Abstract
Purpose of Review
This review discusses movement disorders that occur during pregnancy, the treatment of preexisting movement disorders, and the influence the pregnant state has on movement disorders symptoms, in order to guide clinicians in providing better counseling for female patients who are pregnant or considering pregnancy.
Recent Findings
Unique considerations for movement disorders during pregnancy include investigations and their safety during pregnancy and the impact of treatment on both the pregnant patient and her fetus.
Summary
The most common movement disorders arising in pregnancy are restless leg syndrome and chorea gravidarum. Preexisting movement disorders in women who become pregnant may also be seen.
INTRODUCTION
Neurologists uncommonly encounter patients with serious movement disorders who are in the childbearing years. Therefore, the published evidence for pregnancy and movement disorders are limited.1,2 Furthermore, guidelines do not exist for the treatment of movement disorders in pregnancy. Nonetheless, clinicians require guidance in this area, as several considerations are unique to pregnancy, including the safety of investigations or medications during gestation, labor, and delivery. This review summarizes the published literature on movement disorders and their medical management in pregnant women.
RESTLESS LEGS SYNDROME
Restless legs syndrome (RLS, also known as Ekbom syndrome) is the most common movement disorder of pregnancy. RLS usually is a chronic condition and is believed to have an autosomal dominant pattern of inheritance in most cases. RLS may also be limited to the puerperium. Patients with RLS report discomfort, creepy crawling sensations, or actual restlessness occurring in the legs, with an irresistible urge to move the legs. Once up and walking, the feelings are relieved. RLS can involve the arms. Spread to the arms typically occurs following treatment.3
RLS can occur for the first time during pregnancy, typically in the second and third trimesters (Case 8-1), and usually resolves after delivery. RLS prevalence in pregnancy is estimated to be between 10% and 26%4 compared with a prevalence of 2.5% to 15% of the general population. The increased prevalence of RLS in pregnancy may be linked to iron or folate deficiency, known risk factors for RLS.5
Case 8-1
A 30-year-old woman in her first pregnancy presented with leg cramping during her second trimester. Her only medication was a maternal vitamin. She did not drink caffeine and exercised modestly. No one in her family had similar discomfort. In the past, she had been able to travel in cramped spaces without discomfort and sit through movies with ease.
At the time of the current presentation, she reported that by late afternoon, sitting at her desk was uncomfortable and she often walked around the office to relieve her cramping. Further questioning revealed that the symptoms were worst at night while resting or right before bed and could inhibit sleep onset. Her physical examination was normal and her routine blood chemistry revealed a normal hemoglobin and normal iron saturation.
Comment. This scenario is common in pregnancy-related restless legs syndrome (RLS). The history confirmed that RLS was not present before the patient’s pregnancy (eg, she had been able to sit in cramped spaces), and she had no family history of RLS. This will be important in considering treatment because treatment can be expected to be limited to the puerperium.
Preexisting RLS may be affected by pregnancy. The course of preexisting idiopathic (unknown cause) RLS during pregnancy was studied in a cohort of 606 pregnant women; 59 had preexisting RLS, of whom 36 reported symptomatic worsening while seven had symptomatic improvement during pregnancy. The remaining 17 reported no change.4 Therefore, counseling should be done for women with preexisting RLS to let them know that the majority of women experience worsening during pregnancy, although 12% experience improvement.
Because of the association of RLS with iron deficiency, women with RLS should be tested for iron deficiency and receive replacement therapy if deficiency is confirmed. Unlike idiopathic RLS, iron replacement therapy without iron deficiency has been advocated in pregnant women with RLS, but the utility and safety of this in pregnancy are unknown. The main pitfall in RLS therapy is augmentation. This refers to escalating doses of medication required to control symptoms, the use (often unsuccessful) of polypharmacy, spread to other parts of the body, or symptoms present during the day in addition to nighttime onset.
Dopamine agonists are regarded as first-line agents in the treatment of idiopathic RLS due to lower risk of augmentation compared to other dopaminergic agents such as levodopa.5 Pergolide (no longer available in the United States) and ropinirole have been associated with intrauterine growth retardation, digit malformation, and fetal deaths in animals, while levodopa is thought to be safe in pregnancy. Therefore, in situations of RLS during pregnancy, and given a theoretically lower risk of augmentation during the finite treatment period, clinicians may opt to use levodopa as first-line therapy for safety and efficacy in women who have RLS severe enough to require treatment.
DYSTONIA
Dystonia is the third most common movement disorder in the general population, with a bimodal age of onset peaking during childhood (early onset) or the fifth decade of life. Typically, brain imaging for dystonia is unremarkable. Its pathophysiology has been proposed to be due to decreased inhibition in motor control, aberrant plasticity, and increased cortical excitability.6 Recent evidence has demonstrated that impairments in basal ganglia function may lead to dystonia as well, probably due to abnormalities in synaptic function of striatopallidal or striatonigral terminals in dystonia patients.6 Dystonia can lead to abnormal posture secondary to abnormal sustained muscle contractions. It can further be classified based on location into focal, multifocal, segmental, or generalized. Also based on etiology, dystonia can be idiopathic or symptomatic.
