Abstract
Purpose of Review:
Unruptured intracranial aneurysms are found commonly in the general public, and more frequently in certain populations. This article focuses on the epidemiology, screening strategies, and management options for patients with unruptured aneurysms.
Recent Findings:
Recent epidemiologic studies show the overall prevalence of intracranial aneurysms to be approximately 3%, with higher rates seen in familial aneurysm syndromes and in certain medical conditions, such as autosomal dominant polycystic kidney syndrome. Aneurysm treatment may include surgical or endovascular techniques, with increasing utilization of endovascular strategies over time. Increased aneurysm diameter, certain locations, and other anatomical considerations may be associated with higher risks of aneurysm rupture.
Summary:
Given the high morbidity and mortality associated with aneurysm rupture, screening for unruptured aneurysms is generally recommended for high-risk patients (patients who have at least two first-degree relatives with aneurysms, and patients with autosomal dominant polycystic kidney disease). Screening may be considered for other patients (eg, one first-degree relative with aneurysm) after discussion of the risks and benefits of imaging. Following identification of an aneurysm, decisions regarding observation or treatment should be based on patient characteristics, features of the aneurysm, and provider expertise.
INTRODUCTION
Advances in the availability and resolution of neuroimaging studies have led to more frequent identification of intracranial aneurysms. Rupture of intracranial aneurysms can lead to devastating consequences, namely subarachnoid hemorrhage, but unruptured intracranial aneurysms can also be associated with significant morbidity. Such morbidity can include local compressive symptoms such as headache or cranial nerve palsies, strokes, and seizures, as well as patient and family anxiety regarding the risk of rupture. This article reviews the epidemiology of intracranial aneurysms, recommendations for screening for aneurysms in the general population and in select patient populations, and management options in patients found to have unruptured intracranial aneurysms.
ANEURYSM TYPES AND DEFINITIONS
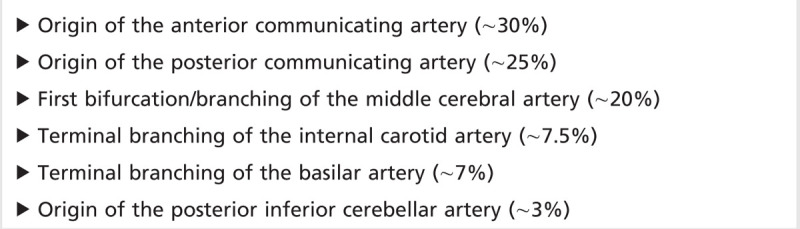
Saccular (berry) aneurysms are the most common type of intracranial aneurysm, consisting of a focal outpouching (ballooning) of the arterial blood vessel wall. Although the exact pathophysiologic mechanism is not clear, these are thought to be due to a congenital weakness in the vessel wall, which then slowly enlarges over time in the setting of arterial pressure. Saccular aneurysms are most frequently seen at the branching points of blood vessels, often larger intracranial vessels in or around the circle of Willis.1 A list of common saccular aneurysm locations is listed in Table 8-1. Despite the predilection for saccular aneurysms to form on larger vessels near the skull base, aneurysms can be found in more distal locations in smaller caliber arteries. For example, distal anterior cerebral artery aneurysms, also known as pericallosal artery aneurysms, are thought to represent 4% to 6% of intracranial aneurysms.2
Table 8-1.
Common Locations of Intracranial Saccular Aneurysms
Although this article focuses on saccular aneurysms, other types of intracranial aneurysms exist. Fusiform aneurysms are seen in association with intracranial atherosclerotic disease or dissections of intracranial arteries and represent longitudinal areas of vessel dilation, as opposed to the focal outpouching seen with saccular aneurysms. Fusiform aneurysmal segments are commonly seen in conjunction with areas of atherosclerotic narrowing, often seen in larger intracranial arteries such as the basilar artery.3 While there are occasional episodes of rupture of dolichoectatic fusiform aneurysms, they are more likely to become symptomatic through (1) local mass effect on adjacent brain structures or (2) thrombus formation, resulting in either occlusion of small perforating vessels or downstream embolization. Mycotic aneurysms result from the deposition of septic emboli in small pial arteries. The underlying infectious agent (often bacteria, in the setting of bacterial endocarditis and bacteremia) causes weakness and outpouching of the blood vessel wall, potentially resulting in subarachnoid or intraparenchymal hemorrhage.4
EPIDEMIOLOGY
Prevalence
Although some past autopsy-based studies quote higher aneurysm identification rates, most population-based studies quote unruptured intracranial aneurysm prevalence rates of 2% to 3%.1,5,6 In a recent meta-analysis encompassing almost 95,000 patients from 68 studies and 21 countries, the prevalence was estimated at 2.8%.6 Subject age, sex, and comorbidity varied across studies; when data were normalized for a population aged 50 years and with equal numbers of men and women, the prevalence was estimated to be 3.2%.6
Risk Factors
Age, sex, and family history are all important nonmodifiable risk factors for unruptured intracranial aneurysms (Table 8-2).7 The prevalence of unruptured intracranial aneurysms increases with patient age, especially among women. Specifically regarding family history, the presence of an aneurysm in one first-degree relative (parent, sibling, or child) increases the risk of aneurysm from 2.3% to 4.0%, while presence in two or more first-degree relatives increases the risk to 8.0% percent.5 Data suggest that not only is the likelihood of developing aneurysms heritable, but aneurysm location is concordant among individuals in families with aneurysms.8 This may have implications regarding the developmental origin of aneurysms, their risk of rupture, and treatment decisions (see section on risk of rupture). The risk of aneurysm rupture may also be increased in familial aneurysm syndromes compared with the overall population’s risk of rupture. Data from the Familial Intracranial Aneurysm study suggest that the risk of aneurysm rupture in patients with a family history of aneurysm was 17 times higher than the risk of rupture based on aneurysm size and location that would be predicted from observational studies.9 The genetics of intracranial aneurysm formation is complex and an area of ongoing research. A large review of more than 116,000 patients included in genetic and genome-wide association studies identified several polymorphisms associated with higher aneurysm incidence; however, this study offered limited insight on other information of relevance for unruptured intracranial aneurysm management (eg, size, location).10
Table 8-2.
