Abstract
Purpose of Review:
This review focuses on aspects of retinal and optic nerve ischemia that may be encountered by neurologists.
Recent Findings:
Recent guidelines have emphasized the similarities between cerebral and retinal ischemia in terms of etiologic workup, acute management, and subsequent stroke risk. However, although ischemic optic neuropathies reflect optic nerve ischemia, they result from local small vessel disease and are not associated with a higher risk of cerebral infarction. Their management is therefore very different from acute cerebral ischemia. It is essential to rule out giant cell arteritis in all patients with acute retinal or optic nerve ischemia.
Summary:
Because the eye is vascularized by branches of the internal carotid artery, retinal ischemic symptoms are common in patients with anterior circulation ischemic strokes. Patients with central retinal artery occlusion, whether permanent or transient (responsible for transient visual loss), need to be evaluated and managed emergently similar to patients with cerebral ischemia, while anterior and posterior ischemic optic neuropathy are more concerning for giant cell arteritis.
Because the eye is vascularized by branches of the internal carotid artery, ocular ischemic symptoms are common in patients with anterior circulation ischemic strokes. The main blood supply to the eye and orbital contents comes from the ophthalmic artery, a branch of the internal carotid artery. Any vascular disease involving the arteries between the heart and the ophthalmic artery may result in ocular ischemia and visual loss. The external carotid artery and its branches also contribute to the vascularization of the eye and orbital contents.
In cases of internal carotid artery stenosis or occlusion, the entire ocular vascularization may originate from the external carotid artery. In this situation, there may be a steal phenomenon from the eye to the brain, and the flow may be reversed in the ophthalmic artery so that most of the blood flow through the eye contributes to the vascularization of the ipsilateral cerebral hemisphere.1
Branches of the ophthalmic artery include the central retinal artery to the inner retina, the short posterior ciliary arteries to the choroid and optic nerve, the long posterior ciliary arteries to the ciliary body and iris, and the anterior ciliary arteries, which arise from the vessels of the rectus muscles. The central retinal artery (first branch of the ophthalmic artery) enters the optic nerve from below approximately 1 cm behind the eyeball and courses within it to the retina, where it gives off multiple branches. The posterior ciliary arteries give off several small branches that supply the orbital optic nerve. The optic nerve head receives its arterial blood supply from an arterial ring (circle of Zinn) formed by anastomoses among branches from the short posterior ciliary arteries. The central retinal artery (for the inner retina) is of relatively large caliber and can be occluded by emboli, which may migrate into its branches (branch retinal arteries); however, the short posterior ciliary arteries (supplying the choroid and optic nerve) are too small and too numerous to be affected by emboli. Occlusion of the ophthalmic artery results in complete ocular ischemia, whereas occlusion of the central retinal artery results in inner retinal ischemia.1 Figure 3-12 illustrates the blood supply to the optic nerve.
Figure 3-1.
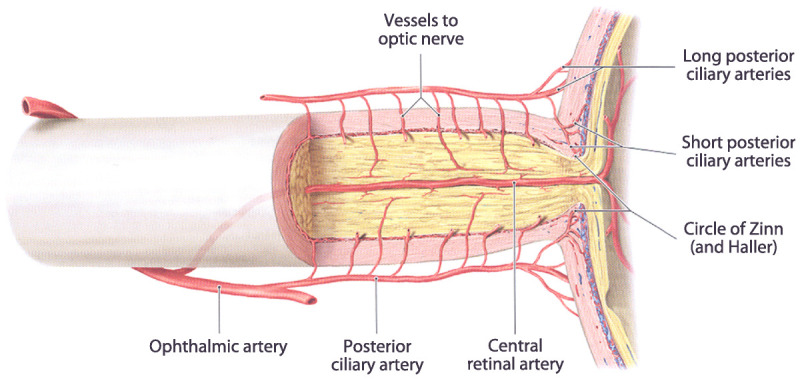
Blood supply to the optic nerve.
Reprinted with permission from Schuenke M, et al, Thieme.2 © 2007 Thieme Medical Publishers, Inc.
RETINAL VASCULAR DISEASES
Occlusion of the retinal arteries or veins produces retinal ischemia with retinal edema and visual loss.
Central Retinal and Branch Retinal Artery Occlusions
Central retinal and branch retinal artery occlusions produce acute monocular visual loss. Unless the retinal vascular occlusion is secondary to giant cell arteritis or to a carotid dissection, the visual loss is painless. Permanent visual loss may have been preceded by one or more episodes of transient monocular visual loss. The visual prognosis is usually poor. These patients are at risk for recurrent ocular or cerebral infarction, and they should be evaluated emergently like patients with cerebral infarctions.3,4
Central retinal artery occlusion produces monocular severe visual loss with an ipsilateral dense relative afferent pupillary defect. Funduscopic examination shows attenuated retinal arteries, sometimes with emboli; retinal edema with a cherry red spot develops after a few hours (Figure 3-2). The fovea appears red by contrast with the surrounding ischemic retina, which is whitish; this cherry red spot appearance is explained by the fact that the retina is very thin at the fovea and the underlying normally vascularized choroid appears red in contrast to the ischemic white surrounding inner retina. The fundus may be almost normal very acutely, and repeat examination may be necessary when a central retinal artery occlusion is suspected.
Figure 3-2.
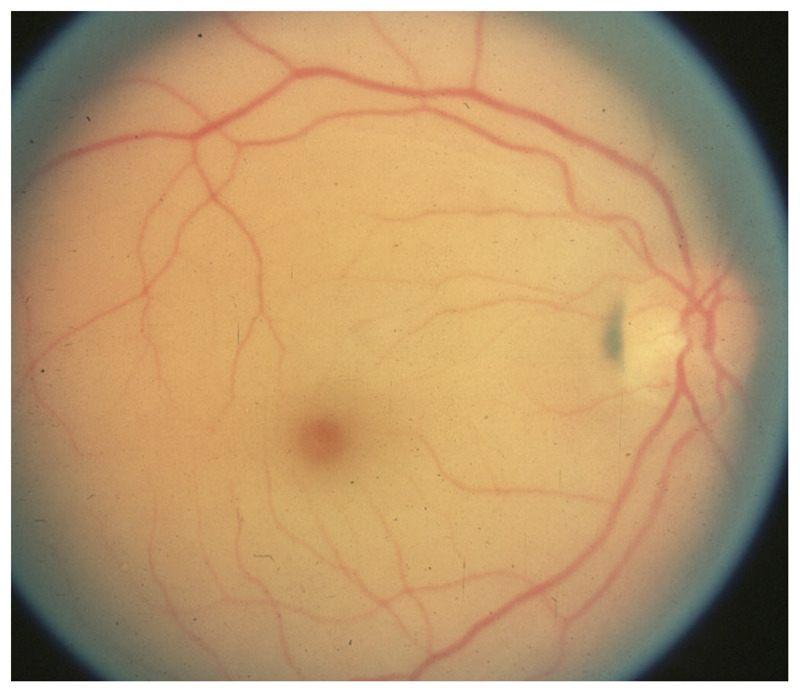
Acute central retinal artery occlusion in the right eye. The patient lost vision acutely 12 hours prior. The retina is diffusely pale, and the fovea appears red by contrast. The arteries are attenuated.
Reprinted with permission from Biousse V, Newman NJ, Thieme.1 © 2009 Thieme Medical Publishers, Inc.
Approximately 30% of individuals have a cilioretinal artery, which originates from the posterior ciliary circulation. In cases of central retinal artery occlusion, the retinal territory vascularized by the cilioretinal artery is not affected and some vision is preserved. If the cilioretinal artery provides the blood supply to the fovea, the central visual acuity remains good.
Branch retinal artery occlusion produces acute monocular partial visual loss depending on the affected retinal artery; because retinal ischemia is localized and usually relatively small, the ipsilateral relative afferent pupillary defect is small. It is possible to visualize the occluded or attenuated branch retinal artery on funduscopic examination, usually with one or more emboli (Figure 3-3). The retinal edema is localized to the ischemic area. A corresponding visual field defect (usually superior or inferior field defect) is present. Branch retinal artery occlusions are most often related to emboli into the central retinal artery. Giant cell arteritis is less likely. The clinical features of the emboli sometimes allow understanding of the nature and the source of the emboli (Table 3-1).
Table 3-1.
Characteristics of Retinal Emboli
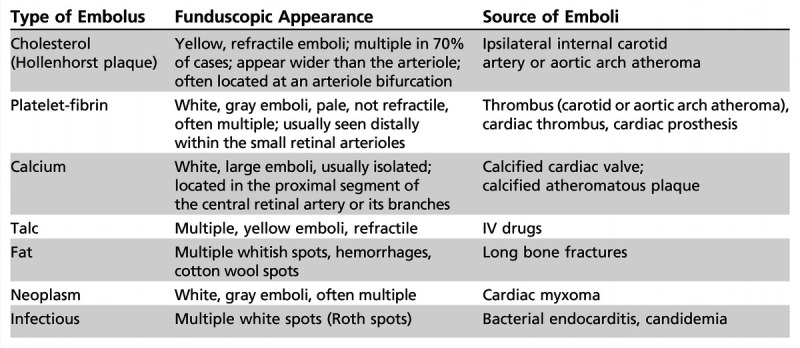
Figure 3-3.