The most common forms of dystonia affecting women in pregnancy are dystonia from Wilson disease, dopa-responsive dystonia, and genetic forms of dystonia beginning in childhood or young adulthood (ie, DYT1).1,2
Some women develop dystonia during their reproductive years, because the mean age of onset for all forms of dystonia is 27 years. Many reports have demonstrated the effect of pregnancy on existing dystonia, which could be generalized or segmental. In a survey of 62 women with dystonia, 27 had at least one pregnancy since dystonia onset, and four of these 27 women developed changes in their symptoms while pregnant.7 Another report described 10 women who had been diagnosed with dystonia before becoming pregnant. Two of the 10 women had worsening of their dystonia, while three women had complete or partial remission during pregnancy. The remaining patients had no notable change. Only one of these 10 women had generalized dystonia, while the rest had focal or segmental dystonia.8 Dystonia gravidarum is a term that has been used for two cases with cervical dystonia not having any identifiable cause, which occurred during pregnancy and resolved before delivery or shortly thereafter.9
Drug-induced dystonia may also occur when neuroleptic agents are used for the treatment of hyperemesis gravidarum. Acute dystonic reactions or oculogyric crises consisting of neck extension with or without arching of the back or extension of the arms can occur. Most of these reactions resolve without treatment. In a few cases, anticholinergics such as benztropine 1 mg to 2 mg were given for rapid relief of dystonic reactions.
Among women with dopa-responsive dystonia, onset is typically in childhood or early adulthood consisting of initial foot dystonia with activity, typically later in the day. With time, dystonia may generalize and also occur throughout the day. The diurnal variation is typical of dopa-responsive dystonia, also known as Segawa disease. This is an autosomal dominant illness. Patients may provide a history of family members with toe walking, scoliosis, or the need for orthopedic surgeries in childhood. Low doses of levodopa (typically up to 300 mg/d) are effective, and response to low doses confirms the diagnosis. Use of levodopa has not resulted in adverse events in pregnancy.10
Another common treatment for focal dystonia is botulinum toxin. Botulinum toxin B has not been linked to fetal harm but is rated US Food and Drug Administration (FDA) pregnancy class C. A survey of physicians’ practice found 16 women received botulinum toxin A during pregnancy. Most of the pregnancies completed to term with no apparent complication. One of the women known to have previous history of spontaneous abortion had a miscarriage, while another woman had her pregnancy medically terminated.11 In another report, a woman had four uncomplicated full-term pregnancies while being treated with botulinum toxin type A for idiopathic cervical dystonia.12 At this point, the product monograph for all forms of botulinum toxin recommends that botulinum toxin not be administered to pregnant women. Therefore, caution is advised in this setting.
Three women with early-onset dystonia previously treated with globus pallidus internus deep brain stimulation (DBS) were able to conceive and successfully complete their pregnancies. DBS did not have any ill effect on their offspring. All of them delivered in the hospital, and there were no DBS-related contraindications to the administration of general or regional anesthesia.13
CHOREA
Chorea Arising During Pregnancy: Chorea Gravidarum
Chorea gravidarum was first described approximately 4 centuries ago, by Horatius, in 1661. The condition was originally associated with rheumatic fever; however, chorea gravidarum is now most closely linked to connective tissue disorders, including systemic lupus erythematosus, antiphospholipid antibody syndrome, syphilis, McLeod neuroacanthocytosis syndrome, and encephalitis.14 The most common cause of chorea is probably drug-induced (Table 8-1). Numerous medications cause chorea (outside of the setting of pregnancy) and presumably can cause chorea during pregnancy. Opioid agents, sympathomimetics such as amphetamine and cocaine, antiepileptic medications, both typical and atypical neuroleptics, and over-the-counter medications such as antihistamines can all cause chorea. Therefore, a careful medication history, including the use of over-the-counter agents and “natural” or herbal substances, should be taken, in conjunction with a toxin screen where appropriate.
Table 8-1.
Chorea Arising in Pregnancy: Differential Diagnosis and Suggested Investigations
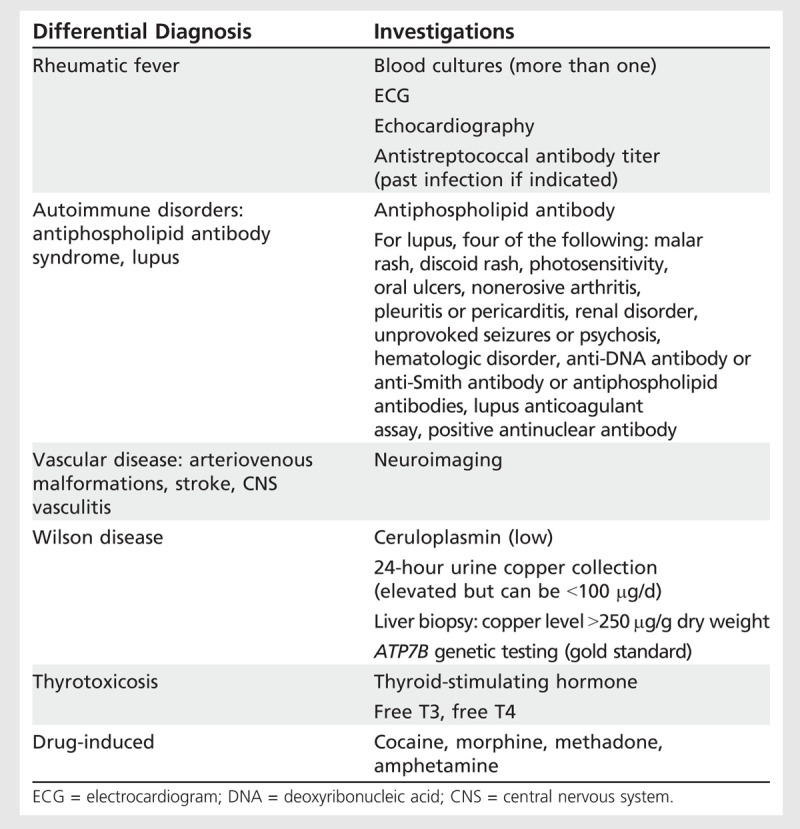
In the case of rheumatic fever resulting in chorea, despite complete resolution, chorea occurs in up to 75% of patients during pregnancy, with the majority noting onset during the first 2 trimesters.15 Chorea gravidarum typically begins in the first trimester and can spontaneously resolve before delivery in up to one-third of cases, with the remainder resolving after delivery. One in five women experience chorea gravidarum recurrence with subsequent pregnancies. Severe forms of chorea gravidarum can cause hyperthermia and rhabdomyolysis and, if not treated aggressively, can result in death.14 Proposed investigations (see Table 8-1) for chorea arising in pregnancy should take into account the issues related to imaging during pregnancy discussed in the article “Neuroradiology in Women of Childbearing Age” by Drs Riley Bove and Joshua Klein in this issue of CONTINUUM.
