose Health Effects.

Rich Leggett is a research scientist in the Environmental Sciences Division at ORNL. His main research interest is in physiological systems modeling, with primary applications to the biokinetics and dosimetry of radionuclides and radiation risk analysis. He is a member of ICRP Committee 2 and the ICRP Task Group on Internal Dosimetry.
Footnotes
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Adams EE, Brues AM. 1980. Breast cancer in female radium dial workers first employed before 1930. J Occup Med. 22:583–587. [PubMed] [Google Scholar]
- Adams EE, Brues AM, Anast GA. 1983. Survey of ocular cataracts in radium dial workers. Health Phys. 44(Suppl 1):73–79. [DOI] [PubMed] [Google Scholar]
- Aub JC, Evans RD, Hemplelmann LH, Martland HS. 1952. The late effects of internally-deposited radioactive materials in man. Medicine. 31(3):221–329. [DOI] [PubMed] [Google Scholar]
- Azizova TV, Bannikova MV, Grigoryeva ES, Rybkina VL, Hamada N. 2020. Occupational exposure to chronic ionizing radiation increases risk of Parkinson’s disease incidence in Russian Mayak workers. Int J Epidemiol. 49(2):435–447. [DOI] [PubMed] [Google Scholar]
- Baverstock KF, Papworth DG. 1989. The UK radium luminizer survey. In: Taylor DM, Mays CW, Gerber GB, Thomas RG, editors. BIR Report 21: Risks from Radium and Thorotrast. London (UK): British Institute of Radiology; p. 72–76. [Google Scholar]
- Bloomfield JJ, Knowles FL. 1933. Health aspects of radium dial painting. II. Occupational environment. J Indust Hygiene. 15:368–382. [Google Scholar]
- Blum T 1924. Osteomyelitis of the mandible and maxilla. J Am Dent Assoc. 11:802–805. [Google Scholar]
- Boice JD, Cohen SS, Mumma MT, Dupree-Ellis E, Eckerman KF, Leggett RW, Boecker B, Brill A, Henderson B. 2006. Mortality among radiation workers at Rocketdyne (Atomics International), 1948–1999. Radiat Res. 166(1):98–115. [DOI] [PubMed] [Google Scholar]
- Boice JD Jr, Cohen SS, Mumma MT, Ellis ED. 2019. The Million Person Study, whence it came and why. Int J Radiat Biol. 1–14. doi: 10.1080/09553002.2019.1589015. [DOI] [PubMed] [Google Scholar]
- Boice JD Jr, Quinn B, Ansari A, Blake PK, Blattnig SR, Caffrey EA, Cohen SS, Golden AP, Held KD, Jokisch DW, Mumma MT, Samuels C, Till JE, Tolmachev SY, Yoder RC, Zhou J, Dauer LT. 2021. A million persons, a million dreams: the study of one million radiation workers and veterans. Health Phys. (submitted) [Google Scholar]
- Bolch WE, Shah AP, Watchman CJ, Jokisch DW, Patton PW, Rajon DA, Zankl M, Petoussi-Henss N, Eckerman KF. 2007. Skeletal absorbed fractions for electrons in the adult male: considerations of a revised 50-mm definition of the bone endosteum. Radiat Prot Dosim. 127(1–4):169–173. [DOI] [PubMed] [Google Scholar]
- Bruenger FW, Lloyd RD. 1989. The influence of age at time of exposure to Ra-226 or Pu-239 on distribution, retention, post-injection survival and bone tumor induction in beagle dogs. In: Thirty-fourth Annual Meeting of the Health Physics Society, Abstracts of Papers Presented at the Meeting Vol. 56 (Suppl. l), 27; June 25–29, 1989; Albuquerque, NM: Albuquerque Convention Center; New York (NY): Pergamon Press. [Google Scholar]
- Bruenger FW, Smith JM, Atherton DR, Jee WSS, Lloyd RD, Stevens W. 1983. Skeletal retention and distribution of Ra-226 and Pu-239 in beagles injected at ages ranging from 2 days to 5 years. Health Phys. 44:513–527. [DOI] [PubMed] [Google Scholar]
