Abstract
Purpose of Review:
This article reviews behavioral signs and symptoms of dementia that can lead to increased mortality, excessive cognitive and functional disability, early institutionalization, and increased caregiver burnout.
Recent Findings:
Almost all patients with a dementia will develop significant behavioral disturbances at some point over the course of their illness. These behavioral signs and symptoms rarely fit into usual diagnostic classifications or meet full criteria for a formal major psychiatric disorder.
Summary:
Treatment of behavioral signs and symptoms of dementia should include both pharmacologic and nonpharmacologic interventions. There are currently no treatments for these disturbances approved by the US Food and Drug Administration. Best judgment should be used in identifying dominant target symptoms and matching them to the most relevant drug class. Implementing nonpharmacologic interventions before the development of neuropsychiatric symptoms may prevent triggers related to a progressively lowered stress threshold and therefore is key in the treatment of all patients with a dementia.
INTRODUCTION TO THE CLINICAL PROBLEM OF NEUROPSYCHIATRIC SIGNS AND SYMPTOMS IN DEMENTIA
Almost all patients with dementia will develop significant behavioral problems at some point in the course of illness.1 Signs and symptoms vary considerably from one person to another, over time, and by type of dementia, and rarely fit into usual diagnostic classifications. It is for this reason that we avoid using typical diagnostic terminology and use more descriptive terms instead. Most fall into four categories:
1. Agitated: verbal or physical aggression toward self, others, or objects; verbal or physical disruptiveness; resistance to care; socially inappropriate behavior
2. Depressive: sad affect, tearfulness, low mood, guilt, self-deprecatory or nihilistic ideation, suicidality, vegetative features, irritability; anxious features almost always co-occur
3. Apathetic: lack of initiative, interest, or pleasure; limited affective response, psychomotor slowing
4. Psychotic: delusions, suspiciousness, persecutory ideation, misidentification, misperception, hallucinations1
Symptom clusters and syndromes can occur alone or in variable combinations and often do not meet criteria for a typical major psychiatric disorder. Left untreated, neuropsychiatric signs and symptoms can lead to increased mortality, early institutionalization, increased caregiver burden, and excess cognitive and functional disability, thereby becoming a major driver of increased cost of care.2
Unmitigated behavioral complications pose a major clinical management challenge. The individual items from the Neuropsychiatric Inventory (NPI) (Table 5-1) can be used as a yes/no checklist to document behavioral issues at baseline and in response to intervention.3 Caregivers will benefit from learning what behaviors to look for and how to label them, which will help them serve as the physician’s “eyes and ears” during treatment. Research using the NPI illustrates how frequent the individual behaviors are at a point in time (Figure 5-1).4
Table 5-1.
The Neuropsychiatric Inventory as a Checklist for Behavioral Issuesa

Figure 5-1.
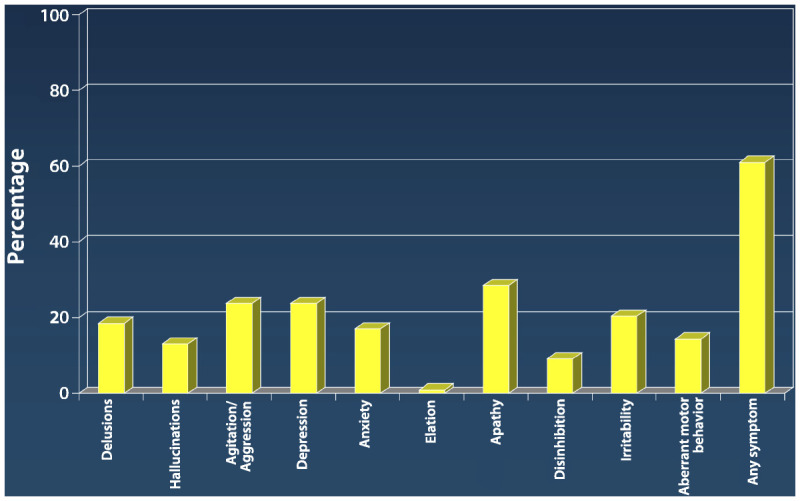
Prevalence of neuropsychiatric symptoms in dementia using the neuropsychiatric inventory.
Data from Lyketsos CG, et al, Am J Psychiatry.4 ajp.psychiatryonline.org/article.aspx?articleid=174106.
Agitation
Agitated behaviors are among the most vexing aspects of dementia to evaluate, manage, and treat. Clinicians should adhere to some basic principles, starting with educating the patient’s family about what behaviors can be expected and how things are likely to change over time. Figure 5-25 shows that, as dementia progresses, the patient’s ability to tolerate social or environmental stressors will diminish. Families should anticipate this and manage accordingly; however, few receive education or training on basic dementia management. Maybe “Dad used to be the life of the party,” but now he gets upset if there are more than five people in the room or if the grandkids are running around. Teaching the family or care partners to reduce these stressors will stave off a great deal of trouble. One or more educational and support options should be offered, such as by providing basic information on dementia and behavioral features; providing resources to help with legal or financial information; addressing family conflict issues; and offering referral to support groups and online resources such as Alzheimer Net and the Alzheimer’s Association. Common precipitants of agitation and therapeutic opportunities are discussed in the section on nonpharmacologic management.
Figure 5-2.