As noted above, chorea can be self-limited, and in general, treatment is safest after the peak period of fetal organogenesis. Treatment should be directed to the etiology (rheumatic heart disease, lupus, Wilson disease, or other inherited dystonias), to control of the chorea itself, or to management of the consequences of chorea (rhabdomyolysis, hyperthermia, pain, weight loss). Therefore, symptomatic treatment is reserved for situations in which the health of either the pregnant woman or her fetus would be adversely affected if the chorea continued. Dopamine receptor–blocking agents (butyrophenones or phenothiazines) and dopamine-depleting agents (tetrabenazines) are mainly the symptomatic treatment of chorea of any cause. Treatment is based on the proposed mechanism of the chorea (heightened dopaminergic activity in the striatum). However, dopamine receptor blockers and dopamine depleting agents are generally contraindicated during the first trimesterof pregnancy and are regarded as FDA category C.
Haloperidol and chlorpromazine have been shown to be helpful in the second and third trimesters and appear to be safe in low doses, such as haloperidol 0.5 mg to 2 mg 2 to 3 times daily. Haloperidol is recommended because it has fewer maternal anticholinergic, hypotensive, and antihistaminergic effectsthan chlorpromazine.16
Pregnancy in Women With Chorea: Huntington Disease
The triad of abnormal movements (chorea, dystonia, or parkinsonism), cognitive impairment, and psychiatric disorder characterizes Huntington disease (HD). HD is caused by an unstable polymorphic cytosine, adenine, and guanine (CAG) trinucleotide repeat unit, coding for glutamine. HD is inherited in an autosomal dominant fashion, usually with complete penetrance when there are over 40 repeats. Intermediate repeat length as low as 27 can confer risk of gene expansion and hence expression of the disease in offspring. Individuals with a repeat length of 26 are definitely not affected and confer no risk of illness to their offspring. The exact role of huntingtin, the gene protein product, is not known.17
Because onset of HD is typically in the fifth to sixth decade, except for patients who have the Westphal variant (juvenile onset), most patients begin experiencing symptoms after childbearing has occurred. However, as the average age at which women give birth continues to rise, pregnancy with symptomatic HD may become more common. Therefore, counseling patients with HD regarding pregnancy has been the current focus, especially the issue of the ability of these patients to care for their children. Since 1986, genetic testing has been available for people at risk for HD, but as few as 5% of at-risk individuals obtain testing, largely because no known cures or neuroprotective treatments exist.17 In a French study of patient choices for genetic testing of HD and autosomal dominant cerebellar ataxia, of the 815 couples at risk of having offspring with HD, only 4.6% (n = 38) of those seeking presymptomatic testing were pregnant during the study. The majority of the HD couples (71%) decided to continue the pregnancy and had presymptomatic testing after delivery; another 21% had presymptomatic testing during the current pregnancy. An even smaller number—11% (4/35)—eventually had prenatal testing.18
Recently, genetic testing for the HD gene has been done for embryos at the eight-cell stage, which have been formed using in vitro fertilization. Thereafter, the embryos that do not have the HD gene are implanted. This gives the advantage of not having to reveal the presymptomatic HD gene status to at-risk parents who do not wish to know their own HD gene status. However, cost and the failure rate of pregnancy with in vitro fertilization may be prohibitive.
HD treatment is mainly symptomatic as it involves treating the disabling chorea with dopamine receptor–blocking agents or dopamine-depleting agents (FDA category C).19 Clozapine has not been associated with malformation, although the safety profile is still under examination (FDA category B). Benzodiazepines may be helpful for chorea and some of the behavioral aspects of HD.20 Psychiatric symptoms, especially depression, are common in HD. Treatment of depression in pregnancy is complex and, given maternal and fetal risk, should be undertaken with a psychiatrist or maternal health specialist when possible. Similarly, treatment of psychosis in HD pregnancy should involve a psychiatrist or maternal health specialist.21
ATAXIA
Ataxia Arising During Pregnancy
Once structural causes are eliminated, the most common cause of ataxia arising during pregnancy is Wernicke encephalopathy secondary to hyperemesis gravidarum causing vitamin B1 deficiency. Ninety percent of Wernicke encephalopathy occurs in the setting of poor nutrition and alcoholism, but it can occur in any setting where nutritional status is compromised. The classic triad of confusion, ataxia, and ophthalmoparesis occurs in less than 10% of all patients with Wernicke encephalopathy. Therefore, a high degree of suspicion is required in the proper clinical context, even with one sign. Thiamine stores are depleted within 3 to 4 weeks of starvation. Neurologic symptoms occur as a result of depleted CNS thiamine stores. This disorder can be fatal. Therefore, early treatment with IV thiamine 200 mg 3 times a day (given slowly over 30 minutes to reduce the risk of anaphylaxis) until symptoms resolve is warranted in any pregnant patient presenting with any of the three signs in the context of hyperemesis. Guidelines do not exist for thiamine deficiency treatment. However, undertreatment is common. IM thiamine treatment with 250 mg/d for 3 to 5 days is an alternative treatment. Initial oral therapy is not recommended. Either regimen (IV or IM) should be followed with 50 mg to 100 mg once daily until proper oral nutrition is maintained.22
Pregnancy in Women With Ataxia
Many autosomal dominant forms of spinocerebellar atrophy can be detected by routine genetic testing. For women with known spinocerebellar atrophy confirmed with genetic testing, prenatal testing or in vitro fertilization using tested embryos resulting in healthy births has been reported.23
Friedreich ataxia is an autosomal recessive neurodegenerative disease.24 A study of 31 women with Friedreich ataxia with 65 pregnancies resulting in 56 live offspring found that spontaneous abortion, preeclampsia, and preterm birth were not increased. The majority of women had normal vaginal deliveries. Women reported unchanged, worse, or better ataxia with pregnancy in equal numbers. Other features of Friedreich ataxia (cardiac conduction block, reduced mobility due to weakness, and diabetes mellitus) should be monitored by the obstetric team or maternal health specialist, and care should be given to prevent deep vein thrombosis and pulmonary embolism. In fact, case reports of deep vein thrombosis occurring despite anticoagulation and of profound weakness occurring with ventilatory compromise after the administration of magnesium for preeclampsia underscore the importance of carefully monitoring treatments. Diabetes mellitus may begin during pregnancy with Friedreich ataxia. Approximately one-third of patients were diagnosed after one pregnancy.24
Vaginal delivery is possible in womenwith Friedreich ataxia even if they are nonambulatory or experiencing reduced proprioception as a result of peripheral neuropathy.
