Abstract
Background
The aim of the study was to provide real-world data on the effectiveness and safety of a new fixed-ratio combination, insulin degludec/liraglutide (IDegLira) injection in Japanese patients with type 2 diabetes mellitus (T2DM).
Methods
The primary endpoint was the change in glycated hemoglobin (HbA1c) level 6 months after the introduction of IDegLira. We also examined the rate of achievement of target HbA1c 7% and the individualized HbA1c targets set for each patient. Baseline characteristics associated with the change in HbA1c were also assessed. Seventy-five patients with T2DM were included in the analysis.
Results
After the initiation of IDegLira, HbA1c decreased significantly from baseline with a change of -1.81% (baseline 9.61% and at 6 months 7.80%; P < 0.001). At baseline, the achievement rate of 7% HbA1c was 2.67% (n = 2), which increased to 36.0% (n = 27) after 6 months of IDegLira introduction (P < 0.05). The attainment rate of individualized HbA1c targets, which were set considering each patient’s characteristics, improved from 2.67% (n = 2) to 49.3% (n = 37) (P < 0.001). Regardless of sex, body mass index, estimated glomerular filtration rate, duration of diabetes, or history of glucagon-like peptide-1 receptor agonist use, IDegLira significantly reduced HbA1c, but a higher C-peptide index was associated with a greater reduction in HbA1c.
Conclusion
In this study, initiation of IDegLira in a real-world clinical setting was beneficial in lowering HbA1c in Japanese T2DM patients with inadequate glycemic control with existing therapy.
Keywords: Insulin degludec/liraglutide, Type 2 diabetes mellitus, C-peptide, Diabetes therapy, Pancreatic β-cell function
Introduction
A cross-sectional nationwide survey conducted in Japan (Japan Diabetes Clinical Data Management study; JDDM [1]) revealed that the target glycated hemoglobin (HbA1c) level is achieved in only half of all cases despite the availability of various diabetes medications [2]. Insulin and glucagon-like peptide-1 receptor agonist (GLP-1RA) are promising treatment intensification options for patients with type 2 diabetes mellitus (T2DM) who fail to achieve target HbA1c level with oral antidiabetic drugs alone [3, 4]. Over the past 20 years, the use of long-acting analog insulin and GLP-1RA has increased in Japanese patients with T2DM [5]. However, increasing the basal insulin level alone is not enough to improve postprandial blood glucose, and it also increases the risk of weight gain and hypoglycemia [6]. It is also important to consider that in real-world practice, complex insulin regimens, hypoglycemia, and weight gain are barriers to achieving HbA1c targets [7, 8]. On the other hand, use of GLP-1RA requires, among other things, that fasting blood glucose not be lowered sufficiently and that there be residual endogenous insulin secretion for the drug to be effective [9, 10]. Gastrointestinal (GI) events caused due to high doses of GLP-1RA are also a barrier to dose escalation [9]. These are the unmet needs for insulin injections and GLP-1RA. Combination formulations of GLP-1RA and basal insulin are now available in clinical practice. In September 2019, a new injectable fixed-ratio combination of insulin degludec and liraglutide (a daily injectable GLP-1RA), i.e., insulin degludec/liraglutide (IDegLira), was launched in Japan. In a global clinical trial involving Japanese patients (the DUAL Program), IDegLira showed superior hypoglycemic effects compared to basal insulin or GLP-1RA monotherapy [11-14]. The pathophysiology of T2DM in East Asians, including the Japanese, is known to be characterized by very low insulin secretion and the insulin resistance is mild compared to T2DM in other countries [15, 16]. Therefore, the evidence from studies conducted overseas, especially in Caucasian populations, may not be entirely applicable to Japanese patients. Moreover, Japanese population with T2DM is getting older [17], and individuals aged over 65 years are known to be at a higher risk of hypoglycemia [18]. The contemporary guidelines recommend individualization of target HbA1c level for elderly patients and the use of simpler and safer methods for reinforcement of treatment in case of inadequate glycemic control [19]. Therefore, there is a need to verify the effectiveness and safety of IDegLira in Japanese T2DM patients, but there is a paucity of real-world data (RWD) in this regard. The purpose of this study was to provide RWD on the efficacy and safety of a new combination injectable formulation, IDegLira, in Japanese patients with T2DM after 6 months of introduction.
Materials and Methods
Study design and participants
This was a single-center retrospective study. Patients with T2DM who were treated with IDegLira at the Jichi Medical University Saitama Medical Center between June 2021 and December 2022 were enrolled. We evaluated the clinical parameters 6 months after the initiation of IDegLira. The inclusion criteria for this study were: 1) Japanese patients with T2DM; 2) patients with an HbA1c level of 6.0% or higher; and 3) patients who were introduced to IDegLira in an outpatient setting. Exclusion criteria were: 1) acute metabolic complications (diabetic ketoacidosis and hyperglycemic hyperosmolar state); 2) patients undergoing treatment for infectious diseases; 3) initiation or reduction of steroids; 4) cancer detection or surgery during follow-up; 5) prescription started during hospitalization; 6) hospitalization for other acute diseases such as myocardial infarction within 6 months; and 7) drug change or addition during the observation period. In all cases, we confirmed that IDegLira was appropriately introduced according to the package insert.