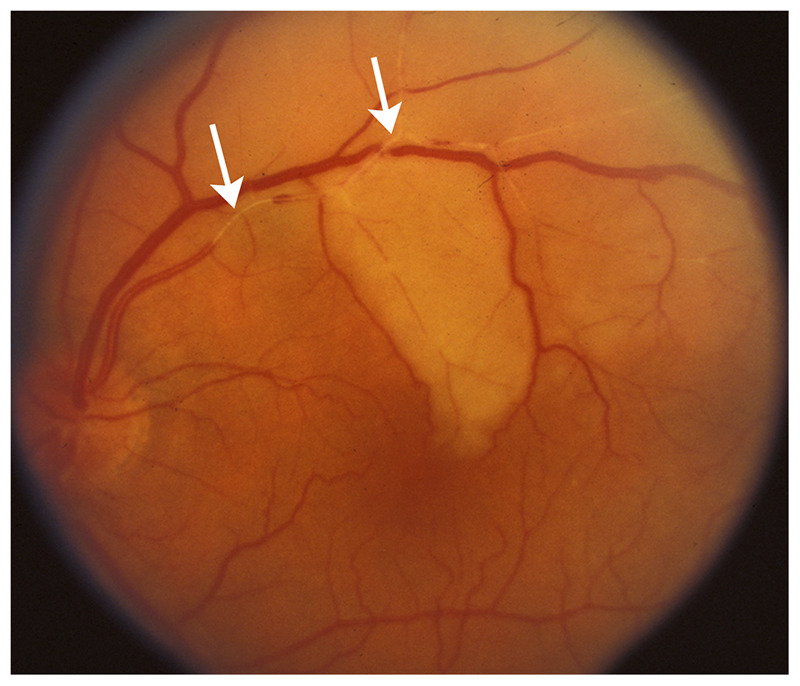
Acute superior branch retinal artery occlusion in the left eye. The superior branch of the central retinal artery is occluded, and emboli are seen in the artery (arrows). The ischemic retina appears white because of retinal edema.
Reprinted with permission from Biousse V, Newman NJ, Thieme.1 © 2009 Thieme Medical Publishers, Inc.
Ophthalmic Artery Occlusion
Ophthalmic artery occlusion has a clinical presentation similar to central retinal artery occlusion. The vision loss is always profound, and, because the underlying choroid is also ischemic, diffuse retinal edema without a cherry red spot is present (Figure 3-4). Often associated mild disc edema from optic nerve ischemia can be seen. Ophthalmic artery occlusions are most often related to internal carotid artery disease with large emboli involving the origin of the ophthalmic artery. Giant cell arteritis should be considered.
Figure 3-4.
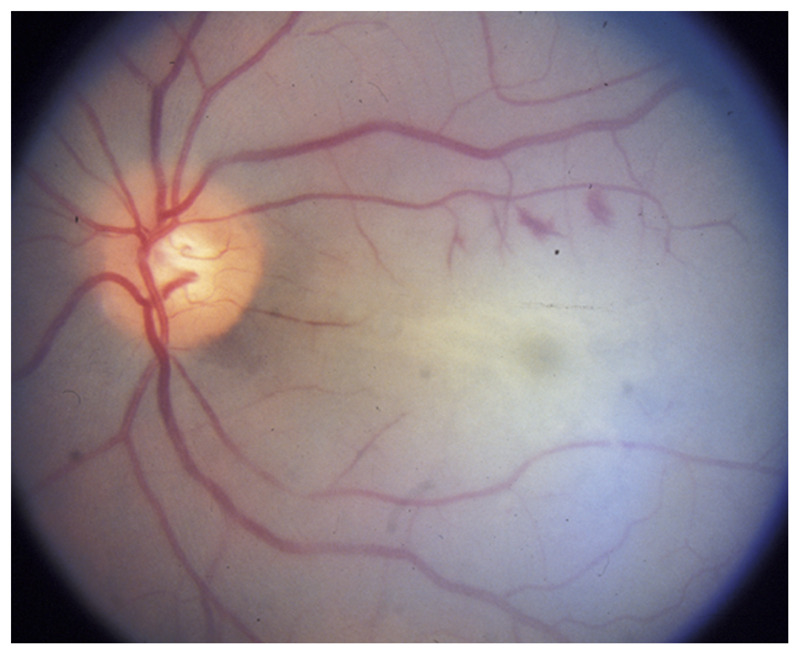
Acute ophthalmic artery occlusion in the left eye. The entire retina is pale because of retinal edema, and there is no cherry red spot because of the associated choroidal ischemia seen in ophthalmic artery occlusion. Mild optic nerve edema is present because of optic nerve ischemia. The arteries are attenuated.
Reprinted with permission from Biousse V, Newman NJ, Thieme.1 © 2009 Thieme Medical Publishers, Inc.
Asymptomatic Retinal Emboli
Asymptomatic retinal emboli, most often cholesterol, are found in 1% to 2% of patients older than 50 years (Figure 3-5 and Figure 3-6). They should prompt an evaluation for atheromatous vascular risk factors, which need to be aggressively controlled.
Figure 3-5.
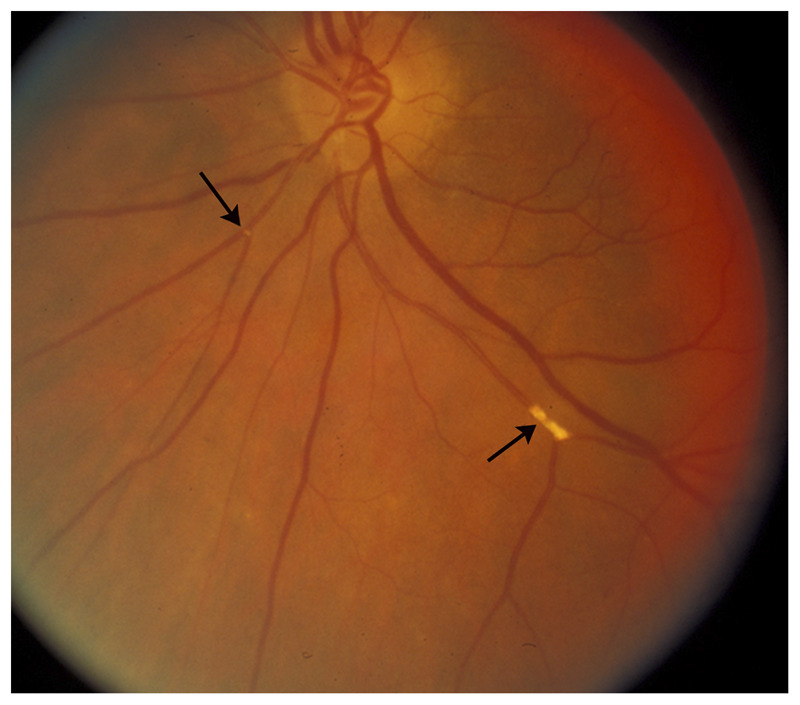
Asymptomatic cholesterol retinal emboli (arrows) from atheromatous carotid stenosis. Multiple yellow, refractile emboli are seen in branches of the central retinal artery. No associated retinal ischemia is present, and the patient is asymptomatic.
Reprinted with permission from Biousse V, Newman NJ, Thieme.1 © 2009 Thieme Medical Publishers, Inc.
Figure 3-6.
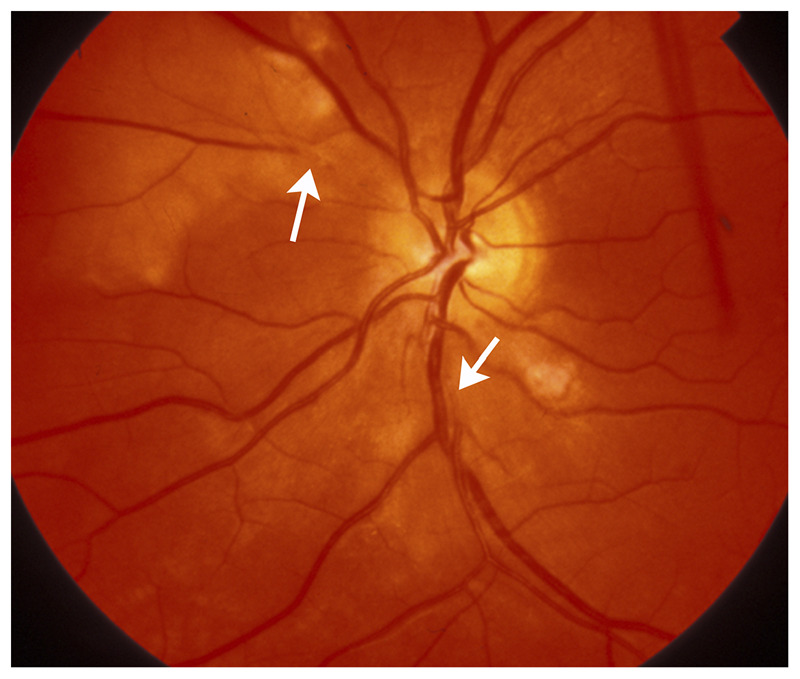
Platelet-fibrin retinal emboli from aortic arch atheroma. Multiple grayish emboli (arrows) are seen in a few branches of the central retinal artery. The whitish areas in the retina correspond to associated retinal ischemia. Although the patient was not aware of visual loss, a visual field test showed multiple small scotomas corresponding to the areas of ischemic retina.
Reprinted with permission from Biousse V, Newman NJ, Thieme.1 © 2009 Thieme Medical Publishers, Inc.
Evaluation of a Patient With Acute Retinal Ischemia
The evaluation of a patient with acute retinal ischemia is very similar to that of the patient with acute cerebral ischemia (Table 3-2). The only difference between retinal and cerebral ischemia is the need to always look for giant cell arteritis in all patients with acute retinal ischemia older than 50, unless a retinal embolus is visible on funduscopic examination. Carotid artery stenosis tends to be more common than other causes of emboli, but the workup is otherwise identical. Recent data have emphasized the need for systematic cerebral imaging acutely, ideally with diffusion-weighted MRI and magnetic resonance angiography of the head and neck, as up to 25% of patients with acute retinal ischemia (whether permanent or transient) will have concomitant acute cerebral ischemia.5,6
Table 3-2.