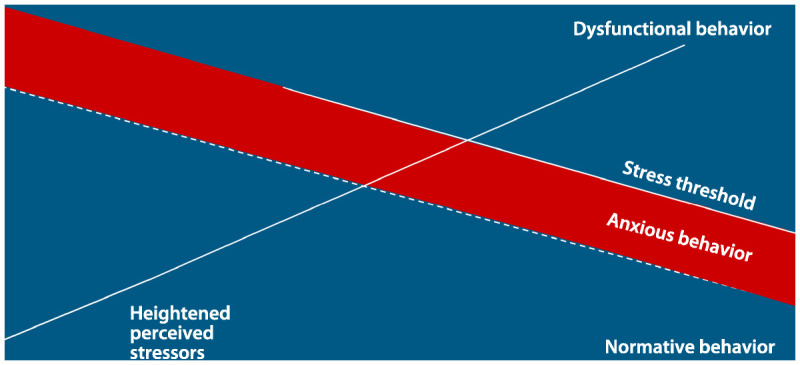
Progressively lowered stress threshold in people with dementia. This figure represents how the individual’s capacity to cope with stress and stimuli diminishes over the course of dementing illness. Over time the capacity to cope decreases, resulting in less calm baseline behavior and increased noncognitive behavioral symptoms (NCBS). Anxiety and increased psychomotor agitation always precede the onset of NCBS. Steps can be taken to intervene when anxiety occurs to thereby prevent NCBS. The x axis represents the timeline for the disease trajectory. The dotted line represents how stress builds throughout any given day to produce anxiety and NCBS. The y axis represents the degree of stress/stimulus the patient is able to manage before developing NCBS.
Reprinted from Hall G, Buckwalter K. Arch Psychiatr Nurs.5 © 1987, with permission from Elsevier.
New-onset agitation should be presumed to be related to delirium until proven otherwise; in as many as 50% of cases, delirium proves to be the culprit. Educating families to recognize the signs of a delirium and to immediately seek medical treatment is essential. At the same time that delirium is being ruled out, nonpharmacologic treatment approaches should continue.
However, when nonpharmacologic interventions are not enough or behaviors result in safety issues, pharmacotherapy may become necessary. The first-line choices of medication treatment, particularly for Alzheimer-related dementia, are the already approved agents for Alzheimer disease (AD), which are typically indicated anyway for treatment of the patient’s dementia. Data from trials suggest that these agents may help relieve behavioral symptoms.6,7 There is even a suggestion that early use may mitigate emergence of behavioral symptoms. Use of these agents in dementias not due to AD has not been studied extensively, but there is some evidence of benefit of cholinesterase inhibitors for dementia due to Lewy body disease and Parkinson disease.8,9
Currently, no psychotropic treatments for agitation in dementia have been approved by the US Food and Drug Administration (FDA). Consensus guidelines have been set forth by the American Geriatrics Society and American Association for Geriatric Psychiatry in their 2004 statement suggesting use of target symptoms to guide selection of drug class (Table 5-2).10 This article more or less follows these guidelines, as shown in the algorithms: drug selection is based on matching the dominant target symptoms to the most relevant drug class. For example, when verbal and physical agitation is present in a patient who is also irritable and negative, has become socially withdrawn, and appears dysphoric, a trial of an antidepressant may be a reasonable initial choice. Patients with aggression and concurrent delusions or hallucinations will likely require initiation of an antipsychotic agent. A mood stabilizer (eg, an anticonvulsant) is sometimes used in a patient displaying agitation in the context of increased motor activity, loud and rapid speech, and affective lability, although evidence from clinical trials for efficacy is inconclusive at best. Although this general approach to drug selection is reasonable, there is limited empirical support for it thus far.2,11 Furthermore, the use of medications to treat any aspect of psychopathology in dementia is not recognized by the FDA and therefore is considered “off-label.” Nonetheless, there is a difference between regulatory and clinical considerations, and clinicians must use their best judgment to decide what treatment a patient needs on a case-by-case basis. Table 5-3 offers some practical tips for using psychotropics.
Table 5-2.
Modified Consensus Guidelines for Treatment of Psychiatric and Behavioral Symptoms in Alzheimer Diseasea

Table 5-3.
Using Psychotropics in Dementia: What is the Clinician to Do?

Antipsychotics. Antipsychotic agents have been used and studied in patients with a wide range of psychopathology. Consensus guidelines recommend them as an appropriate choice for agitation associated with psychosis. There are two main classes of antipsychotics: conventional antipsychotics and newer atypical agents. For practical purposes, side effects typically guide selection of antipsychotics in patients with dementia.
Prior reviews of the conventional agents have concluded that the effects of these agents were consistent but modest, and that no single agent was better than another.12 However, the use of conventional antipsychotics is limited by a side-effect profile that is unacceptable for long-term use, including akathisia, tardive dyskinesia, parkinsonism, peripheral and central anticholinergic effects, orthostatic hypotension, cardiac conduction abnormalities, sedation, and falls; in addition, there is evidence for increased mortality.2,12 Older people treated with conventional antipsychotics have a fivefold to sixfold greater risk of tardive dyskinesia compared with younger populations.13 A clinical issue that is of particular importance is dementia with co-occurring extrapyramidal abnormalities such as dementia with Lewy bodies, where occasional hypersensitivity to conventional agents is seen.14 In such cases, atypical antipsychotics have special utility due to lesser blockade of the dopamine type 2 receptor and reduced risk of extrapyramidal symptoms (EPS).
In light of the considerable toxicity of conventional agents, there was hope that atypical antipsychotics would offer symptom relief in patients with dementia while minimizing side effects seen with older agents. However, meta-analyses show an overall treatment effect (ie, drug response minus placebo response) of about 18%, virtually identical to that seen with conventional agents.15
There have been only four randomized clinical trials investigating typical and atypical antipsychotics in people with dementia: three comparing risperidone with haloperidol, and one studying quetiapine and haloperidol without comparing them directly.2 Only one of these comparator trials found greater efficacy with the atypical than with the typical agent, while the others found no significant difference. However, in all four studies, haloperidol was associated with more EPS than the atypical agent. Atypical antipsychotics were less likely than typical antipsychotics (especially haloperidol) to cause or exacerbate EPS and tardive dyskinesia in patients with dementia, but no other advantages in terms of efficacy or safety have been demonstrated. Although this research indicates that atypical antipsychotics as a class are generally better tolerated than conventional antipsychotics and at least as efficacious, they are not without side effects. Differences in terms of efficacy, tolerability, and side-effect profile exist within the class of atypical antipsychotics that may be useful in treatment selection.