Clinical parameters
Data pertaining to the following baseline demographic and clinical data were obtained from the medical records: age, sex, body mass index (BMI), estimated glomerular filtration rate (eGFR), duration of diabetes, presence of macroangiopathy and cardiovascular disease, concomitant medications, adverse events, and IDegLira dose at 6 months. In addition, we collected fasting serum C-peptide level measured within 6 months of IDegLira introduction. C-peptide index (CPI), as an indicator of pancreatic beta-cell function, was calculated as 100 × fasting C-peptide immunoreactivity (CPR) (ng/mL)/fasting plasma glucose (mg/dL) [20]. CPR was measured using an enzyme immunoassay kit (TOSO, Tokyo, Japan).
Study endpoints and exploratory endpoints
The primary outcome was the change in HbA1c (%) at 6 months after the introduction of IDegLira. The secondary outcome was the percentage of patients achieving an HbA1c of < 7%; for patients aged > 65 years, the percentage of patients achieving the HbA1c target set individually according to the Japanese Clinical Practice Guidelines for Diabetes 2019 and the Japan Diabetes Society (JDS)/Japan Geriatrics Society (JGS) Joint Committee’s “Glycemic control goals in elderly patients with diabetes” [19, 21]. Insulin, sulfonylurea (SU) drugs, and glinide were identified as drugs associated with a risk of hypoglycemia [21]. In this study, HbA1c targets for each age group were set based on the aforementioned guideline [19, 21]; targets for patients aged < 65, ≥ 65 to < 75, and ≥ 75 years were set at < 7.0%, < 7.5%, and 8.0%, respectively. Baseline background factors affecting the change in HbA1c were identified as exploratory outcomes. We also examined the change in HbA1c after the initiation of IDegLira by the use of pre-treatment injection-device users or not. Subsequently, safety information was also evaluated. The incidences of hypoglycemia and GI events were assessed using the medical records filed during the observation period (6 months). Hypoglycemia was defined using self-reported hypoglycemic symptoms and self-measured blood and plasma glucose levels reported at the time of the outpatient visit. The GI events were defined using self-reported GI symptoms (exploratory outcome).
Ethics statement
This study was conducted in accordance with the Declaration of Helsinki of 1964, as revised in 2013. The study was approved by the Ethics Committee of Jichi Medical University Saitama Medical Center (No. S19-005). This study used nonidentifiable data obtained by the treating physicians. Therefore, based on the decision from our local Ethics Committee of Jichi Medical University Saitama Medical Center (No. S19-005), informed consent was not required. Patients had the opportunity to object the usage of their data for retrospective scientific research; however, no patient objected the same. Patient consent was waived due to retrospective collection and analysis of deidentified demographic and medical data.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD) (Table 1) or 95% confidence intervals (CIs) (all figures and supplementary materials) and categorical variables were expressed as frequency (percentage). The Wilcoxon signed-rank sum test was used to compare the parameters before and after initiating IDegLira. Between-group differences in clinical parameters at a single time-point were compared using the Mann-Whitney U-test. Categorical variables were compared by performing McNemar’s test. The Spearman’s rank correlation coefficient was used for correlation analysis. Receiver-operating characteristic (ROC) curve analysis was performed to assess the predictive ability of CPI and CPR for the effectiveness of IDegLira. All statistical analyses were performed using EZR (Jichi Medical University, Saitama Medical Center) [22], a graphical user interface for R (The R Foundation for Statistical Computing), and a modified version of R commander designed to add statistical functions frequently used in biostatistics. P values < 0.05 were considered indicative of statistical significance. Sample size calculations were based on previous clinical studies [23, 24] and assumed a mean (standard deviation) HbA1c reduction of 0.6% (1.5%) 6 months after introduction of IDegLira. At α = 0.05 and 80% power, the total number of patients enrolled in the study was estimated to be at least 52. Assuming that 15-20% meet the exclusion criteria, it was estimated that 65 or more patients would need to be enrolled to achieve a final sample size of 52.
Table 1. Baseline Clinical Characteristics of the Study Population.
| Clinical parameters | Overall (n = 75) |
|---|---|
| Sex, men, n (%) | 41 (54.7) |
| Age (years) | 65.7 (13.5) |
| Body mass index (kg/m2) | 28.0 (4.74) |
| Body weight (kg) | 72.8 (13.9) |
| Duration of diabetes (years) | 16.3 (10.3) |
| Smoking, n (%) | 9 (12.0) |
| HbA1c (%) | 9.61 (2.1) |
| eGFR (mL/min/1.73 m2) | 62.0 (30.4) |
| Fasting plasma glucose (mg/dL) | 171.6 (74.4) |
| Fasting CPR (ng/mL) | 2.43 (1.55) |
| CPI (ng/mL per mg/dL) | 1.47 (0.95) |
| Complications | |
| Dyslipidemia, yes, n (%) | 23 (30.7) |
| Hypertension, yes, n (%) | 34 (45.3) |
| Ischemic heart disease, yes, n (%) | 9 (12.0) |
| Diabetic Retinopathy, yes, n (%) | 16 (21.3) |
| Diabetic nephropathy, yes, n (%) | 43 (57.3) |
| Medications | |
| Sulfonylurea, n (%) | 2 (2.7) |
| Biguanide, n (%) | 27 (36.0) |
| Glinide, n (%) | 15 (20.0) |
| α-Glucosidase inhibitor, n (%) | 8 (10.7) |
| Thiazolidinedione, n (%) | 2 (2.7) |
| SGLT2 inhibitor, n (%) | 33 (44) |
| DPP-4 inhibitor, n (%) | 25 (33.3) |
| OAD only, n (%) | 9 (12.0) |
| Insulin therapy, n (%) | 57 (76.0) |
| IDegAsp, n (%) | 2 (2.7) |
| GLP-1 receptor agonist, n (%) | 36 (48.0) |
| Liraglutide, n (%) | 9 (12.0) |
| Duraglutide, n (%) | 21 (28.0) |
| Lixisenatide, n (%) | 6 (8.0) |
Data presented as frequency (%) for categorical variables and as mean (± standard deviation) for continuous variables. HbA1c: glycated hemoglobin; eGFR: estimated glomerular filtration rate; CPR: C-peptide immunoreactivity; CPI: C-peptide index; eGFR: estimated glomerular filtration rate; SGLT2: sodium-glucose cotransporter 2; DPP-4: dipeptidyl peptidase 4; OAD: oral antidiabetic drug, IDegAsp: insulin degludec/aspart; iGlarLixi: insulin glargine/lixisenatide; GLP-1RA: glucagon-like peptide-1 receptor agonist.