Causes of Acute Retinal Arterial Ischemia and Evaluation of Patientsa
A brain MRI with diffusion-weighted imaging should be obtained immediately, looking for associated asymptomatic cerebral ischemia. Magnetic resonance angiography of the head and neck is usually obtained at the same time.6
Two specific situations need to be considered when evaluating a patient with acute retinal ischemia. (1) Giant cell arteritis: Retinal ischemia can be caused by giant cell arteritis. It is usually associated with optic nerve ischemia or choroidal ischemia. The association of branch or central retinal artery occlusion with anterior ischemic optic neuropathy is highly suggestive of giant cell arteritis. (2) Susac syndrome: Recurrent branch retinal artery occlusions are part of Susac syndrome, which is a rare vasculopathy of unknown cause leading to occlusion of small retinal, cochlear, and cerebral arteries, mostly occurring in young women (Table 3-3).
Table 3-3.
Diagnosis of Susac Syndrome

Treatment of Retinal Arterial Ischemic Occlusions
There is no specific treatment for acute retinal arterial occlusion. A number of options have been tried, mostly in small nonrandomized, noncontrolled studies, without any proof of efficacy.
Intraarterial thrombolysis, with selective catheterization of the ophthalmic artery, or intravenous thrombolysis are sometimes performed with limited benefit in most cases. However, all patients with retinal arterial occlusion seen acutely should be admitted for immediate workup and initiation of secondary prevention similar to what is done for patients with acute cerebral ischemia.3,4
When giant cell arteritis is suspected in a patient with acute visual loss, admission to the hospital for high-dose IV steroids (methylprednisolone 250 mg every 6 hours for 3 to 5 days) followed by oral prednisone (1 mg/kg/d) is warranted.
Ocular Ischemic Syndrome
Chronic hypoperfusion of the eye results in diffuse ocular ischemia. Ocular ischemia develops in patients with severe stenosis or occlusion of the ipsilateral internal carotid artery and poor collateral circulation. These patients often have low blood flow in the ophthalmic artery. The flow is sometimes reversed in the ophthalmic artery in order to protect the ipsilateral brain (there is then a steal phenomenon involving the eye). Patients are usually asymptomatic until they notice episodes of hemodynamic transient monocular visual loss, typically precipitated by standing up, eating, or exposure to bright lights (Case 3-1). They also often develop dull periocular pain, mostly present when standing up and improving when lying down. Examination may show venous stasis retinopathy, which is present early when the patient is still asymptomatic and is characterized by unilateral dilation of the retinal veins, which are tortuous, and retinal blot hemorrhages, mostly in the midperiphery of the retina (Figure 3-7). As the ocular ischemia worsens, the episcleral arteries may be dilated (resulting from collateral circulation to the eye from branches of the external carotid artery), the intraocular pressure is typically low, unless the patient develops neovascular glaucoma with subsequent very high intraocular pressure, and classic pseudointraocular inflammation is present (Figure 3-8). Ultimately, ocular neovascularization may develop in the retina, then the anterior segment of the eye, resulting in neovascular glaucoma (increased intraocular pressure), atonic iris (pupil dilated and unreactive), cataract, and corneal edema.
Figure 3-7.
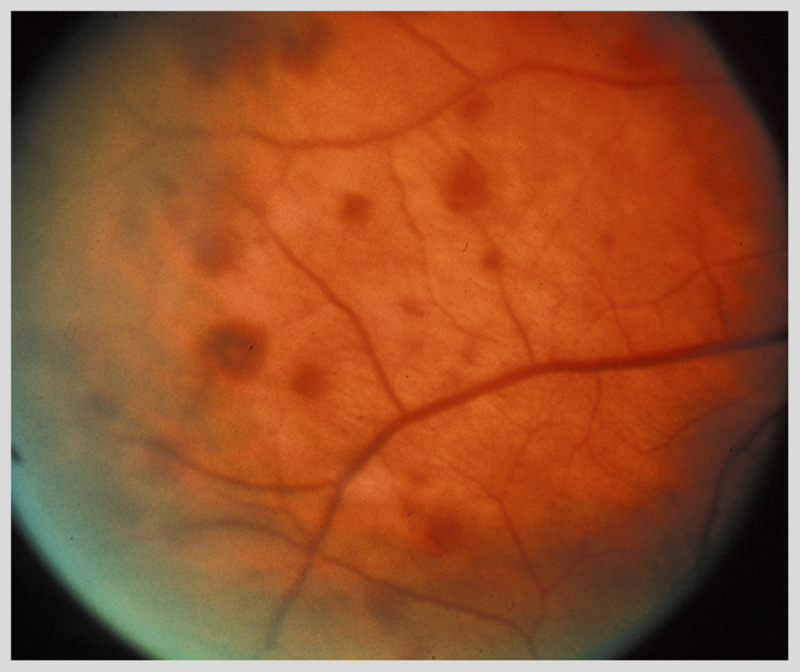
Venous stasis retinopathy in a patient with internal carotid occlusion. Multiple dot-blot retinal hemorrhages are present in the midperiphery of the retina. The veins are dilated.
Reprinted with permission from Biousse V, Newman NJ, Thieme.1 © 2009 Thieme Medical Publishers, Inc.
Figure 3-8.
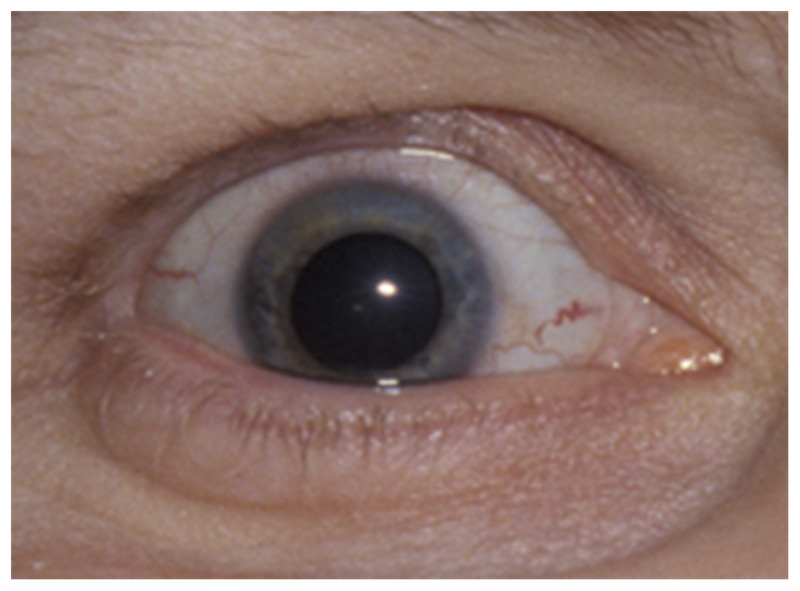
Ischemic ocular syndrome on the right. The episcleral arteries are dilated and the pupil is spontaneously dilated and unreactive. Visual loss in the eye and pain over the right eye are present. This patient has an ipsilateral carotid artery stenosis and poor collateral circulation.
Reprinted with permission from Biousse V, Newman NJ, Thieme.1 © 2009 Thieme Medical Publishers, Inc.
When diagnosed early, revascularization of the eye (eg, by performing a carotid endarterectomy if an ipsilateral internal carotid artery stenosis is present) may result in improvement of the retinopathy. If the eye is not revascularized, ocular ischemic syndrome develops. Local treatments aimed at treating neovascular glaucoma and reducing the ocular neovascularization (eg, similar to what is done for diabetic retinopathy) are moderately useful in the absence of ocular revascularization.
Case 3-1
A 69-year-old man was referred for urgent evaluation of transient visual loss involving the right eye. Three days before he noted a shadow over his right eye for approximately 5 minutes, followed by a grayout of vision in the entire eye lasting about 10 minutes, clearing in turn like a puzzle breaking up over the subsequent 1 to 2 minutes. He had no associated pain, but his right orbital region had been sore for approximately 1 month. He experienced a similar episode in the same eye 1 week earlier. He also noticed difficulty seeing when exposed to bright lights, such as when he was in a mall. He denied focal neurologic symptoms. He otherwise felt well and specifically denied recent weight loss, fever, malaise, scalp tenderness, muscle aches, or discomfort with chewing. His medical problems included chronic stable angina and hypertension. He was a former smoker. On examination, visual acuity was 20/30 in the right eye and 20/25 in the left eye. Color vision was 14 of 14 color plates with both eyes. Pupils were normal without relative afferent pupillary defect. Motility was full. Confrontation visual fields were full. Funduscopic examination showed multiple retinal hemorrhages in the right eye (Figure 3-7). The left fundus was normal. Neurologic examination was unremarkable. No carotid or cranial bruit was audible. Cardiac examination was normal.
Comment. This patient experienced two attacks of transient monocular visual loss in a pattern typical of transient ischemia to the right eye (retinal TIAs). In addition, he reported decreased vision in bright light, suggesting hypoperfusion of the eye. Examination showed retinal hemorrhages consistent with venous stasis retinopathy (Figure 3-7) as observed in patients with severe internal carotid artery stenosis. No systemic symptoms were present to suggest giant cell arteritis; indeed, his erythrocyte sedimentation rate was normal at 15 and his C-reactive protein was also normal. Immediate brain MRI with diffusion-weighted imaging was normal, and magnetic resonance angiography of the head and neck showed internal carotid artery stenosis, estimated at approximately 90% on the right and 40% on the left. The remainder of his stroke workup was normal. He underwent an immediate uncomplicated right carotid endarterectomy with improvement of his visual symptoms. He was discharged on antiplatelet agents, and all his vascular risk factors were appropriately treated.