Atypical antipsychotics have largely replaced conventional agents in the treatment of psychosis, aggression, and agitation in patients with dementia. However, these newer agents continue to have other acute and subacute side effects in elderly patients (eg, sedation, postural hypotension, and falls), especially at higher doses. As is true for younger people, antipsychotic-associated diabetes, obesity, and dyslipidemia are concerns despite the lack of large-scale studies addressing these issues in patients with dementia. In addition, EPS, cognitive impairment, gait disturbance, and anticholinergic effects have been reported in some instances.11
In 2003, the FDA issued a warning titled “Cerebrovascular Adverse Events, Including Stroke, in Elderly Patients with Dementia.”2 It referred to cerebrovascular adverse events (CVAEs) (eg, stroke, TIA), including fatalities, seen in trials of risperidone in elderly patients with dementia-related psychosis or agitation; a significantly higher incidence of CVAEs was noted in patients treated with risperidone compared to those treated with placebo. Shortly thereafter, a similar warning was applied to olanzapine and aripiprazole. The increased risk for CVAEs was determined from research clinical trials and is based on unbiased estimates. It is important to note that subsequent studies, mainly involving large public databases, have found similar or even higher death rates among elders receiving conventional antipsychotics, which resulted in a second, broader “black box” warning for all antipsychotics in 2008.
In 2004, the FDA issued a warning regarding all atypical antipsychotics, indicating that in trials up to 3 months long the risk of death was about 1.6 times greater with active treatment than with placebo (4.5% versus 2.6%, respectively)—findings supported by subsequent academic meta-analyses.2,15 A recent retrospective cohort study suggested that there may be differences in mortality risks among individual antipsychotic agents. While haloperidol use was associated with the highest mortality rates, risperidone and olanzapine also showed increased risk. The least likely to increase mortality was quetiapine. The mortality risk with haloperidol was highest in the first 30 days but decreased sharply thereafter. Among the other agents, mortality risk differences were most significant in the first 120 days and declined in the subsequent 60 days during follow-up. Although these findings were limited to a single study, they indicate the necessity for further investigation of specific mortality risk within this class of agents.
Anticonvulsants. One might consider use of anticonvulsant medications first in patients with features of mania, perhaps as well as in those with prominent impulsivity, lability, or episodic severe aggression. However, the best available evidence, coming from two carbamazepine and five valproate trials for agitation associated with dementia, is conflicting and inconclusive.16 At present the data do not support their widespread use, although they appear to be beneficial in some patients.16 It is likely because of anecdotal experience more than trial data that some consensus statements suggest a possible role as a second- or third-line treatment for agitation and aggressive behaviors in selected patients with dementia. The authors are not aware of any controlled studies of the newer anticonvulsants, including lamotrigine, gabapentin, and topiramate, although a few case reports and case series suggest benefit with gabapentin.17
Based in part on putative neuroprotective effects of valproate discerned in animal studies, the Alzheimer’s Disease Cooperative Study (ADCS) conducted a 2-year, placebo-controlled trial of low doses (about 10 mg/kg/d) of divalproex sodium in over 300 people with AD not yet complicated by agitation or psychosis.18 The primary outcome was survival until incident agitation or psychosis; key secondary measures addressed measures of cognitive, functional, and global progression of dementia. There was no benefit of active treatment for any of the clinical outcomes, and significant brain volume loss was noted in the treated group.
Antidepressants. Patients with agitation accompanied by evidence of depressive features may benefit from initiation of an antidepressant; agitation without depressive features may potentially respond as well.2,17 Most of the studies reporting favorable effects were small or uncontrolled; the largest failed to show clear-cut benefit,19,20,21,22 with further results pending from unpublished citalopram research (clinicaltrials.gov/ct2/show/NCT00898807). These agents have a good tolerability profile, which is a main driver of their use: gastrointestinal distress, loss of appetite and weight, sedation, insomnia, sexual dysfunction, and occasional paradoxical agitation are the main side effects.
Agents for Alzheimer dementia. There is mounting evidence that cholinesterase inhibitors can have positive behavioral effects in terms of either reduction of symptoms or delayed emergence of symptoms. A meta-analysis of the behavioral effects of cholinesterase inhibitors indicated overall benefit,6 which was supported by results from a trial of donepezil in mild-moderate AD that showed improvement in global neuropsychiatric symptoms during open-label treatment for 3 months, followed by symptomatic worsening in patients treated with placebo during the subsequent randomized discontinuation phase of the trial.23 However, donepezil proved ineffective for treatment of severe agitation.24 Similarly, at least one trial7 and a pooled analysis of three placebo-controlled trials’ treatment with memantine in patients with moderate-severe AD showed overall benefit in terms of either symptom reduction or delayed emergence of symptoms,25 while a study in patients with severe agitation did not show benefit.26 Finally, a recent study of cessation of long-term treatment of donepezil in patients with moderate to severe dementia showed behavioral benefit in those who were newly treated with memantine.27 Since these agents are indicated anyway in AD dementia, and the evidence for behavioral benefit is encouraging, our algorithm reflects our view that they should be used as the first line of defense for drug treatment of behavioral features.
Depression
The diagnosis of depression, or at least the recognition of clinically significant depressive features, in dementia can be difficult. Diagnosis is frequently made based on caregiver observations, since patients with dementia may not be able to describe their feelings or give an accurate history of their mood. In general, elders may not meet full criteria for a major depressive disorder since they often tend to present with more somatic complaints. Many symptoms of depression, such as insomnia, fatigue, and difficulty with concentration, may be attributed by clinicians solely to comorbid medical conditions and dementia.
Treatment of depressive symptoms in dementia should include a thorough evaluation of potential environmental and psychosocial triggers, which can exacerbate the condition. Surprisingly, the evidence from clinical trials of antidepressants comes from generally small studies, showing only weak support for their widespread use.28 Anecdotal experience and guidelines typically support use for clinically significant depressive features. If pharmacotherapy is warranted, selective serotonin reuptake inhibitors are recommended as first-line therapy because of their relatively benign side-effect profile. Monoamine oxidase inhibitors and tricyclic antidepressants should be avoided because of their adverse effects on cognition and greater potential for other serious adverse effects.