Results
Primary and secondary outcomes
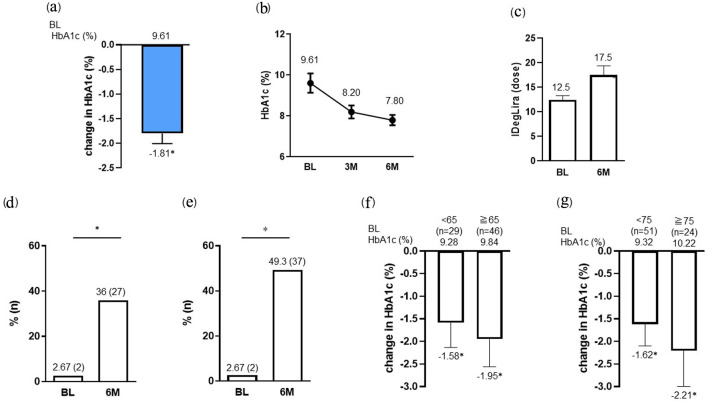
The clinical characteristics of the study population are summarized in Table 1. Out of 78 patients, three patients were excluded (two was hospitalized for treatment of acute infection, and one was hospitalized for surgery for lung cancer). The final analysis included 75 subjects, with a mean (SD) age of 65.7 (13.5) years, mean disease duration of 16.3 (10.3) years, and mean CPI of 1.47 (0.95) ng/mL per mg/dL. At 6 months after the initiation of IDegLira, HbA1c showed a significant decrease from baseline with a change of -1.81% (baseline HbA1c 9.61% versus at 6 months 7.80%; P < 0.001) (primary outcome, Fig. 1a, b). The dose of IDegLira was 12.5 units (12.5 units insulin degludec/0.45 mg liraglutide) at introduction and had increased significantly to 17.5 units (17.5 units insulin degludec/0.63 mg liraglutide) at 6 months (P < 0.01 vs. baseline initial dose) (Fig. 1c). The achievement rate of 7% HbA1c at 6 months after IDegLira introduction (36.0% (n = 27)) was significantly greater than that at baseline (2.67% (n = 2); P < 0.05) (Fig. 1d). Next, we examined the rate of target HbA1c attainment considering the patient’s background. The percentage of patients aged < 65 years (n = 29) achieving target HbA1c (< 7.0%) increased from 0% (n = 0) at baseline to 48.3% (n = 14) at 6 months. Likewise, the percentage of patients aged ≥ 65 to < 75 years (n = 22) achieving target HbA1c (< 7.5%) increased from 9.1% (n = 2) at baseline to 54.5% (n = 12). Furthermore, the percentage of patients aged ≥ 75 years (n = 24) achieving target HbA1c (< 8.0%) increased from 0% (n = 0) at baseline to 45.8% (n = 11). Overall, the target HbA1c attainment rate at 6 months after IDegLira introduction (49.3% (n = 37)) was significantly greater than that at baseline (2.78% (n = 1), P < 0.01) (Fig. 1e). On subgroup analysis (stratified by age under 65 or over and under 75 or over), both subgroups showed a significant reduction in HbA1c from baseline HbA1c after 6 months of IDegLira initiation (Fig. 1f, g) (secondary outcome).
Figure 1.
Change in HbA1c (a, b) and IDegLira dose (c) at 6 months after the initiation of IDegLira. Change in the proportion of patients achieving HbA1c < 7% (d) and the target HbA1c set according to the individual patient’s background (e) at 6 months after the initiation of IDegLira. Change in HbA1c from baseline by subgroups separated by age under 65 or over (f) and under 75 or over (g) years. Data are presented as mean with 95% CIs. *P < 0.01 by Wilcoxon signed-rank sum test (vs. BL HbA1c or dose of IDegLira in a, c, f and g). *P < 0.01 by McNemar’s test (in d and e) (vs. BL). HbA1c: glycated hemoglobin; IDegLira: insulin degludec/liraglutide; CIs: confidence intervals; BL: baseline.
