Abstract
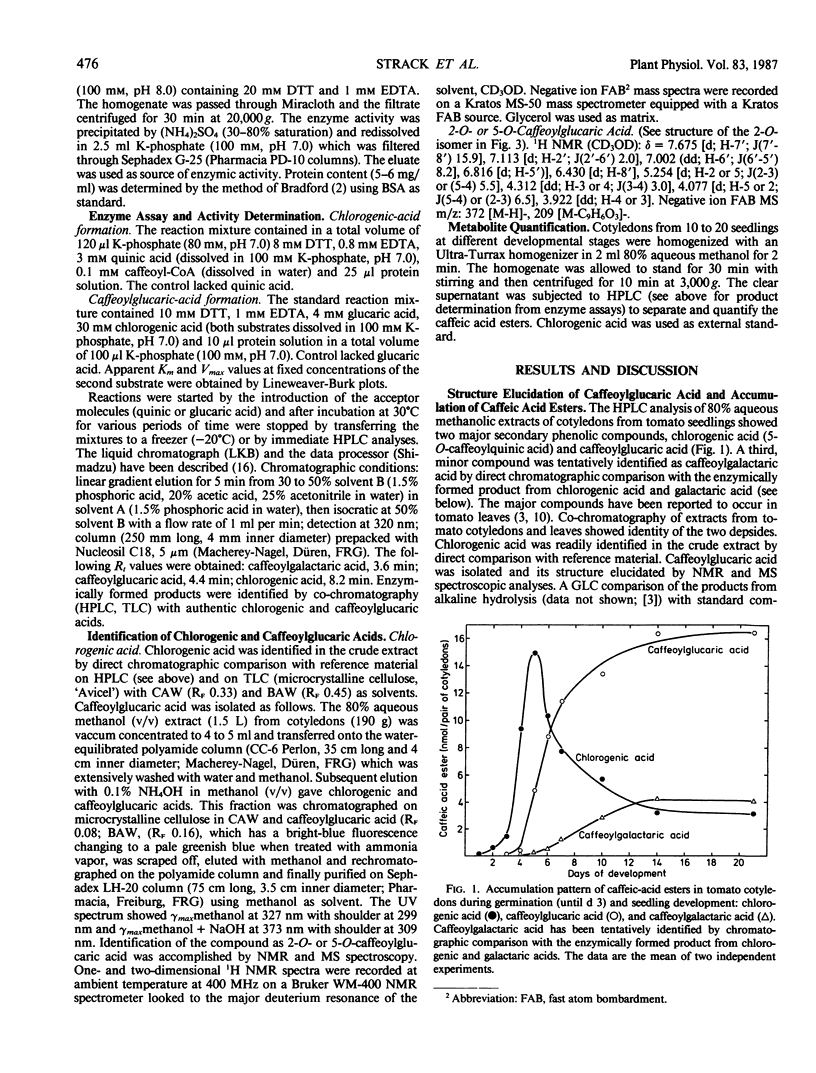
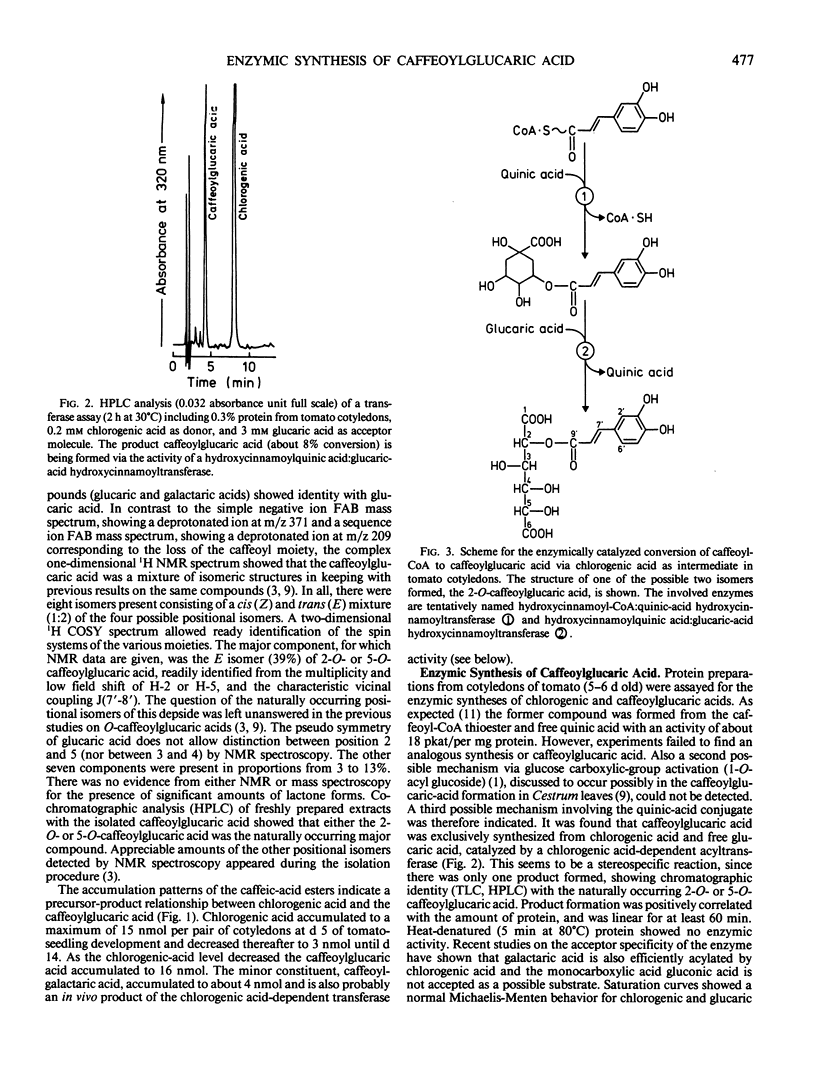
The phenylpropane metabolism of tomato (Lycopersicon esculentum Mill) cotyledons was investigated. The HPLC analysis revealed two hydroxycinnamic-acid conjugates as major components, identified as chlorogenic acid (5-O-caffeoylquinic acid) and caffeoylglucaric acid (2-O- or 5-O-caffeoyl-glucaric acid). Quantitative analyses indicated a precursor-product relationship between the chlorogenic and caffeoylglucaric acids. Protein preparations from tomato cotyledons were found to catalyze the formation of caffeoylglucaric acid with chlorogenic acid as acyl donor and free glucaric acid as acceptor molecule. This enzyme activity, possibly to be classified as hydroxycinnamoylquinic acid:glucaric acid hydroxycinnamoyltransferase, acts together with hydroxycinnamoyl-CoA: quinic acid hydroxycinnamoyltransferase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARONOFF S., PERKINS H. J. Identification of the blue-fluorescent compounds in boron-deficient plants. Arch Biochem Biophys. 1956 Oct;64(2):506–507. doi: 10.1016/0003-9861(56)90293-4. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- HARBORNE J. B., CORNER J. J. Plant polyphenols. 