Abstract
Purpose of Review
Myasthenia gravis (MG) is an acquired autoimmune disorder characterized by fluctuating ocular, limb, or oropharyngeal muscle weakness due to an antibody-mediated attack at the neuromuscular junction. The female incidence of MG peaks in the third decade during the childbearing years. A number of exacerbating factors may worsen MG, including pregnancy. When treatment is needed, it must be carefully chosen with consideration of possible effects on the mother with MG, the pregnancy, and the fetus.
Recent Findings
Decisions are complex in the treatment of women with MG contemplating pregnancy or with presentation during pregnancy. While data is largely observational, a number of characteristic patterns and issues related to risk to the patient, integrity of the pregnancy, and risks to the fetus are recognized. Familiarity with these special considerations when contemplating pregnancy is essential to avoid potential hazards in both the patient and the fetus. Use of immunosuppressive agents incurs risk to the fetus. Deteriorating MG with respiratory insufficiency poses risk to both the mother and the fetus.
Summary
This article reviews available information regarding expectations and management for patients with MG in the childbearing age. Treatment decisions must be individualized based on MG severity, distribution of weakness, coexisting diseases, and welfare of the fetus. Patient participation in these decisions is essential for successful management.
INTRODUCTION
Myasthenia gravis is a chronic disorder manifested by fluctuating weakness and rapid fatigue of voluntary muscles. The estimated prevalence of MG in the United States is 20/100,000 population.1 Acquired MG is an autoimmune disorder in which antibodies at the neuromuscular junction produce impaired neuromuscular transmission and fatigable weakness in skeletal muscle (periocular, limb, or oropharyngeal muscles). Symptoms can present at any age, but the highest incidence in female patients occurs during the third decade of life. MG is more common in women than in men, with a ratio of 3:2. Known triggers for MG include infection, changes in thyroid function, general anesthesia, certain medications, emotional or physical stress, menses, pregnancy, and the postpartum state.2
Caring for the female patient of childbearing age involves anticipation of pregnancy, as well as care throughout pregnancy and the postpartum period. In preparation for pregnancy, women with MG need education and counseling to address special therapeutic issues, including the choice and risks of treating or not treating, effects of MG on the pregnancy, and risks to the fetus and newborn. Women with MG benefit from a personalized interdisciplinary approach to care during pregnancy and the postpartum period, including neuromuscular, high-risk obstetric, and neonatal pediatric specialists.2 Recognizing risks for both the mother and the baby requires careful monitoring and attention during both pregnancy and delivery. Even after the successful delivery of a healthy infant, MG may impact the new mother. A woman with MG should be fully informed and aware that a contemplated pregnancy is a physical commitment that may be affected by MG but also requires additional ability to cope with both the demands of parenting and the ongoing disease. This article discusses the initial approach to female patients of childbearing age with MG, including diagnosis and management of the many challenging questions that arise for the patient and the treating physicians.
DIAGNOSIS OF MYASTHENIA GRAVIS DURING PREGNANCY
MG is unmasked or worsened in approximately one-third of patients during their pregnancy.3,4,5 Symptoms of fluctuating weakness are the hallmark of this condition, and when associated with evident weakness typical of MG (ie, fatigable ptosis, diplopia, dysarthria, dysphagia, and/or limb weakness), they should prompt further diagnostic studies (in the as-yet-undiagnosed patient), including electrodiagnostic studies and acetylcholine receptor (AChR)–binding antibodies. If AChR antibodies are not detectable, anti–muscle-specific kinase (MuSK) should be measured (Case 6-1). Elevated serum levels of anti–AChR-binding antibodies or anti-MuSK antibodies in patients with clinical signs and symptoms of MG confirm the diagnosis. In the seronegative patient electrophysiologic demonstration of an abnormality of neuromuscular transmission establishes the diagnosis. The electrophysiologic tests that demonstrate a defect in neuromuscular transmission are repetitive nerve stimulationstudies and the more sensitive single-fiber EMG, both safely performed in pregnant patients. Patients may undergo chest CT imaging without contrast to assess the thymus gland, however, postponement until after delivery is preferable, particularly in antibody negative patients. The riskof radiation is eliminated with chest MRI, but it does not visualize the anterior mediastinum as well as CT imaging, which is the preferred technique.
Thymoma is uncommon in this age group, particularly if AChR-antibody testing is negative.6,7 The decision to perform thymic imaging can usually be postponed until after delivery in recognition of the potential risk to the fetus, unless there is strong clinical suspicion for thymoma.