Exploratory outcomes
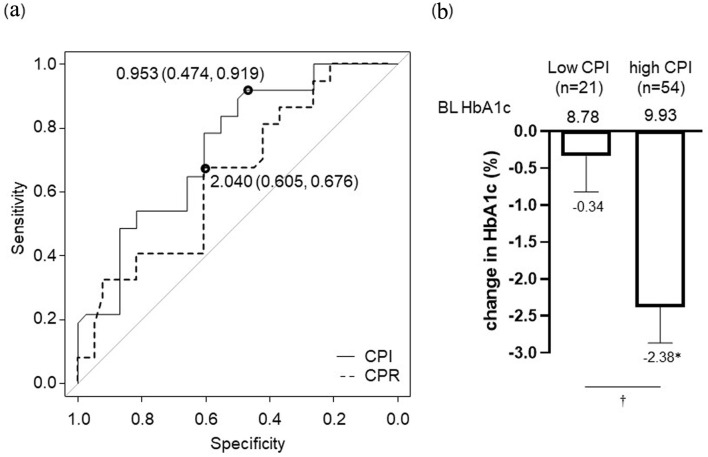
ROC curve analysis was performed to examine the ability of CPR and CPI to predict pancreatic β-cell function for achieving HbA1c set individually for each patient. The area under the curve for CPI (optimal cut-off value: 0.953) was larger than that for CPR (Fig. 2a). Therefore, we divided the patients into two groups: high CPI (CPI ≥ 0.953) and low CPI (CPI < 0.953) groups. Reductions in HbA1c from baseline in the low and high CPI groups were -0.34% and -2.38%, respectively, and the change in the high CPI group was statistically significant (P < 0.01). There was also a significant difference in the change in HbA1c between the low and high CPI groups (P < 0.01) (Fig. 2b) (exploratory outcome). There was no difference in the baseline HbA1c between these two groups (P = 0.07). On correlation analysis, change in HbA1c showed a correlation with baseline HbA1c and residual pancreatic β-cell function (CPR, CPI) (Table 2).
Figure 2.
Pancreatic β-cell function for achieving HbA1c target set individually for each patient (a). Optimal CPI cut-off point: 0.953 ng/mL per mg/dL, area under the receiver-operating characteristic curve: 0.739, 95% CI: 0.627 - 0.851, specificity: 47.4%, sensitivity: 91.9%. Optimal CPR cut-off point: 2.04 ng/mL, AUC: 0.649, 95% CI: 0.524 - 0.774, specificity: 60.5%, sensitivity: 67.6%. Change in HbA1c at 6 months after initiation of IDegLira in high CPI group (CPI ≥ 0.953) and low CPI group (CPI < 0.953) based on the optimal cut-off value (b). Data are shown as mean with 95% CIs. *P < 0.01 by Wilcoxon signed-rank sum test (vs. BL HbA1c). †P < 0.01 for comparison of change in HbA1c between high CPI group vs. low CPI group by Mann-Whitney U-test. ROC: receiver-operating characteristic; CPR: C-peptide immunoreactivity; CPI: C-peptide index; HbA1c: glycated hemoglobin; AUC: area under the curve; CI: confidence interval; IDegLira: insulin degludec/liraglutide; CIs: confidence intervals; BL: baseline.
Table 2. Correlation Between Change in HbA1c (%) and Baseline Clinical Parameters.
| Parameters | r | P-value |
|---|---|---|
| Age (years) | -0.066 | 0.575 |
| Body mass index (kg/m2) | -0.024 | 0.837 |
| Duration of diabetes (years) | 0.187 | 0.109 |
| Baseline HbA1c (%) | -0.725 | < 0.001 |
| eGFR (mL/min/1.73 m2) | -0.013 | 0.912 |
| Fasting plasma glucose (mg/dL) | -0.221 | 0.057 |
| Fasting CPR (ng/mL) | -0.582 | < 0.001 |
| CPI (ng/mL per mg/dL) | -0.468 | < 0.001 |
| IDegLira at 6 months (dose) | 0.110 | 0.345 |
| IDegLira at 6 months (dose/kg) | 0.107 | 0.359 |
HbA1c: glycated hemoglobin; eGFR: estimated glomerular filtration rate; CPR: C-peptide immunoreactivity; CPI: C-peptide index; IDegLira: insulin degludec/liraglutide.
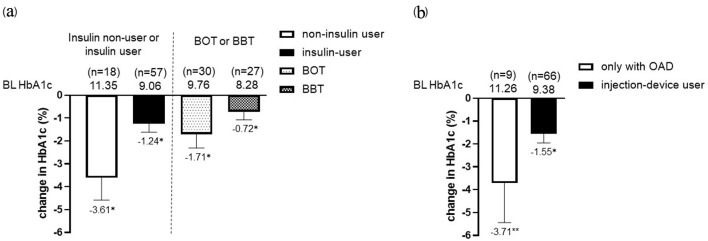
Regardless of sex, BMI, eGFR, duration of diabetes, or prior use of GLP-1RA, IDegLira significantly reduced HbA1c at 6 months after initiation (Fig. 3) (exploratory outcome). HbA1c significantly decreased from baseline in both insulin and non-insulin user groups. In addition, HbA1c significantly decreased from baseline in basal supported oral therapy (BOT) group and basal-bolus therapy (BBT) group. The results were similar for non-injection device users and injection-device users (Fig. 4). After the initiation of IDegLira, bolus insulin dose at 6 months remained unchanged (from 21.5 ± 4.2 to 19.2 ± 5.0, P = 0.14) (exploratory outcome).
Figure 3.
Change in HbA1c by subgroup: sex, BMI, eGFR, diabetes duration, and prior GLP-1RA use after initiation of IDegLira. *P < 0.01 by Wilcoxon signed-rank sum test (vs. BL HbA1c). Data are shown as mean with 95% CIs. HbA1c: glycated hemoglobin; BMI: body mass index; eGFR: estimated glomerular filtration rate; GLP-1RA: glucagon-like peptide-1 receptor agonist; IDegLira: insulin degludec/liraglutide; CIs: confidence intervals; BL: baseline.
