Abstract
Purpose of Review:
This article reviews the various types of visual dysfunction that can result from lesions of the cerebral regions beyond the striate cortex.
Recent Findings:
Patients with dyschromatopsia can exhibit problems with color constancy. The apperceptive form of prosopagnosia is associated with damage to posterior occipital and fusiform gyri, and an associative/amnestic form is linked to damage to more anterior temporal regions. Pure alexia can be accompanied by a surface dysgraphia. New word-length effect criteria distinguish pure alexia from hemianopic dyslexia. Subtler problems with perception of numbers and faces can be seen in patients with pure alexia as well. Also, a developmental form of topographic disorientation, which is due to problems with forming cognitive maps of the environment, has been discovered. In Balint syndrome, added features of decreased flexibility of attention in simultanagnosia include local and global capture. Balint syndrome can affect not just localization in space, but also in time, as manifest in sequence agnosia.
Summary:
Lesions at intermediate levels of a processing hierarchy can cause difficulty with color perception or motion perception. At a higher level, ventral lesions of the occipitotemporal lobes can lead to a variety of problems with object recognition. Dorsal lesions of the occipitoparietal lobes can cause difficulty with spatial localization and guidance.
Most lesions of the visual system from the retina to the striate cortex characteristically cause scotomata, regions of the visual field where vision is lost surrounded by regions where it is preserved. Lesions of extrastriate cortex lead to very different types of deficits, however. Rather than a general loss of vision constrained to a specific location in the visual field, they cause a loss of a specific type of visual function that often is neither localized nor limited to one part of the field.
Diverse types of visual functions are affected by extrastriate lesions, which are grouped into two broad families according to anatomic location and functional relationships.1,2 Lesions of the ventral occipitotemporal cortex affect what has been colloquially called a “what” stream, involved ultimately in object identification. The “intermediate” level of this stream is involved particularly in color processing, and a lesion of this will lead to achromatopsia. Higher-level recognition deficits can lead to diverse types of general visual agnosia or a number of selective agnosias, including prosopagnosia, pure alexia, and topographagnosia. In contrast, lesions of the dorsal occipitoparietal cortex cause problems with spatial processing, in what some view as a “where” stream1 and others view as a focus on preparation for visually guided action.2 The intermediate deficit here is akinetopsia, impaired motion processing. High-level deficits are the components of Balint syndrome, including simultanagnosia, optic ataxia, and ocular motor apraxia, as well as astereognosis.
The lesions that cause the various types of cortical syndromes discussed in this article are the usual suspects for cerebral pathology, eg, stroke, tumors, and encephalitis. In most cases, the temporal pattern of the problem is the key to diagnosing the cause. For syndromes that are more likely to emerge after bilateral lesions, such as achromatopsia or Balint syndrome, etiologies that can have bilateral effects should be considered. Thus, among the different kinds of stroke, postoperative watershed infarctions, embolic showers, and vasculitis would be suspects. Other causes of bilateral lesions include posterior reversible encephalopathy syndrome, multiple cerebral metastases, and herpes simplex encephalitis. Patients with a slower, progressive course of visual dysfunction may have posterior cortical atrophy or the Heidenhain variant of Creutzfeldt-Jakob disease. At the other end of the spectrum from neurodegeneration, increased recognition exists that some people are born with a selective visual agnosia, as has been described, for example, in congenital prosopagnosia3 and developmental topographic disorientation.4 In some cases the deficit appears to run in families, suggesting an inherited genetic defect.
SYNDROMES OF THE VENTRAL STREAM
Achromatopsia
Acquired brain lesions can impair color vision.5 Complete impairment is called achromatopsia, and patients with this condition see the world in grayscale (Case 7-1). Some patients find this unpleasant, as if things look dirty. A partial defect is called dyschromatopsia. With dyschromatopsia, some color perception remains, but the ability to distinguish one color from another is reduced. For some patients, the world takes on a specific tint. Rarely, patients may report illusory spread of colors, in which an object’s color seeps out of its boundaries.
Color vision is processed by a network in the occipitotemporal cortex. Cerebral dyschromatopsia is associated with bilateral lesions of the lingual gyri5 (Figure 7-3); its severity reflects how much of the cortical color network is lost.6 Unilateral lesions may not cause a symptomatic deficit, but when tested carefully, patients with such lesions are shown to have hemiachromatopsia, impaired discrimination of colors in the contralateral hemifield.
An impairment of color perception is often associated with superior field defects and other higher-order problems of recognition, such as prosopagnosia and topographagnosia.
Not all color function is lost in patients with achromatopsia; special tests can show evidence of retained red/green and blue/yellow color opponency that is based on processing by retinal ganglion cells. This residual ability is likely responsible for the fact that such patients can perceive boundaries between areas of different colors, but cannot identify what the respective colors are.7 Clinically, this can lead to the phenomenon in which they cannot read the Ishihara plates up close (at a distance where they can see the dots, and, hence, the visible boundaries are those between each dot and the white background), but can read the plates if they are far enough away that the dots merge, so that the only visible boundaries are those between red and green regions.
More sophisticated deficits of color perception can also be demonstrated in patients with achromatopsia. Color constancy refers to the fact that an individual’s perception of an object’s color remains stable even when the lighting changes. This stability requires the visual system to estimate what the illumination must be from the general coloration of the entire scene and to discount that from the wavelengths of light emanating from the object. “Discounting the illuminant” is a cortical computation that is inaccurate in patients with dyschromatopsia.7
Patients with achromatopsia may still be able to read Ishihara plates, so testing for achromatopsia requires some care. Patients may be unable to name colors, but those with a partial defect may still be able to discern the broad categories of red and blue even though they are impaired in discerning finer distinctions within those color domains. Also, in color anomia, impaired naming of colors exists, although color perception is normal. The best tests for achromatopsia involve sorting colored chips for their hue (red–green) or saturation (red–pink), such as the Farnsworth-Munsell color test (Figure 7-2).
Case 7-1
A 41-year-old man awoke one day with a sense of distortion between the right and left halves of his vision, along with nausea and imbalance. A CT scan showed a left occipital infarction and a right vertebral arterial dissection. The next day in the hospital the patient had a right occipital infarction and noted many visual problems, which slowly improved over time. He had trouble recognizing faces, seeing their shapes, and identifying ethnicity and age of faces. Everyone appeared good-looking. He recognized people by their voices, clothing, physical posture, facial moles, or hairstyles. He could not visualize the faces of well-known actors when he heard their names. Initially the world appeared black and white, but later he saw some color tints, although he had trouble distinguishing colors that were similar to each other, such as blue and green. Although the patient was a sports car enthusiast, all cars looked the same to him. He could not recognize different buildings, which all looked like a “bunch of squares.” He had trouble navigating his neighborhood and got lost following a short but twisting route to the supermarket across the street. His memory for daily events was poor, causing problems.
On examination, the patient’s visual acuity was normal, but he had bilateral superior altitudinal defects (Figure 7-1). The Farnsworth-Munsell color test showed severe difficulties with color discrimination (Figure 7-2). He recognized 47 of 50 words on the Warrington Recognition Memory Test but only 28 of 50 faces. Only 60% of celebrity faces were familiar, even though he recognized 100% of their names. He also performed poorly on a test of his ability to visualize the shapes and features of famous faces.
Figure 7-1.