- Campbell KM, Deck D, Krupski A. 2008. Record linkage software in the public domain: a comparison of Link Plus, The Link King, and a ‘basic’ deterministic algorithm. Health Informatics J. 14(1):5–15. [DOI] [PubMed] [Google Scholar]
- Castle WB, Drinker KR, Drinker CK. 1925. Necrosis of the jaw in workers employed in applying a luminous paint containing radium. J Ind Hyg. 8:371–382. [Google Scholar]
- Cothern CR, Smith JE Jr. 1987. Environmental radon. New York (NY): Springer. [Google Scholar]
- Dauer LT, Williamson MJ, Humm J, O’Donoghue J, Ghani R, Awadallah R, Carrasquillo J, Pandit-Taskar N, Aksnes A-K, Biggin C, et al. 2014. Radiation safety considerations for the use of 112RaCl2 in men with castration-resistant prostate cancer. Health Phys. 106(4):494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Energy (DOE). 2018. DOE 2017 occupational radiation exposure. Washington (DC): US Department of Energy, Office of Environment, Health, Safety and Security, The Radiation Exposure Monitoring System (REMS). [Google Scholar]
- Department of Energy (DOE). 2021. Comprehensive epidemiological data resource (CEDR). [accessed 2020 Dec 18]. https://oriseapps.orau.gov/cedr/default.aspx.
- Department of Labor (DOL). 2011. The Fair Labor Standards Act of 1938, as amended. Washington (DC): US Deptartment of Labor, Wage and Hour Division. [accessed 2021 Jan 12]. http://purl.fdlp.gov/GPO/gpo24709. [Google Scholar]
- Ellis ED, Boice JD, Golden AP, Girardi DJ, Cohen SS, Mumma MT, Shore RE, Leggett RW, Kerr GD. 2018. Dosimetry is key to good epidemiology: workers at Mallinckrodt Chemical Works had seven different source exposures. Health Phys. 114(4):386–397. [DOI] [PubMed] [Google Scholar]
- Flegal KM.2009. Trends in body weight and overweight in the U.S. population. Nutr Rev. 54(4):S97–S100. [DOI] [PubMed] [Google Scholar]
- Fry SA. 1998. Studies of U.S. radium dial workers: an epidemiological classic. Radiat Res. 150(5):S21–S29. [PubMed] [Google Scholar]
- Goans RE, Iddins CJ, Christensen D, Wiley A, Dainiak N. 2015. Appearance of Pseudo Pelger Huet anomaly after exposure to ionizing radiation in vivo. Health Phys. 108(3):303–307. [DOI] [PubMed] [Google Scholar]
- Goans RE, Iddins CJ, Ossetrova NI, Ney PH, Dainiak N. 2017. The Pseudo-Pelger Hu€et cell – a new permanent radiation biomarker. Health Phys. 112(3):252–257. [DOI] [PubMed] [Google Scholar]
- Goans RE, Toohey RE, Iddins CJ, McComish SL, Tolmachev SY, Dainiak N. 2019. The pseudo-Pelger Huët cell as a retrospective dosimeter: analysis of a radium dial painter cohort. Health Phys. 117(2):143–148. [DOI] [PubMed] [Google Scholar]
- Gossner W 2003. Target cells in internal dosimetry. Radiat Prot Dosim. 105: 39–42. [DOI] [PubMed] [Google Scholar]
- Gossner W, Masse R, Stather JW, 2000. Cells at risk for dosimetric modeling relevant to bone tumour induction. Radiat Prot Dosim, 1. 92:209–213. [Google Scholar]
- Gunderman RB, Gonda AS. 2015. Radium girls. Radiology. 274(2): 314–318. [DOI] [PubMed] [Google Scholar]
- Hathaway ML. 1957. Heights and weights of children and youth in the United States. home economics research report No. 2. Washington (DC): US Department of Agriculture. [Google Scholar]
- Hathaway ML, Foard ED. 1960. Heights and weights of adults in the United States. Home Economics Research Report No. 10. Washington (DC): US Department of Agriculture. [Google Scholar]