Retinal Venous Occlusions
Central and branch retinal venous occlusions produce subacute monocular visual loss. The loss of vision is often progressive over a few days and is secondary largely to macular edema and macular ischemia (Figure 3-9).
Figure 3-9.
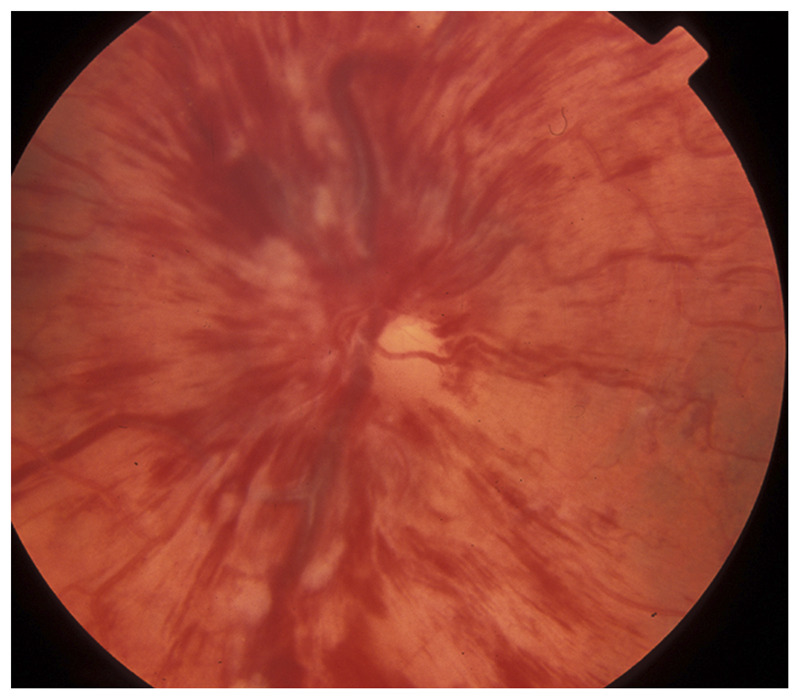
Central retinal vein occlusion in the left eye. Occlusion of the central retinal vein results in diffuse dilation of the retinal veins and numerous retinal hemorrhages with retinal edema responsible for visual loss. The disc is swollen and numerous peripapillary hemorrhages are present.
Reprinted with permission from Biousse V, Newman NJ, Thieme.1 © 2009 Thieme Medical Publishers, Inc.
Relatively prolonged (up to 30 minutes) episodes of transient monocular visual loss may occur before permanent visual loss. The mechanisms and causes of retinal venous occlusions are very different from cerebral venous thrombosis; these patients are always exclusively managed by ophthalmologists and do not need, in most cases, a systemic workup.
Diabetic Retinopathy
Diabetic retinopathy is a common cause of visual loss. Although it is also exclusively managed by ophthalmologists, it can mimic ocular ischemic syndrome and is very common in patients with stroke (Figure 3-10). Retinal ischemia ultimately results in severe visual loss. The ischemia is diffuse, often involves the macula (responsible for central visual loss), and is ultimately complicated by neovascularization of the eye (proliferative diabetic retinopathy).
Figure 3-10.
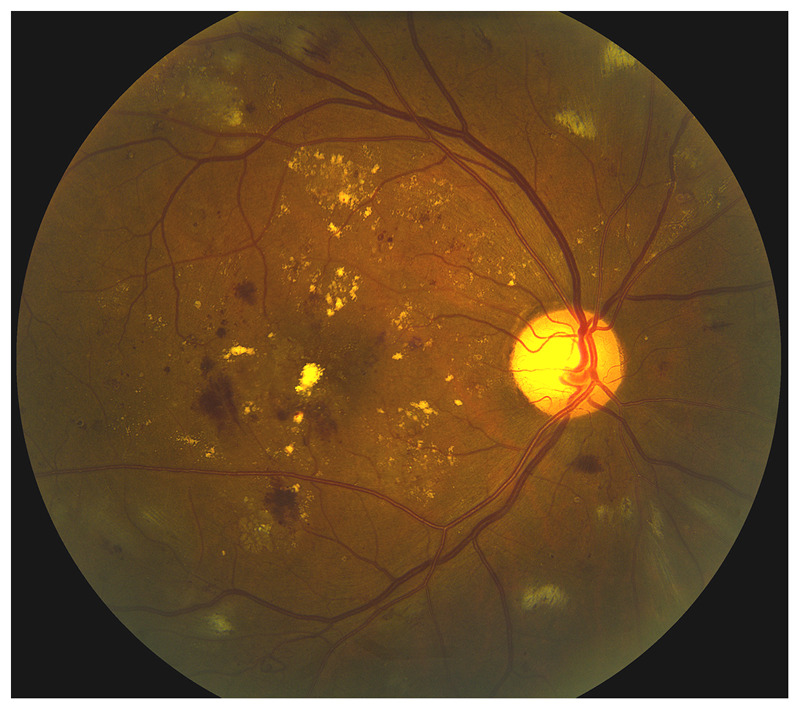
Nonproliferative severe diabetic retinopathy in the right eye. Long-standing poorly controlled diabetes mellitus produces retinal vascular disease responsible for bilateral diabetic retinopathy. The retina is hypoperfused and the arteries are very attenuated. Retinal hemorrhages and lipid exudates with macular edema are responsible for visual loss.
Reprinted with permission from Biousse V, Newman NJ, Thieme.1 © 2009 Thieme Medical Publishers, Inc.
The neovascularization in proliferative diabetic retinopathy is responsible for hemorrhages and traction retinal detachment. Destruction of the peripheral retina with laser (panphotocoagulation) decreases the neovascularization but also results in some degree of night blindness and constriction of the visual field.
Hypertensive Retinopathy
Retinal vascular changes occur as a result of chronically elevated systemic arterial blood pressure, usually bilaterally. Hypertensive retinopathy is associated with a higher risk of cardiovascular disease, including coronary artery disease and stroke. It is a marker of poorly controlled systemic arterial hypertension.
Key features of hypertensive retinopathy include bilateral retinal vascular changes (may be asymmetric), narrowing and irregularity of retinal arteries, arteriovenous nicking (narrowing of retinal veins at arteriovenous crossing sites), retinal hemorrhages, microaneurysms, and cotton wool spots. Moderate chronic hypertensive retinopathy is usually asymptomatic. However, visual loss may occur as a result of macular exudates, macular edema, and chronic retinal ischemia. Acute hypertensive retinopathy describes retinal and choroidal vascular changes occurring as a result of acutely elevated systemic arterial blood pressure. Key features of acute hypertensive retinopathy include various degrees of retinal arteriolar spasm, retinal hemorrhages, cotton wool spots, retinal exudates, serous retinal detachment, and optic nerve edema (Figure 3-11). Because bilateral disc edema may be isolated, without retinal changes, in acute hypertensive crisis, it is essential for neurologists to always measure the blood pressure of patients presenting with headache and bilateral optic nerve edema. Acute hypertensive retinopathy is often complicated by permanent visual loss from choroidal ischemia, retinal pigment epithelium changes, and ischemic optic neuropathy. Other complications include bilateral visual loss from cerebral blindness (ie, posterior reversible encephalopathy syndrome), intracranial hemorrhages, and renal failure.
Figure 3-11.
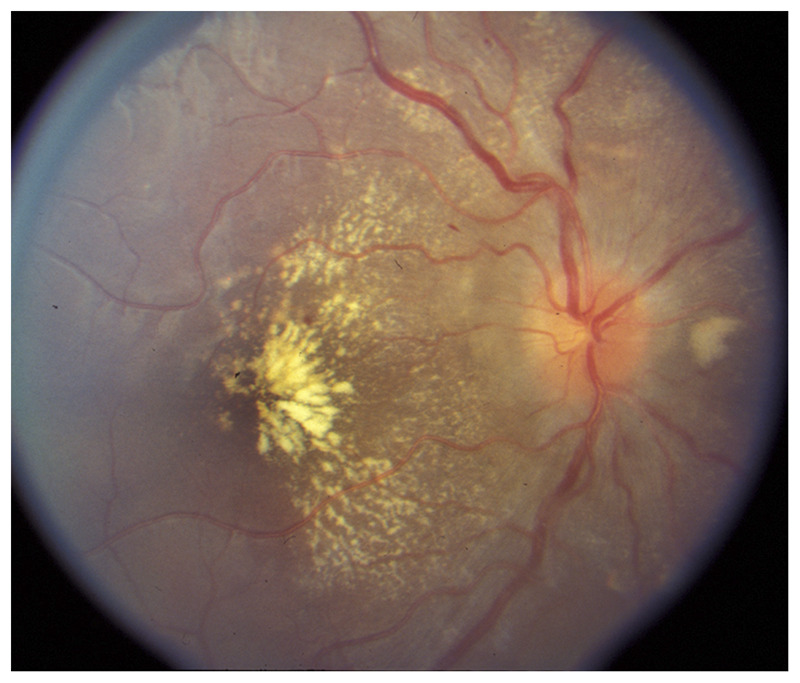
Hypertensive retinopathy in the right eye. Stage IV hypertensive retinopathy is bilateral and associates disc edema with attenuated arteries, retinal hemorrhages, and cotton wool spots, as well as diffuse exudates.
