Abstract
Purpose of Review:
Optimal treatment of women with epilepsy includes consideration of the complex interactions of sex steroid hormones with epilepsy and antiepileptic drugs, and of the potential risks of any antiepileptic drug prescribed during a pregnancy.
Recent Findings:
Clinical studies in women with epilepsy have provided a better foundation of knowledge about the complex relationships between cycling sex steroid hormones, seizure frequency, antiepileptic drugs, contraception, and neuroendocrine abnormalities. Pregnancy registries and observational studies have provided key data that allow for a better estimation of risks to the developing fetus.
Summary:
Understanding these key factors should enable informed treatment recommendations that can reduce adverse health effects in women with epilepsy and improve both seizure control and maternal and fetal outcomes.
INTRODUCTION
Care for women with epilepsy presents several specific challenges toward antiepileptic drug (AED) selection and prescription in light of drug risks during any potential pregnancy, planned or unplanned, and the complex interactions between the AEDs and female sex steroid hormones.
TRIDIRECTIONAL INTERACTIONS BETWEEN EPILEPSY, ANTIEPILEPSY DRUGS, AND SEX STEROID HORMONES
Multidirectional interactions exist between epilepsy and seizures, female sex steroid hormones, and AEDs (Figure 7-1). Female sex steroid hormones and their metabolites act as direct neurosteroids, modulating brain excitability via both short-latency neuronal membrane-mediated effects and long-latency genomic effects.1 Animal studies have consistently suggested that estrogen promotes neuroexcitatory properties. Progesterone instead promotes neuroinhibitory properties, primarily through its metabolite allopregnanolone. Therefore, changes in endogenous and exogenous female sex steroid hormones levels can influence seizure control directly. Changes in estradiol levels can also increase the clearance of some AEDs, and potentially worsen seizure control indirectly through lowering of AED serum concentrations. Additionally, both AEDs and epilepsy itself can adversely affect function of the hypothalamic-pituitary-ovarian axis (Figure 7-21).
Figure 7-1.
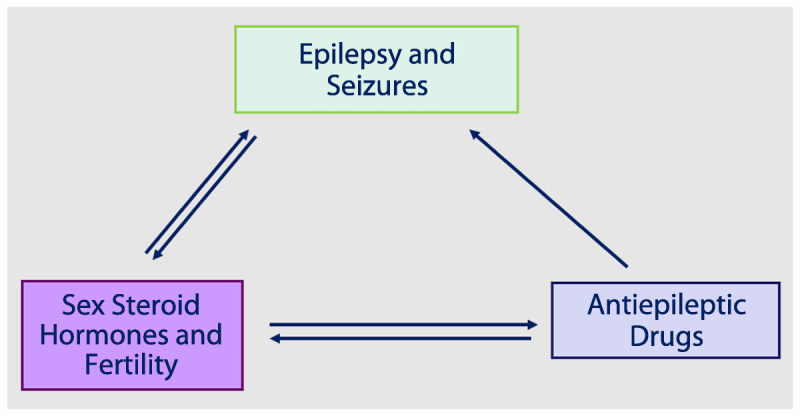
Multidirectional interactions of epilepsy and seizures, female sex steroid hormones and fertility, and antiepileptic drugs and concentrations.
Figure 7-2.