Apathy
Depressive features should be distinguished from apathy, which is a disorder of motivation characterized by reduced goal-directed activity in domains of behavior, cognition, and emotion. Apathy is a frequent symptom of many neuropsychiatric conditions. Unlike apathy, depression involves considerable emotional distress, frequently characterized by tearfulness, sadness, anxiety, agitation, sleep and appetite disturbances, feelings of worthlessness and hopelessness, and recurrent thoughts of death. Clinical features of apathy generally present through behaviors (eg, lack of effort, productivity, structure, and initiative), cognitions (eg, diminished interest, curiosity, concern, insight, planning, and goal-setting), and emotions (eg, decreased emotional responsiveness to both positive and negative events, flat affect). There is insufficient research to dictate best practice regarding drug use. Anecdotal evidence indicates possible benefit in some cases with use of cholinesterase inhibitors, dopaminergic antidepressants (eg, sertraline, bupropion), stimulants (eg, methylphenidate, dextroamphetamine), and dopamine agonists.
PRINCIPLES OF NONPHARMACOLOGIC INTERVENTIONS
The Role and Limitations of Nonpharmacologic Interventions
Families are the primary source of care for people with dementia.29 Over a period of about 10 years, caring for a person with dementia includes meeting instrumental needs such as managing finances, procuring food, maintaining the household, planning activities, maintaining safety, providing for basic needs, and personal care. Because of increasing susceptibility to environmental stimuli, neuropsychiatric signs and symptoms increase in frequency, severity, and presentation with disease progression. These behaviors result in poor outcomes for patients and their families and providers, including caregiver burden, institutionalization, and increased use of psychotropic medications.30,31,32 Neuropsychiatric signs and symptoms are primary barriers to providing care; caregiver response to them is the most common reason for residential placement.33 Therefore, residential facilities (nursing homes and assisted living facilities) are likely to have residents with more challenging neuropsychiatric signs and symptoms. While medications are often prescribed for management of neuropsychiatric signs and symptoms, potentially serious complications mandate minimizing the use of medications in favor of nonpharmacologic measures whenever possible. Thus, nonpharmacologic measures are the primary interventions to use in preventing or relieving neuropsychiatric signs and symptoms (Case 5-1).
Case 5-1
A 76-year-old man presented with a 5-year history of progressive memory and functional loss. He lived at home with his wife and needed supervision or direction with basic activities of daily living. He refused to bathe, shave, clean his teeth, and change into clean clothes more than once a week; he also refused all assistance and became physically aggressive with his wife when she tried to help him.
The patient spent his days watching legal dramas and soap operas and believed that the characters he saw on television lived next door and were committing crimes. He routinely saw imagined silent children in the house.
He usually became agitated starting at 4:00 PM, insisting that the house was not his and refusing his wife’s explanations that it was their home. The patient did not nap during the day and went to bed at about 7:00 PM. He generally woke around 2:00 AM, believed it was morning, and tried to go to work. The patient’s wife was exhausted and, despite her stated goal of keeping the patient at home, was considering residential placement, where she feared the patient would be overmedicated (leading to sedation, falls, and immobility).
Comment. The patient was assessed by an interdisciplinary care team, which found that he was experiencing several behavioral triggers, identification of which led to mitigation strategies:
1. Fatigue—It was recommended that the patient receive a rest period of 30 minutes after his morning activities of daily living, and a 90-minute nap in his recliner after lunch. After his afternoon nap, the patient’s wife has him help her sort family pictures. All activities are kept to 90 minutes or less. These interventions stopped the patient’s 4:00 PM confusion and, with the addition of ice cream at 8:30 PM, keep him awake until 9:30 PM. He now sleeps until 7:30 AM.
2. Loss of meaningful activities—The patient was enrolled in an adult day program for 3 days each week. Initially, he thought he was a volunteer helping the older people, but within 2 weeks settled in and looked forward to attending in order to “spend time with the guys.” The patient now eagerly accepts help with bathing, since he understands that it is a requirement in order to attend the day program, and his wife is considering increasing his participation to 5 days each week. He has also been given some modified chores he can do with his wife, such as sweeping the patio. He reports that he enjoys helping her.
3. Misleading stimuli—The patient was developing illusions, also called pseudohallucinations, from his exposure to television dramas. He no longer watches television except for occasional sports in the evening. His illusions of people in the house have disappeared, as have his worries about the criminal activity next door.
4. Communication issues—The patient’s wife was trained in nonconfrontational communication techniques and now manages by agreeing with him, correcting him only when safety is an issue. Although relearning how to respond has been difficult for her, she acknowledges that it is successful and is no longer considering residential placement for the patient.
While many single interventions have been studied, there are limits on study methods. Few have evidence from randomized controlled trials; however, there are a growing number of caregiver training programs that are theory-based and provide a comprehensive 24/7 view of the person with dementia and the needs of his or her caregivers. When these programs are used consistently, neuropsychiatric signs and symptoms decline significantly.
One problem for providers is that although there is a vast array of such interventions, relatively few are based on randomized controlled trials (RCTs). Most studies were relatively brief and involved small convenience samples.34 As a result, evidence for guiding best practice is viewed as inferior to that available for medications, which have undergone RCTs.35,36 Another problem is that many RCTs were conducted in nursing homes and may not generalize to home environments.
Further, interventions such as bright light therapy, music, aromatherapy, use of pets and animal-like robots in lieu of pets, and multisensory environments are helpful at a “micro” level, meaning they are not part of an overarching 24-hour program.35 Few studies of individual interventions have theoretical underpinnings that point to broader or prescribed use, which leaves caregivers uncertain regarding when to use the intervention and for what duration. Moreover, few individual interventions have been studied in the context of relieving or preventing neuropsychiatric signs and symptoms.36
Comprehensive evidence-based programs. There are comprehensive evidence-based caregiver training programs available nationwide. Evidence-based caregiver training programs have been evaluated for efficacy and are cataloged by the Rosalyn Carter Institute for Caregiving for consumer use, for community program development, and to provide frameworks for future research. These programs range from several hours of caregiver training to 6 months with an average of 8 to 9 weeks (www.rosalynncarter.org/caregiver_intervention_database/).37 The database provides information on how to access authors or investigators for information and materials for implementation.