Case 6-1
Five weeks post partum, a 29-year-old woman developed proximal upper extremity weakness. The weakness lasted for 4 weeks and was not associated with changes in sensation. Pregnancy, cesarean delivery, and the immediate postpartum period were uncomplicated. Two years later, she developed fatigable right ptosis and horizontal, binocular diplopia that lasted 1 to 2 weeks. Neuro-ophthalmic evaluation noted ptosis and extraocular motility abnormalities. Myasthenia gravis (MG) did not appear to have been considered at that time, and the patient was diagnosed with a right Horner syndrome. MRI of the brain and magnetic resonance angiogram of the intracranial and extracranial vessels were negative.
A year later she became pregnant and had an uneventful pregnancy. On the morning of her planned cesarean delivery, she was hypertensive, and possible preeclampsia was treated with magnesium without event. She delivered a healthy daughter without weakness, feeding difficulty, or respiratory distress. Two weeks post partum, she developed nasal dysarthria and dysphagia followed by right ptosis. Over the next few weeks, she had gradual worsening of generalized weakness.
Examination showed severe bilateral fatigable ptosis and limited bilateral eye abduction with diplopia. Eye closure, cheek puff, tongue protrusion, and the palate showed fatigable weakness. Neck flexion, proximal arm, and hip flexion were weak bilaterally. Electrodiagnostic studies showed abnormal decrement on 3-Hz repetitive nerve stimulation consistent with MG. AChR-binding antibodies were elevated. Given the distribution of weakness with oropharyngeal muscle predominance, intervention seemed necessary. Prednisone was considered but not chosen as patients often worsen in the first 2 weeks of therapy before receiving benefit, and worsening of her oropharyngeal weakness may have required intubation. She received five sessions of plasma exchange without complication and with significant improvement of her condition.
Comment. This patient represents a case of MG unmasked by her pregnancies. A high index of suspicion with focused history taking, paying special attention to the patient’s medical history, and correlation with the patient’s physical examination should prompt the diagnosis. Appropriate management includes caution with some medications that may contribute to worsening of MG symptoms.
CHANGES IN MYASTHENIA GRAVIS DURING PREGNANCY
Pregnancy may change the course of MG, often in unpredictable ways.8 The severity of weakness at the beginning of pregnancy does not predict either remission or exacerbation,3,4 and in fact disease exacerbations, myasthenic crisis, or even disease remission may each occur during pregnancy. Patients can develop hypoventilation secondary to respiratory muscle weakness. The growing fetus may also restrict the diaphragm and compromise respiratory function; late in the pregnancy, increased abdominal pressure and diaphragm elevation reduce the capacity for the lungs to inflate fully. At some point during pregnancy, approximately 20% of patients develop respiratory crisis requiring mechanical ventilation.Close monitoring for respiratory difficulties is essential throughout pregnancy to maintain the welfare of both mother and fetus.
Rare complications of MG during pregnancy, including bone marrow suppression, have been reported. It has been postulated that suppression could be due to an autoimmune reaction against the megakaryocyte colony-forming unit.9,10 Patients on immunosuppressive therapy also may develop infections secondary to decreased immunity.
During labor, the uterine smooth muscle is not compromised, because unlike striated muscle, it is not affected by AChR antibodies. Patients may develop worsening weakness especially during the second stage of labor, when striated muscle is involved; some may become exhausted and require assistance for delivery.
An association between MG and preeclampsia has been suggested. If treatment is indicated, magnesium sulfate should be used with extreme caution due to its direct deleterious effect on neuromuscular transmission.11,12 Exacerbation of weakness may require respiratory support.
FETUS DURING PREGNANCY AND DELIVERY
Rare circumstances affecting the fetus also occur in pregnant women with MG. Transplacental passage of maternal autoantibodies may lead to fetal muscle weakness in utero, thus reducing fetal movements, producing polyhydramnios, and resulting in stillbirth. Fetal difficulty has been described even in mothers with mild or asymptomatic disease, who produce antibodies against the fetal AChRs. In rare cases, babies of mothers with MG may develop arthrogryposis multiplex congenita, a disorder characterized by multiple joint contractures and other anomalies.13 This condition most likely is secondary to decreased fetal movement in utero, which can be monitored with ultrasound. Having a child affected with neonatal complications of MG may be predictive of subsequent offspring being affected. No evidence has been published that babies born tomothers with MG have any increased risk of developing autoimmune-mediated MG.14,15
Approximately 10% to 20% of infants born to mothers with MG develop transient neonatal MG.4 While more common with AChR-positive mothers, transient neonatal MG may occur with anti-MuSK antibody–positivemothers16,17 and rarely even with seronegative mothers. Maternal antibodies are presumed to transfer across the placenta to the infant. Although most infants have detectable maternal antibodies, only a small percentage of infants develop symptoms. Common symptoms include generalized hypotonia as well as respiratory, feeding, and swallowing problems. Symptoms of transient neonatal MG usually develop a few hours after birth and typically resolve within 1 month (range of 1 to 7 weeks).18 Treatment is supportive, including ventilator support and nasogastric feedings, when needed. Pyridostigmine (0.5 mg/kg to 1.0 mg/kg) in divided doses administered 30 minutes before feeding may be useful to improve suck and reduce risk of aspiration.