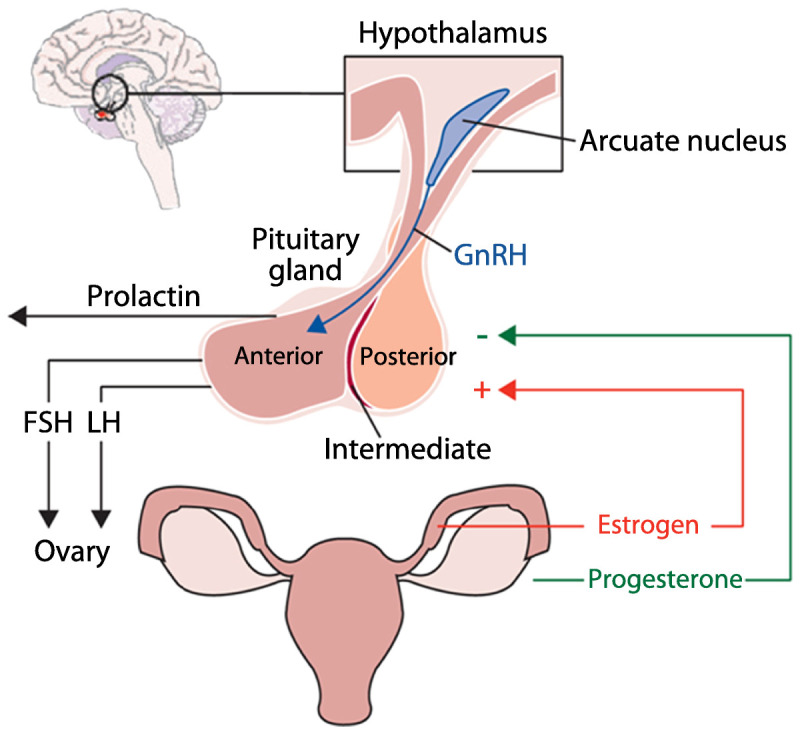
Hypothalamic-pituitary-ovarian axis. 2.GnRH = gonadotropin-releasing hormone; FSH = follicle-stimulating hormone; LH = luteinizing hormone. Adapted from Harden CL, Pennell PB, Lancet Neurol.1 © 2013, with permission from Elsevier. www.sciencedirect.com/science/article/pii/S1474442212702399.
CATAMENIAL EPILEPSY
The term “catamenial epilepsy” denotes that seizure frequency increases during certain phases of the menstrual cycle. A catamenial pattern can occur with any seizure type or epilepsy syndrome, but it has been most commonly reported and studied in focal epilepsies.
The average menstrual cycle is 28 days, with day 1 being the first day of menses and ovulation occurring on day 14 (also designated as day -14 to adjust for cycle lengths other than 28 days). The menstrual cycle has two major phases: the follicular phase (days 1 through 14) and the luteal phase (days -14 to -1). Figure 7-31 displays the fluctuating levels of estradiol and progesterone in normal menstrual cycling, as well as in anovulatory menstrual cycling with an inadequate luteal phase. Catamenial seizures most commonly worsen when levels of estrogen are relatively higher than progesterone or when levels are rapidly changing.
Figure 7-3.
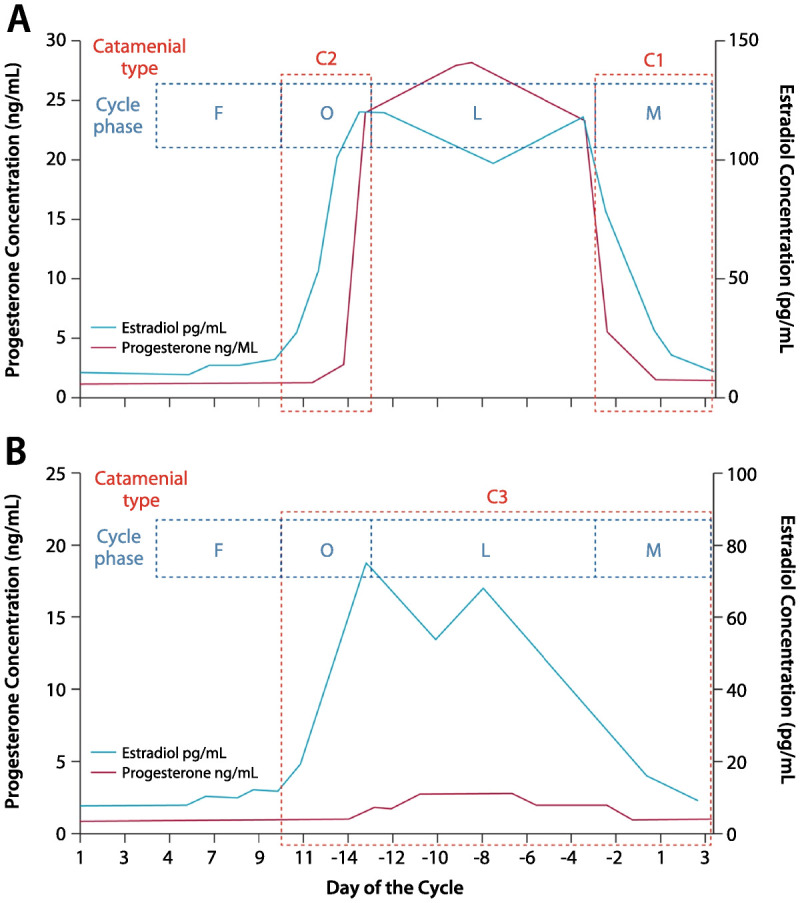
Patterns of catamenial epilepsy. Day 1 is the first day of menstrual flow and day −14 is the day of ovulation. A, Normal cycle with normal ovulation. C1 pattern is associated with exacerbation of seizures in the perimenstrual phase (day −3 to day +3 of next cycle), and C2 pattern is associated with exacerbation of seizures in the periovulatory phase (day +10 to day −13). B, Inadequate luteal phase cycle with anovulation. The C3 pattern is associated with exacerbations during the entire inadequate luteal phase (day +10 to day +3 of the next cycle). C = catamenial seizure pattern; F = follicular phase; O = periovulatory phase; L = luteal phase; M = perimenstrual phase.Reprinted from Harden CL, Pennell PB, Lancet Neurol.1 © 2013, with permission from Elsevier. www.sciencedirect.com/science/article/pii/S1474442212702399.