Figure 4.
Change in HbA1c from baseline in the insulin non-user (n = 18) and user (n = 57) group (BOT; n = 30, BBT; n = 27) after the initiation of IDegLira (a). Change in HbA1c from baseline in only with OAD group and injection-device user group (insulin and GLP-1RA users) after the initiation of IDegLira (b). Data are presented as mean with 95% CIs. *P < 0.01 by Wilcoxon signed-rank sum test (vs. BL HbA1c). HbA1c: glycated hemoglobin; BOT: basal supported oral therapy; BBT: basal-bolus therapy; GLP-1RA: glucagon-like peptide-1 receptor agonist; OAD: oral antidiabetic drug; IDegLira: insulin degludec/liraglutide; CIs: confidence intervals; BL: baseline.
Safety
Hypoglycemia developed in three patients (4.0%) in this study, but no GI events were reported. These three patients were on insulin treatment. No hypoglycemia was observed in patients newly introduced to IDegLira who used GLP-1RA or oral antidiabetic drug (OAD). No GI events were reported (exploratory outcome).
Discussion
In this study, initiation of IDegLira was found to be useful in lowering HbA1c in patients with inadequate glycemic control with existing therapy. IDegLira also improved the rate of achievement of individualized HbA1c targets set in the real-world clinical setting.
DUAL I Japan was conducted in Japanese patients with T2DM who had inadequate glycemic control with oral antidiabetic drugs and IDegLira reduced the HbA1c by 2.38% from baseline (8.52%) [12]. In our cohort, the reduction was 1.81% (Fig. 1). One reason for this difference may be that DUAL I Japan study was conducted in injection-naive patients [12], whereas 76% patients in our study were insulin users and 48% were GLP-1RA users, most of whom were receiving injectable drugs and were poorly controlled.
Moreover, in DUAL I Japan, the study started with 10 doses of IDegLira, and the final dose was 27.75 doses after 52 weeks [12], whereas in our study, the dose was 17.5 doses (Fig. 1c) at the end of the observation (after 6 months), about 10 doses less. This may be due to the fact that each physician adjusted the IDegLira dose according to the patient’s condition, and that the observation period was 6 months, unlike DUAL I Japan [12], which was conducted for 52 weeks in a treat-to-target study, and some patients did not receive an adequate IDegLira dose increase. Age subgroup analysis of the DUAL I Japan/DUAL II Japan study showed greater HbA1c reductions with IDegLira compared to comparator drugs in subgroups aged < 65 years and ≥ 65 years [13]. Similarly, in our study, HbA1c-lowering effect was observed regardless of age group. Furthermore, subgroup analyses by sex, renal function, and BMI also suggested that IDegLira improved HbA1c regardless of baseline differences. The results suggest that IDegLira may be useful in patients with a wide range of background characteristics.
The average age of the subjects in this study was 65.7 years, and many of them were insulin users, a population that requires treatment with consideration for hypoglycemia. The life expectancy of the Japanese patients with T2DM is gradually lengthening [17, 25], and these patients require safe and simple intensified therapy to reduce the risk of hypoglycemia [19]. IDegLira is a single injection of basal insulin and liraglutide and may be well tolerated by the elderly [26]. Hypoglycemia was identified in three patients in this study, but no GI events were reported. The low incidence of GI symptoms events was likely attributable to the fact that 48% of the patients were already on GLP-1RA, a population with relatively high tolerability to GLP-1RA, and IDegLira doses were introduced starting at less than 10 doses for some patients. Post-hoc analysis of the DUAL I Japan/DUAL II Japan showed a higher rate (%) of treatment-induced hypoglycemia in patients aged ≥ 65 years than in those aged < 65 years, but the rate of hypoglycemia was consistently lower in the IDegLira group than in the insulin degludec (IDeg) group [13]. In our study, IDegLira significantly reduced HbA1c from baseline even in patients older than 75 years, and hypoglycemia occurred only in one patient on BBT (the other two patients: < 75 years). We observed an improvement in HbA1c with the initiation of IDegLira in patients on insulin therapy, irrespective of whether their previous therapy was BBT or BOT. Furthermore, improvement in HbA1c was observed after initiating IDegLira, regardless of whether the patient was a non-injection device or injection-device user. These results suggested that introducing IDegLira might be useful for improving HbA1c when previously used injection-based therapy did not help in achieving HbA1c target. Recent studies using continuous glucose monitoring have shown that IDegLira improves mean glucose and glycemic variability without increasing the risk of hypoglycemia compared to the combination of IDeg and dipeptidyl peptidase-4 inhibitors [27]; in addition, it is characterized by a greater increase in time in range compared to insulin degludec/insulin aspart (IDegAsp) [28]. Elderly patients on insulin therapy are known to be at risk of hypoglycemia regardless of their HbA1c level, and the risk increases with the duration of disease [29, 30]. The previously reported RWD [27, 28] in Japanese patients with T2DM and the one reported in the present study suggest that IDegLira may be a means of treatment intensification with less hypoglycemic risk for patients who have had an inadequate response to existing treatments, including insulin therapy.