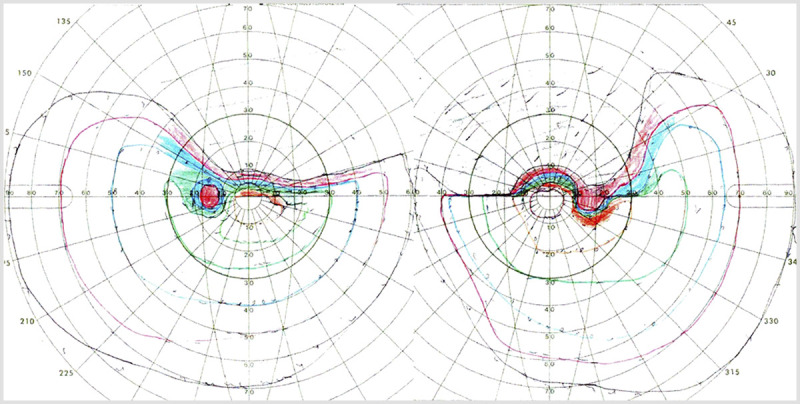
Goldmann perimetry of the patient in Case 7-1 showing bilateral superior altitudinal defects.
Figure 7-2.
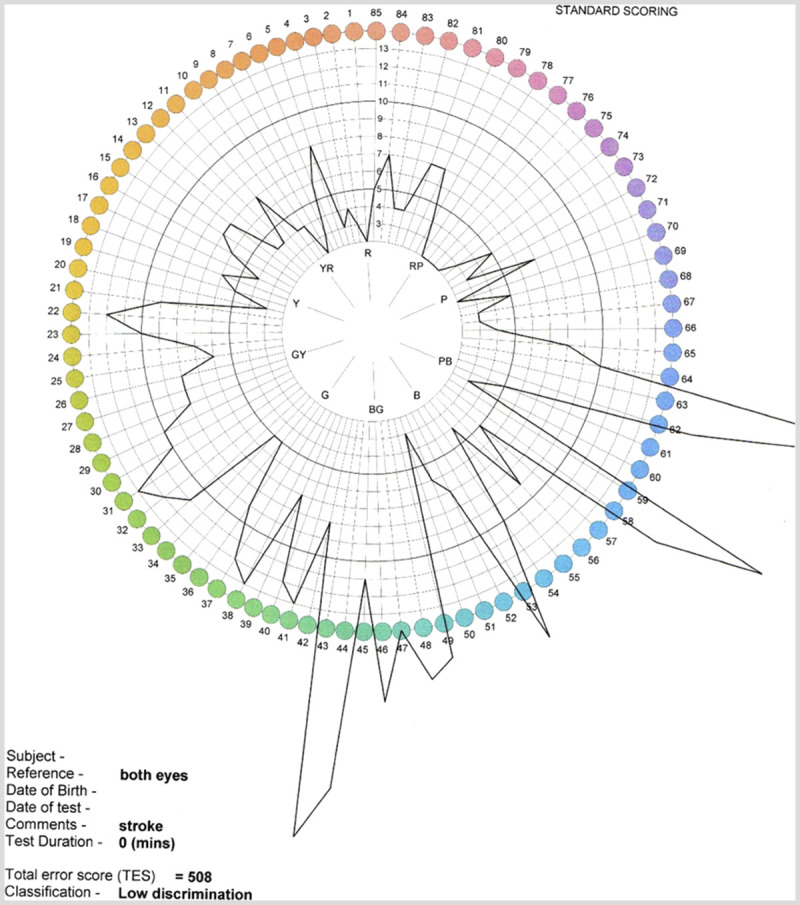
Farnsworth-Munsell color test results of the patient with cerebral dyschromatopsia in Case 7-1. The black lines indicate the magnitude of the patient’s error for color sorting at each hue location. The greater distance away from the center of the disc, the greater the error. The patient has difficulty with all hues, and particularly with green and blue. These findings are consistent with the patient’s own observations from daily life.
MRI showed bilateral occipitotemporal ischemic lesions involving the lingual and fusiform gyri (Figure 7-3), and functional MRI confirmed the loss of both right and left fusiform face areas.
Figure 7-3.
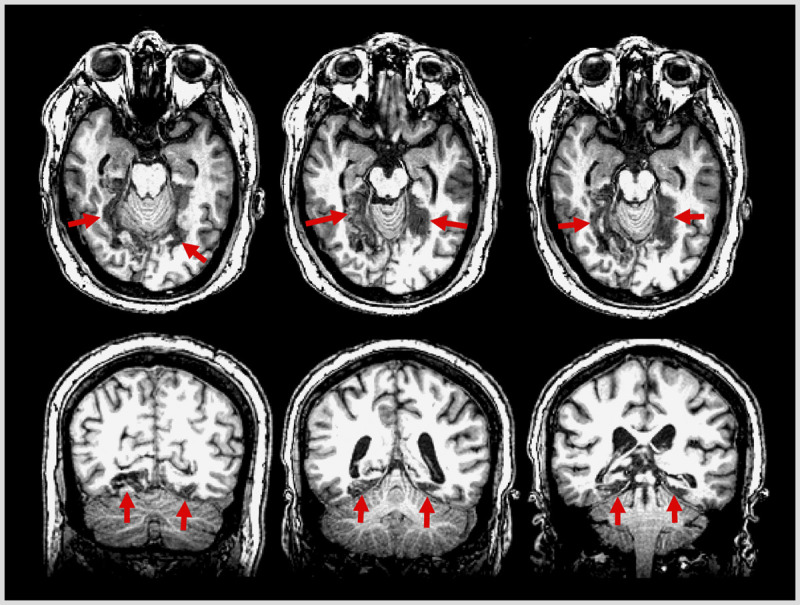
Axial and coronal T1-weighted MRI from the patient in Case 7-1 showing bilateral lesions of the fusiform and lingual gyri (arrows).
Comment. This man had a typical history and findings due to bilateral occipitotemporal lesions, which caused the constellation of dyschromatopsia, prosopagnosia, topographic disorientation, and bilateral superior quadrantanopia.
General Visual Agnosia
Patients with general visual agnosia have trouble recognizing objects.8 This is often particularly true if the image of an object is impoverished (eg, a two-dimensional line drawing) and less so for real three-dimensional objects, although the latter can also cause patients difficulty. If the object they cannot name is made to emit a sound or given to them to touch, they will recognize it, showing that the problem is limited to vision.
Another important diagnostic consideration is that the patient should have sufficient basic vision (ie, visual acuity and central visual field) to support object recognition. Sometimes diffuse visual deficits can mimic object agnosia.
As with many complex functions, object recognition involves a hierarchy of processing stages and a network of occipitotemporal regions. A fundamental and traditional distinction lies between apperceptive and associative forms of agnosia. Patients with apperceptive visual agnosia are unable to form accurate visual representations of objects in their brain, and without such information they cannot determine what the objects are. Patients with associative visual agnosia are able to form fairly accurate visual representations, but they have difficulty matching this information to stored knowledge about what objects look like or their use. Further fractionating distinctions have been proposed in a taxonomy of agnosia.9
A number of types of apperceptive visual agnosia exist. In visual form agnosia, the ability to perceive the shape of objects is impaired8; patients may have difficulty identifying even elementary geometric forms, eg, triangles and squares. Severity varies: some patients see simple shapes but not more complex ones. Their difficulties likely stem from their inability to see the basic building blocks of shape, such as curvature, surface, and volume. If asked to copy a drawing of an object, these patients fail miserably. This type of visual agnosia has often been associated with carbon monoxide poisoning, which likely causes a diffuse array of lesions affecting the occipital cortex, as well as object-processing areas such as the lateral occipital cortex. It is also seen with posterior cortical atrophy (Case 7-2 and Figure 7-4) and bilateral occipital ischemia.
Figure 7-4.