The comprehensive programs focus primarily on caregiver education about the disease process, caregiver behavior, environmental modification, communication skills, and suggestions for meeting patient needs in order to minimize neuropsychiatric signs and symptoms.38,39,40,41 The focus of these programs is to meet patient needs and minimize neuropsychiatric signs and symptoms by creating a supportive environment and through modified communication. Caregiver counseling programs result in statistically significant declines in patients’ psychosis and delusions.42,43 These findings were significant among groups of white, Latino, and African American families.31,44 A secondary goal of these programs is to reduce caregiver burden, hopefully permitting the patient to stay at home longer.
For purposes of brevity and provider usefulness, this discussion will focus on one evidence-based theoretical model, the Progressively Lowered Stress Threshold.5,41,45 The model hypothesizes that the majority of neuropsychiatric signs and symptoms are responses to stress resulting from the interaction of neurodegenerative changes with the environment. With dementia, the patient’s capacity for dealing with stress diminishes over time as the disease progresses, and stress responses occur with increasing frequency. The stress leads to anxiety and, if unrelieved, neuropsychiatric signs and symptoms. Figure 5-2 demonstrates this concept.5 The model identifies six triggers that increase stress leading to neuropsychiatric signs and symptoms. Caregivers learn that by avoiding or minimizing these triggers, they can both prevent and relieve neuropsychiatric signs and symptoms:
1. Fatigue
2. Change of routine, environment, or caregiver
3. Excessive demand to achieve activity beyond limitations
4. Excessive or misleading stimulus
5. Perception of losses resulting in depression or anger (at loss of cherished activities or at inadequate activities)
6. Physical changes causing delirium, such as acute illness, pain, medication reactions, or infections
Table 5-4 demonstrates interventions used to prevent triggers for excess disability in the categories identified above. When used by family or professional caregivers, this approach results in significant declines in neuropsychiatric signs and symptoms including falls, pacing and fidgeting, refusing care, late day confusion, wandering, aggression, and psychosis.5,41,45 The model outlines the basic knowledge caregivers need to provide for planning days throughout the illness.
Table 5-4.
Triggers for Behavioral Symptoms
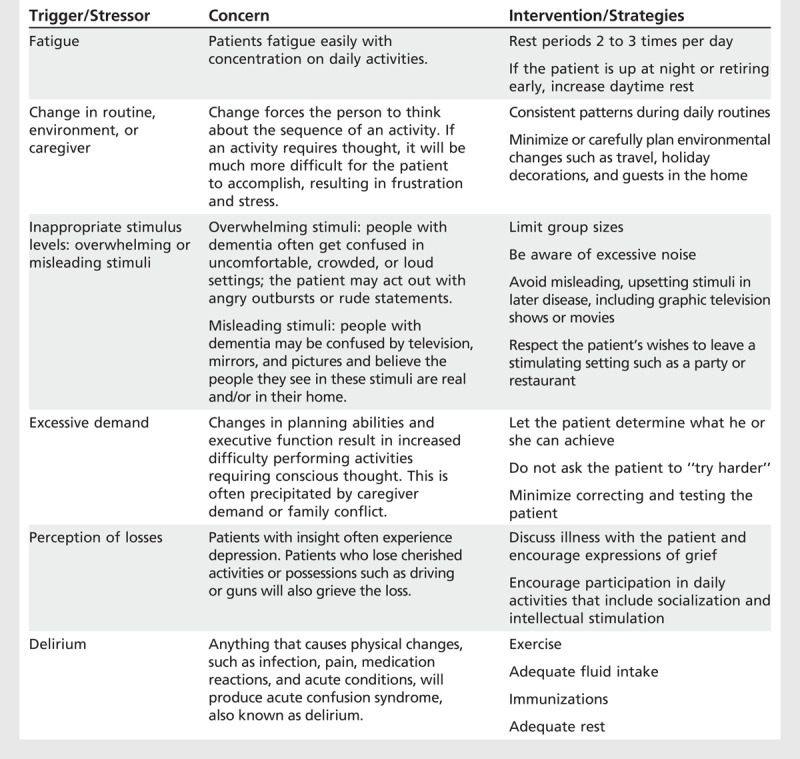
One key to minimizing the triggers for stress is to understand the symptoms of dementia that complicate the presentation of memory loss. Providers must understand that the following symptoms are critically important to the patient’s sense of well-being in their environment: altered visual perception, including loss of depth perception, and altered ability to perceive moving objects; diminished sense of time; inability to plan, initiate, and carry through to a goal (activities that require thought); increased self-absorption; progressively lowered insight; and gradual diminishment of endurance.
Activities
People who remain active and engaged in pleasurable activities tend to be functional longer, have less depression, and manifest fewer problems with neuropsychiatric signs and symptoms. Activities are thought to be the most important aspect of care for patients with dementia. A variety of activities is important; however, they must be modified and individualized continuously as the disease progresses.35 This may include varied and, at times, mildly challenging activities. In moderate dementia, each activity should not exceed 90 minutes.
Activities that are enjoyable, safe, and hold the patient’s attention should be encouraged. Many books are available for caregivers on how to plan activities for people with dementia. It is important for caregivers and the patient’s family members to understand that the time the patient is able to spend engaged in activities will be shorter as the disease progresses because of fatigue and diminished attention span. Occupational therapists, recreational therapists, and adult day programs can be sources of help in modifying or finding new activities.39,40
Communication
Many neuropsychiatric signs and symptoms are correlated with problematic communication techniques.46 Dementia produces progressive losses in language function, rendering the patient more vulnerable to nonverbal communications such as gestures, facial expression, body language, and vocal tone. Caregivers using less than optimal communication styles, including “elder-speak,” a pejorative, childlike approach, will elicit more neuropsychiatric signs and symptoms.46 Escape/avoidance approaches such as ignoring the patient also increase neuropsychiatric signs and symptoms, especially agitation and aggression.46,47 Evidence-based training programs are available nationally to help caregivers develop techniques to avoid confrontation.