Our study showed that residual endogenous insulin secretion is a requirement for achieving the target HbA1c by IDegLira (CPI ≥ 0.953). Some studies in Japanese patients with T2DM have shown that good response to liraglutide requires residual endogenous insulin secretion. In a detailed analysis by Usui et al, the CPI cut-off level for achieving < 7% HbA1c after 1 year of treatment with the combination of basal insulin and liraglutide was 1.103 [31]. In contrast, the CPI required to achieve HbA1c < 7.0% after 54 weeks of liraglutide initiation was higher (CPI 1.86) in patients with T2DM who started liraglutide monotherapy or sulfonylurea combination therapy [32]. Our results showed that CPI cut-off level to achieve HbA1c of < 7.0% after initiation of IDegLira (at 6 months) was approximately 1.51 (Supplementary Material 1, www.jocmr.org). Although sufficient endogenous insulin secretion is required to achieve good glycemic control with GLP-1RA alone, using the combination of basal insulin and liraglutide at a CPI of 1.0 - 1.1 may be a simpler approach to achieve the target HbA1c than using BBT, as shown in this study and the study conducted by Usui et al [31]. Our study was conducted for a relatively short time (6 months after the introduction of IDegLira). If we follow up these patients for more than 1 year, we can possibly increase IDegLira dose and probably reduce HbA1c below 7% even if the CPI is approximately 1.0. The results of our study indicate that target HbA1c may be achieved with the introduction of IDegLira in a relatively short period of time (6 months) based on the patient’s background. Notably, 30 patients used long-acting GLP-1RA (liraglutide, nine patients; dulaglutide, 21 patients) before IDegLira initiation. Long-acting GLP-1RA stimulates endogenous insulin secretion [33], thereby increasing fasting CPR and consequently increasing fasting CPI. The utility of assessing CPI during long-acting GLP-1RA administration should be assessed in the future. In this study, CPI was significantly higher in long-acting GLP-1RA users (CPI: 1.90 ± 1.27) than in non-users (CPI: 1.19 ± 0.50) (P = 0.03), but there was no significant difference in the change in HbA1c 6 months after IDegLira administration between the two groups (P = 0.31). Moreover, evaluation of CPI while using long-acting GLP-1RA may be a useful predictor of HbA1c improvement caused by the introduction of IDegLira, if values are retained to CPI around 1.0. In patients with and without diabetic nephropathy (DN), CPR was significantly higher in patients with DN, but CPI was not different. There was no difference in HbA1c change from baseline 6 months after introduction of IDegLira by presence of nephropathy (Supplementary Material 2, www.jocmr.org). Since there were few patients with advanced renal impairment in this study, it is possible that DN status (degree of eGFR and proteinuria) did not affect the effect of IDegLira.
Some limitations of this study should be considered. First, this was a single-armed retrospective study with a small sample size and no comparator drug. The potential confounding effect of some of the patient background characteristics could not be accounted for in the analysis. Adverse events such as hypoglycemia and GI events may also have been underestimated. Second, data on the changes in body weight were missing and could not be examined. Third, we could not collect physical activity. The degree of physical activity might also affect the effectiveness of IDegLira, but could not be examined. Finally, endogenous insulin secretory capacity is not necessarily limited to the data immediately before the introduction of IDegLira, but insulin secretory capacity may have changed at the time of IDegLira introduction due to persistent hyperglycemia. Lack of adequate data on non-fasting CPR and urinary CPR prevented in-depth assessment of pancreatic β-cell function. In conclusion, this study showed that the initiation of IDegLira was useful in lowering HbA1c in Japanese patients with inadequate glycemic control with existing therapies (including insulin and GLP-1RA) in a real-world clinical setting.
Supplementary Material
Pancreatic β-cell function for achieving HbA1c under 7%. Optimal CPI cut-off point: 1.507 ng/mL per mg/dL, AUC: 0.712, 95% CI: 0.589 - 0.835, specificity: 85.4%, sensitivity: 51.9%. ROC: receiver-operating characteristic; CPR: C-peptide immunoreactivity; CPI: C-peptide index; HbA1c: glycated hemoglobin; AUC: area under the curve; CI: confidence interval.
Pancreatic β-cell function (CPR and CPI) in with or without diabetic nephropathy (DN) (a, b). Change in HbA1c in with DN and without DN after initiation of IDegLira (c). Data are presented as mean with 95% CIs. P-value for comparison of CPR and CPI between with DN or not by Mann-Whitney U-test. *P < 0.001 by Wilcoxon signed-rank sum test (vs. BL HbA1c). CPR: C-peptide immunoreactivity; CPI: C-peptide index; HbA1c: glycated hemoglobin; IDegLira: insulin degludec/liraglutide; CIs: confidence intervals; BL: baseline; DN: diabetic nephropathy.
Acknowledgments
None to declare.
Funding Statement
This work was supported by a Grant-in-Aid for Young Scientists (JSPS KAKENHI Grant Number 19K18012) and Saitama City Community Medical Research Subsidy Project (2023) to H.Y.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Informed Consent
Not applicable.