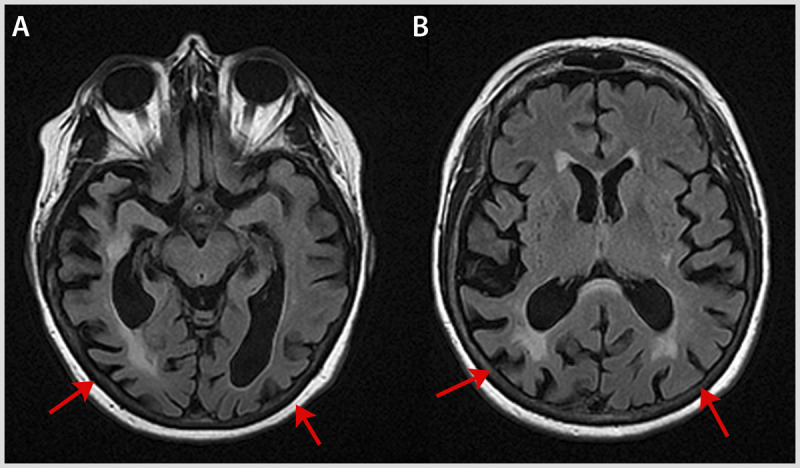
MRI from the patient in Case 7-2, with visual agnosia. Axial fluid-attenuated inversion recovery (FLAIR) images show marked atrophy (arrows) of occipitotemporal (A) and occipitoparietal (B) cortex, more pronounced on the right.
Patients with integrative agnosia, on the other hand, perceive shape reasonably well10; they can match similar shapes. They copy drawings slowly and proceed painstakingly, part by part, but can be relatively accurate. Their problem lies in linking or grouping the parts of an object to see the whole item, especially if the object is large and complex. These patients can get derailed by the intersections of lines in drawings with overlapping figures, and they may not appreciate the incongruities in impossible M. C. Escher figures and false animals created by joining the parts of different beasts together. Like visual agnosia, integrative agnosia can occur with posterior cortical atrophy or bilateral occipital infarctions.
Patients with transformation agnosia fail to recognize objects that are shown from unusual viewpoints; they may have difficulty creating or using a mental representation of the three-dimensional shape of an object. Fortunately for these patients, this difficulty may not create too many problems in real life.
Among the associative visual agnosias, in which knowledge about objects is lost or rendered inaccessible, interesting distinctions can be made between knowledge of object form and knowledge of object utility. The latter can be probed by tests such as the Pyramid and Palm Trees Test, which requires that patients determine which two of three pictured items are related (eg, hammer, screwdriver, hockey stick). A patient with associative visual agnosia has trouble relating items with pictures, but can do so if told the names of the objects instead. Another interesting distinction is that recognition of living organisms can be more affected than that of inanimate objects. Associative agnosia is linked to left or bilateral lesions of the parahippocampal, fusiform, and lingual gyri.11
Case 7-2
A 71-year-old woman reported that she had noted visual problems for several years. She had great difficulty finding things and tended to misidentify objects. She could recognize that a face was familiar, although she often could not recall the names of people. Reading was difficult for her even with large print, and she had trouble playing dominoes. She got confused while dressing; her husband often had to help her finish. She could walk her dog by herself along well-known paths in her neighborhood, but often did not recognize where she was when being driven in a car.
On examination, her visual acuity was 20/20, but attaining this result required showing her one letter at a time. She identified a square and a circle, and said a triangle was a tent. She had more difficulty with three-dimensional shapes such as cubes and pyramids. From line drawings of objects she identified a chair but not a key, hammer, glasses, or glove. With real objects, she stated that a ruler was a thermometer, but when the ruler was placed in her hand, she realized it was a ruler. She knew that the ruler was used to draw straight lines, but did not know what edible product came from maple trees. When she viewed the cookie theft picture, she identified “a smaller woman on a ladder, the other woman looking out a window.” Her brain MRI showed diffuse atrophy of both the occipitotemporal and occipitoparietal cortex consistent with posterior cortical atrophy, a focal neurodegenerative form of dementia (Figure 7-4).
Comment. This patient had an apperceptive problem that was manifest with slightly more complex three-dimensional drawings, and worse for line drawings of objects (eg, she mistook the chair in the cookie theft picture for a ladder). The patient had some deficits in semantic knowledge as well; these could also have affected her object recognition.
Prosopagnosia
Prosopagnosia is the inability to recognize faces that were either previously known to the patient or recently learned.12 Patients with prosopagnosia learn to recognize people by other cues (Case 7-1). They can recognize some faces by an unusual feature, such as a distinctive nose or mustache. They may also use other visual cues, such as hairstyle (although not entirely reliable because hair is easily changed), or body motion, such as a distinctive gait. Almost all patients report that they rely heavily on recognizing voices. The ability to glean other sorts of information from faces seems to vary among patients. This information includes the ability to perceive emotional expression in faces, the age or gender of the person, and attractiveness.
To diagnose prosopagnosia, the clinician should first confirm, by administering a basic object recognition test, that the patient does not have a general visual agnosia. The Boston Naming Test can be used for this purpose. Next, the examiner should confirm that the patient has preserved knowledge about people. If culturally appropriate, patients can be asked to give the occupations and biographic details of celebrities. Ideally, a voice recognition test could verify the patients’ assertion that they can recognize people by voice, but a standard test for this has not yet been developed. Patients who cannot recognize voices or faces and have reduced semantic knowledge about people have a multimodal person semantic deficit that is usually associated with anterior temporal lesions,13 not just prosopagnosia.
To test face recognition, a clinician can use a famous faces test to probe for long-term recognition.12 Patients are presented with images of celebrities and anonymous people and asked to indicate which faces look familiar. A patient with prosopagnosia has reduced ability to make this distinction. If patients can state which faces are familiar but have trouble providing the names belonging to those faces, the diagnosis is prosopanomia, a much rarer condition.
The problem with tests that use famous faces is that they assume that the patient knows the celebrities, which may not be the case for a recent immigrant or a recluse. The validity of the test can be verified by also presenting the patient with a list of names that includes the celebrities already used in the famous face test, and having the patient indicate which names are famous and which are not.12 An alternate approach is to assess short-term recognition for faces by showing a patient a series of faces, and then, after an interval, presenting these faces again mixed with new faces and asking the patient which ones were seen the first time around. This is the strategy of the Warrington Recognition Memory Test, which has as a useful contrast a second component that involves words rather than faces, and the Cambridge Face Memory Test, which is available online.
Prosopagnosia is caused by lesions of the fusiform gyri or anterior temporal lobe12; these are generally right or bilateral (Figure 7-3 and Figure 7-5). Unilateral left lesions are unusual and tend to occur in left-handed patients. These findings are consistent with functional neuroimaging results showing that the face-processing network is asymmetric, with greater involvement of right hemispheric regions.
Figure 7-5.
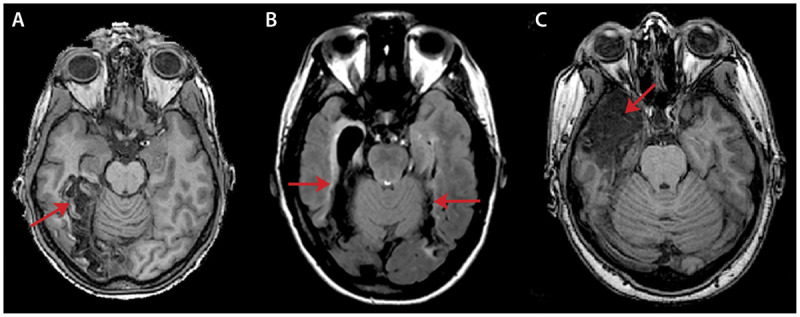
Axial MRIs of lesions in three different patients with prosopagnosia. A, T1-weighted MRI of a 58-year-old man who had a carotid dissection causing a right occipitotemporal infarction (arrow) because of a persistent fetal circulation pattern. B, Fluid-attenuated inversion recovery (FLAIR) MRI of a 20-year-old woman who had herpes encephalitis at age 10 and has bilateral fusiform lesions (arrows). C, T1-weighted MRI of a 30-year-old woman who presented with a seizure and confusion because of herpes encephalitis at age 25, which caused right anterior temporal encephalomalacia (arrow).