- Hine GJ. 1977. The inception of photoelectric scintillation detection commemorated after three decades. J Nucl Med. 18(9):867–871. [PubMed] [Google Scholar]
- Hoffman FL. 1925. Radium (mesothorium) necrosis.JAMA. 85(13): 961–965. [Google Scholar]
- International Commission on Radiological Protection (ICRP). 1973. Alkaline earth metabolism in adult man (ICRP Publication 20). Health Phys. 24(2):125–221. [PubMed] [Google Scholar]
- International Commission on Radiological Protection (ICRP). 1975. Report of the task group on reference man (ICRP Publication 23). Oxford (UK): Pergamon Press. [Google Scholar]
- International Commission on Radiological Protection (ICRP). 1979. Limits for intakes of radionuclides by workers (ICRP Publication 30). Ann ICRP. 2(3/4):1–116. [PubMed] [Google Scholar]
- International Commission on Radiological Protection (ICRP). 1993. Age-dependent doses to members of the public from intake of radionuclides: Part 2, ingestion dose coefficients (ICRP Publication 67). Ann ICRP. 23(3/4):1–167. [PubMed] [Google Scholar]
- International Commission on Radiological Protection (ICRP). 2008. Nuclear decay data for dosimetric calculations (ICRP Publication 107). Ann ICRP. 38(3):1–96. [DOI] [PubMed] [Google Scholar]
- International Commission on Radiological Protection (ICRP). 2009. Adult reference computational phantoms (ICRP Publication 110). Ann ICRP. 39(2):1–110. [DOI] [PubMed] [Google Scholar]
- International Commission on Radiological Protection (ICRP). 2016. The ICRP computational framework for internal dose assessment for reference adults: specific absorbed fractions (ICRP Publication 133). Ann ICRP. 45(2):1–74. [DOI] [PubMed] [Google Scholar]
- International Commission on Radiological Protection (ICRP). 2017. Occupational intakes of radionuclides: Part 3 (ICRP Publication 137). Ann ICRP. 46(3/4):1–486. [DOI] [PubMed] [Google Scholar]
- International Commission on Radiological Protection (ICRP). 2019. Radiological protection in therapy with radiopharmaceuticals (Publication 140). Ann ICRP. 48(1):1–102. [DOI] [PubMed] [Google Scholar]
- International Commission on Radiological Protection (ICRP). 2020a. Paediatric reference computational phantoms (ICRP Publication 143). Ann ICRP. 49(1):1–299. [DOI] [PubMed] [Google Scholar]
- International Commission on Radiological Protection (ICRP). 2020b. Dose coefficients for external exposures to environmental sources (ICRP Publication 144). Ann ICRP. 49(2):1–147. [DOI] [PubMed] [Google Scholar]
- Jurcic JG, Rosenblat TL. 2014. Targeted alpha-particle immunotherapy for acute myeloid leukemia. Am Soc Clin Oncol Educ Book, 34. e126–131, e131. [DOI] [PubMed] [Google Scholar]
- Kathren RL, Tolmachev SY. 2019. The US Transuranium and Uranium Registries (USTUR): a five-decade follow-up of plutonium and uranium workers. Health Phys. 117(2):118–132. [DOI] [PubMed] [Google Scholar]
- Keane AT, Holtzmann RB, Rundo J. 1994. Prevalence of technical mesothorium in self-luminous compounds used by New Jersey radium dial workers (ANL/ER/CP–81143). Argonne (IL): Argonne National Laboratory. [Google Scholar]
- Keane AT, Schlenker RA. 1987. Long-term loss of radium in 63 subjects exposed at ages 6–46. In: Gerger H, Metivier H, Smith H, editors. Age-related factors in radionuclide metabolism and dosimetry. Proceedings of a workshop in Angers, November 26–28, 1986. Dordrecht (NL): Martinus Nijhoff Publishers; p. 127–135. [Google Scholar]