TREMOR
Tremor may occur in pregnancy as a result of enhanced physiologic tremor, drug-induced tremor from sympathomimetic agents, or essential tremor (Table 8-2 25). Essential tremor, one of the most common inherited movement disorders, is characterized by postural and kinetic tremor typically affecting the hands. Although it is bilateral, typically one hand is more affected. Worsening with caffeine, improvement with alcohol, and a positive family history are common in essential tremor. Essential tremor prevalence is estimated to be 1.7%. For those over 40 years of age, prevalence is 5.5%, affecting women and men equally.26 It is inherited in an autosomal dominant pattern, although genetic testing is currently only conducted for research purposes.
Table 8-2.
Medications Causing Tremora
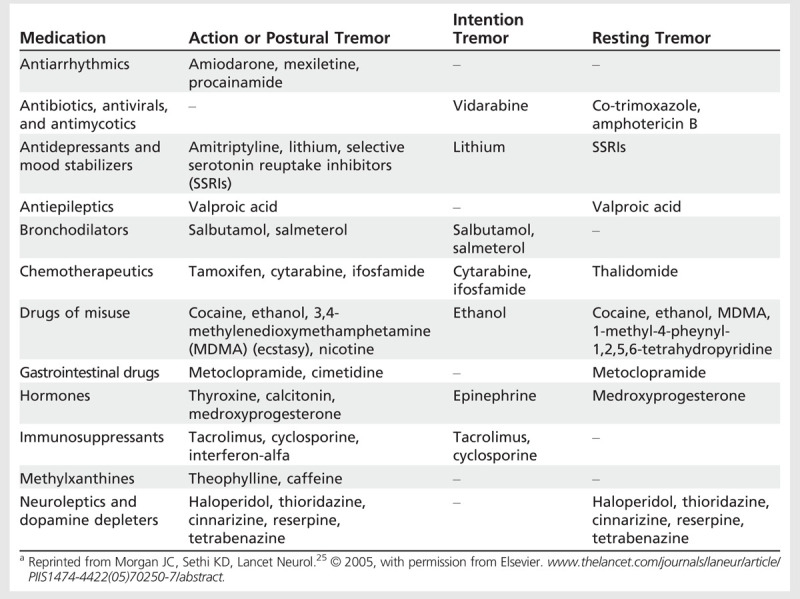
Anticipation can occur, with children displaying tremor before onset in their parents. Thus, a young woman may note tremor before her parent is diagnosed. Essential tremor can begin at any stage of life, with slow progression. Typically, disability will only occur after a prolonged duration of decades.
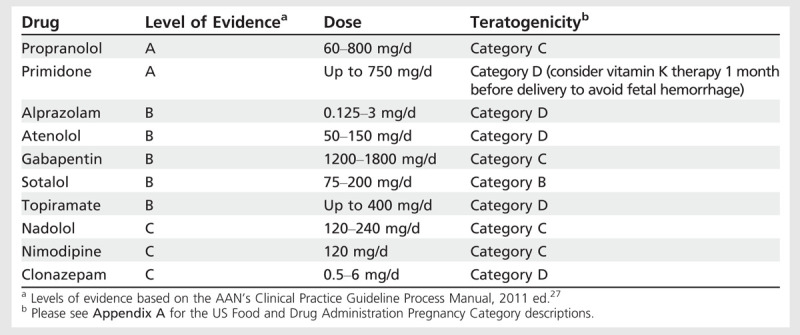
Table 8-3 lists the medications with best evidence for efficacy in essential tremor with FDA-assigned risks duringpregnancy where known. Given the evidence of risk during the first trimester or throughout pregnancy for all agents, clinicians should counsel their patients appropriately. Only patients with disabling tremor should consider treatment.28
Table 8-3.
Treatment for Essential Tremor
PARKINSONISM
Parkinson disease (PD) is a neurodegenerative disease characterized by cogwheel rigidity, bradykinesia, resting tremor, and later imbalance. The occurrence of pregnancy in patients with PD is rare. Evidence regarding the effects of estrogen on PD is conflicting. Improvement, no change, or worsening motor symptoms during pregnancy have been reported.