As with general visual agnosia, subtypes of prosopagnosia likely exist. An apperceptive variant is associated with fusiform lesions.14 Patients with this variant have trouble perceiving the complex three-dimensional geometry of the face, particularly in the eye region. This can be assessed with tests that require patients to match anonymous faces, particularly across changes in the point of view or lighting, as with the Benton Facial Recognition Test or the Cambridge Face Perception Test. By contrast, patients with the associative/amnestic variant have accurate perception and score well on those tests. They have difficulty matching what they see to what they know, however, because their facial memories have either been destroyed or disconnected from perceptual processing, which appears to be the case in patients with anterior temporal damage. Clinicians can test the status of facial memories with a face imagery test that requires patients to imagine the faces of two celebrities and answer a question about the faces, such as which face is thinner or has the bigger nose.15 Whether anterior temporal lesions cause a recognition impairment that is truly face specific or also affects voices has been questioned.13
Is prosopagnosia really just about faces? Can a lesion cause a perceptual deficit that is so specific that it affects only faces? This debate has been ongoing for many decades. Some researchers claim to have identified patients who appear to be the holy grail of the field: patients with pure prosopagnosia with completely intact recognition of objects other than faces. This would be consistent with the claims of some neuroimaging researchers that regions like the fusiform face area may be modules dedicated only to face processing. Others suggest that prosopagnosia is merely the most dramatic example of the difficulty in seeing the fine and subtle distinctions between different examples of the same type of object. Thus, a patient with prosopagnosia may struggle telling not just one face from another face, but also one car from another car and one dog from another dog.
Settling this debate is not easy. Given the untidy nature of pathologic lesions, the trouble that a patient with prosopagnosia has differentiating various cars can always be attributed to a lesion that may have affected not just face-processing areas but adjacent cortex involved in perceiving other objects. On the other hand, if a patient with prosopagnosia passes a car recognition test, is that enough evidence to prove normal perception of all other object categories? In addition to the issue of the number of various types of objects needed for testing to be confident on this point, the vexing issue of prior patient expertise remains. The skill of seeing fine differences between objects of the same type may depend on experience; differentiating one type of hawk from another by its shape may be trivial for a veteran bird watcher but challenging for a novice. Adjustment for premorbid expertise has rarely been done in studies of object recognition in prosopagnosia. One study that did use a test with two phases first tested subjects on their verbal knowledge about cars; the assessment of their car expertise was used to adjust their scores on the second phase, visual recognition of cars. The results showed that most patients with prosopagnosia recognized fewer cars than would be predicted from their verbal knowledge.16
Patients with prosopagnosia often have other deficits. Those with fusiform lesions often have superior field defects, topographagnosia, and dyschromatopsia if their lesions are bilateral. Those with anterior temporal lesions may have some amnestic defects, although too mild to account for their failure to recognize people.
Prosopagnosia is usually a permanent deficit, although some patients notice some improvement in the first few months if the problem was of sudden onset, as with stroke. However, it rarely resolves completely. Ongoing research efforts use perceptual learning approaches to try to improve face recognition in patients with prosopagnosia.
Pure Alexia (Alexia Without Agraphia)
Like face recognition, reading is a highly developed expert skill in humans. The parallel is also anatomic: functional neuroimaging studies show that reading activates a network very similar to the one activated by face perception. The key difference is that the reading network depends more heavily on the left hemisphere and the face network depends more heavily on the right hemisphere. Just as the right fusiform face area is a dominant component of the face network, the left visual word-form area is a key region for reading. Whereas lesions of the right fusiform gyrus cause prosopagnosia, lesions of the left fusiform gyrus cause alexia.
Alexia is the loss of prior reading skill. Assessment of reading in these patients must take into account their previous reading competency. In pure alexia, reading is the only verbal skill affected. These patients can comprehend speech, converse with others normally, and can even write. Other names for pure alexia include alexia without agraphia and pure word blindness. As with many things, purity is an ideal not often achieved in real life. Some patients with alexia have a mild degree of anomia. Detailed testing of their writing shows that, while the dramatic problems with writing that are seen in alexia with agraphia are absent, most patients with pure alexia have what is known as surface dysgraphia.17 They make spelling mistakes when writing irregular words such as yacht and colonel; these mistakes show that they are guided by sound-to-spelling correspondences rather than knowledge of the word, as if they had lost a mental dictionary in which they could look up words with unusual spelling.
As a complex skill, reading depends on the integrity of a number of other processes. Before concluding that a patient who reports difficulty with reading has pure alexia, the clinician should evaluate for other conditions. Reading may be impaired by ocular disorders that impair central vision in both eyes, such as macular degeneration and cataracts, but these disorders should be evident by reduced acuity and depression of the central visual field on perimetry. Likewise, hemianopia that encroaches upon the central 5 degrees of vision can cause a hemianopic dyslexia, particularly if the right hemifield is affected.18 Impaired attention in patients with simultanagnosia or hemineglect will disrupt reading. Patients with left hemineglect have a characteristic pattern of errors involving the left side of words or the left side of pages, and usually these attentional defects are also apparent for other visual stimuli. Eye movements also play a key role in efficient reading. Unstable fixation because of saccadic intrusions or nystagmus interferes with reading, as do inaccurate saccades caused by cerebellar disease. These ocular motor impairments should be evident on examination.
While pure alexia is generally regarded as a problem of visual processing, some alexic syndromes reflect difficulty with linguistic processing.19 These are most often described in neurodegenerative conditions. Patients with surface alexia have lost a mental lexicon, so they rely on grapheme-to-phoneme rules to read, which works fine if words are regular in spelling or even made up, but not if words are irregular in spelling. Patients with phonologic alexia have the opposite problem. They have their mental dictionary but not the spelling rules. They can read regular or irregular words correctly but are unable to guess how a made-up word they have never seen before would be pronounced, as it does not have a mental dictionary entry. Deep dyslexia is more bizarre, as patients make semantic errors when reading, for example seeing the word piano and reading it aloud as orchestra. At least one patient with alexia with agraphia from a parietal lesion showed this pattern in his reading.20
Pure alexia has a range of severity; an extreme case was Monsieur C, a patient Dejerine described who could not read even single letters. These patients are classified as having letter dyslexia. Other patients have apparently intact letter identification but read slowly. A characteristic feature of these patients is the high correlation between the time it takes to read a word and the number of letters in the word. In healthy individuals, the word-length effect is trivial, around several milliseconds per letter. In patients with hemianopic dyslexia, it does not exceed 160 milliseconds per letter,18 while in alexic patients, it can be several hundred to several thousand milliseconds per letter. Some patients even read aloud each letter of a word before identifying the word; another term for this disorder is letter-by-letter reading. Even this strategy fails some patients; despite having laboriously identified each letter, they still cannot identify the word.
Pure alexia is always associated with left occipitotemporal lesions. Previous notions interpreted alexia as a disconnection syndrome. Since reading is dependent on centers in the left hemisphere, visual input to these centers can be disrupted by a combination of a left occipital lesion causing right hemianopia and a callosal lesion disrupting transfer of information from the intact right occipital cortex. Indeed, some cases exist in which disconnection seems the only plausible explanation of alexia; however, most patients have extensive damage to the occipitotemporal cortex in addition to possible damage to callosal fibers. This often involves the fusiform gyrus, site of the visual word form area. Hence, in many patients a selective visual agnosia is an equally plausible explanation (Case 7-3). In this view pure alexia is functionally conceptualized as a damaged whole-word–processing mechanism, so patients resort to reading each word serially by its component letters, combined with variably damaged processes that encode those single letters, which leads to letter dyslexia in extreme cases and very prolonged word-length effects in the rest.
As with faces, debate continues as to whether the alexic defect is specific for reading. In the past, reports noted that some patients also had problems reading musical notation or map symbols. A new review suggests that reading of numbers is affected in most patients.21 Also, recent studies show subtle perceptual deficits in alexic patients,22 even for faces.23 Again, it remains uncertain whether these deficits are related to the mechanism causing the alexic defect and therefore inextricably linked to it, or merely associated through damage to neighboring cortex.