Rarely, patients with transient neonatal MG may develop more permanent complications, including persistent bulbar and facial weakness and hearing loss. Inactivation of the fetal subunit of the AChR during a critical period of fetal muscle development has been proposed as the cause of this phenotype.19 The maternal fetal/adult AChR antibody ratio was reported as useful in predicting the severity of these manifestations.19,20,21 Case reports suggest that plasma exchange and possibly prednisone during pregnancy may reduce phenotypic severity in offspring, but further studies are needed.19,22
TREATMENT DECISIONS BEFORE PREGNANCY
In general, the severity and distribution of weakness should guide therapy decisions for women with MG who are planning a pregnancy. Patients with recently diagnosed ocular or mild MG have an increased risk of conversionto severe generalized MG, particularly within the first 2 years after onset of symptoms. Also, the MG patient currently using immunosuppressive medication presents another challenge. Initiation of immunosuppressive agents other than prednisone before or during pregnancy is typically avoided. Table 6-1 lists medications used in the treatment of MG with their associated US Food and Drug Administration (FDA) pregnancy category and reported teratogenic risks.23
Table 6-1.
Therapeutic Interventions in Myasthenia Gravis
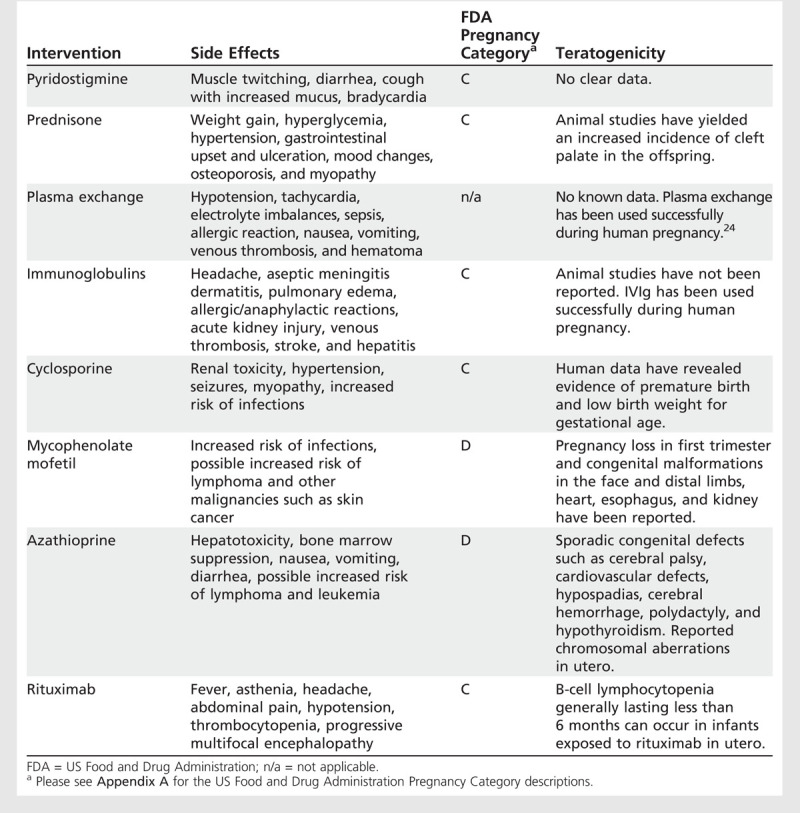
The risk of generalized MG is highest in the first 2 to 3 years after onset. During these years, it is advisable for a patient to delay pregnancy, thereby reducing potential worsening provoked by pregnancy and clarifying her severity and response to treatment. In some women with severe disease, pregnancy would be dangerous and is therefore considered to becontraindicated. Abandoning the idea of pregnancy and continuing immunosuppressive treatment may be contrary to the patient’s desires and necessitates a trusting physician-patient relationship. Without patient acceptance, this recommendation may lead to patient abandonment of care. Consideration of pregnancy must be dealt with sympathetically, and the patient should be reassured that regardless of her decision, she will receive our care. With close monitoring, an otherwise healthy woman with well-controlled MG can have an uneventful pregnancy.