The most widely recognized programs to enhance caregiver communications are the Resources for Enhancing Alzheimer’s Caregiver Health (REACH) programs. Developed to assist caregivers with modifying their own behavior and responses to their patients’ neuropsychiatric signs and symptoms, the REACH programs have been developed and tested in many sites with group education and telephone-based educational programs.31,48 REACH interventions include provision of information, didactic instruction, role-playing exercises, problem-solving strategies, skills training, stress management, and telephone support groups. These programs were developed using twelve 90-minute sessions over 6 months, in addition to resource notebooks with educational materials and telephones linked to a computer-integrated system. Interventions were developed based on findings that active techniques are more effective at improving outcomes than passive techniques.
Bathing
Caregivers often encounter issues when attempting to bathe patients with AD. Many patients with AD may refuse to bathe or say they have already bathed when they have not, which can be frustrating, especially if the person develops body odor. Many older adults are modest about disrobing; those with AD may also become afraid of bathwater or the shower or feel overwhelmed by the complexity of the task. A new evidence-based practice guideline has been issued outlining effective bathing practices, including towel baths.49 It is available from www.nursing.uiowa.edu/sites/default/files/documents/hartford/EBP_Catalog2012.pdf.
CONCLUSION
Behavioral disturbances are common, morbid, and distressing for patients with dementia and their caregivers. There are no FDA-approved psychotropic treatments for these disturbances. Nonpharmacologic management can often be effective; however, when this is not enough, pharmacotherapy may be necessary to minimize patient and caregiver distress and assure safety. Although there are a substantial number of clinical trials of some of these agents, they are not sufficient to define best practice. Clinicians can be guided in part by available evidence combined with their own experience, advice from experienced colleagues, and consensus guidelines such as those of the American Geriatrics Society and American Association for Geriatric Psychiatry, or the American Psychiatric Association.10,11 Best judgment should be used in identifying dominant target symptoms and matching them to the most relevant drug class. Practice models need to evolve to allow the time and expertise to offer the best behavioral approaches. Treating AD dementia before behavioral features are evident may be the best treatment for behavioral symptoms in the long run, and agents now in development will hopefully prove to alter the course of the illness. In the meantime, the guiding principles depicted in Table 5-5 are recommended; there is always something that can be done to help, regardless of the problem or stage of illness. Giving this message to patients and families is by itself therapeutic.
Table 5-5.
Principles of Care for People With Dementia

KEY POINTS
Neuropsychiatric signs and symptoms in dementia are common, morbid, and distressing and occur in predictable clusters.
The Neuropsychiatric Inventory trigger questions may be useful for detecting and tracking neuropsychiatric signs and symptoms in dementia.
Neuropsychiatric signs and symptoms in dementia occur more readily as dementia progresses because of progressively lowered stress threshold.
A systematic approach to help evaluate, manage, and treat neuropsychiatric signs and symptoms in dementia is helpful.
No medication for treatment of neuropsychiatric signs and symptoms in dementia is approved by the US Food and Drug Administration, although this does not preclude clinician judgment regarding clinical necessity.
Cholinesterase inhibitors and memantine are approved by the US Food and Drug Administration for treatment of dementia due to Alzheimer disease and may mitigate neuropsychiatric signs and symptoms in dementia.
There is no first-line recommendation for medications to treat agitation without psychosis.
Atypical antipsychotics may be first-line treatment for clinically significant psychosis but should be discontinued if ineffective.
Predictable triggers for neuropsychiatric signs and symptoms in dementia can be identified.
Refer to basic precepts for care of people with dementia.
Footnotes
Relationship Disclosure: Dr Burke reports no disclosure. Dr Hall has served as an expert witness for a legal case regarding a resident care facility. Dr Tariot has served as a consultant for Abbott Laboratories; AC Immune; Adamas Pharmaceuticals, Inc; Allergan, Inc; AstraZeneca; Avanir Pharmaceuticals, Inc; Avid Radiopharmaceuticals; Boehringer Ingelheim; Bristol-Myers Squibb Company; Eisai Co, Ltd; Elan Corporation; Eli Lilly and Company; Genentech, Inc; GlaxoSmithKline; MedAvante, Inc; Medivation, Inc; Merck & Co, Inc; Novartis AG; Otsuka Pharmaceutical Co, Ltd; Pfizer Inc; Sanofi-Aventis; Toyama Pharmaceutical Association; and Worldwide Clinical Trials, Inc. Dr Tariot holds stock options in Adamas Pharmaceuticals, Inc, recently forfeited options in MedAvante, Inc, and is a contributor to a patent titled “Biomarkers of Alzheimer’s Disease.” Dr Tariot receives research support from Abbott Laboratories; Alzheimer’s Association; Arizona Department of Health Services, AstraZeneca; Avid Radiopharmaceuticals; Bristol-Myers Squibb Company; Elan Corporation; Eli Lilly and Company; Genentech, Inc; GlaxoSmithKline; Medivation, Inc; Merck & Co, Inc; National Institute on Aging; National Institute of Mental Health; Pfizer Inc; and Toyama Pharmaceutical Association.
Unlabeled Use of Products/Investigational Use Disclosure: Dr Burke discusses the unlabeled use of atypical antipsychotics, antidepressants, and anticonvulsants for the treatment of agitation in dementia. Dr Hall reports no disclosure. Dr Tariot discusses the unlabeled use of cholinesterase inhibitors and memantine for people with dementia, as well as the unlabeled use of antipsychotics, antidepressants, and anticonvulsants. Information on drugs is provided for general purposes only and not relied on for prescribing. Before prescribing any of the drugs discussed, the physician should be knowledgeable about the full prescribing information that can be obtained from the manufacturers.