Alexia is often persistent, but the few longitudinal studies that exist show that after sudden onset, the deficit can improve substantially. In particular, improvements can be seen in the word-length effect. A number of rehabilitative approaches have been tried,24 some emphasizing letter identification, others trying to force whole-word recognition instead of the letter-by-letter strategy. These approaches can improve reading speed, although with variable transfer to words that were not part of the training set and with variable effects on the word-length effect. In general, it appears that training must continue for 6 months or more for any improvement to ensue.
Case 7-3
A 41-year-old right-handed man awoke with numbness of the right side of his face and right leg and trouble speaking, all of which recovered over a few days. He had trouble finding the names of objects, but this ability also slowly recovered over months. He was left with a right hemianopia and trouble reading. He read letter by letter and did better with words related to his work and with vertically oriented text. He tried a number of strategies, including scrolling text and tracing letters, without much success. He could identify individual letters and easily distinguish them from other objects, and he could write well. He recognized familiar faces but had trouble retrieving the names of people. He also had difficulty recognizing his work tools until they were placed in his hand.
Visual field testing confirmed a complete right hemianopia. He identified single letters quickly. Assessment of his reading of words showed good accuracy, but he was quite slow, taking on average 6.5 seconds to read a word. Testing of his word-length effect showed that he took an extra 1.5 seconds for every additional letter in a word. MRI showed a small left occipital lesion involving the region of the left visual word-form area (Figure 7-6).
Figure 7-6.
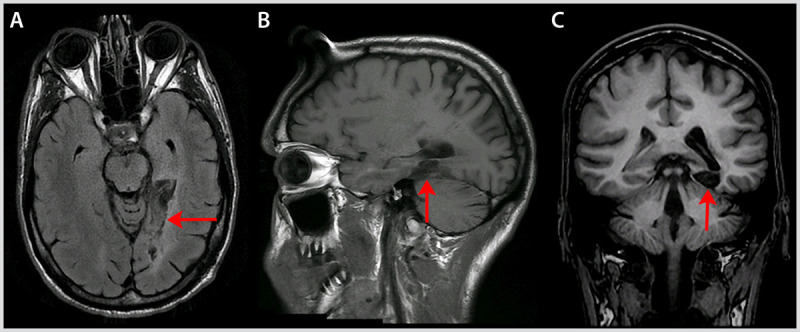
Axial fluid-attenuated inversion recovery (FLAIR) (A), sagittal T1-weighted (B), and coronal T1-weighted (C) MRI of the patient with pure alexia in Case 7-3 showing the stroke in his left fusiform gyrus (arrows).
Comment. This man had pure alexia and right hemianopia. A right hemianopia alone can account for patients taking up to 160 milliseconds more for each additional letter in a word, but this patient’s word-length effect was 10 times that. His disorder was on the milder end of alexia since his ability to read single letters appeared to be preserved. Interestingly, he also had a subtle perceptual problem with other objects, namely his tools. This was consistent with the arguments advanced by some that alexia is not completely specific to words.
Topographagnosia
Patients with topographagnosia get lost in familiar surroundings. As with all complex tasks, multiple cognitive skills are involved in orientation and route finding. Furthermore, navigation problems may be solved in a number of ways. Thus, it is not surprising that topographic disorientation encompasses a family of disorders with different origins.25
The most flexible and useful means of navigation is the formation of a mental image of the environs, a cognitive map of the relationships of pathways, buildings, and other landmarks to each other. This may be derailed if the ability to recognize landmarks is impaired. Landmark agnosia has been associated with medial occipitotemporal lesions, particularly on the right, and may account for many cases of topographagnosia that accompany prosopagnosia (Case 7-1). Functional imaging shows that images of buildings and places activate a region just medial to the fusiform face area called the parahippocampal place area. The difficulty with recognizing landmarks following lesions in this region can be considered another type of selective visual object agnosia. As with faces, this can be tested by seeing if patients can recognize images of famous places or, more informally (although perhaps better), with images from their own town.
On the other hand, it is possible for patients to recognize landmarks but have trouble encoding the spatial relationships between them in the cognitive map. This can now be tested with computer simulations, some of which are available online (www.gettinglost.ca). Cognitive map formation and its use are associated with activity in the hippocampi and retrosplenial cortex. A deficit in forming cognitive maps may account for some cases of developmental topographic disorientation, a recently described congenital form of selective agnosia.26
One can also navigate without a mental map by using a sequence of moves, much like following verbal directions after stopping at a gas station (eg, go three blocks and turn right, then left again after one block). Such directions are not as useful as a cognitive map because they are not helpful if one deviates from the prescribed path. This type of navigation requires a sense of how one is oriented spatially relative to the environment; loss of this ability is called egocentric disorientation.25
It is not known whether it is possible to improve the navigational deficits of patients with topographagnosia through training. Fortunately, smartphones with global positioning system capability provide these patients with an easy, available substitute strategy.
SYNDROMES OF THE DORSAL STREAM
Akinetopsia
Motion perception was one of the first selective visual functions localized to a specific cortical area, namely the middle temporal area, or visual cortical area 5 (V5). Not long after, there was a report of a patient who had impaired motion perception and bilateral lesions of the lateral occipitotemporal cortex, which presumably destroyed the human homologues of V5.27 For this patient, water poured from a pitcher seemed to be frozen and objects appeared to jump rather than move; the patient also had trouble judging the approach of oncoming cars. A second patient with similar symptoms and similar lesions was later found. No other patients have since surfaced, however, underscoring the extreme rarity of this condition in its full-blown bilateral variant. Patients with unilateral lesions have been described28 but are usually asymptomatic, requiring specialized computerized motion tests to demonstrate their impairments, which are localized to the contralateral hemifield.
Balint Syndrome
The classic formulation of Balint syndrome is the triad of simultanagnosia, optic ataxia, and ocular motor apraxia caused by bilateral occipitoparietal lesions. However, these components are only loosely associated. Each can be found in patients without the others, indicating that they result from damage to independent processing networks that have in common a spatial analysis of vision. The fact that these deficits can occur together indicates that their networks are in proximity. The traditional definition should be expanded to include other notable consequences of dorsal lesions, such as astereognosis and smooth pursuit deficits.
Diagnosing Balint syndrome requires first and foremost careful perimetry to establish that the patient’s loss of vision is not responsible for the lack of awareness of objects, misreaching, or failure to make accurate saccades to objects. This is not always easy; it requires a setting free of distraction and an examiner with patience. A patient with only a keyhole of central vision remaining after bilateral striate lesions can behave very much like a patient with Balint syndrome. The impact of disturbances of attention, spatial localization, and action guidance can be extremely disabling (Case 7-4), as is also described in an account of the life of two patients with Balint syndrome.29
Case 7-4
A 49-year-old woman was referred because of visual difficulties. Three months earlier she had experienced simultaneous bilateral strokes (Figure 7-7) due to primary cerebral angiitis. Visual field testing during her hospitalization showed a left inferior quadrantanopia. After discharge from the hospital she had trouble seeing multiple objects in photographs on pages of books, and both arms were initially very clumsy in reaching for items in the right or left hemispace. The clumsiness of her right arm improved within several weeks, but she still felt her left arm was not functioning quite right when she tried to use utensils. She had so much trouble stumbling into things while walking that she had to use a wheelchair.
On examination, her visual fields were normal. She had trouble relating the items of the cookie theft picture to one another. In line drawings she could identify a key, a glove, a chair, and a cactus. When shown a picture of a baby face, she reported seeing only the eyes. She was able to recognize famous faces. She read short sentences of small words well, but with longer sentences had trouble maintaining the words in their correct sequence. She read the word phantom correctly, but when asked to read its letters, she omitted the a and the n. When shown Navon letters (Figure 7-8) she often did not see the global letter, although she always reported the small local letters. When shown Arcimboldo pictures, she knew that the images depicted faces but did not realize that the faces were made up of other objects, such as fruits or vegetables. Her reaching for objects was erratic, and she did not align her grasp to the orientation of the object.