Reprinted with permission from Biousse V, Newman NJ, Thieme.1 © 2009 Thieme Medical Publishers, Inc.
Retinal Vasculitis
Vasculitis of the retinal vasculature can involve the arteries, the veins, or both, and may result in visual loss. As with cerebral vasculitis, the term retinal vasculitis implies that inflammation is present in or around the vessel wall. It therefore should be reserved for those disorders that are definitely secondary to inflammation (eg, Susac syndrome is associated with retinal vasculopathy, not vasculitis). Causes of retinal vasculitis are numerous and include all classic causes of cerebral and systemic vasculitides. Recognition of whether the vasculitis involves the arteries or the veins, and associated ocular findings, are helpful in understanding the cause of retinal vasculitis. Indeed, retinal vasculitis from Behçet disease involves the veins, is usually extensive, and is classically associated with anterior chamber inflammation. Periphlebitis associated with multiple sclerosis only involves the veins, affects the peripheral retina, and is asymptomatic. Conversely, vasculitis from sarcoidosis usually involves both arteries and veins and may be subtle and isolated, or extensive and associated with retinal hemorrhages and infiltrates and anterior chamber inflammation. The involved vessels are attenuated (appear small) with sheathing (whitish line along the vessels) and sometimes are occluded (Figure 3-12). Retinal fluorescein angiography demonstrates occlusion of the small vessels and leakage of fluorescein.
Figure 3-12.
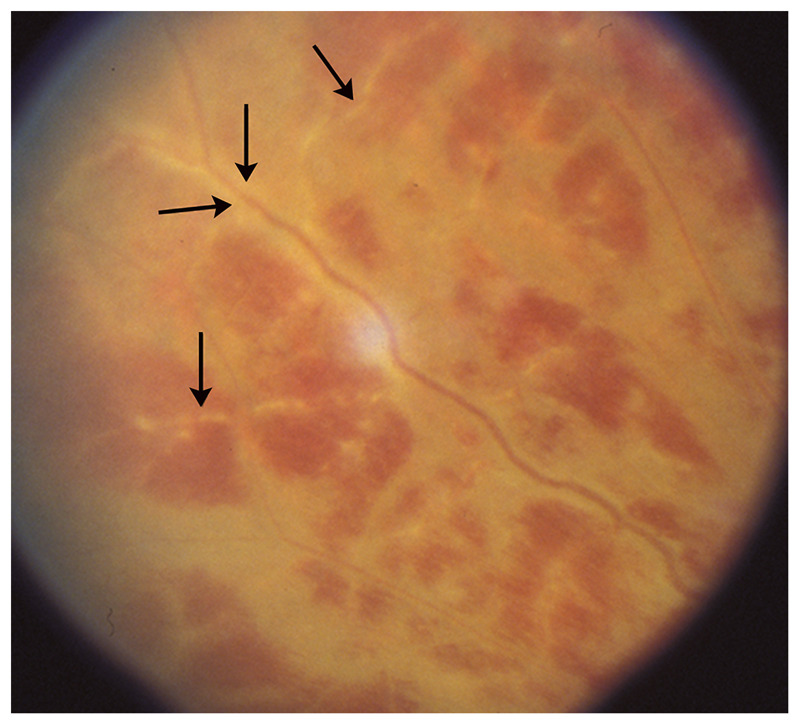
Retinal vasculitis from sarcoidosis. The inflamed arteries are surrounded by whitish sheathing (arrows), and the resultant retinal ischemia produces retinal hemorrhages. This patient has ocular inflammation in the setting of untreated sarcoidosis.
Reprinted with permission from Biousse V, Newman NJ, Thieme.1 © 2009 Thieme Medical Publishers, Inc.
Radiation Retinopathy
Chronic progressive retinal vasculopathy induced by radiation can be responsible for progressive, painless visual loss. It may be unilateral or bilateral depending on the type of radiation. It typically develops months or years after radiotherapy and is more common in patients with underlying retinal vascular disease, such as hypertension or diabetes mellitus. Key features of radiation retinopathy include retinal hemorrhages, retinal microaneurysms, retinal exudates, cotton wool spots, and macular edema (Figure 3-13). Similar to any other chronic ocular hypoperfusion, progressive visual loss develops secondary to severe retinal ischemia, retinal neovascularization, vitreous hemorrhage, traction retinal detachment, neovascularization of the anterior segment, and optic atrophy.
Figure 3-13.
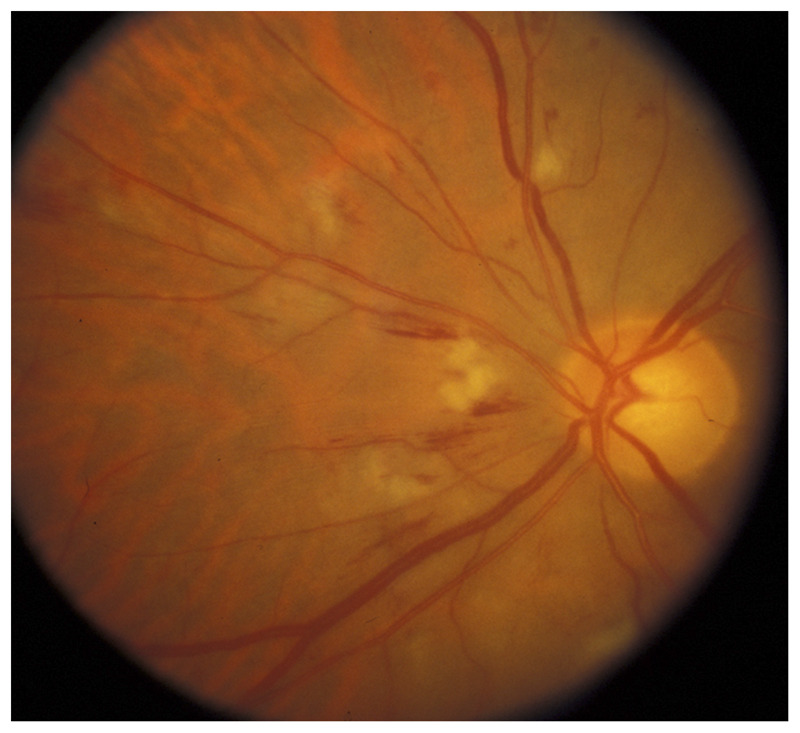
Radiation retinopathy in a patient who had received brain and eye radiation therapy for CNS lymphoma. Radiation retinopathy is insidious and causes chronic retinal ischemia with attenuated arteries, cotton wool spots, and retinal hemorrhages.
Reprinted with permission from Biousse V, Newman NJ, Thieme.1 © 2009 Thieme Medical Publishers, Inc.
Purtscher Retinopathy
Bilateral peripapillary retinal infarctions with numerous cotton wool spots may occur after trauma, acute pancreatic disorders, or amniotic fluid embolism. It is called Purtscher retinopathy and has a dramatic and distinctive funduscopic appearance (Figure 3-14).
Figure 3-14.
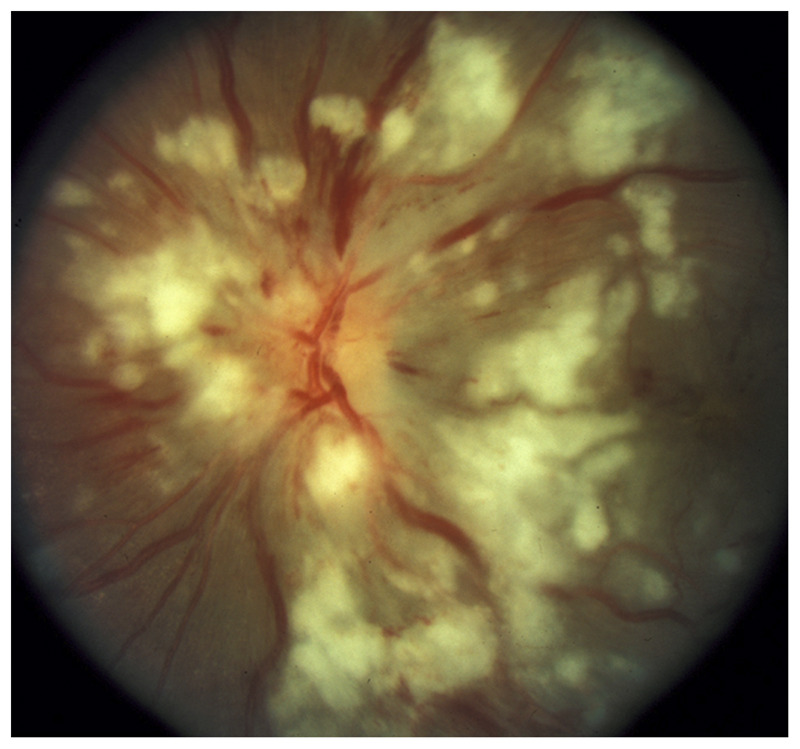
Bilateral Purtscher disease in a patient with acute pancreatitis. In the left eye, numerous cotton wool spots and large white exudates cover the optic nerve and the retina. This developed in the setting of acute pancreatitis and is consistent with Purtscher disease.
Reprinted with permission from Biousse V, Newman NJ, Thieme.1 © 2009 Thieme Medical Publishers, Inc.