REFERENCES
- 1.Profenno L,, Tariot PN,, Loy R, et al.. Treatments for behavioral and psychological symptoms in Alzheimer’s disease and other dementia. In: Burns A,, Ames D,, O’Brien J, editors. Dementia 3rd ed. London, England: Edward Arnold Limited, 2006; 38: 482–498. [Google Scholar]
- 2.Jeste DV,, Blazer D,, Casey D, et al.. ACNP white paper: update on use of antipsychotics drugs in elderly persons with dementia. Neuropsychopharmacology 2008; 33 (5): 957–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cummings JL,, Mega M,, Gray K, et al.. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994; 44 (12): 2308–2314. [DOI] [PubMed] [Google Scholar]
- 4.Lyketsos CG,, Steinberg M,, Tschanz JT, et al.. Mental and behavioral disturbances in dementia: findings from the Cache County Study on Memory in Aging. Am J Psychiatry 2000; 157 (5): 708–714. [DOI] [PubMed] [Google Scholar]
- 5.Hall G,, Buckwalter K. A conceptual model for planning and evaluating care of the client with Alzheimer’s disease. Arch Psychiatr Nurs 1987; 1 (6): 399–406. [PubMed] [Google Scholar]
- 6.Trihn NH,, Hoblyn J,, Mohanty S,, Yaffe K. Efficacy of cholinesterase inhibitors in the treatment of neuropsychiatric symptoms and functional impairment in Alzheimer disease: a meta-analysis. JAMA 2003; 289 (2): 210–216. [DOI] [PubMed] [Google Scholar]
- 7.Cummings JL,, Schneider E,, Tariot PN, Graham SMfor the MEM-MD-02 Study Group. Behavioral effects of memantine in Alzheimer’s disease patients receiving donepezil. Neurology 2006; 67 (1): 57–63. [DOI] [PubMed] [Google Scholar]
- 8.Emre M,, Tsolaki M,, Bonuccelli U, et al.11018 Study Investigators. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2010; 9 (10): 969–977. [DOI] [PubMed] [Google Scholar]
- 9.Wild R,, Pettit T,, Burns A. Cholinesterase inhibitors for dementia with Lewy bodies. Cochrane Database Syst Rev 2003; (3): CD003672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alexopoulos GS,, Streim J,, Carpenter C,, Docherty JP. Using antipsychotic agents in older patients. J Clin Psychiatry 2004; 65 (suppl 2): 5–104. [PubMed] [Google Scholar]
- 11.APA Work Group on Alzheimer’s Disease and other Dementias, Rabins PV, Blacker D, Rovner BW, et al.. American Psychiatric Association practice guideline for the treatment of patients with Alzheimer’s disease and other dementias. Second edition. Am J Psychiatry 2007; 164 (12): 5–56. [PubMed] [Google Scholar]
- 12.Schneider LS,, Pollock VE,, Lyness SA. A metaanalysis of controlled trials of neuroleptic treatment in dementia. J Am Geriatr Soc 1990; 38 (5): 553–563. [DOI] [PubMed] [Google Scholar]
- 13.Jeste DV,, Caligiuri MP,, Paulsen JS, et al.. Risk of tardive dyskinesia in older patients. A prospective longitudinal study of 266 outpatients. Arch Gen Psychiatry 1995; 52 (9); 756–765. [DOI] [PubMed] [Google Scholar]
- 14.Ballard C,, Grace J,, McKeith I,, Holmes C. Neuroleptic sensitivity in dementia with Lewy bodies and Alzheimer’s disease. Lancet 1998; 351 (1908): 1032–1033. [DOI] [PubMed] [Google Scholar]
- 15.Schneider LS,, Dagerman K,, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry 2006; 14 (3): 191–210. [DOI] [PubMed] [Google Scholar]
- 16.Konovolov S,, Muralee S,, Tampi RR. Anticonvulsants for the treatment of behavioral and psychological symptoms of dementia: a literature review. Int Psychogeriatr 2008; 20 (2): 293–308. [DOI] [PubMed] [Google Scholar]
- 17.Sink KM,, Holden KF,, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA 2005; 293 (5): 596–608. [DOI] [PubMed] [Google Scholar]
- 18.Tariot PN,, Schneider LS,, Cummings J, et al.. Chronic divalproex sodium to attenuate agitation and clinical progression of Alzheimer disease. Arch Gen Psychiatry 2011; 68 (8): 853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teri L,, Logsdon RG,, Peskind E, et al.. Treatment of agitation in AD: a randomized, placebo-controlled clinical trial. Neurology 2000; 55 (9): 1271–1278. [DOI] [PubMed] [Google Scholar]
- 20.Pollock BG,, Mulsant BH,, Rosen J, et al.. Comparison of citalopram, perphenazine, and placebo for the acute treatment of psychosis and behavioral disturbances in hospitalized, demented patients. Am J Psychiatry 2002; 159 (3): 460–465. [DOI] [PubMed] [Google Scholar]
- 21.Finkel SI,, Mintzer JE,, Dysken M, et al.. A randomized, placebo-controlled study of the efficacy and safety of sertraline in the treatment of the behavioral manifestations of Alzheimer’s disease in outpatients treated with donepezil. Int J Geriatr Psychiatry 2004; 19 (1): 9–18. [DOI] [PubMed] [Google Scholar]
- 22.Pollock BG,, Mulsant BH,, Rosen J, et al.. A double-blind comparison of citalopram and risperidone for the treatment of behavioral and psychotic symptoms associated with dementia. Am J Geriatr Psychiatry 2007; 15 (11): 942–952. [DOI] [PubMed] [Google Scholar]
- 23.Holmes C,, Wilkinson D,, Dean C, et al.. The efficacy of donepezil in the treatment of neuropsychiatric symptoms in Alzheimer disease. Neurology 2004; 63 (2): 214–219. [DOI] [PubMed] [Google Scholar]
- 24.Howard RJ,, Juszczak EJ,, Ballard CG, et al.. Donepezil for the treatment of agitation in Alzheimer’s disease. N Engl J Med 2007; 375 (14): 1382–1392. [DOI] [PubMed] [Google Scholar]
- 25.Wilcock GK,, Ballard CG,, Cooper JA,, Loft H. Memantine for agitation/aggression and psychosis in moderately severe to severe Alzheimer’s disease: a pooled analysis of three studies. J Clin Psychiatry 2008; 69 (3): 341–348. [DOI] [PubMed] [Google Scholar]
- 26.Fox C,, Crugel M,, Maidment I, et al.. Efficacy of memantine for agitation in Alzheimer’s dementia: a randomised double-blind placebo controlled trial. PLoS One 2012; 7 (5): e35185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howard R,, McShane R,, Lindesay J, et al.. Donepezil and memantine for moderate-to-severe Alzheimer’s disease. N Engl J Med 2012; 366 (10): 893–903. [DOI] [PubMed] [Google Scholar]
- 28.Bains J,, Birks J,, Dening T. The efficacy of antidepressants in the treatment of depression in dementia. Cochrane Database Syst Rev 2002; (4): CD003944. [DOI] [PubMed] [Google Scholar]
- 29.Alzheimer’s Association. Alzheimer’s Association annual report: fiscal year 2011. www.alz.org/annual_report/overview.asp. Accessed July 17, 2012.