4. Hydroxycinnamic acid-sugar derivatives. Biochem J. 1961 Nov;81:242–250. doi: 10.1042/bj0810242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IUPAC Commission on the Nomenclature of Organic Chemistry (CNOC) and IUPAC-IUB Commission on Biochemical Nomenclature (CBN). Nomenclature of cyclitols. Recommendations, 1973. Biochem J. 1976 Jan 1;153(1):23–31. doi: 10.1042/bj1530023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knogge W., Weissenböck G., Strack D. Application of liquid chromatography to a study on 4-coumarate: coenzyme A ligase activity. Z Naturforsch C. 1981 Mar-Apr;36(3-4):197–199. doi: 10.1515/znc-1981-3-402. [DOI] [PubMed] [Google Scholar]
- Stöckigt J., Zenk M. H. Chemical syntheses and properties of hydroxycinnamoyl-coenzyme A derivatives. Z Naturforsch C. 1975 May-Jun;30(3):352–358. doi: 10.1515/znc-1975-5-609. [DOI] [PubMed] [Google Scholar]
- Stöckigt J., Zenk M. H. Enzymatic synthesis of chlorogenic acid from caffeoyl coenzyme A and quinic acid. FEBS Lett. 1974 Jun 1;42(2):131–134. doi: 10.1016/0014-5793(74)80769-6. [DOI] [PubMed] [Google Scholar]
- Villegas R. J., Kojima M. Purification and characterization of hydroxycinnamoyl D-glucose. Quinate hydroxycinnamoyl transferase in the root of sweet potato, Ipomoea batatas Lam. J Biol Chem. 1986 Jul 5;261(19):8729–8733. [PubMed] [Google Scholar]