Treatment is stepwise and depends on the clinical scenario. With only minimal manifestations of the disease, pyridostigmine for symptomatic treatment before contemplating pregnancy may be considered. Given the uncertainty of the course of MG, therapy in an asymptomatic myasthenic patient is not suggested. Patients with moderate weakness may benefit from steroids, a medication with lower teratogenic profile. A prior response to steroids or other comorbid conditions aids in the decision to choose this therapy. However, if a patient is on other therapy (eg, steroid-sparing immunosuppressive agents), she may have had a previous incomplete response to steroids. Depending on the distribution of weakness and severity of involvement, steroid use may be a reasonable alternative. Side effects should be closely monitored. If thymectomy is considered, it should be performed before pregnancy or after a stable postpartum period because of the delayed therapeutic effect and surgical risks.
Another, less desirable alternative would be to continue immunosuppressive therapy while attempting pregnancy, during pregnancy, and delivery. The risk of precipitating myasthenic exacerbation or crisis by withdrawing immunosuppressive therapy must be weighed against potential harm to the fetus. In this scenario, the mother will have the greatest likelihood of maintaining her strength and overall health, but risk of teratogenicity to the fetus is increased. If benefits are significant enough to outweigh the risk, it is important that the parents be well informed and the patient be registered in the appropriate drug-risk pregnancy registry once pregnancy is confirmed (eg, www.mycophenolatepregnancyregistry.com).
TREATMENT OPTIONS DURING PREGNANCY
During pregnancy, MG improves in approximately 30% to 40% of patients, remains unchanged in 30% to 40%, and worsens in 20% to 30%.3,5,8 The greatest percentages of exacerbations occur during the first trimester, in the final 4 weeks of gestation, or puerperium. Patients with only mild disease may not require treatment but need close follow-up with assessment for weakness. When weakness is mild, no treatment may be necessary. When needed, medications that have less teratogenic effects are recommended. Potential treatment alternatives for symptomatic relief, including pyridostigmine, can be used safely in recommended doses during pregnancy. Anticholinesterase medications are pregnancy category C. Because of the changes in intestinal absorption and renal function during pregnancy, the dose may need frequent adjustments. The overuse of cholinesterase inhibitors may induce uterine contractions, premature labor, and increase oral secretions, which can be difficult for patients with oropharyngeal weakness.
Corticosteroids, plasma exchange, and IV immunoglobulin (IVIg) have been used safely during pregnancy and are agents often chosen for treatment of exacerbation of weakness. These treatments are generally very well tolerated, although they are not innocuous. Prednisone, prednisolone, and IVIg are pregnancy category C. All have been used frequently during pregnancy in many other autoimmune diseases. However, a small increase in cleft palate with use of prednisone in the first trimester is reported.25 In addition, high doses of prednisone have been associated with premature rupture of membrane. With plasma exchange or IVIg, a theoretical risk of inducing abortion during the post–24-hour periodof coagulopathy is present.26 Given this risk, their use is reserved for the management of more severe MG symptoms or myasthenic crisis. When using IVIg, hyperviscosity and volume overload should be monitored carefully. Hypotension is a serious side effect associated with plasma exchange. To protect against hypotension, the patient should be placed in a left lateral decubitus position and her fluid status carefully monitored during treatment. During the third trimester, fetal monitoring is recommended during plasmapheresis. Benefit from plasma exchange or IVIg is short-lived, and retreatment may be required.
Other medications routinely used in MG pose a greater risk to pregnant mothers, and their use is usually discouraged during pregnancy.27 Although cyclosporine is pregnancy category C, its use during pregnancy is not recommended because of increased risk of spontaneous abortions, prematurity, and low birth weight. Azathioprine and mycophenolate mofetil are pregnancy category D, and methotrexate is category X. The use of these medications is not recommended in pregnancy because they pose significant risk to the fetus.23 Case 6-2 demonstrates decisions in the treatment of a patient with MG throughout pregnancy.
Case 6-2
A 17-year-old girl was diagnosed with oculobulbar myasthenia gravis (MG) based on clinical presentation, elevated acetylcholine receptor antibodies, abnormal repetitive stimulation, and single-fiber EMG. Her first symptoms were ptosis and diplopia followed by dysphagia. Mycophenolate mofetil therapy was initiated and pre-pregnancy counseling was provided. She responded well with only minimal stable signs of MG. Mycophenolate mofetil therapy was continued.
At 26 years of age, she was referred after discovering that she was in her fifth week of gestation. She reported recent decreased energy but denied weakness. Physical examination demonstrated mild-moderate ptosis accentuated by upgaze, minimal weakness of bilateral eye closure, and mild-moderate weakness of cheek puff. Extraocular muscles were intact. She had no dysphagia or limb weakness.
After a long discussion, she agreed to discontinue mycophenolate mofetil. Counseling regarding the natural history of MG during pregnancy was provided. She was prescribed pyridostigmine 30 mg 3 times daily for her symptoms. She had frequent follow-up assessments and remained stable. She was enrolled in the mycophenolate mofetil pregnancy registry and followed in a high-risk obstetric clinic.