Risk Factors for Intracranial Aneurysms

Certain connective tissue disorders are associated with an increased risk of aneurysms, such as Marfan syndrome and Ehlers-Danlos syndrome type IV.1 A single study from the Mayo Clinic found higher than expected rates of unruptured intracranial aneurysms in adults with coarctation of the aorta (10% versus 2%), although the number of aneurysms was small (10 aneurysms among 100 studied patients).11 Intracranial aneurysms can be seen in association with fibromuscular dysplasia,12,13 although it remains unclear whether the pathophysiology is related to arterial dissection (commonly seen in fibromuscular dysplasia) or other mechanisms causative of unruptured intracranial aneurysm development in other populations.
Although it is not a purely connective tissue disorder, autosomal dominant polycystic kidney disease is strongly associated with aneurysm development. In a recent study from China, patients with autosomal dominant polycystic kidney disease were found to have a 12.4% prevalence of unruptured intracranial aneurysms, significantly elevated over the general population rate.14 The risk increased with age (23.3% risk in patients aged 60 to 69 years) and was twice as high in patients with a family history of aneurysm.14 Risk of aneurysms is possibly also increased among patients with the less common but related condition of autosomal recessive polycystic kidney disease.15
Tobacco use, excessive alcohol consumption, and hypertension are important modifiable risk factors for aneurysm growth. While a congenital weakness may underlie the development of an aneurysm, tobacco or alcohol abuse and hypertension can lead to further vascular injury/weakness and increased aneurysm size, which are factors associated with a higher risk of aneurysm rupture.16
SIGNS AND SYMPTOMS OF ANEURYSMS
Subarachnoid hemorrhage (SAH) secondary to aneurysm rupture is the most feared complication of intracranial aneurysms. Common signs and symptoms of SAH should be well known to neurologists and can include sudden severe headache, headache associated with concerning symptoms (loss of consciousness, meningeal symptoms), or coma without other obvious cause.17
In contrast to the presentation of SAH, most unruptured intracranial aneurysms are neurologically silent. Many aneurysms will be identified when patients are imaged for an unrelated neurologic indication, such as headache (Case 8-1). Unless it has grown to a significant size, an unruptured intracranial aneurysm would be unlikely to cause headache, and alternative explanations should be considered. However, when an aneurysm is found, it is important to inquire about possible symptoms of prior aneurysmal rupture, such as an especially severe headache, headache associated with meningeal symptoms, or headache associated with loss of consciousness. The presence of these symptoms may suggest that a presumed unruptured intracranial aneurysm may instead be an aneurysm that has previously ruptured, and this may affect decision-making regarding treatment.
Rarely, unruptured aneurysms can be associated with focal neurologic symptoms, often from compression of adjacent structures such as cranial nerves. Classic examples include oculomotor nerve palsies related to posterior communicating artery aneurysms or compression of the third, fourth, fifth, or sixth cranial nerves from aneurysms of the carotid artery within the cavernous sinus. Giant aneurysms (measuring more than 25 mm in diameter) can result in mass effect on nearby brain parenchyma and cause various symptoms depending on the specific location; such mass effect can also result in partial seizures. The turbulent, nonlaminar flow within aneurysms can result in thrombus formation; downstream embolization and stroke may occur.