Criteria for Catamenial Epilepsy
For women with epilepsy, prevalence estimates for catamenial epilepsy vary between 12.5% and 75%, but using the most accepted definition of a consistent doubling of seizure frequency during at least one of the three specific portions of the menstrual cycle (Figure 7-31), approximately one-third of women with focal epilepsy may be classifiedas having catamenial seizures.2
Treatment Options
Most reported treatments for catamenial epilepsy have been in women with a C1 pattern. A C1 catamenial pattern is associated with exacerbation of seizures in the perimenstrual phase (day -3 to day +3 of next cycle). Case reports or small series suggest that acetazolamide or clobazam given during the menstrual phase of seizure worsening may be helpful.1 Medroxyprogesterone acetate, a synthetic progestin-only contraceptive agent, can also reduce seizure frequency3 but may be associated with side effects of weight gain, bone density loss, and delayed return of fertility.
The only double-blind, placebo-controlled, randomized trial for catamenial epilepsy involved adjunctive progesterone lozenges during menstrual days 14 to 28. Although the primary outcomes were not significant, benefit was demonstrated for a subgroup of women who had a threefold or greater seizure frequency increase during the C1 phase (days -3 to +3).4
PERIMENOPAUSE AND MENOPAUSE
Women with epilepsy may be at risk for early onset of menopause. In one study, 14% of women with epilepsy had premature ovarian failure compared with 4% of healthy control women, and the age of menopause and seizure frequency have been negatively correlated.5,6
Small studies suggest that women with epilepsy may experience increased seizures during the perimenopausal transition and decreased seizures after menopause is complete, especially if they had a catamenial pattern during earlier reproductive years.7 During perimenopause, estrogen levels can rise steadily, remain stable, or become erratic with surges until menopause is complete, while cyclic luteal phase progesterone elevation gradually decreases during anovulatory cycles.