Figure 7-7.
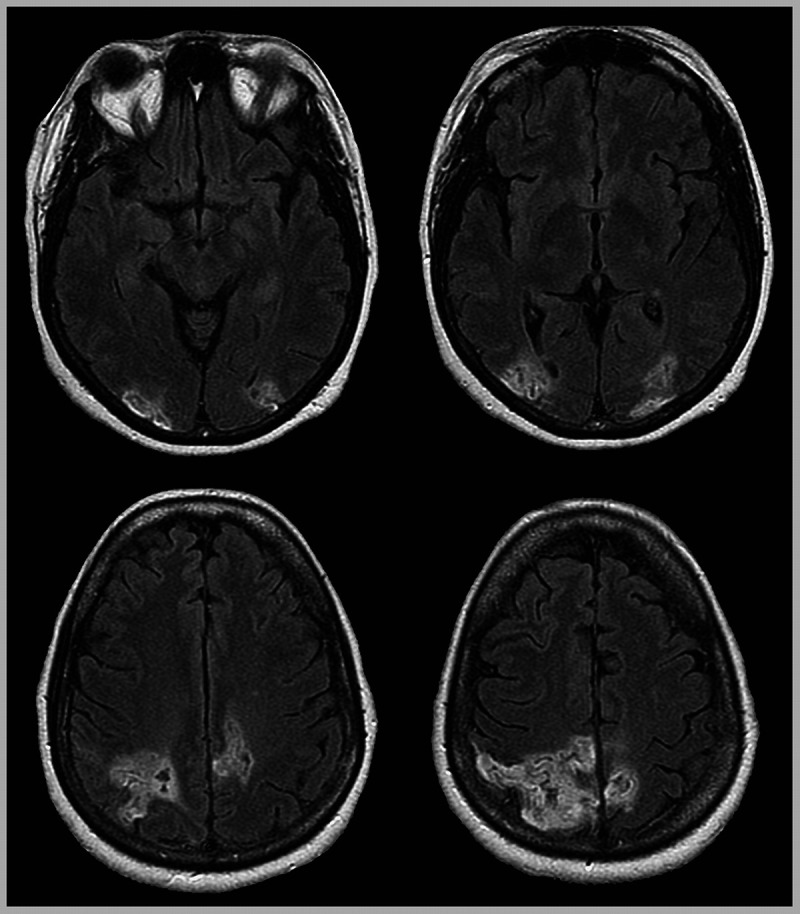
Axial fluid-attenuated inversion recovery (FLAIR) MRI of the patient with Balint syndrome in Case 7-4 showing bilateral lesions in lateral occipital and parietal cortex.
Figure 7-8.
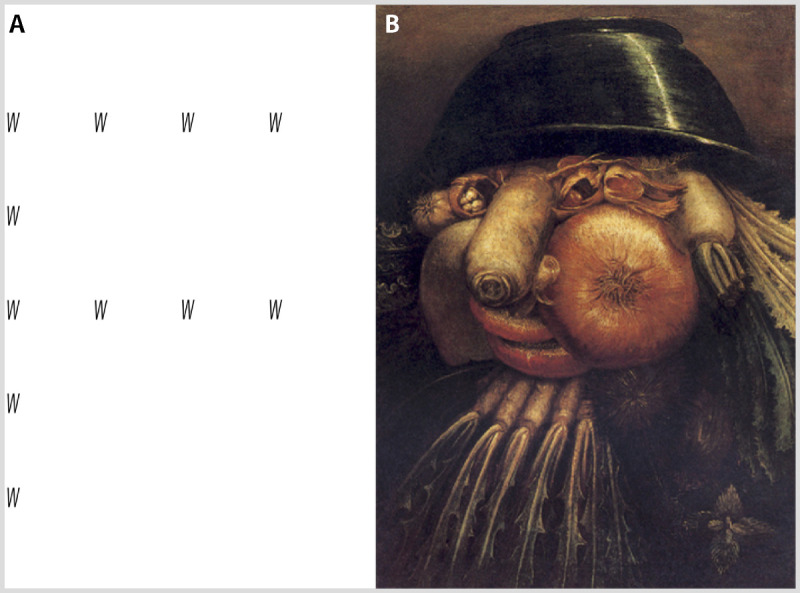
Examples of stimuli with global and local elements. A, a Navon letter with sparsely distributed Ws that at the global level form an F. A patient with Balint syndrome sees only the Ws. B, an Arcimboldo painting of a face composed of vegetables. The patient with Balint syndrome reports the face but not the vegetables.
Comment. This patient displayed classic problems with reaching to visual objects, seeing multiple objects simultaneously and relating those objects to each other correctly in space, as demonstrated in her reading. She also showed both local and global capture.
Simultanagnosia
Simultanagnosia is a deficit of spatial attention.30 Primarily it is a limitation in capacity; it is classically defined as a failure of the ability to pay attention to more than one object at a time. When a display contains two items, the patient with simultanagnosia may be aware of only one, even if both are located in the same place. If the examiner holds up a pen placed on top of a finger, the patient may be aware of the pen but not the finger. The lack of awareness in this condition can be as disabling as blindness: patients bump into things when walking, struggle with reading, and certainly should not drive. Their limitation makes searching for objects difficult. Patients also have trouble sustaining attention over large displays with many items. A classic test is the starry night display, where small bright dots appear or disappear in random locations; patients with simultanagnosia struggle to detect these occurrences.31
The reduction in attentional capacity of patients with simultanagnosia is accompanied by a lack of flexibility in shifting attention. This is reflected in the phenomena of local and global capture. Some stimuli have both local and global structures. A typical example is the Navon letter, in which small local letters are grouped so that they make up a large global letter (Figure 7-8). When the global form is weak because the local letters are few and far between, patients with simultanagnosia report seeing the small local letters only, which is an example of local capture.32 When the global form is strong, as with the faces that are composed of vegetables or other items in Arcimboldo pictures (Figure 7-8), patients may report seeing only the faces, but not the vegetables, which is an example of global capture.32 Healthy individuals have no difficulty moving between the local and global frames to see the forms present in both, but patients with simultanagnosia tend to have difficulty at one or the other level.
To diagnose simultanagnosia, the clinician simply needs displays that have multiple elements and to ask patients to report what they see. The cookie theft picture from the Boston Diagnostic Aphasia Examination is commonly employed (but probably could use updating). Simultanagnosia is linked to lesions of the medial occipitoparietal junction, cuneus, and intraparietal sulcus, as well as a number of white matter tracts linking a visual attention network.33
Optic Ataxia
Optic ataxia refers to inaccurate reaching to targets under visual guidance. Unlike patients with cerebellar ataxia, patients with optic ataxia should be able to reach to targets whose spatial coordinates are derived from other senses, eg, reaching parts of their own bodies. This is not always the case, however; in some patients reaching can be inaccurate to somatosensory targets such as parts of their own body. This is not surprising given that the parietal cortex contains regions that perform multimodal spatial computations. Optic ataxia is likely the result of degraded analyses of the position of objects in space. Patients with Balint syndrome therefore also have trouble judging how far apart objects are from each other. Functional neuroimaging provides evidence that these computations involve the inferior parietal lobule. Misreaching with either limb results from bilateral lesions, but a unilateral lesion may cause misreaching with only the contralateral arm or only for visual targets in the contralateral hemifield. Tests for optic ataxia should therefore assess accurate pointing to objects to the right or left of center and with the right and the left hand. In particular, reaching may be more impaired for targets seen in the peripheral rather than the central visual field.34 Having the patient reach without looking directly at the object may be a more sensitive, although less natural, test for optic ataxia. The deficit is also more specific to rapid reaching for objects and may not be apparent if the patient is allowed to delay reaching for a few seconds.