Case 8-1
A 52-year-old woman was seen in the office 1 week after going to an emergency department for a syncopal episode. At the time of her presentation, she denied headache, nausea, neck stiffness, and any other neurologic symptoms. Her overall medical examination and neurologic examination in the emergency department had been unremarkable except for mild orthostasis, and subsequent cardiologic evaluation was unrevealing. Additional history in the office disclosed that her loss of consciousness had been very brief and occurred in the setting of recent dehydration, and her current neurologic examination was normal. Intravascular depletion at the time of the syncopal episode was suspected as the primary contributor to her event. Review of her evaluation in the emergency department, however, included a head CT that showed a possible enlargement in the area of the basilar bifurcation (Figure 8-1A). CT angiography followed by catheter angiography confirmed an 8 mm aneurysm at the bifurcation of the basilar artery (CT angiogram image shown in Figure 8-1B). Following discussion of the risks and benefits of treatment, she underwent coil embolization of the aneurysm. After 5 years of follow-up, there were no signs of aneurysm recurrence.
Figure 8-1.
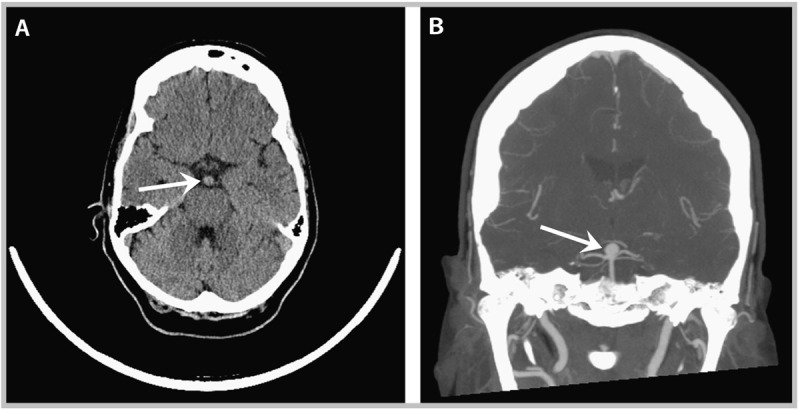
Imaging studies taken of the patient in Case 8-1 at presentation to the emergency department. A, Noncontrast CT of the brain showing enlargement of the basilar artery near the bifurcation (arrow). B, CT angiogram (coronal view) showing basilar apex aneurysm (arrow).
Comment. As is often the case, this aneurysm was discovered incidentally, following a CT scan of the brain that was ordered for other purposes (syncope, in this case). Given the patient’s relatively young age, the aneurysm size being greater than 7 mm, and the posterior circulation location of the aneurysm, the decision to treat via endovascular coiling (as opposed to open surgery or observation) seems appropriate. She received adequate follow-up, both clinically and radiographically, to ensure there had been no recurrence of the aneurysm at the treated site or new aneurysms forming elsewhere.
GUIDELINES FOR SCREENING
Which Patients to Assess for an Unruptured Intracranial Aneurysm
All patients with symptoms of a possible symptomatic unruptured aneurysm described in the above section should be candidates for cerebrovascular imaging to evaluate for aneurysm.
Regarding asymptomatic unruptured intracranial aneurysms, the American Heart Association and American Stroke Association18 have established guidelines about specific patient populations in which screening of asymptomatic individuals should be considered. Patients with two first-degree relatives with known intracranial aneurysms (ruptured or unruptured) are recommended to undergo screening imaging (Case 8-2). Patients with a single first-degree relative affected by aneurysms may be candidates for screening imaging based on a higher risk of unruptured intracranial aneurysms in this setting; however, the potential risks of aneurysm detection (eg, anxiety, risks of subsequent testing, difficulty obtaining life insurance, occupational concerns) need to be discussed with the patient along with the potential benefits (earlier detection and possible treatment). Patients with autosomal dominant polycystic kidney disease are also recommended to undergo screening.5
Case 8-2
A 33-year-old man was seen in clinic to discuss screening for intracranial aneurysms. He had no current neurologic symptoms and no active medical problems. He had never been prone to headache, and his history was completely unremarkable for any events suggestive of subarachnoid hemorrhage (eg, severe or sudden unexplained headache, headache with loss of consciousness, headache associated with meningeal symptoms). His family history was notable for an unruptured intracranial aneurysm in his sister, diagnosed at 40 years of age after she underwent an MRI of the brain to evaluate her migraine headaches; this was subsequently treated with surgical clipping. In addition, his father died suddenly of unknown causes at 51 years of age; an autopsy was not performed. The patient’s mother was alive and in good health, as were two other siblings (aged 34 and 37 years) and his one daughter (aged 4 years). To his knowledge, his other first-degree relatives had not undergone intracranial imaging for any reason.
Comment. This patient may be a reasonable candidate for noninvasive imaging to screen for unruptured intracranial aneurysms. He has at least one first-degree relative with an intracranial aneurysm (his sister). In addition, his father’s sudden death may have been related to subarachnoid hemorrhage secondary to aneurysm rupture. It would be important to inquire about other possible causes of his father’s death (eg, cardiovascular disease, substance abuse); if none are present, noninvasive cerebrovascular imaging with either CT angiography or magnetic resonance angiography should be considered for aneurysm screening purposes.