She delivered a healthy son without complications, who had no difficulties in the postnatal period. On no therapy, the mother had no symptoms of MG until 1 year after delivery, when she began to develop ptosis and diplopia. One consideration was to restart mycophenolate mofetil at that time. However, she was not using contraception and had no plans to do so. Given the unknown risks of fetal malformation secondary to mycophenolate mofetil, corticosteroid therapy was initiated. She also resumed pyridostigmine 60 mg 3 times daily. With good clinical response, prednisone was gradually tapered to 5 mg/d.
Comment. This case typifies management decisions that arise in a patient with known MG on immunosuppressive therapy who becomes pregnant. Vast knowledge of medication side effects and potential teratogenic effects is needed for appropriate therapeutic management in patients with MG during childbearing age. Prepregnancy counseling is important for the care of both mother and fetus. Education and counseling may need reinforcement at subsequent visits.
LABOR AND DELIVERY
MG typically does not hinder the early stages of labor, as smooth muscle contraction is involved in the first stage of labor. In the second stage of labor, fatigability may be pronounced as striated muscle contraction becomes more important. The obstetrician should be prepared to assist the delivery with vacuum or forceps when necessary. Cholinesterase inhibitors can minimize fatigable weakness during labor. No correlation has been proven between the mode of pregnancy delivery and the rate of exacerbation in the puerperium.3
During labor, regional anesthesia can be used safely and lessens the risk of medication-induced neuromuscular blockade from nondepolarizing anesthetic or curare-like agents (Table 6-2). It is also recommended for patients undergoing cesarean delivery. Those who receive a high level of spinal or epidural anesthesia may experience decreased respiratory function, especially if they have had previous respiratory weakness or significant oropharyngeal symptoms. Nondepolarizing agents worsen neuromuscular transmission and are therefore avoided in MG. Immediate-acting drugs, carefully titrated, are recommended if general anesthesia is needed.28
Table 6-2.
Medications That May Exacerbate Myasthenia Gravis

Treating eclampsia with magnesium sulfate in a woman with MG should be approached with caution. Magnesium blocks calcium entry at the nerve terminal and inhibits acetylcholine release, further disrupting neuromuscular transmission. If the potential benefit of administering magnesium sulfate outweighs the risks, the physician and patient should be prepared for potential worsening of MG and be prepared to provide ventilator support. Phenytoin is an accepted alternative for the treatment of eclampsia.29
Infections, electrolyte disturbances, and numerous drugs have been found to unmask latent MG or trigger a myasthenic crisis. Additionally, the issue of appropriate vaccines may arise. As a general rule, live virus vaccines should be avoided in any patient with MG, particularly in the setting of immunosuppressive therapy.30,31 Table 6-2 summarizes medications that may exacerbate MG.
THE POSTPARTUM PERIOD
Symptoms may worsen in the puerperium, typically within 6 to 8 weeks after delivery. Close follow-up for potential worsening is recommended. Treatment selection during lactation may pose another challenge. Most of the medications for the treatment of MG can be secreted through the milk and therefore pose a potential risk to the newborn.32,33 The American Academy of Pediatrics considers pyridostigmine, prednisone, and prednisolone compatible with lactation.32 Pyridostigmine is excreted into human breast milk. Conclusions from very limited data have estimated that infants would ingest less than 0.1% of the maternal dose, so adverse effects in the infant are unlikely. Manufacturers for prednisone recommend that caution be used when administering prednisone to nursing women.
Cyclosporine is excreted in human breast milk. Because of potential effects in a nursing infant such as immunosuppression, neutropenia, growth retardation, and potential carcinogenesis, cyclosporine is considered contraindicated by the American Academy of Pediatrics. Azathioprine and methotrexate are unsafe during breast-feeding. The safety of mycophenolate mofetil, rituximab, or IVIg (human) during lactation is not known. No data have been published on the excretion of mycophenolic acid (the active metabolite of mycophenolate mofetil) in human breast milk.32,33 Rituximab is secreted in the milk of lactating cynomolgus monkeys, and IgG is excreted in human breast milk. Table 6-3 summarizes the safety of the medications used in MG during lactation.
Table 6-3.
Safety of Medications Used in Myasthenia Gravis During Lactation

Even after a successful pregnancy, the care of the newborn poses new challenges that may have an effect on the mother with MG. Patients may develop worsening of symptoms due to fatigue induced by reduced sleep, frequent feedings, and increased physical exertion related to caring for the baby. Symptoms during this period can be transient and managed by conservative treatment, including engagement of a support system.