Combining reports in the literature, of a total of 36 pregnancies in 27 women diagnosed with PD, 47% had worsening or the appearance of new PD symptoms during pregnancy or shortly after delivery.29 Potential explanations for worsening motor symptoms include changes in the volume of distribution for antiparkinsonian medications, metabolic changes of pregnancy, or the effects of changing levels of estrogen particularly in women noting prepregnancy effects between ovulation and menstruation (when estrogen increases). The data regarding treatment of PD in pregnant patients has been obtained from small retrospective series and case reports (Table 8-4). Patients with PD may have worsening of their motor symptoms during pregnancy, although women report return to baseline in follow-up. One report of twin pregnancy in an inherited form of parkinsonism (DJ1) using levodopa resulted in normal offspring at 2-year follow-up.
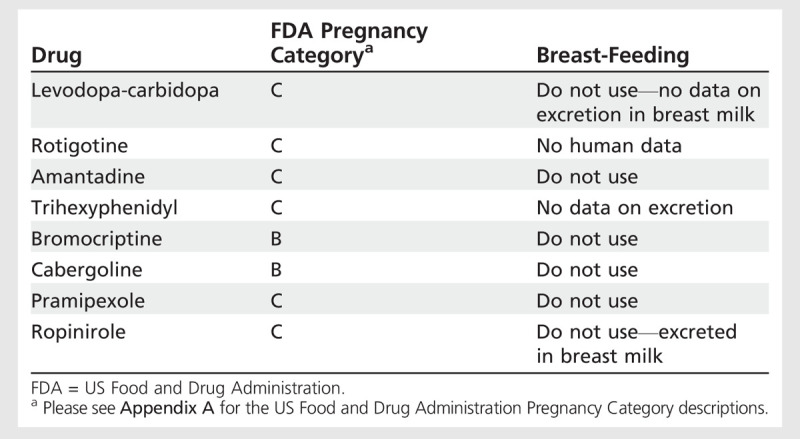
Table 8-4.
Medications for Treatment of Parkinson Disease in Pregnant and Breast-Feeding Women
Only one case has been reported of a woman who developed signs of new-onset parkinsonism of unknown reason at 11 weeks’ gestational age. She had a miscarriage even though she had received depot progesterone for a threatened abortion. Her symptoms resolved completely without requiring any antiparkinsonian treatment.30
TICS OR TOURETTE SYNDROME
Tics are sudden, repetitive, nonrhythmic motor movements or vocalizations, often associated with a premonitory sensation that is relieved by completing the movement. Tics areclassified as either simple or complex, and motor or phonic. Tourette syndrome is a movement disorder characterized by chronic motor and vocal tics beginning before 18 years of age. The condition is often lifelong, but tic severity frequently diminishes with age. Tourette syndrome is often associated with obsessive-compulsive disorder and attention deficit disorder. Family history of tics, obsessive compulsive disorder, or learning disorders are common, although a definite genetic cause has not been identified. Males are 3 to 4 times more commonly affected than females.31
Most tics do not result in disability. In patients with nondisabling tics, medication withdrawal should be attempted before pregnancy. In a series of eight women with 11 total pregnancies, five of the pregnancies had symptomatic improvement, while three had symptomatic worsening. The remainder had no change.32 Medications used to treat tics or Tourette syndrome consist of neuroleptics (category C), dopamine depleting agents (tetrabenazine, category C), and benzodiazepines (category B). Clonidine (category C) is also used to treat tics. One case of pimozide use (with fluoxetine) during pregnancy reported no untoward effects on the fetus.
WILSON DISEASE
Wilson disease, also known as hepatolenticular degeneration, is an autosomal recessive neurodegenerative disorder secondary to abnormal copper metabolism leading to copper accumulation in the liver, brain, and cornea. The gene locus is on chromosome 13 and codes for P-type copper-transporting adenosine triphosphatase (ATPase). The age of onset of Wilson disease ranges from 5 to 50 years. The clinical picture consists of liver cirrhosis, chorea, tremor, psychiatric disturbances, and Kayser-Fleischer rings. In most cases, hepatic disease predates the neuropsychiatric component of the disease by 5 to 10 years in adolescents, while young adults typically present with neuropsychiatric illness.33
Wilson disease predisposes pregnant patients to miscarriages, while pregnancy does not change the course of the disease. In a series of 16 patients with 59 pregnancies, three stillbirths and 24 miscarriages occurred.34 One case has been reported of a 23-year-old pregnant woman who at 23 weeks’ gestation developed pedal edema, thrombocytopenia, coagulopathy, and liver cirrhosis. At 35 weeks’ gestation, the diagnosis of Wilson disease was made. She underwent elective cesarean delivery at 36 weeks’ gestation. The ceruloplasmin level in the umbilical serum was decreased by half compared with normal levels, while the copper concentration was twice the normal value. The neonate demonstrated hepatomegaly and elevated liver enzymes, which persisted even after a year, despite normal development. This report predated genetic testing, and therefore the child’s genetic status was not known.35
Wilson disease treatment with chelating agents (D-penicillamine, zinc, trientine, and tetrathiomolybdate) is recommended throughout pregnancy because untreated pregnant patients would potentially develop fatal liver disease or hemolytic anemia, while the fetus would develop liver damage and copper accumulation. D-penicillamine has been considered teratogenic as there are case reports that associate D-penicillamine use with diffuse cutis laxa, severe micrognathia, contractures of all limbs, CNS abnormalities (including agenesis of the corpus callosum), and transient myelosuppression. Hypothyroidism and dyshormonogenesis has been reported as well in a woman taking penicillamine.36 Healthy infants may be born to women with treated Wilson disease (penicillamine 500 mg/d), as evidenced by a meta-analysis of18 Wilson disease patients who had 29 pregnancies while using D-penicillamine that did not show adverse outcomes.37
While chelation is necessary for patients with Wilson disease during pregnancy, given these concerns with D-penicillamine, chelation may be most safely achieved using zinc acetate therapy alone (maintenance dose 50 mg 3 times per day). Zinc acetate is FDA category A during pregnancy; however, it should be avoided during lactation, as taking zinc acetate may result in copper deficiency in the infant. Trientine is FDA category C, and therefore caution should be used.