- 30.Cohen-Mansfield J,, Mintzer JE. Time for change: the role of nonpharmacological interventions in treating behavior problems in nursing home residents with dementia. Alzheimer Dis Assoc Disord 2005; 19 (1): 37–40. [DOI] [PubMed] [Google Scholar]
- 31.Belle SH,, Burgio L,, Burns R, et al.. Enhancing the quality of life of dementia caregivers from different ethnic or racial groups: a randomized, controlled trial. Ann Intern Med 2006; 145 (10): 727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nazir A,, Arling G,, Perkins AJ,, Boustani M. Monitoring quality of care for nursing home residents with behavioral and psychological symptoms related to dementia. J Am Med Dir Assoc 2011; 12 (9): 660–667. [DOI] [PubMed] [Google Scholar]
- 33.de Vugt ME,, Stevens F,, Aalten P, et al.. A prospective study of the effects of behavioral symptoms on the institutionalization of patients with dementia. Int Psychogeriatr 2005; 17 (4): 577–589. [DOI] [PubMed] [Google Scholar]
- 34.Logsdon RG,, McCurry SM,, Teri L. Evidence-based psychological treatments for disruptive behaviors in individuals with dementia. Psychol Aging 2007; 22 (1): 28–36. [DOI] [PubMed] [Google Scholar]
- 35.Avila R,, Belmin J,, Camp C, et al. Non-pharmacologic therapies: evaluation methodology. Salamanca consensus white paper 2, draft 29. wisdem.org/sites/default/files/manifestos/evaluation_methodology_manifesto.pdf. Accessed November 27, 2012.
- 36.Ayalon L,, Gum AM,, Feliciano L, et al.. Effectiveness of nonpharmacological interventions for the management of neuropsychiatric symptoms in patients with dementia: a systematic review. Arch Intern Med 2006; 166 (20): 2182–2188. [DOI] [PubMed] [Google Scholar]
- 37.Rosalynn Carter Institute for Caregiving. Caregiver intervention database. www.rosalynncarter.org/caregiver_intervention_database/. Accessed February 8, 2013.
- 38.Czaja SJ,, Gitlin LN,, Schulz R, et al.. Development of the risk appraisal measure: a brief screen to identify risk areas and guide interventions for dementia caregivers. J Am Geriatr Soc 2009; 57 (6): 1064–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gitlin LN,, Winter L,, Dennis MP, et al.. Targeting and managing behavioral symptoms in individuals with dementia: a randomized trial of a nonpharmacological intervention. J Am Geriatr Soc 2010; 58 (8): 1465–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gitlin LN,, Winter L,, Burke J, et al.. Tailored activities to manage neuropsychiatric behaviors in persons with dementia and reduce caregiver burden: a randomized pilot study. Am J Geriatr Psychiatry 2008; 16 (3): 229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerdner LA,, Buckwalter KC,, Reed D. Impact of a psychoeducational intervention on caregiver response to behavioral problems. Nurs Res 2002; 51 (6): 363–374. [DOI] [PubMed] [Google Scholar]
- 42.Nobili A,, Riva E,, Tettamanti M, et al.. The effect of a structured intervention on caregivers of patients with dementia and problem behaviors: a randomized controlled pilot study. Alzheimer Dis Assoc Disord 2004; 18 (2): 75–82. [DOI] [PubMed] [Google Scholar]
- 43.Herman RE,, Williams KN. Elderspeak’s influence on resistiveness to care: focus on behavioral events. Am J Alzheimers Dis Other Demen 2009; 24 (5): 417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bank AL,, Arguelles S,, Rubert M, et al.. The value of telephone support groups among ethnically diverse caregivers of persons with dementia. Gerontologist 2006; 46 (1): 134–138. [DOI] [PubMed] [Google Scholar]
- 45.Hall G. Testing the PLST model with community-based caregivers. Doctoral dissertation. Iowa, IA: The University of Iowa, 1998. [Google Scholar]
- 46.Savundranayagam MY,, Hummert ML,, Montgomery RJ. Investigating the effects of communication problems on caregiver burden. J Gerontol B Psychol Sci Soc Sci 2005; 60 (1): S48–S55. [DOI] [PubMed] [Google Scholar]
- 47.Mausbach BT,, Aschbacher K,, Patterson TL, et al.. Avoidant coping partially mediates the relationship between patient problem behaviors and depressive symptoms in spousal Alzheimer caregivers. Am J Geriatr Psychiatry 2006; 14 (4): 299–306. [DOI] [PubMed] [Google Scholar]
- 48.Holland JM,, Currier JM,, Gallagher-Thompson D. Outcomes from the Resources for Enhancing Alzheimer’s Caregiver Health (REACH) program for bereaved caregivers. Psychol Aging 2009; 24 (1): 190–202. [DOI] [PubMed] [Google Scholar]
- 49.Hall G,, Gallagher M,, Hoffman-Snyder C. Evidence-based practice guideline: Bathing persons with dementia. Gerontological Nursing Interventions Research Center, Research Translational and Dissemination Core (RTDC). Iowa, IA: The University of Iowa, 2012. [Google Scholar]