Author Contributions
Conceptualization and methodology: Hodaka Yamada, Masashi Yoshida and Kazuo Hara. Investigation: Hodaka Yamada, Jun Morimoto, Shiori Tonezawa, Asuka Takahashi, Shunsuke Funazaki, Shuichi Nagashima, and Kazuo Hara. Formal analysis: Hodaka Yamada, Kazuo Hara. Writing-review and editing: Masashi Yoshida, Hodaka Yamada and Kazuo Hara.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Kobayashi M, Yamazaki K, Hirao K, Oishi M, Kanatsuka A, Yamauchi M, Takagi H. et al. The status of diabetes control and antidiabetic drug therapy in Japan—a cross-sectional survey of 17,000 patients with diabetes mellitus (JDDM 1) Diabetes Res Clin Pract. 2006;73(2):198–204. doi: 10.1016/j.diabres.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Yokoyama H, Oishi M, Takamura H, Yamasaki K, Shirabe SI, Uchida D, Sugimoto H. et al. Large-scale survey of rates of achieving targets for blood glucose, blood pressure, and lipids and prevalence of complications in type 2 diabetes (JDDM 40) BMJ Open Diabetes Res Care. 2016;4(1):e000294. doi: 10.1136/bmjdrc-2016-000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouchi R, Kondo T, Ohta Y, Goto A, Tanaka D, Satoh H, Yabe D. et al. A consensus statement from the Japan Diabetes Society: A proposed algorithm for pharmacotherapy in people with type 2 diabetes. J Diabetes Investig. 2023;14(1):151–164. doi: 10.1111/jdi.13960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies MJ, Aroda VR, Collins BS, Gabbay RA, Green J, Maruthur NM, Rosas SE. et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2022;65(12):1925–1966. doi: 10.1007/s00125-022-05787-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yokoyama H, Araki SI, Yamazaki K, Kawai K, Shirabe SI, Oishi M, Kanatsuka A. et al. Trends in glycemic control in patients with insulin therapy compared with non-insulin or no drugs in type 2 diabetes in Japan: a long-term view of real-world treatment between 2002 and 2018 (JDDM 66) BMJ Open Diabetes Res Care. 2022;10(3):e002727. doi: 10.1136/bmjdrc-2021-002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson SL, Trujillo JM. Basal insulin use with GLP-1 receptor agonists. Diabetes Spectr. 2016;29(3):152–160. doi: 10.2337/diaspect.29.3.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carver C. Insulin treatment and the problem of weight gain in type 2 diabetes. Diabetes Educ. 2006;32(6):910–917. doi: 10.1177/0145721706294259. [DOI] [PubMed] [Google Scholar]
- 8.Hex N, Bartlett C, Wright D, Taylor M, Varley D. Estimating the current and future costs of Type 1 and Type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabet Med. 2012;29(7):855–862. doi: 10.1111/j.1464-5491.2012.03698.x. [DOI] [PubMed] [Google Scholar]
- 9.Lyseng-Williamson KA. Glucagon-like peptide-1 receptor analogues in type 2 diabetes: their use and differential features. Clin Drug Investig. 2019;39(8):805–819. doi: 10.1007/s40261-019-00826-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenstock J, Rodbard HW, Bain SC, D'Alessio D, Seufert J, Thomsen AB, Svendsen CB. et al. One-year sustained glycemic control and weight reduction in type 2 diabetes after addition of liraglutide to metformin followed by insulin detemir according to HbA1c target. J Diabetes Complications. 2013;27(5):492–500. doi: 10.1016/j.jdiacomp.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Harris S, Abrahamson MJ, Ceriello A, Charpentier G, Evans M, Lehmann R, Liebl A. et al. Clinical considerations when initiating and titrating insulin degludec/liraglutide (IDegLira) in people with type 2 diabetes. Drugs. 2020;80(2):147–165. doi: 10.1007/s40265-019-01245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaku K, Araki E, Tanizawa Y, Ross Agner B, Nishida T, Ranthe M, Inagaki N. Superior efficacy with a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with insulin degludec and liraglutide in insulin-naive Japanese patients with type 2 diabetes in a phase 3, open-label, randomized trial. Diabetes Obes Metab. 2019;21(12):2674–2683. doi: 10.1111/dom.13856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Komatsu M, Watada H, Kaneko S, Ross Agner BF, Nishida T, Kaku K. Efficacy and safety of the fixed-ratio combination of insulin degludec and liraglutide by baseline glycated hemoglobin, body mass index and age in Japanese individuals with type 2 diabetes: A subgroup analysis of two phase III trials. J Diabetes Investig. 2021;12(9):1610–1618. doi: 10.1111/jdi.13525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watada H, Kaneko S, Komatsu M, Agner BR, Nishida T, Ranthe M, Nakamura J. Superior HbA1c control with the fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with a maximum dose of 50 units of insulin degludec in Japanese individuals with type 2 diabetes in a phase 3, double-blind, randomized trial. Diabetes Obes Metab. 2019;21(12):2694–2703. doi: 10.1111/dom.13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seino Y, Kuwata H, Yabe D. Incretin-based drugs for type 2 diabetes: Focus on East Asian perspectives. J Diabetes Investig. 2016;6(Suppl 1):102–109. doi: 10.1111/jdi.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yabe D, Kuroe A, Watanabe K, Iwasaki M, Hamasaki A, Hamamoto Y, Harada N. et al. Early phase glucagon and insulin secretory abnormalities, but not incretin secretion, are similarly responsible for hyperglycemia after ingestion of nutrients. J Diabetes Complications. 2015;29(3):413–421. doi: 10.1016/j.jdiacomp.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura J, Kamiya H, Haneda M, Inagaki N, Tanizawa Y, Araki E, Ueki K. et al. Causes of death in Japanese patients with diabetes based on the results of a survey of 45,708 cases during 2001-2010: Report of the Committee on Causes of Death in Diabetes Mellitus. J Diabetes Investig. 