Transient Monocular Visual Loss
Acute reversible retinal ischemia manifests as episodes of transient visual loss in one eye only. As emphasized in the article “Diagnostic Approach to Vision Loss,” by these same authors in this issue of CONTINUUM, many ocular disorders can present as fluctuating vision or transiently blurred vision. Vascular visual loss is usually profound, described as a black or dark shade that spreads across the visual field of the affected eye, or complete blackout of vision, disappearing after a few minutes. It may result from emboli into the retinal circulation (central and branch retinal arteries), from hypoperfusion of the eye (hemodynamic transient visual loss often associated with ocular ischemic syndrome), rarely from transient optic nerve ischemia secondary to posterior ciliary arteries vasculitis (usually in the setting of giant cell arteritis), or from arterial vasospasm involving the central retinal artery. Although the ocular evaluation is often normal in patients with transient monocular visual loss, it is essential to obtain an ophthalmic examination immediately to rule out an ocular cause and to look for a Horner syndrome that would point to a carotid dissection (Figure 3-15), retinal emboli (Figure 3-5 and Figure 3-6), ocular ischemic syndrome (Figure 3-7 and Figure 3-8), or disc edema or any other sign of giant cell arteritis.5,6,7,8
Figure 3-15.
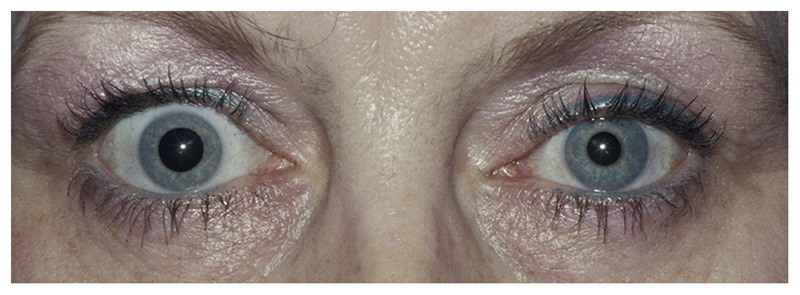
Left Horner syndrome from a left internal carotid artery dissection. This patient presented with pain over the left eye and had one episode of sudden visual loss in the left eye, lasting a few minutes. A left Horner syndrome is present with mild decreased left palpebral fissure from mild left upper lid ptosis. The left pupil is smaller than the right pupil, and the amount of anisocoria increases in the dark.
Reprinted with permission from Biousse V, Newman NJ, Thieme.1 © 2009 Thieme Medical Publishers, Inc.
Except for giant cell arteritis, transient retinal ischemia (retinal TIA) is similar to a cerebral TIA. Guidelines from the American Heart Association and the American Stroke Association emphasize that these patients need to be evaluated emergently in a specialized center in order to prevent a devastating subsequent stroke, myocardial infarction, or even death, with the same protocol recommended for patients with recent cerebral TIA.4
ISCHEMIC OPTIC NEUROPATHIES
Ischemic optic neuropathies are the most common acute optic neuropathies in patients over 50 years of age. The term ischemic optic neuropathy is used as a general term to refer to all presumed ischemic causes of optic neuropathy.
The posterior ciliary arteries provide the blood supply to the optic nerve (short posterior ciliary arteries to the optic nerve head and long posterior ciliary arteries to the retrobulbar optic nerve). They originate from the ophthalmic artery. These are small arteries that may be affected by a number of local disorders, such as atherosclerosis and vasculitis. Emboli do not usually reach these arteries. Because of the anatomy of the posterior ciliary arteries, optic nerve ischemia often results in superior (more often) or inferior segmental optic nerve involvement; ultimately pallor affects only the superior or inferior portion of the optic nerve. This explains why altitudinal defects are common with ischemic optic neuropathies. Superior segmental optic nerve involvement results in an inferior altitudinal defect (Figure 3-16 and Figure 3-17).1
Figure 3-16.
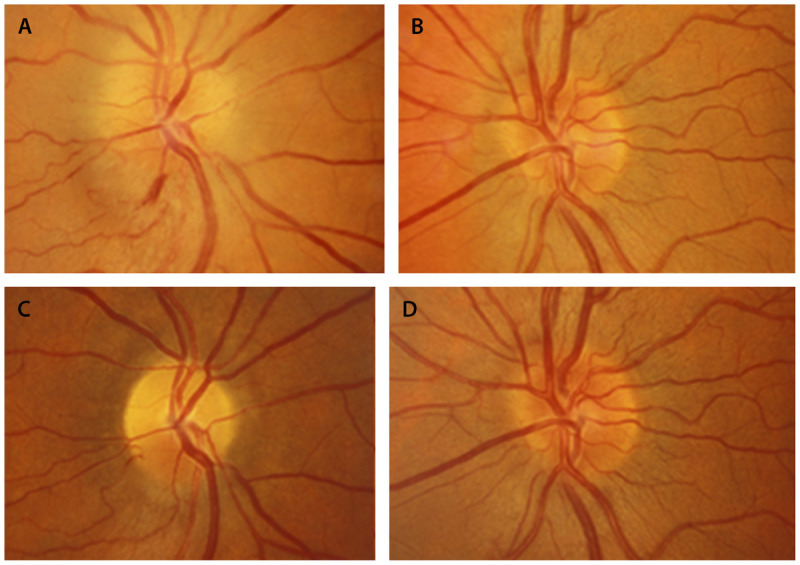
Nonarteritic anterior ischemic optic neuropathy in the right eye. A (right), B (left), Optic nerves 1 week after visual loss in the right eye. The right optic nerve is swollen with a small peripapillary hemorrhage. The left eye has a disc at risk for nonarteritic anterior ischemic optic neuropathy, with a small cup-disc ratio. C (right), D (left), Optic nerves 8 weeks later. In the right eye, the swelling has resolved, and segmental superior optic nerve head pallor is present in the right eye.
Reprinted with permission from Biousse V, Newman NJ, Thieme.1 © 2009 Thieme Medical Publishers, Inc.
Figure 3-17.
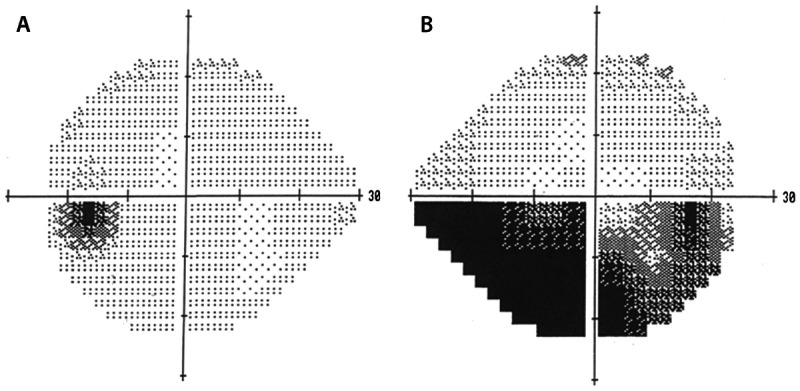
Inferior altitudinal defect in nonarteritic anterior ischemic optic neuropathy in the right eye. Humphrey visual field tests (A, left eye; B, right eye) showing an inferior altitudinal defect (in dark) in the right eye of the same patient in Figure 3-16.
Reprinted with permission from Biousse V, Newman NJ, Thieme.1 © 2009 Thieme Medical Publishers, Inc.
Classification
Ischemic optic neuropathies include anterior ischemic optic neuropathy (AION), in which disc edema is always present, and posterior ischemic optic neuropathy (PION), in which the optic nerve appears normal acutely. AION is much more common than PION, accounting for 90% of cases of optic nerve ischemia.
Ischemic optic neuropathies are categorized as nonarteritic ischemic optic neuropathy and arteritic ischemic optic neuropathy (usually in the setting of giant cell arteritis). Patients with giant cell arteritis and ischemic optic neuropathy are in danger of catastrophic, irreversible, bilateral total blindness that may be prevented by prompt treatment with corticosteroid therapy.
It is essential to correctly diagnose patients with ischemic optic neuropathies. Indeed, inflammatory optic neuritis is often overdiagnosed in patients with acute optic neuropathy. A brain MRI is usually obtained in these patients, who may be found to have nonspecific hyperintensities on the T2-weighted images. This inappropriately may prompt a diagnosis of optic neuritis and presumed multiple sclerosis, with dramatic consequences for the patients’ life and treatment. In addition, when ischemic optic neuropathy is missed, giant cell arteritis may also be overlooked.
Nonarteritic Anterior Ischemic Optic Neuropathy
Nonarteritic AION is the most common cause of acute optic neuropathy in white patients older than 50 years. Clinical presentation includes acute, painless monocular loss of vision, which may progress over several hours or days. As with all unilateral optic neuropathies, an ipsilateral relative afferent pupillary defect is present. There is always optic disc swelling acutely, frequently with peripapillary hemorrhages (Figure 3-16) (Case 3-2). Gradually, over weeks, the optic disc develops pallor and the edema resolves. Occasionally, disc swelling may be seen prior to visual loss (incipient AION). The typical visual field defect is an altitudinal or arcuate defect, especially inferiorly. All affected patients have an underlying crowded optic nerve that likely contributes to the pathophysiology of nonarteritic AION. In rare cases, AION may occur in a patient with optic nerve head drusen or papilledema. The drusen or the papilledema makes the optic nerve head more crowded and may “choke” the optic nerve.
Although nonarteritic AION results from vascular occlusive disease of small vessels supplying the anterior portion of the optic nerve, its exact cause remains unclear. Anatomic factors, such as a congenitally small and crowded optic nerve head with a small cup-disc ratio (so-called disc at risk), may mechanically contribute to the vascular event. Because it is a disease of the small vessels, nonarteritic AION is not associated with ipsilateral internal carotid artery stenosis, and embolic AION is extremely rare. Most patients have atheromatous vascular risk factors and are older than age 50, although nonarteritic-AION may occur at any age (Table 3-4).
Table 3-4.