STIFF PERSON SYNDROME
Stiff person syndrome, also known as Moersche-Woltman syndrome or stiff man syndrome, is a rare progressive disorder of fluctuating trunk and limb stiffness, painful muscle spasms, and exaggerated startle response that can also be triggered by voluntary movement or tactile or emotional stimuli. Symptoms occur predominantly in axial musculature. Disability and reduced quality of life are common; therefore, the majority of patients require treatment. The prevalence is estimated to be 1 in 1,000,000, and two-thirds of patients are female. Age of onset peaks around 45 years, and symptoms develop over months or years.
Stiff person syndrome is thought to be an autoimmune disorder because more than two-thirds of patients have glutamic acid decarboxylase antibodies. A paraneoplastic variant is also recognized. High titers of antibodies directed against a 65-kD isoform of glutamic acid decarboxylase (GAD65) are found in serum or CSF in 85% of patients. These antibodies theoretically result in impaired inhibition of motor neurons and thus the presence of continuous motor unit activity. Patients may have antiamphiphysin antibodies, antigephyrin and anti–γ-aminobutyric acid type A (GABA[A]) receptor-associated protein antibody, diabetes mellitus, thyroiditis, vitiligo, or pernicious anemia. Anti-GABA(A) receptor-associated protein occurs in approximately 70% of patients and can disrupt the synaptic stability and surface expression of GABA(A) receptors. Antigephyrin antibodies are directed at the postsynaptic cellular protein that clusters glycine receptors in the spinal cord and GABA(A) receptors in the brain. Amphiphysin is involved in endocytosis of the vesicle membrane after exocytosis of GABA from axonal terminals and will therefore result in decreased inhibition. This particular antibody is characteristic of the paraneoplastic form of stiff person syndrome. Diagnosis according to the Dalakas criteria requires “stiffness of axial muscles leading to fixed deformity (hyperlordosis); superimposed painful spasms precipitated by unexpected noises, emotional stress, tactile stimuli; confirmation of continuous motor unit activity in agonist and antagonist muscles by EMG; absence of neurological or cognitive impairments that could explain the stiffness; positive serology for GAD65 or amphiphysin antibodies by immunocytochemistry, western blot, or radioimmunoassay; and response to diazepam.”38,39 Treatment outside of pregnancy includes diazepam, 5 mg/d to 100 mg/d in divided doses (FDA category D); clonazepam, 1 mg/d to 20 mg/d in divided doses (category D); baclofen, 5 mg/d to 60 mg/d in divided doses (category C); levetiracetam, up to 2000 mg/d in divided doses (category C); plasma exchange, 5 to 6 exchanges (safe in case reports and given to pregnant women with other medical conditions); gabapentin, up to 3600 mg/d in divided doses (category B); or IV immunoglobulin, 2 g/kg body weight divided over 2 to 5 days (safe in case reports and given to pregnant women with other medical conditions).40
The occurrence of stiff person syndrome in pregnancy is rare. The following cases have been reported in the literature:
A 41-year-old patient with stiff limb syndrome had a full-term pregnancy and delivered by forceps-assisted vaginal delivery. During episiotomy, she had a generalized spasm. During pregnancy, her symptoms improved in the second trimester as she required less baclofen and diazepam, although her symptoms worsened postpartum.41
A 36-year-old primigravida with stiff person syndrome was treated with diazepam from the third trimester onward. She delivered a healthy neonate by scheduled cesarean delivery and reported remission of her symptoms by 2 weeks postpartum.42
Finally, a 27-year-old woman was diagnosed with stiff person syndrome 2 months before pregnancy. During the pregnancy, she continued on prednisone and gabapentin, while diazepam was discontinued. Her muscle spasms increased without the diazepam; therefore, she received baclofen 10 mg 3 times daily. In the 40th week of gestation, she began labor, and a healthy neonate was delivered via urgent cesarean delivery. On postpartum day 1, diazepam was reintroduced and led to resolution of symptoms.43
SUMMARY
The most common movement disorder in pregnancy is RLS followed by chorea gravidarum. All movement disorders may occur before pregnancy, requiring a review of medications for safety during pregnancy and with breast-feeding. Movement disorders may also occur de novo in pregnancy. Chorea, dystonia, parkinsonism, and stiff person syndrome require investigation to rule out underlying causes. The potential risks to mother and child of various pharmacologic agents are often unknown in pregnancy, and this should be discussed with patients. Treatment following delivery may not always be necessary (as in the case of RLS, which typically resolves).
KEY POINTS
Restless legs syndrome is the most common movement disorder of pregnancy.
Counseling should be done for women with preexisting restless legs syndrome to let them know that the majority of women experience worsening during pregnancy, although 12% experience improvement.
Clinicians may opt to use levodopa as first-line therapy for safety and efficacy in women who have restless legs syndrome severe enough to require treatment.
Drug-induced dystonia may also occur when neuroleptic agents are used for the treatment of hyperemesis gravidarum.
Botulinum toxin B has not been linked to fetal harm but is rated US Food and Drug Administration pregnancy class C.
Chorea gravidarum is now most closely linked to connective tissue disorders.
The most common cause of chorea is probably drug-induced.
A careful medication history, including the use of over-the-counter agents and “natural“ or herbal substances, should be taken, in conjunction with a toxin screen where appropriate.