All women of childbearing potential (including pubertal girls and perimenopausal women) who begin or restart an immunosuppressive regimen must receive contraceptive counseling and use effective contraception. The patient should begin using her chosen contraceptive method 4 weeks before starting therapy for MG and continue contraceptive use during therapy. When discontinuing immunosuppressive therapy, effective contraception should be continued for 6 months before attempting pregnancy. Mycophenolate mofetil reduces blood levelsof the hormones in oral contraceptive pills and could theoretically reduce its effectiveness. Two reliable forms of contraception must be used simultaneously for this particular medication unlessabstinence is the chosen method. Case 6-3 demonstrates counseling and treatment decisions in a childbearing female patient before pregnancy and follow-up management after pregnancy.
Case 6-3
A 23-year-old woman presented with diplopia, ptosis, then generalized weakness over several months. The diagnosis of myasthenia gravis (MG) was established by an abnormal single-fiber EMG. She underwent thymectomy with partial improvement and had further benefit with azathioprine. Prepregnancy counseling was provided.
She decided to become pregnant and presented to discuss discontinuation of azathioprine. She reported some fatigue and difficulty using her arms over her head. Her examination was normal. After counseling, she agreed to discontinue azathioprine and continue birth control pills for several months to provide time for azathioprine clearance. She understood the possibility of her MG worsening and recognized the risks associated with pregnancy. She was also informed regarding possible intervention with prednisone, pyridostigmine, or plasma exchange during the pregnancy, depending on her symptoms. The patient became pregnant and was followed throughout her pregnancy with no significant complications apart from minimal weakness of her upper extremities. She also was followed by a high-risk pregnancy obstetric service. Her fetus remained very active and was delivered without difficulty.
She did very well through the immediate postpartum period, and therefore, no medications were reinstituted. Her baby had slight head lag and was floppy. He had good suck, good grasp, and a robust cry and showed no signs of difficulty breathing. He could bear weight on his legs while supported. He had minimal manifestations of neonatal MG that did not require treatment and resolved after a few weeks.
At 9 months post partum, the patient experienced a recurrence of symptoms and signs, and azathioprine was restarted. She was informed about the risk of breast-feeding with this medication. Her symptoms resolved within 3 months with no further complications.
Comment. This case highlights management in a patient before, during, and after pregnancy. Patients should avoid pregnancy during the first 6 months after discontinuing immunosuppression. Close follow-up in this special population is partnered with the guidance of a high-risk obstetric team. The patient should be aware of potential treatment interventions with less teratogenic potential for the fetus. During the postpartum period, the mother and the baby are followed closely to determine whether additional treatment is indicated. If immunosuppressive therapy needs to be initiated, adequate counseling regarding breast-feeding and contraception must be provided.
CONCLUSION
MG can first present during pregnancy or the postpartum period. Exacerbations may also occur in patients with preexisting MG. Pregnancy may affect the course of MG in an unpredictable way, but worsening symptoms most frequently occur in the first trimester or in the first 3 to 4 weeks postpartum. The effect of one pregnancy on MG does not predict the effect in subsequent pregnancies. Clinical status does not reliably predict the course of MG during pregnancy. Close follow-up of women with MG in the childbearing age is essential. Frequent evaluation during and before pregnancy allows therapy modification based on changes in severity. Table 6-4 summarizes important aspects that require attention and monitoring to ensure adequate management in patients with MG during the childbearing age.
Table 6-4.
Issues for Women of Childbearing Age Who Have Myasthenia Gravis

Immunosuppressive medications have potential teratogenic effects, and preferably their use should be discontinued 4 to 6 months before conceiving. Corticosteroids, plasma exchange, and IVIg have a lower potential risk, have been used safely during pregnancy, and therefore are more preferred choices for treatment of MG exacerbation during pregnancy. An individualized and interdisciplinary approach to care is needed throughout pregnancy and the postpartum period of patients with MG and their newborns.
KEY POINTS
Women with myasthenia gravis benefit from a personalized interdisciplinary approach to care during pregnancy and the postpartum period, including neuromuscular, high-risk obstetric, and neonatal pediatric specialists.
Myasthenia gravis is unmasked or worsened in approximately one-third of patients during their pregnancy.
Elevated serum levels of anti–acetylcholine receptor-binding antibodies or anti–muscle-specific kinase (MuSK) antibodies in patients with clinical signs and symptoms of myasthenia gravis confirm the diagnosis. In the seronegative patient, electrophysiologic demonstration of an abnormality of neuromuscular transmission establishes the diagnosis.
Patients may undergo chest CT imaging without contrast to assess the thymus gland; however, postponement until after delivery is preferable, particularly in antibody-negative patients.
The severity of weakness at the beginning of pregnancy does not predict either remission or exacerbation, and, in fact, disease exacerbations, myasthenic crisis, or even disease remission may each occur during pregnancy.
Close monitoring for respiratory difficulties is essential throughout pregnancy to maintain the welfare of both mother and fetus.
No evidence has been published that babies born to mothers with myasthenia gravis have any increased risk of developing autoimmune-mediated myasthenia gravis.