Risk Factors for Nonarteritic Anterior Ischemic Optic Neuropathy

Unlike inflammatory optic neuropathy, the vision loss is usually permanent in nonarteritic AION. It ranges from excellent visual acuity (and a visual field defect) to devastating visual loss. Up to 50% of patients retain visual acuity of 20/60 or better. Although recurrences in the same eye are rare, less than 5%, subsequent involvement of the fellow eye is about 15% at 5 years in patients with a disc at risk.
No established treatment for nonarteritic AION is available. The clinician’s primary role is to exclude giant cell arteritis, control vascular risk factors (including obstructive sleep apnea), treat anemia when present, and prevent hypotension when appropriate (in the setting of dialysis, for example). The use of oral steroids for a few weeks remains debated in nonarteritic AION and is not routinely recommended. No data are available to support anticoagulation, and, although antiplatelet agents are usually prescribed for primary prevention of vascular events, they have no obvious impact on visual outcome.
Case 3-2
A 50-year-old white man reported sudden visual loss in the right eye, worsening over the next 2 days. His past medical history was remarkable for well-controlled hypertension. He had been smoking for the past 30 years. He denied pain on eye movement, other eye pain, headache, scalp tenderness, jaw claudication, muscle aches, fevers, or fatigue. He had no history of previous focal neurologic deficits or visual loss.
On examination, he appeared healthy. Blood pressure was 132/84 mm Hg. Visual acuity was 20/60 in the right eye and 20/20 in the left eye. A right relative afferent pupillary defect was present. Motility was full. Visual field examination showed a full field in the left eye and an inferior altitudinal defect in the right eye (Figure 3-17). The left fundus was normal with a cup-disc ratio of less than 0.1, but disc edema was present in the right eye (Figure 3-16). The remainder of the neurologic examination was normal.
The patient was encouraged to stop smoking. Aspirin was recommended because of his age and vascular risk factors. One month later, his visual acuity in the right eye had improved to 20/40, and the visual field had not changed. The disc edema resolved over several weeks; he ultimately was left with superior optic atrophy of the right disc (Figure 3-16).
Comment. This patient exhibited the classic presentation of an anterior ischemic optic neuropathy (AION). He had vascular risk factors, and his optic nerve heads were congenitally small, as indicated by the small cup in the unaffected eye. He had no pain on eye movement or central acuity loss to suggest an alternative diagnosis of optic neuritis. He had no symptoms to suggest a diagnosis of giant cell arteritis; further, he was unusually young for that disease.
Diabetic Papillopathy
Diabetic papillopathy is believed to be an atypical form of AION that usually occurs in young patients with insulin-dependent diabetes mellitus. It is distinguished from typical nonarteritic AION by the slight degree (or even absence) of visual loss, the frequency of bilateral involvement (50%), the long duration of disc edema, and the generally good visual outcome.
Nonarteritic Posterior Ischemic Optic Neuropathy
Nonarteritic PION is extremely rare and usually occurs during lengthy spine surgery in the prone position or in patients with acute systemic hypotension. It is usually a diagnosis of exclusion made only after compression of the posterior optic nerve is ruled out by a good quality MRI of the orbits with contrast and an extensive systemic workup looking for an underlying systemic inflammatory disorder (Table 3-5). PION in patients older than 50 years is usually indicative of giant cell arteritis.
Table 3-5.
Risk Factors for Nonarteritic Posterior Ischemic Optic Neuropathy
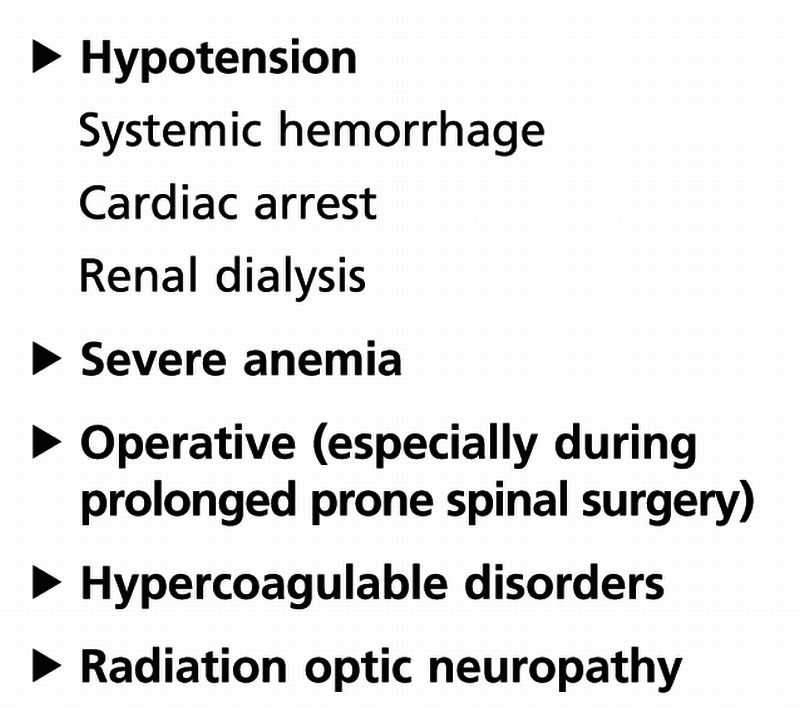
Because the posterior part of the optic nerve is involved first, there is no visible disc edema acutely. Patients with nonarteritic PION report acute, painless monocular loss of vision, which may progress over several hours or days. A relative afferent pupillary defect in unilateral forms is present, and the optic disc is normal acutely. Gradually the optic disc develops pallor over the ensuing 4 to 6 weeks.
Arteritic Ischemic Optic Neuropathy
Anterior ischemic optic neuropathy is the most common ophthalmic manifestation of giant cell arteritis, and giant cell arteritis is the most common cause of PION (Table 3-6). Giant cell arteritis therefore should be suspected and systematically ruled out in all patients older than 50 years presenting with an ischemic optic neuropathy (Case 3-3).
Table 3-6.
Causes of Arteritic Ischemic Optic Neuropathy

The clinical presentation of giant cell arteritis varies widely, with as many as 25% of patients having isolated visual loss and no systemic symptoms of giant cell arteritis (such as weight loss, fatigue, myalgias, headaches, or jaw claudication). Patients are usually older, typically in their seventies and eighties, and present usually with severe unilateral or bilateral visual loss with acuities reduced to no light perception, light perception only, or hand motion. Visual loss is often bilateral and can be associated with a concurrent retinal or choroidal infarction (Figure 3-18). Visual loss may be preceded by recurrent episodes of transient monocular visual loss or transient diplopia. The optic nerve swelling appears pale, even acutely at the time of visual loss (Figure 3-19). The peripapillary retina is often pallid, suggesting associated choroidal and retinal ischemia. Cotton wool spots are common and indicate diffuse ocular ischemia when associated with AION.
Figure 3-18.
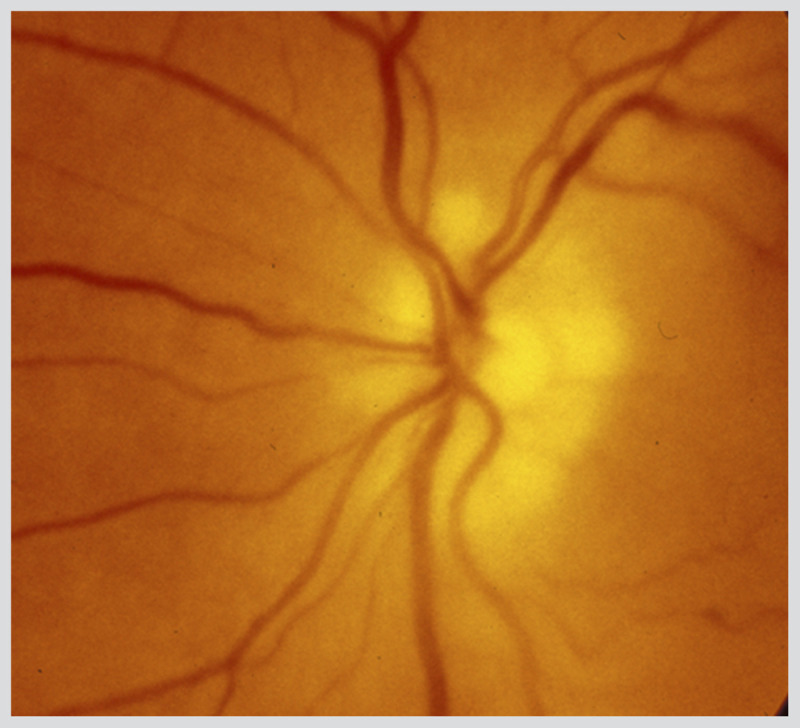
Left anterior ischemic optic neuropathy in giant cell arteritis. Pallid disc swelling acutely occurs in arteritic anterior ischemic optic neuropathy. The nerve is very pale acutely and cotton wool spots are present.
Reprinted with permission from Biousse V, Newman NJ, Thieme.1 © 2009 Thieme Medical Publishers, Inc.
Figure 3-19.