When to Screen and Rescreen
Aneurysms are uncommon in patients younger than 20 years,19 so screening is typically not recommended for children and adolescents except in unusual circumstances. A screening imaging test that fails to show an aneurysm does not rule out the possibility of subsequent aneurysm formation, especially since the prevalence of aneurysms increases with age. In high-risk subpopulations (eg, patients who have two or more first-degree relatives with aneurysms, patients with autosomal dominant polycystic kidney disease), it may be reasonable to repeat noninvasive imaging every 5 years, since studies show the risk of aneurysm identification following an initial negative study may be as high as 7%.20
Which Imaging Modality to Use
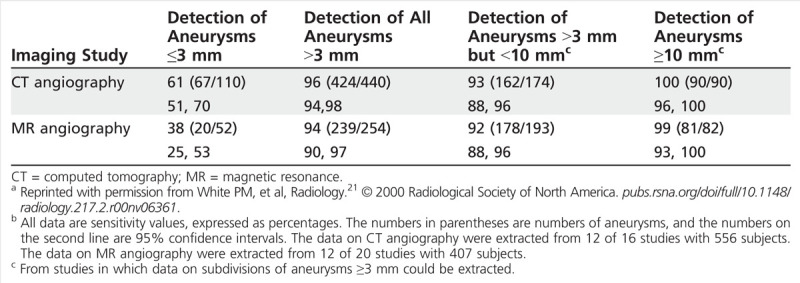
Catheter angiography remains the gold standard for aneurysm detection; although its risks are low, they are not inconsequential, and so typically noninvasive imaging options are preferred for initial screening. CT angiography and magnetic resonance (MR) angiography are both associated with high specificity in the detection of unruptured intracranial aneurysms. Sensitivity values range across studies, but CT angiography (sensitivity of 0.77 to 0.97) and MR angiography (0.69 to 0.99) are both felt to have acceptable diagnostic test characteristics (see Table 8-3 for examples of sensitivities from one systematic review).1,21,22,23 It is important to note that the sensitivity of both modalities drops considerably in the investigation of small aneurysms (less than 3 mm in diameter),23 although aneurysms of this size also have low rupture rates. The selection of the appropriate test should incorporate patient factors (eg, renal disease) and institutional factors (eg, availability) as well; if repeated screening is necessary, it may be reasonable to consider MR angiography because of concerns about repeated radiation exposure associated with CT.
Table 8-3.
Sensitivities of CT Angiography and MR Angiography for Aneurysm Detection Based on Aneurysm Sizea,b
A recent cost-effectiveness analysis attempted to address some of these potential questions for patients with at least two first-degree relatives with unruptured intracranial aneurysms or SAH (ie, individuals who appeared to be good candidates for screening imaging). This analysis used MR angiography as the initial noninvasive technique, followed by catheter angiography if needed to identify false positives and to better characterize the aneurysm. After varying the age to begin screening, the age to end screening, and the interval between imaging studies, the authors determined that the optimal cost-effectiveness strategy was to begin screening at age 20 years and reimage every 7 years until reaching 80 years of age.24
MANAGEMENT OF THE UNRUPTURED ANEURYSM
Risk of Rupture
While other possible symptoms of unruptured intracranial aneurysms were discussed in an earlier section, the major goal of unruptured aneurysm management is minimizing the risk of rupture and subsequent SAH. There is a strong association between aneurysm size and risk of rupture, shown in two large observational studies. The International Study of Unruptured Intracranial Aneurysms (ISUIA)25 included a prospective observational cohort of 1692 patients in whom aneurysm treatment (surgery or endovascular treatment) was not performed. Over 5 years, the risk of aneurysm rupture among aneurysms in the anterior circulation (internal carotid, middle cerebral, and anterior cerebral arteries) was 0% in aneurysms less than 7 mm in diameter, 2.6% for those 7 mm to 12 mm in diameter, 14.5% for those 13 mm to 24 mm in diameter, and 40% for diameters greater than 25 mm. Across all diameter sizes, rupture risks were higher for aneurysms in the posterior circulation: 2.5% for less than 7 mm; 14.5% for 7 mm to 12 mm; 18.4% for 13 mm to 24 mm; and 50% for greater than 25 mm.25 Criticisms of the ISUIA data include the fact that aneurysms of the cavernous segment of the carotid artery were included despite the fact that their intradural location prevents the formation of SAH, as well as concerns regarding selection biases and lower than expected rupture rates.26
A large cohort of Japanese patients27 with unruptured intracranial aneurysms (6697 aneurysms in 5720 patients) showed some similarities to the ISUIA results but also some important differences. As in the ISUIA study, a clear association was found between aneurysm size and risk of rupture. Specifically, using aneurysms of 3 mm to 4 mm in diameter as a reference, the hazard ratio for aneurysm rupture was 3.35 for those 7 mm to 9 mm in diameter, 9.09 for those measuring 10 mm to 24 mm in diameter, and 76.26 for aneurysms 25 mm or larger in diameter; no significant increase in risk was seen for aneurysms between 5 mm and 6 mm in diameter (Figure 8-2).27 However, unlike ISUIA, this study did not find an elevated risk of rupture for aneurysms in the posterior circulation compared with those of similar size occurring in the anterior circulation. The study did show that, compared with unruptured intracranial aneurysms involving the middle cerebral artery, aneurysms involving the anterior communicating or posterior communicating arteries were more prone to rupture (hazard ratios 2.02 and 1.90, respectively). Finally, this cohort study also showed an association between rupture and the presence of a daughter sac, an irregular protrusion from the aneurysmal wall, as opposed to a smooth wall (hazard ratio 1.63).27 Because this study involved only patients from Japan, the ability to generalize these results to other populations is uncertain.