2017;8(3):397–410. doi: 10.1111/jdi.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Namba M, Iwakura T, Nishimura R, Akazawa K, Matsuhisa M, Atsumi Y, Satoh J. et al. The current status of treatment-related severe hypoglycemia in Japanese patients with diabetes mellitus: a report from the committee on a survey of severe hypoglycemia in the Japan Diabetes Society. Diabetol Int. 2018;9(2):84–99. doi: 10.1007/s13340-018-0346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Araki E, Goto A, Kondo T, Noda M, Noto H, Origasa H, Osawa H. et al. Japanese Clinical Practice Guideline for Diabetes 2019. Diabetol Int. 2020;11(3):165–223. doi: 10.1007/s13340-020-00439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saisho Y, Kou K, Tanaka K, Abe T, Kurosawa H, Shimada A, Meguro S. et al. Postprandial serum C-peptide to plasma glucose ratio as a predictor of subsequent insulin treatment in patients with type 2 diabetes. Endocr J. 2011;58(4):315–322. doi: 10.1507/endocrj.k10e-399. [DOI] [PubMed] [Google Scholar]
- 21.Japan Diabetes Society /Japan Geriatrics Society Joint Committee on Improving Care for Elderly Patients; Haneda M, Ito H. Glycemic targets for elderly patients with diabetes. Diabetol Int. 2016;7(4):331–333. doi: 10.1007/s13340-016-0293-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fadini GP, Buzzetti R, Fittipaldi MR, D'Incau F, Da Porto A, Girelli A, Simoni L. et al. IDegLira for the real-world treatment of type 2 diabetes in Italy: protocol and interim results from the REX observational study. Diabetes Ther. 2022;13(8):1483–1497. doi: 10.1007/s13300-022-01287-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melzer-Cohen C, Chodick G, Naftelberg S, Shehadeh N, Karasik A. Metabolic control and adherence to therapy in type 2 diabetes mellitus patients using IDegLira in a real-world setting. Diabetes Ther. 2020;11(1):185–196. doi: 10.1007/s13300-019-00725-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaneto H, Koshida R, Baxter M. Fixed-ratio combination of basal insulin and glucagon-like peptide-1 receptor agonists in the treatment of Japanese people with type 2 diabetes: An innovative solution to a complex therapeutic challenge. Diabetes Obes Metab. 2020;22(Suppl 4):24–34. doi: 10.1111/dom.14095. [DOI] [PubMed] [Google Scholar]
- 26.Perreault L, Rodbard H, Valentine V, Johnson E. Optimizing fixed-ratio combination therapy in type 2 diabetes. Adv Ther. 2019;36(2):265–277. doi: 10.1007/s12325-018-0868-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oe Y, Nomoto H, Nakamura A, Kuwabara S, Takahashi Y, Yasui A, Izumihara R. et al. Switching from insulin degludec plus dipeptidyl peptidase-4 inhibitor to insulin degludec/liraglutide improves glycemic variability in patients with type 2 diabetes: a preliminary prospective observation study. J Diabetes Res. 2022;2022:5603864. doi: 10.1155/2022/5603864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawaguchi Y, Miyamoto S, Hajika Y, Ashida N, Hirota T, Masumoto K, Sawa J. et al. Efficacy of IDegLira versus IDegAsp therapy in patients with type 2 diabetes: a randomized crossover study by isCGM. Adv Ther. 2022;39(6):2688–2700. doi: 10.1007/s12325-022-02138-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis KL, Wei W, Meyers JL, Kilpatrick BS, Pandya N. Association between different hemoglobin A1c levels and clinical outcomes among elderly nursing home residents with type 2 diabetes mellitus. J Am Med Dir Assoc. 2014;15(10):757–762. doi: 10.1016/j.jamda.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 30.ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS. et al. 13. Older adults: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S216–S229. doi: 10.2337/dc23-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Usui R, Sakuramachi Y, Seino Y, Murotani K, Kuwata H, Tatsuoka H, Hamamoto Y. et al. Retrospective analysis of liraglutide and basal insulin combination therapy in Japanese type 2 diabetes patients: The association between remaining beta-cell function and the achievement of the glycated hemoglobin target 1 year after initiation. J Diabetes Investig. 2018;9(4):822–830. doi: 10.1111/jdi.12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Usui R, Yabe D, Kuwata H, Murotani K, Kurose T, Seino Y. Retrospective analysis of safety and efficacy of liraglutide monotherapy and sulfonylurea-combination therapy in Japanese type 2 diabetes: Association of remaining beta-cell function and achievement of HbA1c target one year after initiation. J Diabetes Complications. 2015;29(8):1203–1210. doi: 10.1016/j.jdiacomp.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 33.Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art. Mol Metab. 2021;46:101102. doi: 10.1016/j.molmet.2020.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pancreatic β-cell function for achieving HbA1c under 7%. Optimal CPI cut-off point: 1.507 ng/mL per mg/dL, AUC: 0.712, 95% CI: 0.589 - 0.835, specificity: 85.4%, sensitivity: 51.9%. ROC: receiver-operating characteristic; CPR: C-peptide immunoreactivity; CPI: C-peptide index; HbA1c: glycated hemoglobin; AUC: area under the curve; CI: confidence interval.
Pancreatic β-cell function (CPR and CPI) in with or without diabetic nephropathy (DN) (a, b). Change in HbA1c in with DN and without DN after initiation of IDegLira (c). Data are presented as mean with 95% CIs. P-value for comparison of CPR and CPI between with DN or not by Mann-Whitney U-test. *P < 0.001 by Wilcoxon signed-rank sum test (vs. BL HbA1c). CPR: C-peptide immunoreactivity; CPI: C-peptide index; HbA1c: glycated hemoglobin; IDegLira: insulin degludec/liraglutide; CIs: confidence intervals; BL: baseline; DN: diabetic nephropathy.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.