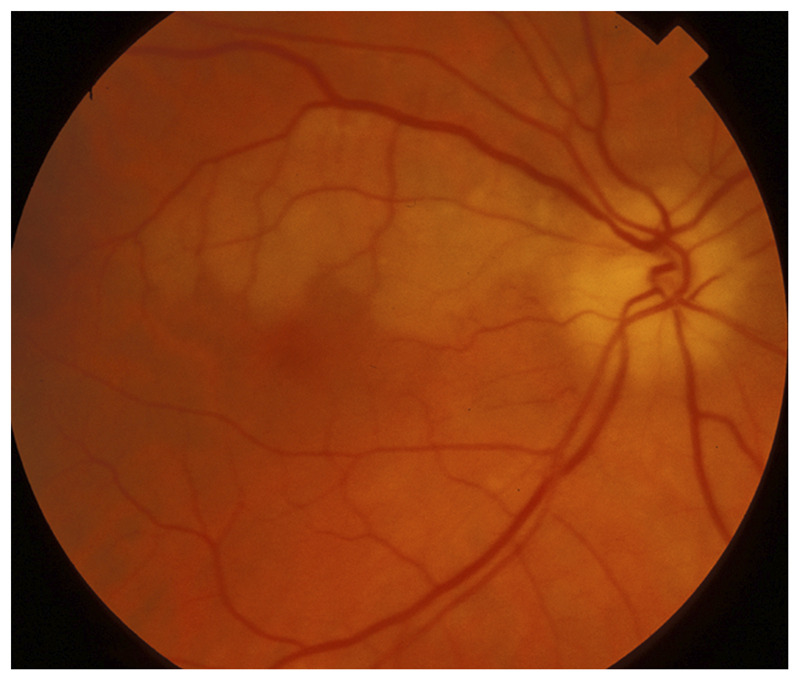
Right branch retinal artery occlusion with anterior ischemic optic neuropathy in giant cell arteritis. This older patient had profound acute visual loss in the right eye and was found to have disc edema suggestive of anterior ischemic optic neuropathy associated with a superior branch retinal artery occlusion in the same eye. The association of anterior ischemic optic neuropathy and branch artery retinal occlusion in the same eye is very suggestive of vasculitis, such as giant cell arteritis.
Reprinted with permission from Biousse V, Newman NJ, Thieme.1 © 2009 Thieme Medical Publishers, Inc.
Elevation of the erythrocyte sedimentation rate and C-reactive protein is highly suggestive of the disease, and the diagnosis is proven by finding granulomatous inflammation with giant cells and disruption of the internal elastic lamina, on biopsy of a superficial temporal artery. In the absence of treatment, vision deteriorates and there is a high risk of involvement of the second eye within days or weeks.
Arteritic AION requires emergency treatment to prevent complete blindness. Systemic corticosteroid therapy should be instituted immediately upon presumed diagnosis and should not be delayed awaiting results of the erythrocyte sedimentation rate or temporal artery biopsy. Patients with acute visual loss from suspected giant cell arteritis are usually treated with IV methylprednisolone (1 g/d for 3 to 5 days) followed by oral prednisone (1 mg/kg/d), whereas patients with long-standing symptoms of giant cell arteritis or no ischemic complications are most often treated with oral prednisone alone. In all cases, the oral prednisone is tapered very slowly on a monthly basis if the patient remains asymptomatic and no active biologic inflammatory syndrome is present. Most patients need to be treated for approximately 2 years.
Case 3-3
A 79-year-old white woman was seen in the emergency department (ED) for visual loss. Her past medical history was remarkable for breast cancer treated with left mastectomy 15 years ago and cataract surgery in both eyes 8 years ago. She had no vascular risk factors.
She was in her usual state of health until 3 months before referral, at which time she had neck pain, neck stiffness, and shoulder pain. She felt as if she had the flu and was evaluated by her primary physician. She remained stable until four weeks ago when she developed three episodes of horizontal binocular diplopia lasting approximately 15 minutes each. She reported headaches, but had no other neurologic or ocular symptoms. Two weeks ago, she awoke with a “shade across the right eye.” She was evaluated by an ophthalmologist, who obtained a head CT, which was normal, and blood tests, including complete blood cell count (normal), erythrocyte sedimentation rate (32 mm/h), and glucose (179 mg/dL).
On the morning of presentation to the ED, her visual acuity dramatically decreased in her right eye, accompanied by slight pain. Examination in the ED showed that her visual acuity was “hand motion” in the right eye and 20/40 in the left eye. She had a right relative afferent pupillary defect. Motility was full. Funduscopic examination showed a swollen, pale optic nerve in the right eye (Figure 3-18) and was unremarkable in the left eye. The diagnosis of giant cell arteritis was suspected, and the patient was admitted to the hospital where she received 3 days of high-dose IV steroids (1 g of methylprednisolone per day). She was discharged on prednisone 80 mg per day. A temporal artery biopsy was performed 2 days after admission and confirmed the diagnosis of giant cell arteritis. One month later, her visual function had not improved in her right eye. Her left eye was never affected.
Comment. This patient had a typical presentation for giant cell arteritis. Painful episodes of diplopia should suggest this diagnosis in elderly patients. Transient monocular visual loss preceded by diplopia is almost diagnostic of giant cell arteritis. Unfortunately, the diagnosis was made only after the patient developed permanent visual loss in one eye. The erythrocyte sedimentation rate was normal, and the ophthalmologist did not notice that the C-reactive protein, which had been obtained at the same time, was elevated (3.9 mg/dL; normal below 0.8 mg/dL). It should have prompted emergent treatment with steroids at that time to prevent visual loss. Up to 12% of patients with giant cell arteritis have a normal erythrocyte sedimentation rate. In these patients, other biological parameters suggesting inflammation such as elevated platelets, low hemoglobin, or elevated C-reactive protein should be present.
KEY POINTS
Acute central retinal artery occlusion is a vascular emergency. Once giant cell arteritis is ruled out, an immediate stroke workup is necessary, similar to what is done for patients with acute cerebral ischemia.
Giant cell arteritis needs to be ruled out in all patients older than 50 years of age presenting with acute retinal or optic nerve ischemia.
Approximately 25% of patients with acute retinal ischemia also have acute cerebral ischemia on brain diffusion-weighted images.
There is no accepted or proven treatment for acute central retinal artery occlusion. The most important management is the secondary prevention of stroke.
Most retinal vascular disorders have specific ocular complications that need to be managed by an ophthalmologist. Neurologists evaluating patients with acute or chronic retinal vascular disorders need to work closely with a retina specialist.
Vascular transient monocular visual loss is a retinal TIA and must be evaluated emergently like any cerebral TIA.
Ischemic optic neuropathies are the most common acute optic neuropathies in patients over 50 years of age. The term ischemic optic neuropathy is used as a general term to refer to all presumed ischemic causes of optic neuropathy.
Nonarteritic anterior ischemic optic neuropathy typically occurs in the setting of a disc at risk (small crowded optic nerve with a small cup-disc ratio). The absence of a disc at risk in a patient with anterior ischemic optic neuropathy should suggest giant cell arteritis or another underlying disorder.
Although ischemic optic neuropathies are considered the equivalent of a stroke of the optic nerve, they cannot be directly compared with cerebral infarctions. The causes and mechanisms of ischemic optic neuropathies are different, and patients with an ischemic optic neuropathy do not need the same workup as those with a retinal or a cerebral infarction.
Nonarteritic anterior ischemic optic neuropathy (AION) is not an embolic disorder. Evaluation of the internal carotid artery is not indicated. Patients with anterior ischemic optic neuropathy have not been found to have a definite increased risk of cerebrovascular disease, but vascular risk factors are common and should be controlled.
Posterior ischemic optic neuropathy is extremely rare and is most often related to giant cell arteritis or postoperative complications. Nonarteritic posterior ischemic optic neuropathy is a diagnosis of exclusion and should only be made after all other causes of acute optic neuropathy have been ruled out.
In a patient with suspected ischemic optic neuropathy, the first step should always be to consider giant cell arteritis.
Footnotes
Relationship Disclosure: Dr Biousse receives book royalties from Elsevier, Thieme, and Up-to-Date, and her institution receives a grant from Research to Prevent Blindness.
Dr Newman receives book royalties from Elsevier and Thieme, serves as a consultant for Trius Therapeutics, Inc, and has provided expert testimony for multiple law firms.
Unlabeled Use of Products/Investigational Use Disclosure: Drs Biousse and Newman report no disclosures.
REFERENCES
- 1.Biousse V,, Newman NJ. Neuro-ophthalmology illustrated. New York, NY: Thieme, 2009. [Google Scholar]
- 2.Schuenke M,, Schulte E,, Schumacher U, et al. THIEME atlas of anatomy: head and neuroanatomy. New York, NY: Thieme, 2007. [Google Scholar]
- 3.Johnston SC,, Albers GW,, Gorelick PB, et al. National Stroke Association recommendations for systems of care for transient ischemic attack. Ann Neurol 2011; 69 (5): 872–877. [DOI] [PubMed] [Google Scholar]
- 4.Furie KL,, Kasner SE,, Adams RJ, et al.; American Heart Association Stroke Council, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Interdisciplinary Council on Quality of Care and Outcomes Research. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42 (1): 227–276. [DOI] [PubMed] [Google Scholar]
- 5.Biousse V,, Trobe JD. Transient monocular visual loss. Am J Ophthalmol 2005; 140 (4): 717–721. [DOI] [PubMed] [Google Scholar]
- 6.Biousse V. Acute retinal arterial ischemia: an emergency often ignored. Am J Ophthalmol 2014; 157 (6): 1119–1121. [DOI] [PubMed] [Google Scholar]
- 7.Streifler JY,, Eliasziw M,, Benavente OR, et al. The risk of stroke in patients with first-ever retinal vs hemispheric transient ischemic attacks and high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial. Arch Neurol 1995; 52 (3): 246–249. [DOI] [PubMed] [Google Scholar]
- 8.Benavente O,, Eliasziw M,, Streifler JY, et al.; North American Symptomatic Carotid Endarterectomy Trial Collaborators. Prognosis after transient monocular blindness associated with carotid-artery stenosis. N Engl J Med 2001; 345 (15): 1084–1090. [DOI] [PubMed] [Google Scholar]