Haloperidol and chlorpromazine have been shown to be helpful in the second and third trimesters and appear to be safe in low doses, such as haloperidol 0.5 mg to 2 mg 2 to 3 times daily.
Genetic testing for the Huntington disease gene has been done for embryos at the eight-cell stage.
Once structural causes are eliminated, the most common cause of ataxia arising during pregnancy is Wernicke encephalopathy secondary to hyperemesis gravidarum causing vitamin B1 deficiency.
Patients with Parkinson disease may have worsening of their motor symptoms during pregnancy, although women report return to baseline in follow-up.
Wilson disease treatment with chelating agents (D-penicillamine, zinc, trientine, and tetrathiomolybdate) is recommended throughout pregnancy because untreated pregnant patients would potentially develop fatal liver disease or hemolytic anemia, while the fetus would develop liver damage and copper accumulation.
Footnotes
Relationship Disclosure: Dr Miyasaki has served as a speaker or on advisory boards for Novartis Corporation and Teva Pharmaceuticals. Dr Miyasaki has received research support from the Canadian Agency for Drugs and Technologies in Health, the Canadian Institute for Health Research, the Michael J. Fox Foundation for Parkinson’s Research, the National Center for Complementary and Alternative Medicine, the National Parkinson Foundation, the NIH, the Ontario Drug Benefits Program, and the Ontario Ministry of Health and Long-Term Care. The Movement Disorders Centre at Toronto Western Hospital has received research support from Teva Pharmaceuticals. Dr AlDakheel reports no disclosure.
Unlabeled Use of Products/Investigational Use Disclosure: Drs Miyasaki and AlDakheel report no disclosures.
REFERENCES
- 1.Bordelon Y,, Smith M. Movement disorders in pregnancy. Semin Neurol 2007; 27 (5): 467–475. [DOI] [PubMed] [Google Scholar]
- 2.Kranick S,, Mowry E,, Colcher A, et al. Movement disorders and pregnancy: a review of the literature. Mov Disord 2010; 25 (6): 665–671. [DOI] [PubMed] [Google Scholar]
- 3.Earley CJ. Clinical practice. Restless legs syndrome. N Engl J Med 2003; 348 (21): 2103–2109. [DOI] [PubMed] [Google Scholar]
- 4.Manconi M,, Govoni V,, De Vito A, et al. Restless legs syndrome and pregnancy. Neurology 2004; 63 (6): 1065–1069. [DOI] [PubMed] [Google Scholar]
- 5.Chesson AL, Jr,, Wise M,, Davila D, et al. Practice parameters for the treatment of restless legs syndrome and periodic limb movement disorder. An American Academy of Sleep Medicine Report. Standards of Practice Committee of the American Academy of Sleep Medicine. Sleep 1999; 22 (7): 961–968. [DOI] [PubMed] [Google Scholar]
- 6.Prescott IA,, Dostrovsky JO,, Moro E, et al. Reduced paired pulse depression in the basal ganglia of dystonia patients. Neurobiol Dis 2013; 51: 214–221. [DOI] [PubMed] [Google Scholar]
- 7.Gwinn-Hardy KA,, Adler CH,, Weaver AL, et al. Effect of hormone variations and other factors on symptom severity in women with dystonia. Mayo Clin Proc 2000; 75 (3): 235–240. [DOI] [PubMed] [Google Scholar]
- 8.Rogers JD,, Fahn S. Movement disorders and pregnancy. In: Devinsky O, ed. Advances in neurology: Neurological complications of pregnancy. Vol 64, New York: Raven Press, 1994: 163–178. [PubMed] [Google Scholar]
- 9.Lim EC,, Seet RC,, Wilder-Smith EP,, Ong BK. Dystonia gravidarum: a new entity? Mov Disord 2006; 21 (1): 69–70. [DOI] [PubMed] [Google Scholar]
- 10.Nomoto M,, Kaseda S,, Iwata S, et al. Levodopa in pregnancy. Mov Disord 1997; 12 (2): 261. [PubMed] [Google Scholar]
- 11.Morgan JC,, Iyer SS,, Moser ET, et al. Botulinum toxin A during pregnancy: a survey of treating physicians. J Neurol Neurosurg Psychiatry 2006; 77 (1): 117–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newman WJ,, Davis TL,, Padaliya BB, et al. Botulinum toxin type A therapy during pregnancy. Mov Disord 2004; 19 (11): 1384–1385. [DOI] [PubMed] [Google Scholar]
- 13.Paluzzi A,, Bain PG,, Liu X, et al. Pregnancy in dystonic women with in situ deep brain stimulators. Mov Disord 2006; 21 (5): 695–698. [DOI] [PubMed] [Google Scholar]
- 14.Robottom BJ,, Weiner WJ. Chorea gravidarum. Handb Clin Neurol 2011; 100: 231–235. [DOI] [PubMed] [Google Scholar]
- 15.Maia DP,, Fonseca PG,, Camargos ST, et al. Pregnancy in patients with Sydenham’s Chorea. Parkinsonism Relat Disord 2012; 18 (5): 458–461. [DOI] [PubMed] [Google Scholar]
- 16.American Academy of Pediatrics Committee on Drugs. Use of psychoactive medication during pregnancy and possible effects on the fetus and the newborn. Pediatrics 2000; 105 (4 pt 1): 880–887. [DOI] [PubMed] [Google Scholar]
- 17.Walker FO. Huntington’s disease. Lancet 2007; 369 (9557): 218–228. [DOI] [PubMed] [Google Scholar]