- Larson SM, Carrasquillo JA, Cheung N-KV, Press OW. 2015. Radioimmunotherapy of human tumours. Nat Rev Cancer. 15(6): 347–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann M, Nosske D. 2013. Dosimetry of 223Ra-chloride: dose to normal organs and tissues.Eur J Nucl Med Mol Imaging. 40(2): 207–212. [DOI] [PubMed] [Google Scholar]
- Leggett RW. 1992. A generic age-specific biokinetic model for calcium-like elements. Rad Prot Dosim. 41:183–198. [Google Scholar]
- Leggett RW, Tolmachev SY, Boice JD Jr. 2018. Potential improvements in brain dose estimates for internal emitters. Int J Rad Biol. 4:1–54. doi: 10.1080/09553002.2018.1554923. [DOI] [PubMed] [Google Scholar]
- Lloyd RD, Bruenger FW, Jones CW, Taylor GN, Mays CW. 1983a. Retention in mature beagles injected at 5 years of age. Radiat Res. 94(1):210–216. [PubMed] [Google Scholar]
- Lloyd RD, Bruenger FW, Mays CW, Jones CW. 1983b. Skeletal radon-to-radium ratios in neonatal, juvenile and mature beagles and in adult St. Bernards. Health Phys. 44:61–63. [PubMed] [Google Scholar]
- Lloyd RD, Jones CW, Bruenger FW, Atherton DR, Mays CW. 1983c. Radium retention and dosimetry in juvenile beagles. Radiat Res. 94(2):295–304. [PubMed] [Google Scholar]
- Lloyd RD, Taylor GN, Jones CW, Mays CW. 1983d. Radium retention and dosimetry in the St. Bernard. Radiat Res. 95(1):150–157. [PubMed] [Google Scholar]
- Lloyd RD, Mays CW, Atherton DR. 1976a. Distribution of injected Ra-226 and Sr-90 in the beagle skeleton. Health Phys. 30:183–189. [DOI] [PubMed] [Google Scholar]
- Lloyd RD, Mays CW, Atherton DR, Taylor GN, Van Dilla MA. 1976b. Retention and skeletal dosimetry of injected Ra-226, Ra-228, and Sr-90 in beagles. Radiat Res. 66(2):274–287. [PubMed] [Google Scholar]
- Lloyd RD, Mays CW, Taylor GN, Atherton DR, Bruenger FW, Jones CW. 1982. Radium-224 retention, distribution, and dosimetry in beagles. Radiat Res. 92(2):280–295. [PubMed] [Google Scholar]
- Marazziti D, Baroni S, Catena-Dell’Osso M, Schiavi E, Ceresoli D, Conversano C, Dell’Osso L, Picano E. 2012. Cognitive, psychological and psychiatric effects of ionizing radiation exposure.CMC. 19(12): 1864–1869. [DOI] [PubMed] [Google Scholar]
- Martland HS. 1926. Histopathology of certain anemias due to radio-activity. Proc NY Pathological Soc NS. 26:1–5. [Google Scholar]
- Martland HS. 1929. Occupational poisoning in manufacture of luminous watch dials. JAMA. 92(6):466. [Google Scholar]
- Martland HS. 1931. The occurrence of malignancy in radioactive persons. Am J Cancer. 15:2435. [Google Scholar]
- Martland HS, Conlon P, Knef JP. 1925. Some unrecognized dangers in the use and handling of radioactive substances: with especial reference to the storage of insoluble products of radium and mesothorium in the reticulo-endothelial system.JAMA. 85(23):1769–1776. [Google Scholar]
- Martland HS, Humphries RE. 1929. Osteogenic sarcoma in dial painters using luminous paint. Arch Path Lab Med. 7:406–417. [Google Scholar]
- Monthly Labor Review (MLR). 1926. Effects of use of radioactive sub-stances on the health of workers. MLR. 22(5):18–31. [Google Scholar]
- Moore K 2017. The radium girls: the dark story of America’s shining women. Naperville (IL): Sourcebooks. [Google Scholar]
- Mumma MT, Cohen SS, Sirko JL, Ellis ED, Boice JD Jr. 2018. Obtaining vital status and cause of death on a million persons. Int J Radiat Biol. 9:1–21. doi: 10.1080/09553002.2018.1539884. [DOI] [PubMed] [Google Scholar]