Hormone replacement therapy should be used with caution in women with epilepsy. Seizure frequency dose-dependently increased during a 3-month controlled trial of a combination estrogen-medroxyprogesterone hormone-replacement therapy regimen.8
REPRODUCTIVE FUNCTION
Increased rates of polycystic ovarian syndrome, decreased libido, infertility, and early menopause have been described in women with epilepsy. The effects of epilepsy, seizures, underlying brain abnormalities, and interictal epileptiform discharges are difficult to distinguish from AED effects on female sex steroid hormones. Hypothalamic hypogonadism has been described in both women and men with epilepsy.
Gonadotropin-releasing hormone (GnRH) is produced by a small number of cells located in the preoptic area of the hypothalamus and controls ovarian activity via pulsatile secretion and stimulation of pituitary hormone release (follicle-stimulating hormone [FSH] and luteinizing hormone [LH]). This GnRH cell population can be injured by seizures in a rodent model.1 Human studies demonstrated that temporal lobe epilepsy lateralization may be associated with different patterns of reproductive dysfunction. In an investigation of unilateral temporal lobe epilepsy, left temporal lobe discharges were associated with polycystic ovary syndrome, and right-sided discharges with hypothalamic hypogonadism.9
Polycystic ovarian syndrome is a major cause of infertility and appears more frequently in women with epilepsy. Criteria for diagnosis include the presence of hyperandrogenism and ovarian dysfunction (oligo-anovulation, polycystic ovaries, or both).10 Polycystic ovarian syndrome has been more frequently reported in association with genetic (idiopathic) generalized than focal epilepsy syndromes, although more women with generalized epilepsy received valproate treatment. In one study, many metabolic abnormalities of polycystic ovarian syndrome were reduced by discontinuing valproate.11 A randomized prospective study of women with epilepsy reported that polycystic ovarian syndrome features developed more frequently during valproate than lamotrigine treatment, especially in women with epilepsy who began treatment before 26 years of age.12
Effects of Antiepileptic Drugs on Reproductive Hormones
In general, hepatic enzyme-inducing AEDs directly alter female sex steroid hormone levels (Table 7-1) and induce production of sex hormone–binding globulin, thereby reducing both endogenous biologically active (free) reproductive hormone serum levels and exogenous contraceptive hormone levels, as discussed further below.1 AED effects on circulating sex steroid hormone levels can also affect sexual function. In a study of sexual function and hormones in women with epilepsy, women receiving enzyme-inducing AEDs had statistically higher sexual dysfunction and lower sexual arousal compared to controls.13 Questions about sexual function should be part of the routine evaluation in the outpatient clinic. Sexual dysfunction in women with epilepsy has multifactorial causes, but AED type is one contributing and potentially modifiable factor, and available evidence suggests that non–enzyme-inducing AEDs show more favorable profiles.13
Table 7-1.
Antiepileptic Drugs: Degree of Induction of Metabolism of Sex Steroid Hormones (Endogenous Sex Steroid Hormones and Exogenous Hormonal Contraceptives)

Fertility and Childbirth Rates
While birth rates encompass psychosocial factors such as choosing whether to bear children, infertility refers to a couple not using contraceptive methods for 1 year and not conceiving. The Kerala Registry of Epilepsy and Pregnancy reported a 38% infertility rate among younger women with epilepsy actively trying to conceive after marriage, with higher infertility rates in those receiving AED polytherapy.14 Other studies of women with epilepsy have been inconsistent but suggest decreased fertility rates. Another study also reported that the reduced rate of childbirth holds in married women with epilepsy (69% of expected number of live-born children).15 A population-based Finnish cohort study of newly diagnosed women withepilepsy reported slightly lower childbirth rates than in a reference cohort,16 whereas a comparable Icelandic study suggested no difference in childbearing rates in women with epilepsy.17
Several biological reasons could cause decreased fertility in women with epilepsy, including central dysregulation of the hypothalamic-pituitary-ovarian axis, premature ovarian failure, polycystic ovarian syndrome, and effects of enzyme-inducing AEDs.1 In addition to these factors, geographic and cultural differences are likely substantial factors that influence self-directed decisions. The “Ideal World” survey of Epilepsy Action UK women found that 33% of women with epilepsy were considering not having children because of their epilepsy.18
CONTRACEPTION
Effective contraception in women with epilepsy is essential to allow for preconception planning and to implement the measures known to improve pregnancy outcomes. However, concomitant use of AEDs and hormonal contraceptives is complicated because of bidirectional pharmacokinetic interactions, pharmacodynamic consequences, and potential effects on seizure control.