The risk of generalized myasthenia gravis is highest in the first 2 to 3 years after onset. During these years, it is advisable for a patient to delay pregnancy, thereby reducing potential worsening provoked by pregnancy and clarifying her severity and response to treatment.
Treatment is stepwise and depends on the clinical scenario. With only minimal manifestations of the disease, pyridostigmine for symptomatic treatment before contemplating pregnancy may be considered.
Corticosteroids, plasma exchange, and IV immunoglobulin have been used safely during pregnancy and are agents often chosen for treatment of exacerbation of weakness.
Azathioprine and mycophenolate mofetil are US Food and Drug Administration pregnancy category D, and methotrexate is category X. The use of these medications is not recommended in pregnancy because they pose significant risk to the fetus.
Myasthenia gravis typically does not hinder the early stages of labor, as smooth muscle contraction is involved in the first stage of labor.
During labor, regional anesthesia can be used safely and lessens the risk of medication-induced neuromuscular blockade from nondepolarizing anesthetic or curare-like agents.
Infections, electrolyte disturbances, and numerous drugs have been found to unmask latent myasthenia gravis or trigger a myasthenic crisis.
The American Academy of Pediatrics considers pyridostigmine, prednisone, and prednisolone compatible with lactation.
All women of childbearing potential (including pubertal girls and perimenopausal women) who begin or restart an immunosuppressive regimen must receive contraceptive counseling and use effective contraception.
Footnotes
Relationship Disclosure: Dr Massey has received educational grants from Allergan, Inc; and Merz Pharma. Dr De Jesus-Acosta reports no disclosure.
Unlabeled Use of Products/Investigational Use Disclosure: Drs Massey and De Jesus-Acosta discuss the use of drugs for the treatment of myasthenia gravis, none of which are labeled by the US Food and Drug Administration for use in pregnancy.
REFERENCES
- 1.Myasthenia Gravis Foundation of America. www.myasthenia.org. Accessed October 16, 2013.
- 2.Ciafaloni E,, Massey J. Myasthenia gravis and pregnancy. Neurol Clin 2004; 22 (4): 771–782. [DOI] [PubMed] [Google Scholar]
- 3.Schlezinger NS. Pregnancy in myasthenia gravis and neonatal myasthenia gravis. Am J Med 1955; 19 (5): 718–720. [DOI] [PubMed] [Google Scholar]
- 4.Plauche WC. Myasthenia gravis in mothers and their newborns. Clin Obstet Gynecol 1991; 34 (1): 82–99. [PubMed] [Google Scholar]
- 5.Djelmis J,, Sostarko M,, Mayer D,, Ivanisevic M. Myasthenia gravis in pregnancy: report on 69 cases. Eur J Obset Gynecol Reprod Biol 2002; 104 (1): 21–25. [DOI] [PubMed] [Google Scholar]
- 6.Maggi L,, Andreetta F,, Antozzi C, et al. Two cases of thymoma-associated myasthenia gravis without antibodies to the acetylcholine receptor. Neuromuscul Disord 2008; 18 (8): 678–680. [DOI] [PubMed] [Google Scholar]
- 7.Choi DeCroos E,, Hobson-Webb LD,, Juel VC, et al. Do acetylcholine receptor and striated muscle antibodies predict the presence of thymoma in patients with myasthenia gravis? [published online ahead of print April 27, 2013]. Muscle Nerve 2013. doi:10.1002/mus.23882. [DOI] [PubMed] [Google Scholar]
- 8.Batocchi A,, Majolini L,, Evoli A, et al. Course and treatment of myasthenia gravis during pregnancy. Neurology 1999; 52 (3): 447–452. [DOI] [PubMed] [Google Scholar]
- 9.Ellison J,, Thomson AJ,, Walker ID,, Greer IA. Thrombocytopenia and leucopenia precipitated by pregnancy in a woman with myasthenia gravis. BJOG 2000; 107 (8): 1052–1054. [DOI] [PubMed] [Google Scholar]
- 10.Igarashi S,, Yamauchi T,, Tsuji S, et al. A case of myasthenia gravis complicated by cyclic thrombocytopenia [in Japanese]. Rinsho Shinkeigaku 1992; 32 (3): 321–323. [PubMed] [Google Scholar]
- 11.Duff GB. Preeclampsia and the patient with myasthenia gravis. Obstet Gynecol 1979; 54 (3): 355–358. [PubMed] [Google Scholar]