Figure 8-2.
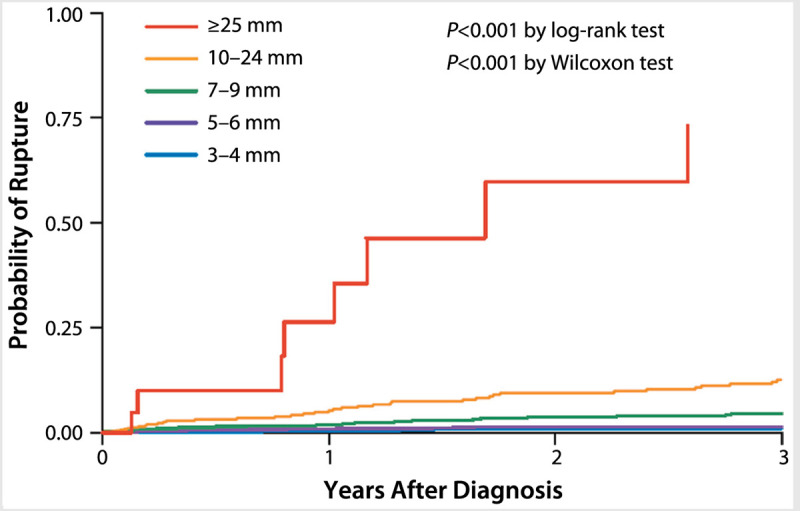
Probability of aneurysm rupture based on aneurysm size, as seen in a large prospective cohort of Japanese patients. Reprinted with permission from UCAS Japan Investigators, et al, N Engl J Med.27 © 2012 Massachusetts Medical Society. http://pubs.rsna.org/doi/full/10.1148/radiology.217.2.r00nv06361.
Despite these two large prospective studies, it still remains difficult to predict aneurysm rupture rates within individual patients. In general, risks increase with larger unruptured intracranial aneurysm size, although rupture rates were not zero for smaller aneurysms in the Japanese cohort (0.36% annual risk for aneurysms measuring 3 mm to 4 mm in diameter).27 Aneurysms affecting the middle cerebral artery may have the lowest risk of rupture, and atypical wall features are likely to increase risk.
In addition to the presence of a daughter sac, other anatomical features of aneurysms that possibly predict rupture are needed to guide decisions regarding treatment. Recent studies have looked at not only aneurysm diameter but also the ratio of the aneurysm size to the size of the parent vessel.28,29 This size ratio was found to be especially predictive of aneurysm rupture in smaller aneurysms, which are common and have a low but not 0% rate of rupture.29
Medical Management
No high-quality randomized clinical trials have been done to guide the medical management of patients with unruptured intracranial aneurysms. Given the associations between (1) hypertension and unruptured intracranial aneurysm growth and (2) larger unruptured intracranial aneurysm size and aneurysm rupture, treatment of hypertension is generally recommended. National guidelines from the Seventh Report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure should be followed.30 Similarly, use of tobacco or illicit drugs and excessive alcohol consumption can lead to vascular injury and weaken blood vessel walls. As part of good practice, patients with unruptured intracranial aneurysms should be counseled to avoid these habits.