Enzyme-inducing AEDs lead to rapid clearance of female sex steroid hormones and may allow ovulation in women taking oral contraceptives or other hormonal forms of birth control (eg, vaginal ring, patch). Table 7-1 categorizes the different AEDs according to the degree of female sex steroid hormones induction. These data are derived primarily from pharmacokinetic interaction studies between the AED and oral contraceptive formulations. Other authors have recommended high-dose oral contraceptives with enzyme-inducing AEDs, assuming that enzyme induction will lower oral contraceptive levels. Oral contraceptives with higher doses of ethinyl estradiol and progestin remain available but are infrequently used in practice for healthy women. No direct evidence supports contraceptive efficacy in women with epilepsy receiving enzyme-inducing AEDs. The US Centers for Disease Control and Prevention (CDC) Medical Eligibility Criteria for Contraceptive Use classified certain AEDs (phenytoin, carbamazepine, phenobarbital, primidone, topiramate, and oxcarbazepine) as Category 3, meaning that potential risks (eg, birth control failure) generally outweigh benefits of use with oral contraceptives.19 Other contraceptives should be encouraged for women with epilepsy who are long-term users of these AEDs.
Oral contraceptives, as well as patches, rings, and implants, are no longer first-line contraceptive methods for women with epilepsy who use enzyme-inducing AEDs (Table 7-1). For these women, a long-acting reversible contraceptive (LARC), such as the progestin implant or an intrauterine device (IUD), is an excellent choice. The concentration of progestin delivered by the implant is high enough that efficacy is maintained with concomitant use of an enzyme-inducing AED. The levonorgestrel IUD prevents pregnancy by local hormonally mediated changes in cervical mucus and is not likely to be impacted by enzyme-inducing AEDs. One reassuring prospective UK registry study demonstrated a pregnancy rate of 1.1 per 100 person-years for 56 women using the levonorgestrel IUD with enzyme-inducing AEDs.20 IM medroxyprogesterone acetate is another LARC but is not a first-line option because it is associated with weight gain, bone density loss, and delayed return of fertility.
PREGNANCY
Introduction
Epilepsy is the most common neurologic disorder that requires continuous treatment during pregnancy, and AEDs are one of the most frequent chronic teratogen exposures. Approximately one-half million women with epilepsy are of childbearing age in the United States, and three to five births per thousand will be to women with epilepsy.21 However, the total number of children exposed in utero to AEDs is likely considerably greater, given AED use for headache, pain, and mood disorders.
AED treatment during pregnancy is a precarious balancing act between teratogenic risks to the fetus and maintaining maternal seizure control. However, pregnancy registries and other prospective studies have provided invaluable information toward optimizing treatment regimens and regarding the safety of breast-feeding. Previous study results and the recent American Academy of Neurology (AAN) and American Epilepsy Society (AES) Practice Parameter updates (available at www.neurology.org/content/73/2/133.full.pdf, www.neurology.org/content/73/2/142.full.pdf, and www.neurology.org/content/73/2/126.full.pdf) are key considerations when counseling and treating women with epilepsy during their reproductive years.21,22,23
Major Congenital Malformations and Minor Anomalies
Offspring of women with epilepsy on AEDs are at an increased risk for major congenital malformations and minor anomalies. Minor anomalies are defined as structural deviations from the norm that do not constitute a threat to health. Minor anomalies affect 6% to 20% of infants born to women with epilepsy, approximately 2.5-fold the rate of the general population. Although not of direct health consequence, the finding of a minor anomaly should lead to enhanced vigilance about the child’s health and neurodevelopment.