- 18.Lesca G,, Goizet C,, Durr A. Predictive testing in the context of pregnancy: experience in Huntington’s disease and autosomal dominant cerebellar ataxia. J Med Genet 2002; 39 (7): 522–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armstrong MJ,, Miyasaki JM. Evidence-based guideline: the treatment of chorea in Huntington disease. Neurology 2012; 79 (6): 597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oyebode F,, Rastogi A,, Berrisford G,, Coccia F. Psychotropics in pregnancy: safety and other considerations. Pharmacol Ther 2012; 135 (1): 71–77. [DOI] [PubMed] [Google Scholar]
- 21.Byatt N,, Deligiannidis KM,, Freeman MP. Antidepressant use in pregnancy: a critical review focused on risks and controversies. Acta Psychiatr Scand 2013; 127 (2): 94–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isenberg-Grzeda E,, Kutner HE,, Nicolson SE. Wernicke-Korsakoff-syndrome: under-recognized and under-treated. Psychosomatics 2012; 53 (6): 507–516. [DOI] [PubMed] [Google Scholar]
- 23.Moutou C,, Nicod JC,, Gardes N,, Viville S. Birth after pre-implantation genetic diagnosis (PGD) of spinocerebellar ataxia 2 (Sca2). Prenat Diagn 2008; 28 (2): 126–130. [DOI] [PubMed] [Google Scholar]
- 24.Friedman LS,, Paulsen EK,, Schadt KA, et al. Pregnancy with Friedreich ataxia: a retrospective review of medical risks and psychosocial implications. Am J Obstet Gynecol 2010; 203 (3):224. e1–e5. [DOI] [PubMed] [Google Scholar]
- 25.Morgan JC,, Sethi KD. Drug-induced tremors. Lancet Neurol 2005; 4 (12): 866–876. [DOI] [PubMed] [Google Scholar]
- 26.Tanner CM,, Goldman SM. Epidemiology of movement disorders. Curr Opin Neurol 1994; 7 (4): 340–345. [DOI] [PubMed] [Google Scholar]
- 27.American Academy of Neurology. Clinical practice guideline process manual, 2011 ed. St Paul, MN: American Academy of Neurology, 2011. [Google Scholar]
- 28.Zesiewicz TA,, Elble RJ,, Louis ED, et al. Evidence-based guideline update: treatment of essential tremor. Neurology 2011; 77 (19): 1752–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott M,, Chowdhury M. Pregnancy in Parkinson’s disease: unique case report and review of the literature. Mov Disord 2005; 20 (8): 1078–1079. [DOI] [PubMed] [Google Scholar]
- 30.Demirkiran M,, Aslan K,, Bicakci S, et al. Transient parkinsonism: induced by progesterone or pregnancy? Mov Disord 2004; 19 (11): 1382–1384. [DOI] [PubMed] [Google Scholar]
- 31.Bruun RD,, Budman CL. The natural history of Tourette syndrome. Adv Neurol 1992; 58: 1–6. [PubMed] [Google Scholar]
- 32.Stern JS,, Orth M,, Robertson MM. Gilles de la Tourette syndrome in pregnancy: a retrospective series. Obstet Med 2009; 2: 128–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosencrantz R,, Schilsky M. Wilson disease: pathogenesis and clinical considerations in diagnosis and treatment. Semin Liver Dis 2011; 31 (3): 245–259. [DOI] [PubMed] [Google Scholar]
- 34.Sinha S,, Taly AB,, Prashanth LK, et al. Successful pregnancies and abortions in symptomatic and asymptomatic Wilson’s disease. J Neurol Sci 2004; 217 (1): 37–40. [DOI] [PubMed] [Google Scholar]
- 35.Oga M,, Matsui N,, Anai T, et al. Copper deposition of the fetus and placenta in a patient with untreated Wilson’s disease. Am J Obstet Gynecol 1993; 169 (1): 196–198. [DOI] [PubMed] [Google Scholar]
- 36.Hanukoglu A,, Curiel B,, Berkowitz D, et al. Hypothyroidism and dyshormonogenesis induced by D-penicillamine in children with Wilson’s disease and healthy infants born to a mother with Wilson’s disease. J Pediatr 2008; 153 (6): 864–866. [DOI] [PubMed] [Google Scholar]
- 37.Scheinberg IH,, Sternlieb I. Pregnancy in penicillamine-treated patients with Wilson’s disease. N Engl J Med 1975; 293 (25): 1300–1302. [DOI] [PubMed] [Google Scholar]
- 38.Hadavi S,, Noyce AJ,, Leslie RD,, Giovannoni G. Stiff person syndrome. Pract Neurol 2011; 11 (5): 272–282. [DOI] [PubMed] [Google Scholar]
- 39.Dalakas MC. Stiff person syndrome: advances in pathogenesis and therapeutic interventions. Curr Treat Options Neurol 2009; 11 (2): 102–110. [DOI] [PubMed] [Google Scholar]
- 40.Elovaara I,, Apostolski S,, van Doorn P, et al. EFNS guidelines for the use of intravenous immunoglobulin in treatment of neurological diseases: EFNS task force on the use of intravenous immunoglobulin in treatment of neurological diseases. Eur J Neurol 2008; 15 (9): 893–908. [DOI] [PubMed] [Google Scholar]
- 41.Weatherby SJ,, Woolner P,, Clarke CE. Pregnancy in stiff-limb syndrome. Mov Disord 2004; 19 (7): 852–854. [DOI] [PubMed] [Google Scholar]
- 42.Cerimagic C,, Bilic E. Stiff-person syndrome first manifesting in pregnancy. Gynecol Obstet Invest 2009; 67 (2): 134–136. [DOI] [PubMed] [Google Scholar]
- 43.Goldkamp J,, Blaskiewicz R,, Myles T. Stiff person syndrome and pregnancy. Obstet Gynecol 2011; 118 (2 pt 2): 454–457. [DOI] [PubMed] [Google Scholar]