If a conservative strategy is chosen, follow-up noninvasive imaging should be performed on a periodic basis to ensure that the aneurysm is not rapidly enlarging, concerning features such as daughter sacs are not developing, and other aneurysms are not identified. Again, highly regarded studies to guide imaging in this setting are lacking, but a reasonable strategy may include noninvasive imaging 6 to 12 months after initial discovery of an unruptured intracranial aneurysm, repeated annually until the treatment team is confident that the aneurysm has not changed significantly.31
Surgical/Endovascular Management
Full discussion of the surgical and endovascular options for unruptured intracranial aneurysm management go beyond the scope of this article, but basic premises are reviewed here. Historically, craniotomy followed by surgical clipping was the preferred option, but endovascular aneurysm treatment is now more common than surgery—from 2001 through 2008, the proportion of unruptured intracranial aneurysms treated by endovascular techniques increased from 20% to 63%.32 Figure 8-3 shows an example of an aneurysm treated by endovascular coiling. Both options have their relative benefits and drawbacks, and certain aneurysms may be best addressed by one strategy over the other. For example, aneurysms of the middle cerebral artery bifurcation are more readily accessible via craniotomy and may be more amenable to surgery compared with aneurysms in deeper locations (such as the basilar artery apex). The diameter of the aneurysm neck may also play a role in treatment options, with wider-necked aneurysms thought to be better treated via surgical clipping; however, the development of newer endovascular techniques such as stent-assisted coiling now offer additional alternatives for wide-necked aneurysms.
Figure 8-3.
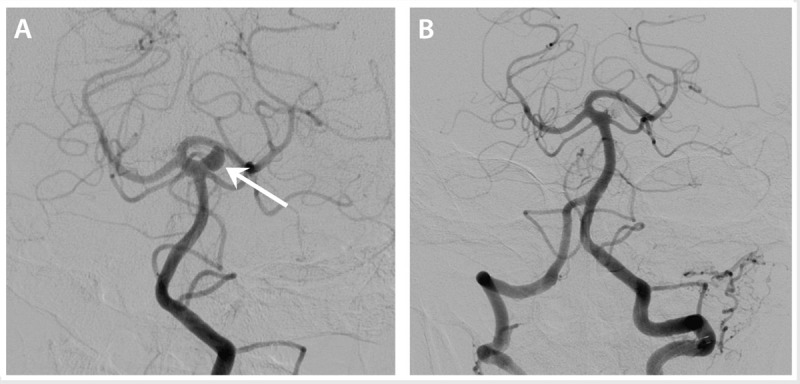
Catheter angiogram images showing a 6 mm aneurysm arising from the origin of the left superior cerebellar artery, prior to treatment (A, arrow) and following an endovascular coiling procedure (B).
To further complicate decision making, data regarding neurologic and other functional outcomes after unruptured intracranial aneurysm treatment are of limited quality because no randomized controlled trials have compared surgical and endovascular techniques for unruptured intracranial aneurysms. Retrospective or nonrandomized data are available from the ISUIA study, analyses of the Nationwide Inpatient Sample (a large database of hospitalizations at institutions throughout the United States),33 and other sources. In the ISUIA study, mortality rates at 1 year were 2.3% in the surgical group and 3.1% in the endovascular group; significant morbidity rates at 1 year were 9.8% among surgical patients and 6.4% among patients treated by endovascular means.25 Treatment assignment was not randomized, and it is difficult to make strong conclusions on these values given this fact, as well as some differences in baseline characteristics between groups. Specifically, patients in the endovascular arm were older, had larger aneurysm sizes, and were more likely to have basilar artery aneurysms; tobacco use and a family history of aneurysms were seen more commonly in surgically treated patients.
A retrospective analysis of the Nationwide Inpatient Sample found higher inpatient mortality among patients treated with surgery compared with those treated with endovascular techniques (1.2% versus 0.6%, respectively).32 The rate at which patients were admitted to a long-term nursing facility following hospitalization was also considerably higher for surgically treated patients than for those treated by endovascular means (14.0% versus 4.9%, respectively).32 Detailed information regarding patient-level and aneurysm-specific characteristics was not included in this study.
Although not 100% effective, surgical clipping may lead to a more definitive eradication of the aneurysm, assuming clip placement is appropriate, while aneurysm coils may become compacted over time and result in flow within the aneurysm neck again. Although the International Subarachnoid Aneurysm Trial (ISAT) was a study of outcomes following ruptured as opposed to unruptured aneurysms, late rebleeding events in this study were seen more commonly in patients treated by endovascular coiling than in those treated with clipping.34
The costs of either endovascular or surgical treatment of unruptured intracranial aneurysms are considerable: an analysis of Medicare patients from 2001 to 2008 showed that without complications, endovascular treatment was associated with approximately $26,000 in costs, versus approximately $23,500 for surgical treatment (in 2008 US dollars).35 However, given the significant morbidity and mortality associated with aneurysmal rupture, an analysis found treatment to be cost-effective for aneurysms with estimated annual rupture rates as low as 0.3%.36
CONCLUSION
Unruptured aneurysms are fairly prevalent and commonly identified through noninvasive brain imaging studies. Certain populations, especially patients who have multiple family members with aneurysms and patients with polycystic kidney disease, are at especially high risk of unruptured intracranial aneurysms and should be screened with either CT angiography or MR angiography. Once an aneurysm is identified, management options include observation or surgical/endovascular treatment; if surgical or endovascular options are being contemplated, referral to a comprehensive cerebrovascular center with expertise in these treatment options should be considered.