Other spatial coding that may be defective includes the assessment of the orientation of objects in space and in relation to oneself. If asked to grasp an object with a dominant long axis, such as a pen, patients with optic ataxia may fail to orient their grasp to match the axis of the object.
Poor spatial coding may not be the only localization problem in these patients. One study showed that localizing events in time can also be affected. For example, a patient was shown a square, a triangle, and another triangle in sequence and could not tell whether the square had been the first, second, or third in the sequence, even though the patient could report how many squares and triangles had been shown. It was proposed that the patient had trouble assigning the correct temporal “location” or epoch to each stimulus, which was called sequence agnosia.35
The occipitoparietal lesions that cause optic ataxia likely involve the junction between the inferior parietal lobule and the superior occipital cortex.36 Similar deficits have been reported with lesions of the premotor cortex and occipital-frontal white matter connections, however.
Acquired Ocular Motor Apraxia
Bilateral lesions can also cause a defect in the initiation and guidance of saccades to visual targets. When severely affected with acquired ocular motor apraxia, patients may have difficulty generating any saccades to a visual stimulus on command, although on occasion saccades can be facilitated by a blink; this difficulty is sometimes called psychic paralysis of gaze. A target that suddenly and unexpectedly appears may still trigger a reflexive saccade. If the defect is less severe, the patient may merely take longer than expected to make a saccade to a target. This delay can be accentuated if the object at which the patient was initially looking when the target appeared remains visible. Some refer to this as spasm of fixation.37 In the mildest of cases, prolonged saccadic latencies may be apparent only with formal ocular motor recordings.
In addition to problems with initiation, the saccades of patients with acquired ocular motor apraxia may be inaccurate, reflecting, as with optic ataxia, a deficit in obtaining the correct coordinates of visual objects in space. In severe cases, the eyes may appear to wander until they stumble upon the object; even then they may fail to stay with the object, demonstrating an impersistence of gaze. As a result, these patients have chaotic eye movements when searching for objects.37
Ocular motor apraxia is associated with bilateral lesions of the parietal eye fields, the frontal eye fields, or both.38 A severe inability to make any saccades can improve with time, particularly if the lesions do not involve both the frontal and the parietal regions. Unilateral parietal lesions are unlikely to cause symptomatic deficits, but ocular motor recordings can show similar deficits in a patient’s planning and guidance of saccades in the direction contralateral to the lesion.
Although saccades dominate the discussion about ocular motor apraxia, smooth pursuit is often also defective in these patients because cortical areas involved in generating smooth pursuit are adjacent to the parietal and frontal eye fields. These regions occupy the medial superior temporal sulcus and the fundus of the arcuate sulcus in monkeys. Unilateral lesions of these structures in humans can lead to reduced pursuit of targets moving toward the side of the lesion, and sometimes even excessive pursuit (ie, the eye moving faster than the target) for targets moving in the other direction. These pursuit defects are asymptomatic and detected only by close observation or ocular motor recordings.
Astereognosis
Perception of the position of objects in depth is another type of spatial computation. The most well-known process that is involved in depth perception is stereopsis. Two objects located at different distances from the observer have a different relationship to each other in the retinal image of the right versus the left eye. The power of this depth cue can be appreciated by trying to thread a needle with one eye closed and then with both eyes open. Loss of stereopsis can occur with bilateral occipitoparietal lesions,39 and can be demonstrated with common tests of stereovision used in eye clinics, such as the Titmus stereo fly test or Randot Stereo Test, which require the patient to wear polarized glasses and present slightly different images to each eye.
Stereopsis is not the only cue to depth relationships, to which any one-eyed person or painter can attest. Relative size and saturation are strong pictorial cues to distance; moving one’s head can give depth cues from optic flow patterns and motion parallax. It is not known whether the cerebral lesions that cause astereopsis also affect the ability of a patient to derive depth from these monocular cues.
CONCLUSION
Deficits of cortical visual function are unusual but varied and intriguing. They are important because they offer opportunities to study how the brain makes sense of the visual world. This review provides a framework for understanding and categorizing these different types of deficit, as well as for understanding and utilizing the diagnostic measures and insights gained in recent years.
USEFUL WEBSITES
For Prosopagnosia
The Cambridge Face Memory Test and Cambridge Face Perception Tests www.faceblind.org/facetests/
The test of expertise-adjusted car recognition http://www.ubcneuroophthalmology.ca/JBarton/Frperception.html
For Topographagnosia
Tests of landmark recognition and cognitive map formation gettinglost.ca/content.php?159-Take-The-Online-Tests
For Hemianopic Dyslexia
A therapy using scrolling text to promote larger rightward saccades www.readright.ucl.ac.uk/
For Simultanagnosia
The cookie theft picture
www.ling.ohio-state.edu/~hana/x201m/other/11-cookieTheft.jpg
Giuseppe Arcimboldo images
KEY POINTS
Cortical visual syndromes can be grouped into problems with various types of object recognition with ventral occipitotemporal lesions, and problems with spatial analysis and attention with dorsal occipitoparietal lesions.
Cerebral dyschromatopsia is best diagnosed by tests that require patients to sort colored chips by either their hue or saturation, rather than by having patients name colors.
A taxonomy of general visual object agnosia is divided into apperceptive and associative variants, each with further subtypes.
To diagnose prosopagnosia, the examiner must show that a patient does not recognize faces either long known or recently seen as familiar. The patient should be familiar with or even name the person through other visual or nonvisual cues.
When tested for recognition of objects such as cars, patients with prosopagnosia tend to have difficulty if their performance is adjusted for their level of premorbid expertise.
Pure alexia may not be entirely without a degree of agraphia. Patients with pure alexia tend to make errors when writing irregularly spelled words, indicating a surface dysgraphia.
Pure alexia is characterized by a marked word-length effect, with reading time determined by the number of letters in a word: 160 milliseconds per letter is the diagnostic criterion separating pure alexia from hemianopic dyslexia.
Getting lost can be the result of a number of different problems, including the inability to recognize landmarks, failure to form a cognitive map with these landmarks, or poor orienting of oneself to the environment.
Attention in simultanagnosia is characterized both by reduced capacity, causing failure to detect multiple stimuli, and lack of flexibility, causing difficulty seeing both the trees and the forest simultaneously.
Balint syndrome may include problems not only localizing objects in space, but also localizing them in time.
The ocular motor deficits with occipitoparietal lesions include difficulty with initiating saccades, accurate targeting of saccades, and accurate matching of eye to target speed during smooth pursuit.
Footnotes
Relationship Disclosure: Dr Barton receives personal compensation for serving on the advisory board of Vycor Medical, Inc, and for speaking at the LAUNCH review course for residents provided by EMD Serono, Inc. Dr Barton has also provided expert review for several law firms and receives book royalties from Springer and UpToDate. Dr Barton’s laboratory is supported by grants from the Canadian Institutes of Health Research and Natural Sciences and Engineering Research Council of Canada.
Unlabeled Use of Products/Investigational Use Disclosure: Dr Barton reports no disclosure.