- 12.Mueksch JN,, Stevens WA. Undiagnosed myasthenia gravis masquerading as eclampsia. Int J Obstet Anesth 2007; 16 (4): 379–382. [DOI] [PubMed] [Google Scholar]
- 13.Polizzi A,, Huson S,, Vincent A. Teratogen update: maternal myasthenia gravis as a cause of congenital arthrogryposis. Teratology 2000; 62 (5): 332–341. [DOI] [PubMed] [Google Scholar]
- 14.Guidon AC,, Massey EW. Neuromuscular disorders in pregnancy. Neurol Clin 2012; 30 (3): 889–911. [DOI] [PubMed] [Google Scholar]
- 15.Hoff JM,, Daltveit AK,, Gilhus NE. Myasthenia gravis in pregnancy and birth: identifying risk factors, optimising care. Eur J Neurol 2007; 14 (1): 38–43. [DOI] [PubMed] [Google Scholar]
- 16.O’Carroll P,, Bertorini TE,, Jacob G,, Mitchell CW,, Graff J. Transient neonatal myasthenia gravis in a baby born to a mother with new-onset anti-MuSK-mediated myasthenia gravis. J Clin Neuromuscul Dis 2009; 11 (2): 69–71. [DOI] [PubMed] [Google Scholar]
- 17.Niks EH,, Verrips A,, Semmekrot BA, et al. A transient neonatal myasthenic syndrome with anti-MUSK antibodies. Neurology 2008; 70 (14): 1215–1216. [DOI] [PubMed] [Google Scholar]
- 18.Ahlsten G,, Lefvert AK,, Osterman PO, et al. Follow-up study of muscle function in children of mothers with myasthenia gravis during pregnancy. J Child Neurol 1992; 7 (3): 264–269. [DOI] [PubMed] [Google Scholar]
- 19.Oskoui M,, Jacobson L,, Chung WK, et al. Fetal acetylcholine receptor inactivation syndrome and maternal myasthenia gravis. Neurology 2008; 71 (24): 2010–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoff J,, Daltveit A,, Gilhus N. Myasthenia gravis: consequences for pregnancy, delivery, and the newborn. Neurology 2003; 61 (10): 1362–1366. [DOI] [PubMed] [Google Scholar]
- 21.Gardnerova M,, Eymard B,, Morel E, et al. The fetal/adult acetylcholine receptor ratio in mothers with myasthenia gravis as a marker for transfer of the disease to the newborn. Neurology 1997; 48 (1): 50–54. [DOI] [PubMed] [Google Scholar]
- 22.Wen JC,, Liu TC,, Chen YH, et al. No increased risk of adverse pregnancy outcomes for women with myasthenia gravis: a nationwide population-based study. Eur J Neurol 2009; 16 (8): 889–894. [DOI] [PubMed] [Google Scholar]
- 23.US Food and Drug Administration pregnancy categories, drug safety and availability. www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm. Accessed December 9, 2013.
- 24.Watson WJ,, Katz VL,, Bowes WA, Jr. Plasmapheresis during pregnancy. Obstet Gynecol 1984; 76 (3 pt 1): 451–457. [PubMed] [Google Scholar]
- 25.Rodriguez-Pinilla E,, Martinez-Frias ML. Corticosteroid during pregnancy and oral clefts: a case-control study. Teratology 1998; 58 (1): 2–5. [DOI] [PubMed] [Google Scholar]
- 26.Kaaja R,, Julkunen A,, Ammala P, et al. Intravenous immunoglobulin treatment of pregnant patients with recurrent pregnancy losses associated with antiphospholipid antibodies. Acta Obtet Gynecol Scand 1993; 72 (1): 63–66. [DOI] [PubMed] [Google Scholar]
- 27.Armenti VT,, Radomski JS,, Moritz MJ, et al. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl 2001; 97–105. [PubMed] [Google Scholar]
- 28.Kuczkowski KM. Labor analgesia for the parturient with neurological disease: what does an obstetrician need to know? Arch Gynecol Obstet 2006; 274 (1): 41–46. [DOI] [PubMed] [Google Scholar]
- 29.Carr SR,, Gilchrist JM,, Abuelo DN,, Clark D. Treatment of antenatal myasthenia gravis. Obstet Gynecol 1991; 78 (3 pt 2): 485–489. [PubMed] [Google Scholar]
- 30.Zhang J,, Xie F,, Delzell E, et al. Association between vaccination for herpes zoster and risk of herpes zoster infection among older patients with selected immune-mediated diseases. JAMA 2012; 308 (1): 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. Recommended adult immunization schedule—United States, 2012. JAMA 2012; 308 (1): 22–23. [PubMed] [Google Scholar]
- 32.American Academy of Pediatrics Committee on Drugs. Transfer of drugs and other chemicals into human breast milk. Pediatrics 2001; 108 (3): 776–789. [DOI] [PubMed] [Google Scholar]
- 33.Drugs.com. Pregnancy and breastfeeding warnings. www.drugs.com/pregnancy. Accessed December 9, 2013.