KEY POINTS
The prevalence of aneurysms in the general population is estimated at approximately 3%.
A family history of aneurysms is a strong predictor of aneurysm development, and familial aneurysms may have a higher risk of rupture than aneurysms of similar size and location in nonfamilial syndromes.
Autosomal dominant polycystic kidney disease is a strong predictor of the presence of unruptured intracranial aneurysms.
Most unruptured intracranial aneurysms are discovered when asymptomatic, although other presenting symptoms include compressive cranial neuropathies, seizures, or ischemic stroke.
Patients with two or more first-degree relatives with unruptured intracranial aneurysms or subarachnoid hemorrhage, or patients with autosomal dominant polycystic kidney disease, are candidates for intracranial imaging to screen for unruptured intracranial aneurysms.
Both CT angiography and magnetic resonance angiography are reasonable options for noninvasive screening imaging for unruptured intracranial aneurysms, although catheter angiography remains the gold standard for aneurysm identification.
The risk of aneurysm rupture appears to increase as aneurysm size increases.
Data on the risk of aneurysm rupture based on location are inconsistent across studies.
Certain anatomic features, such as the presence of a daughter sac, may increase the risk of aneurysm rupture.
If indicated, interventional options for unruptured intracranial aneurysm management include endovascular and surgical approaches; large randomized trial data to support one strategy over the other are lacking.
Footnotes
Relationship Disclosure: Dr Kelly reports no disclosure.
Unlabeled Use of Products/Investigational Use Disclosure: Dr Kelly reports no disclosure.
REFERENCES
- 1.Brisman JL,, Song JK,, Newell DW. Cerebral aneurysms. N Engl J Med 2006; 355 (9): 928–939. [DOI] [PubMed] [Google Scholar]
- 2.Lehecka M,, Dashti R,, Lehto H, et al Distal anterior cerebral artery aneurysms. Acta Neurochir Suppl 2010; 107: 15–26. [DOI] [PubMed] [Google Scholar]
- 3.Lou M,, Caplan LR. Vertebrobasilar dilatative arteriopathy (dolichoectasia). Ann N Y Acad Sci 2010; 1184 (1): 121–133. [DOI] [PubMed] [Google Scholar]
- 4.Kannoth S,, Thomas S. Intracranial microbial aneurysm (infectious aneurysm): current options for diagnosis and management. Neurocrit Care 2009; 11 (1): 120–129. [DOI] [PubMed] [Google Scholar]
- 5.Rinkel GJE. Intracranial aneurysm screening: indications and advice for practice. Lancet Neurol 2005; 4 (2): 122–128. [DOI] [PubMed] [Google Scholar]
- 6.Vlak MH,, Algra A,, Brandenburg R,, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol 2011; 10 (7): 626–636. [DOI] [PubMed] [Google Scholar]
- 7.Rinkel GJE,, Djibuti M,, Algra A,, van Gijn J. Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke 1998; 29 (1): 251–256. [DOI] [PubMed] [Google Scholar]
- 8.Mackey J,, Brown RD, Jr,, Moomaw CJ, et al Familial intracranial aneurysms: is anatomic vulnerability heritable? Stroke 2013; 44 (1): 38–42. [DOI] [PubMed] [Google Scholar]
- 9.Broderick JP,, Brown RD,, Sauerbeck L, et al Greater rupture risk for familial as compared to sporadic unruptured intracranial aneurysms. Stroke 2009; 40 (6): 1952–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alg VS,, Sofat R,, Houlden H,, Werring DJ. Genetic risk factors for intracranial aneurysms: a meta-analysis in more than 116,000 individuals. Neurology 2013; 80 (23): 2154–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connolly HM,, Huston J, 3rd,, Brown RD, Jr, et al Intracranial aneurysms in patients with coarctation of the aorta: a prospective magnetic resonance angiographic study of 100 patients. Mayo Clin Proc 2003; 78 (12): 1491–1499. [DOI] [PubMed] [Google Scholar]
- 12.Touze E,, Oppenheim C,, Trystram D, et al Fibromuscular dysplasia of cervical and intracranial arteries. Int J Stroke 2010; 5 (4): 296–305. [DOI] [PubMed] [Google Scholar]
- 13.Slovut DP,, Olin JW. Fibromuscular dysplasia. N Engl J Med 2004; 350 (18): 1862–1871. [DOI] [PubMed] [Google Scholar]
- 14.Xu HW,, Yu SQ,, Mei CL,, Li MH. Screening for intracranial aneurysm in 355 patients with autosomal-dominant polycystic kidney disease. Stroke 2011; 42 (1): 204–206. [DOI] [PubMed] [Google Scholar]
- 15.Chalhoub V,, Abi-Rafeh L,, Hachem K, et al Intracranial aneurysm and recessive polycystic kidney disease: the third reported case. JAMA Neurol 2013; 70 (1): 114–116. [DOI] [PubMed] [Google Scholar]