Major congenital malformations are defined as an abnormality of an essential anatomical structure present at birth that interferes significantly with function or requires major intervention. The reported major congenital malformation rates in the general population vary between 1.6% to 3.2%, and women with epilepsy who are not receiving AEDs show similar major congenital malformation rates. The average major congenital malformation rates among all AED exposures vary between 3.1% to 9%, approximately two- to threefold higher than the general population.24
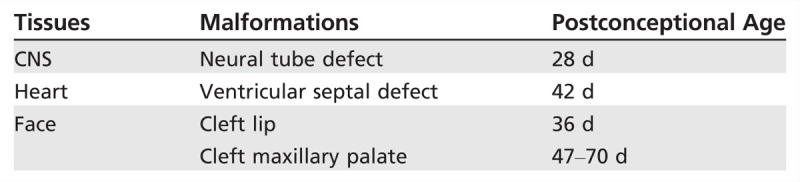
Major congenital malformations most commonly associated with AED exposure include congenital heart disease, cleft lip/palate, urogenital defects, and neural tube defects. Since neural tube closure usually occurs between the third and fourth weeks of gestation, it is usually too late to adjust AEDs to avoid malformations by the time most women realize they are pregnant (Table 7-2).
Table 7-2.
Relative Timing and Developmental Pathology of Certain Malformations
Antiepileptic Drug Monotherapies
Information concerning the risks of specific AEDs for major congenital malformations has increased dramatically over the last 2 decades. Worldwide data obtained from large prospective pregnancy registries have demonstrated remarkably consistent findingsfor many of the AEDs. The 2009 AAN-AES Practice Parameter updates on management issues for women with epilepsy—focus on pregnancy21 offered the following important conclusions about intrauterine first-trimester exposure and risk for major congenital malformations (with parenthetical confidence of conclusion): (1) valproate exposure has higher risk of major congenital malformations compared to carbamazepine (highly probable) and compared to phenytoin or lamotrigine (possible); (2) compared to untreated women with epilepsy, valproate as part of a polytherapy regimen (probable) and as monotherapy (possible) contributes to the development of major congenital malformations; (3) AED polytherapy as compared to monotherapy regimens contributes to the development of major congenital malformations (probable); (4) carbamazepine does not substantially increase the risk of major congenital malformations in the offspring of women with epilepsy (probable); and (5) there is a relationship between the dose of valproate and lamotrigine and the risk of development of major congenital malformations in the offspring of women with epilepsy (probable). Additionally, for specific types of major congenital malformations, findings included the following: (1) phenytoin contributes to the risk of cleft palate (possible); (2) carbamazepine contributes to the risk of posterior cleft palate (possible); (3) valproate contributes to neural tube defects and facial clefts (probable) and to hypospadias (possible); and (4) phenobarbital exposure in utero contributes to cardiac malformations (possible).
Since this evidence-based review of the literature, several large, worldwide prospective pregnancy registries have consistently demonstrated a pattern of amplified risk for the association of valproate exposure during pregnancy and development of major congenital malformations, and provided additional information on other AEDs that help further refine estimates for teratogenicity risk in women with epilepsy (see Case 7-1).
Case 7-1
A 30-year-old, right-handed woman presented for epilepsy management during the first trimester of pregnancy. Seizure onset was at 8 years old, with habitual clinical seizures characterized by an aura of depersonalization, followed by speech arrest, altered awareness, and variable progression to a generalized tonic-clonic seizure, then headache and lethargy. Seizures tended to occur only with antiepileptic drug (AED) tapering, nonadherence, or sleep deprivation. Her most recent seizure was 2 years ago. She received valproate monotherapy. At an infertility evaluation with a gynecologist, the diagnosis of pregnancy was made with estimated 4 weeks gestational age. She was started on 1 mg of folic acid and a multivitamin. Her local neurologist stopped valproate suddenly, and the patient had a generalized tonic-clonic seizure 3 days later. Lamotrigine was begun at 25 mg twice a day. Eight days later, she was hospitalized for Stevens-Johnson syndrome. After urgent consultation, levetiracetam extended release was begun at 500 mg twice a day and continued throughout pregnancy. The patient had another generalized tonic-clonic seizure at 20 weeks gestational age due to nonadherence. Levetiracetam concentrations were followed for therapeutic drug monitoring and doses were increased, eventually reaching 1500 mg twice a day by delivery. She gave birth to a healthy baby boy without complications; birth weight was 6 lbs, 6 oz, and Apgar scores were 8/8 at 1 and 5 minutes, respectively. She breast-fed, and levetiracetam doses were decreased back to 750 mg twice a day over the first month postpartum.