REFERENCES
- 1.Ungerleider L,, Mishkin M. Two cortical visual systems. In: Ingle DJ,, Mansfield RJW,, Goodale MS, eds. The analysis of visual behaviour. Cambridge, MA: MIT Press, 1982: 549–586. [Google Scholar]
- 2.Milner A,, Goodale M. The visual brain in action. Oxford, UK: Oxford University Press, 1995. [Google Scholar]
- 3.Susilo T,, Duchaine B. Advances in developmental prosopagnosia research. Curr Opin Neurobiol 2013; 23 (3): 423–429. [DOI] [PubMed] [Google Scholar]
- 4.Iaria G,, Barton JJ. Developmental topographical disorientation: a newly discovered cognitive disorder. Exp Brain Res 2010; 206 (2): 189–196. [DOI] [PubMed] [Google Scholar]
- 5.Zeki SM. A century of cerebral achromatopsia. Brain 1990; 113 (pt 6): 1721–1777. [DOI] [PubMed] [Google Scholar]
- 6.Beauchamp M,, Haxby J,, Rosen A,, DeYoe E. A functional MRI case study of acquired cerebral dyschromatopsia. Neuropsychologia 2000; 38 (8): 1170–1709. [DOI] [PubMed] [Google Scholar]
- 7.Kentridge RW,, Heywood CA,, Cowey A. Chromatic edges, surfaces and constancies in cerebral achromatopsia. Neuropsychologia 2004; 42 (6): 821–830. [DOI] [PubMed] [Google Scholar]
- 8.Farah MJ. Visual agnosia. 2 ed. Cambridge, UK: MIT Press, 2004. [Google Scholar]
- 9.Humphreys GW,, Riddoch MJ,, Donnelly N, et al. Intermediate visual processing and visual agnosia. In: Farah M,, Ratcliff G, eds. The neuropsychology of high-level vision. Hillsdale, NJ: Lawrence Erlbaum Associates, 1994: 63–101. [Google Scholar]
- 10.Riddoch M,, Humphreys G. A case of integrative visual agnosia. Brain 1987; 110 (pt 6): 1431–1462. [DOI] [PubMed] [Google Scholar]
- 11.Feinberg TE,, Schindler RJ,, Ochoa E, et al. Associative visual agnosia and alexia without prosopagnosia. Cortex 1994; 30 (3): 395–412. [DOI] [PubMed] [Google Scholar]
- 12.Barton JJ. Structure and function in acquired prosospagnosia: lessons from a series of ten patients with brain damage. J Neuropsych 2008; 2 (pt 1): 197–225. [DOI] [PubMed] [Google Scholar]
- 13.Gainotti G,, Marra C. Differential contribution of right and left temporo-occipital and anterior temporal lesions to face recognition disorders. Front Hum Neurosci 2011; 5: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barton JJ,, Press DZ,, Keenan JP,, O’Connor M. Lesions of the fusiform face area impair perception of facial configuration in prosopagnosia. Neurology 2002; 58 (1): 71–78. [DOI] [PubMed] [Google Scholar]
- 15.Barton JJ,, Cherkasova M. Face imagery and its relation to perception and covert recognition in prosopagnosia. Neurology 2003; 61 (2): 220–225. [DOI] [PubMed] [Google Scholar]
- 16.Barton JJ,, Hanif H,, Ashraf S. Relating visual to verbal semantic knowledge: the evaluation of object recognition in prosopagnosia. Brain 2009; 132 (pt 12): 3456–3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rapcsak SZ,, Beeson PM. The role of left posterior inferior temporal cortex in spelling. Neurology 2004; 62 (12): 2221–2229. [DOI] [PubMed] [Google Scholar]
- 18.Sheldon CA,, Abegg M,, Sekunova A,, Barton JJ. The word-length effect in acquired alexia, and real and virtual hemianopia. Neuropsychologia 2012; 50 (5): 841–851. [DOI] [PubMed] [Google Scholar]
- 19.Black S,, Behrmann M. Localization in alexia. In: Kertesz A,, Cermark LS, eds. Localization and neuroimaging in neuropsychology. San Diego, CA: Academic Press, 1994: 331–376. [Google Scholar]
- 20.Sheldon CA,, Malcolm GL,, Barton JJ. Alexia with and without agraphia: an assessment of two classical syndromes. Can J Neurol Sci 2008; 35 (5): 616–624. [DOI] [PubMed] [Google Scholar]
- 21.Starrfelt R,, Behrmann M. Number reading in pure alexia: a review. Neuropsychologia 2011; 49 (9): 2283–2298. [DOI] [PubMed] [Google Scholar]
- 22.Starrfelt R,, Habekost T,, Gerlach C. Visual processing in pure alexia: a case study. Cortex 2010; 46 (2): 242–255. [DOI] [PubMed] [Google Scholar]
- 23.Behrmann M,, Plaut DC. Bilateral hemispheric processing of words and faces: evidence from word impairments in prosopagnosia and face impairments in pure alexia. Cereb Cortex 2014; 24 (4): 1102–1118. [DOI] [PubMed] [Google Scholar]
- 24.Starrfelt R,, Olafsdottir RR,, Arendt IM. Rehabilitation of pure alexia: a review. Neuropsychol Rehabil 2013; 23 (5): 755–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aguirre G,, D’Esposito M. Topographical disorientation: a synthesis and taxonomy. Brain 1999; 122: 1613–1628. [DOI] [PubMed] [Google Scholar]
- 26.Iaria G,, Bogod N,, Fox CJ,, Barton JJ. Developmental topographical disorientation: case one. Neuropsychologia 2009; 47 (1): 30–40. [DOI] [PubMed] [Google Scholar]
- 27.Zihl J,, von Cramon D,, Mai N. Selective disturbance of movement vision after bilateral brain damage. Brain 1983; 106 (pt 2): 313–340. [DOI] [PubMed] [Google Scholar]
- 28.Barton J,, Sharpe J,, Raymond J. Retinotopic and directional defects in motion discrimination in humans with cerebral lesions. Ann Neurol 1995; 37 (5): 665–675. [DOI] [PubMed] [Google Scholar]
- 29.Cuomo J,, Flaster M,, Biller J. Right brain: a descriptive account of two patients’ experience with and adaptations to Bálint syndrome. Neurology 2012; 79 (11): e95–e96. [DOI] [PubMed] [Google Scholar]
- 30.Dalrymple KA,, Barton JJ,, Kingstone A. A world unglued: simultanagnosia as a spatial restriction of attention. Front Hum Neurosci 2013; 7: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rizzo M,, Robin DA. Simultanagnosia: a defect of sustained attention yields insights on visual information processing. Neurology 1990; 40 (3 pt 1): 447–455. [DOI] [PubMed] [Google Scholar]
- 32.Dalrymple KA,, Kingstone A,, Barton JJ. Seeing trees OR seeing forests in simultanagnosia: attentional capture can be local or global. Neuropsychologia 2007; 45 (4): 871–875. [DOI] [PubMed] [Google Scholar]
- 33.Chechlacz M,, Rotshtein P,, Hansen PC,, Riddoch JM,, Deb S,, Humphreys GW. The neural underpinings of simultanagnosia: disconnecting the visuospatial attention network. J Cogn Neurosci 2012; 24 (3): 718–835. [DOI] [PubMed] [Google Scholar]
- 34.Himmelbach M,, Karnath HO,, Perenin MT,, Franz VH,, Stockmeier K. A general deficit of the ’automatic pilot’ with posterior parietal cortex lesions? Neuropsychologia 2006; 44 (13): 2749–2756. [DOI] [PubMed] [Google Scholar]
- 35.Malcolm GL,, Barton JJ. “Sequence Agnosia” in Bálint syndrome: defects in visuotemporal processing after bilateral parietal damage. J Cogn Neurosci 2007; 19 (1): 102–108. [DOI] [PubMed] [Google Scholar]
- 36.Karnath HO,, Perenin MT. Cortical control of visually guided reaching: evidence from patients with optic ataxia. Cereb Cortex 2005; 15 (10): 1561–1569. [DOI] [PubMed] [Google Scholar]
- 37.Nyffeler T,, Pflugshaupt T,, Hofer H, et al. Oculomotor behaviour in simultanagnosia: a longitudinal case study. Neuropsychologia 2005; 43 (11): 1591–1597. [DOI] [PubMed] [Google Scholar]
- 38.Pierrot-Deseilligny C,, Gray F,, Brunet P. Infarcts of both inferior parietal lobules with impairment of visually guided eye movements, peripheral inattention and optic ataxia. Brain 1986; 109 (pt 1): 81–97. [DOI] [PubMed] [Google Scholar]
- 39.Rizzo M,, Damasio H. Impairment of stereopsis with focal brain lesions. Ann Neurol 1985; 18: 147. [Google Scholar]