- 16.van Gijn J,, Kerr RS,, Rinkel GJ. Subarachnoid haemorrhage. Lancet 2007; 369 (9558): 306–318. [DOI] [PubMed] [Google Scholar]
- 17.Suarez JI,, Tarr RW,, Selman WR. Aneurysmal subarachnoid hemorrhage. N Engl J Med 2006; 354 (4): 387–396. [DOI] [PubMed] [Google Scholar]
- 18.Bederson JB,, Awad IA,, Wiebers DO, et al Recommendations for the management of patients with unruptured intracranial aneurysms: a statement for healthcare professionals from the Stroke Council of the American Heart Association. Circulation 2000; 102 (18): 2300–2308. [DOI] [PubMed] [Google Scholar]
- 19.Jordan LC,, Johnston SC,, Wu YW, et al The importance of cerebral aneurysms in childhood hemorrhagic stroke: a population-based study. Stroke 2009; 40 (2): 400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wermer MJ,, Rinkel GJ,, van Gijn J. Repeated screening for intracranial aneurysms in familial subarachnoid hemorrhage. Stroke 2003; 34 (12): 2788–2791. [DOI] [PubMed] [Google Scholar]
- 21.White PM,, Wardlaw JM,, Easton V. Can noninvasive imaging accurately depict intracranial aneurysms? A systematic review. Radiology 2000; 217 (2): 361–370. [DOI] [PubMed] [Google Scholar]
- 22.Chappell ET,, Moure FC,, Good MC. Comparison of computed tomographic angiography with digital subtraction angiography in the diagnosis of cerebral aneurysms: a meta-analysis. Neurosurgery 2003; 52 (3): 624–631; discussion 630–631. [DOI] [PubMed] [Google Scholar]
- 23.White PM,, Teasdale EM,, Wardlaw JM,, Easton V. Intracranial aneurysms: CT angiography and MR angiography for detection prospective blinded comparison in a large patient cohort. Radiology 2001; 219 (3): 739–749. [DOI] [PubMed] [Google Scholar]
- 24.Bor AS,, Koffijberg H,, Wermer MJ,, Rinkel GJ. Optimal screening strategy for familial intracranial aneurysms: a cost-effectiveness analysis. Neurology 2010; 74 (21): 1671–1679. [DOI] [PubMed] [Google Scholar]
- 25.Wiebers DO,, Whisnant JP,, Huston J, 3rd, et al Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003; 362 (9378): 103–110. [DOI] [PubMed] [Google Scholar]
- 26.White PM,, Wardlaw J. Unruptured intracranial aneurysms: prospective data have arrived. Lancet 2003; 362 (9378): 90–91. [DOI] [PubMed] [Google Scholar]
- 27.UCAS Japan Investigators; Morita A,, Kirino T,, Hashi K, et al The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med 2012; 366 (26): 2474–2482. [DOI] [PubMed] [Google Scholar]
- 28.Dhar S,, Tremmel M,, Mocco J, et al Morphology parameters for intracranial aneurysm rupture risk assessment. Neurosurgery 2008; 63 (2): 185–196; discussion 185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kashiwazaki D,, Kuroda S. Size ratio can highly predict rupture risk in intracranial small (<5 mm) aneurysms. Stroke 2013; 44 (8): 2169–2173. [DOI] [PubMed] [Google Scholar]
- 30.Lenfant C,, Chobanian AV,, Jones DW,, Roccella EJ. Seventh report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7): resetting the hypertension sails. Hypertension 2003; 41 (6): 1178–1179. [DOI] [PubMed] [Google Scholar]
- 31.Williams LN,, Brown RD. Management of unruptured intracranial aneurysms. Neurol Clin Pract 2013; 3 (2): 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brinjikji W,, Rabinstein AA,, Nasr DM, et al Better outcomes with treatment by coiling relative to clipping of unruptured intracranial aneurysms in the United States, 2001-2008. AJNR Am J Neuroradiol 2011; 32 (6): 1071–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Healthcare Cost and Utilization Project. Overview of the Nationwide Inpatient Sample (NIS). www.hcup-us.ahrq.gov/nisoverview.jsp. Updated December 11, 2013. Accessed December 26, 2013.
- 34.Molyneux AJ,, Kerr RSC,, Birks J, et al Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): long-term follow-up. Lancet Neurol 2009; 8 (5): 427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brinjikji W,, Kallmes DF,, Lanzino G,, Cloft HJ. Hospitalization costs for endovascular and surgical treatment of unruptured cerebral aneurysms in the United States are substantially higher than medicare payments. AJNR Am J Neuroradiol 2012; 33 (1): 49–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greving JP,, Rinkel GJ,, Buskens E,, Algra A. Cost-effectiveness of preventive treatment of intracranial aneurysms: new data and uncertainties. Neurology 2009; 73 (4): 258–265. [DOI] [PubMed] [Google Scholar]