- Muth H, Glöbel B. 1983. Age dependent concentration of Ra-226 in human bone and some transfer factors from diet to human tissues. Health Phys. 44:113–121. [DOI] [PubMed] [Google Scholar]
- National Academies/National Research Council (NA/NRC). 1988. Health risks of radon and other internally deposited alpha-emitters: BEIR IV. Washington (DC): National Academies Press. [PubMed] [Google Scholar]
- National Council on Radiation Protection and Measurements (NCRP). 2009. Report No. 164: uncertainties in internal radiation dose assessment. Bethesda (MD): NCRP. [Google Scholar]
- National Council on Radiation Protection and Measurements (NCRP). 2018. Report No. 178: deriving organ doses and their uncertainty for epidemiologic studies (with a focus on the one million U.S. workers and veterans study of low-dose radiation health effects). Bethesda (MD): NCRP. [Google Scholar]
- National Council on Radiation Protection and Measurements (NCRP). 2019. Report No. 183: Radiation exposures in space and the potential for central nervous system effects: phase II. Bethesda (MD): NCRP. [Google Scholar]
- National Council on Radiation Protection and Measurements (NCRP). 2021. NCRP Draft SC 6–12 Commentary: development of kinetic and anatomical models for brain dosimetry for internally deposited radionuclides. Bethesda (MD): NCRP. [accessed 2021 February 20]. https://ncrponline.org/wp-content/themes/ncrp/Docs_in_Review/SC6-12_Council%20Review%20Draft_2021%2002%2016.pdf [Google Scholar]
- Nelson BJ, Andersson JD, Wuest F.2020. Targeted alpha therapy: progress in radionuclide production, radiochemistry, and applications. Pharmaceuticals. 13(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New York Times. 1928. Radium paint takes its inventor’s life. Obituary. New York Times TimesMachine, 15 November 1928, p. 29, column 5. [accessed 2021 Mar 11]. https://timesmachine.nytimes.com/ [Google Scholar]
- Norris WP, Speckman TW, Gustafson PF. 1955. Studies of the metabolism of radium in man. Am J Roentgen. 73:785–802. [PubMed] [Google Scholar]
- Nuclear Regulatory Commission (NRC). 2019. Radiation exposure information and reporting system (REIRS). US Nuclear Regulatory Commission. [accessed 2020 Feb 7]. https://www.reirs.com/. [DOI] [PubMed] [Google Scholar]
- Pandit-Taskar N, Batraki M, Divgi CR. 2004. Radiopharmaceutical therapy for palliation of bone pain from osseous metastases. J Nucl Med. 45(8):1358–1365. [PubMed] [Google Scholar]
- Pandit-Taskar N, Larson SM, Carrasquillo JA. 2014. Bone-seeking radiopharmaceuticals for treatment of osseous metastases, Part 1: alpha therapy with 223Ra-dichloride. J Nucl Med. 55(2):268–274. [DOI] [PubMed] [Google Scholar]
- Parihar VK, Allen BD, Caressi C, Kwok S, Chu E, Tran KK, Chmielewski NN, Giedzinski E, Acharya MM, Britten RA, Baulch JE, Limoli CL. 2016. Cosmic radiation exposure and persistent cognitive dysfunction. Sci Rep, 16:34774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar VK, Angulo MC, Allen BD, Syage A, Usmani MT, Passerat de la Chapelle E, Amin AN, Flores L, Lin X, Giedzinski E, Limoli CL. 2020. Sex-specific cognitive deficits following space radiation exposure. Front Behav Neurosci. 14:535885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fosså SD, Chodacki A, Wiechno P, Logue J, Seke M, Widmark A, 2013. Alpha emitter radium-223 and survival in metastatic prostate cancer.N Engl J Med. 369(3):213–223. [DOI] [PubMed] [Google Scholar]
- Parks NJ, Keane AT. 1983. Consideration of age-dependent radium retention in people on the basis of the beagle model. Health Phys. 44(Suppl 1):103–112. [DOI] [PubMed] [Google Scholar]
- Parks NJ, Pool RR, Williams JR, Wolf HG. 1978. Age and dosage-level dependence of radium retention in beagles. Radiat Res. 75(3): 617–632. [PubMed] [Google Scholar]
- Pasqual E, Boussin F, Bazyka D, Nordenskjold A, Yamada M, Ozasa K, Pazzaglia S, Roy L, Thierry-Chef I, de Vathaire F, Benotmane MA, Cardis E. 2021. Cognitive effects of low dose of ionizing radiation – lessons learned and research gaps from epidemiological and biological studies. Environ Int. 147:106295. [DOI] [PubMed] [Google Scholar]
- Polednak AP. 1978a. Bone cancer among female radium dial workers. Latency periods and incidence rates by time after exposure: brief communication. J Natl Cancer Inst. 60(1):77–82. [DOI] [PubMed] [Google Scholar]
- Polednak AP. 1978b. Long-term effects of radium exposure in female dial workers: differential white blood cell count. Environ Res. 15(2):252–261. [DOI] [PubMed] [Google Scholar]
- Polednak AP. 1980. Fertility of women after exposure to internal and external radiation. J Environ Pathol Toxicol. 4:457–470. [PubMed] [Google Scholar]
- Polednak AP. 1986. Thyroid tumors and thyroid function in women exposed to internal and external radiation. J Environ Pathol Toxicol Oncol. 7:53–64. [PubMed] [Google Scholar]
- Polednak AP, Stehney AF, Rowland RE. 1978. Mortality among women first employed before 1930 in the U.S. radium dial-painting painting industry. A group ascertained from employment lists. Am J Epidemiol. 107(3):179–195. [DOI] [PubMed] [Google Scholar]
- Priest ND. 1989. Alpha-emitters in the skeleton: an evaluation of the risk of leukaemia following intakes of plutonium 239. In: Taylor DM, Mays CW, Gerber GH, Thomas RG, editors. BIR report 21: risks from radium and thorotrast. London (UK): British Institute of Radiology; p. 159–165. [Google Scholar]
- Rowland RE. 1993. Low-level radium retention by the human body: a modification of the ICRP publication 20 retention equation. Health Phys. 65(5):507–513. [DOI] [PubMed] [Google Scholar]
- Rowland RE. 1994. Radium in humans. A review of U.S. studies. Report ANUER-3. Argonne (IL): Argonne National Laboratory. [Google Scholar]
- Rowland RE, Lucas HF Jr. 1982. Radium dial workers. Conference on radiation carcinogenesis: epidemiology and biological significance, Washington, DC, USA, 24 May 1982. [Google Scholar]
- Rowland RE, Lucas HF Jr. 1984. Radium-dial workers. In: Boice JD Jr. and Fraumeni JF Jr., editors. Radiation carcinogenesis: epidemiology and biological significance. New York (NY): Raven Press; p. 231–240. [Google Scholar]
- Rowland RE, Lucas HF, Schlenker RA. 1989. External radiation doses received by female radium dial painters. In: Taylor DM, Mays CW, Gerber GB, Thomas RG, editors. BIR report 21: risks from radium and thorotrast. London (UK): British Institute of Radiology; p. 67–72. [Google Scholar]
- Rowland RE, Stehney AF, Brues AM, Littman MS, Keane AT, Patten BC, Shanahan MM. 1978. Current status of the study of 226Ra and 228Ra in humans at the Center for Human Radiobiology. Health Phys. 35:159–166. [DOI] [PubMed] [Google Scholar]
- Rowland RE, Stehney AF, Lucas HF Jr. 1978. Dose-response relationships for female radium dial workers. Radiat Res. 76(2):368–383. [PubMed] [Google Scholar]
- Rowland RE, Stehney AF, Lucas HF. 1983. Dose-response relationships for radium induced bone sarcomas. Health Phys. 44(Suppl I):15–31. [DOI] [PubMed] [Google Scholar]
- Schieve LA, Davis F, Roeske J, Handler A, Freels S, Stinchcomb T, Keane A. 1997. Evaluation of internal alpha-particle radiation exposure and subsequent fertility among a cohort of women formerly employed in the radium dial industry. Radiat Res, 2147:236–244. [PubMed] [Google Scholar]
- Schlenker RA, Keane AT, Holtzman RB. 1982. The retention of 226Ra in human soft tissue and bone: Implications for the ICRP 20 alkaline earth model. Health Phys. 42:671–693. [DOI] [PubMed] [Google Scholar]
- Schwarz BC, Godwin WJ, Wayson MB, Dewji SA, Jokisch DW, Lee C, Bolch WE. 2021a. Specific absorbed fractions for a revised series of the UF/NCI pediatric reference phantoms: internal electron sources. Phys Med Biol. 66:035005. [DOI] [PubMed] [Google Scholar]
- Schwarz BC, Godwin WJ, Wayson MB, Dewji SA, Jokisch DW, Lee C, Bolch WE. 2021b. Specific absorbed fractions for a revised series of the UF/NCI pediatric reference phantoms: internal photon sources. Phys Med Biol. 66:035006. [DOI] [PubMed] [Google Scholar]
- Sgouros G, Roeske JC, McDevitt MR, Palm S, Allen BJ, Fisher DR, Brill AB, Song H, Howell RW, Akabani G, In oration with the SNM MIRD Committee: Bolch Wesley E., Brill A. Bertrand, Fisher Darrell R., Howell Roger W., Meredith Ruby F., Sgouros (Chair) George, Wessels Barry W., and Zanzonico Pat B.. 2010. MIRD pamphlet No. 22 (abridged): radiobiology and dosimetry of alpha-particle emitters for targeted radionuclide therapy. J Nucl Med. 51(2):311–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe WD. 1978. The New Jersey radium dial painters: a classic in occupational carcinogenesis. Bull Hist Med. 52(4):560–570. [PubMed] [Google Scholar]
- Spiers FW, Lucas HF, Rundo J, Anast GA. 1983. Leukemia incidence in the U.S. dial workers. Health Phys. 44(Suppl. 1):65–72. [DOI] [PubMed] [Google Scholar]
- Stebbings JH. 2001. Health risks from radium in workplaces: an unfinished story. Occup Med. 16:259–270. [PubMed] [Google Scholar]
- Stebbings JH, Lucas HF, Stehney AF. 1984. Mortality from cancers of major sites in female radium dial workers.Am J Ind Med. 5(6): 435–459. [DOI] [PubMed] [Google Scholar]
- Stewart E 1929. Radium poisoning. Industrial poisoning from radio-active substances. MLR. 28(6):20–95. [Google Scholar]
- Stoudt HW, Damon A, McFarland RA. 1960. Heights and weights of white Americans. Hum Biol. 32(4):331–341. [Google Scholar]
- Stram DO, Sokolnikov M, Napier BA, Vostrotin VV, Efimov A, Preston DL. 2021. Lung cancer in the Mayak workers cohort: risk estimation and uncertainty analysis. Radiat Res. 195:334–346. [DOI] [PubMed] [Google Scholar]
- Tolmachev SY, Swint MJ, Bistline RW, McClellan RO, McInroy JF, Kathren RL, Filipy RE, Toohey RE. 2019. USTUR special sessions roundtable: United States Transuranium and Uranium Registries (USTUR): a five-decade follow-up of plutonium and uranium workers. Health Phys. 117(2):211–222. [DOI] [PubMed] [Google Scholar]
- Yoder RC, Balter S, Boice JD Jr, Grogan H, Mumma M, Rothenberg L, Passmore C, Vetter R, Dauer L. 2021. Using personal monitoring data to derive organ doses for medical radiation workers in the Million Person Study – considerations regarding NCRP Commentary No. 30. J Radiol Prot. 41:118–128. [DOI] [PubMed] [Google Scholar]