Comment. Lack of appropriate preconception planning and good medical advice can lead to an unnecessarily unstable situation and increased risk for both the mother and developing baby. Lamotrigine cannot be started quickly during pregnancy, and if a conversion to lamotrigine was contemplated, it should have been done before conception. Recent evidence supports a low relative risk of major congenital malformations with levetiracetam, which may be a reasonable alternative when AEDs need to be introduced quickly during pregnancy.
A recent report from the European and International Registry of Antiepileptic Drugs in Pregnancy (EURAP) confirmed that both AED type and amount of exposure are important factors for teratogenic risk during structural organogenesis.25 Major congenital malformation rates in pregnancies exposed to carbamazepine, lamotrigine, valproate, and phenobarbital were analyzed by dose at time of conception. The lowest rates of major congenital malformations occurred with lamotrigine at less than 300 mg/d (2.0%; 95% confidence interval [CI] 1.19–3.24), which served as the comparator group. Risks of major congenital malformations were higher with valproate and phenobarbital at all doses, and with carbamazepine at greater than 400 mg/d. Additionally, an increase in major congenital malformation rates was observed with increasing doses for all of the four AEDs (Figure 7-426).
Figure 7-4.
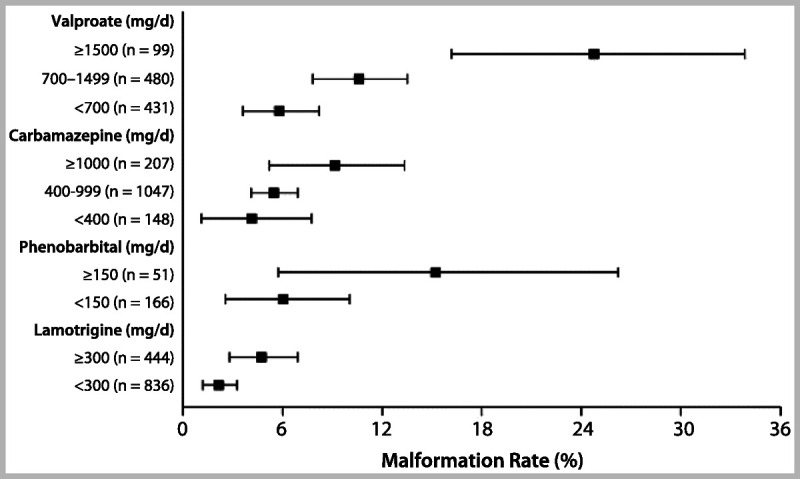
Rates of major congenital malformations at 1 year after birth in relation to exposure to AED monotherapy according to data from the International Registry of Antiepileptic Drugs and Pregnancy. Bars represent 95% confidence interval.Reprinted from Tomson T, Battino D, et al, Lancet Neurol.26 © 2011, with permission from Elsevier. www.sciencedirect.com/science/article/pii/S1474442211701077.
The UK Epilepsy and Pregnancy Register reported on topiramate use in 178 live births.27 Although the confidence intervals (CIs) were wide, this preliminary information noted a major congenital malformation rate of 4.8% for monotherapy use and an even higher rate for use of topiramate in polytherapy regimens. They also noted a particularly higher rate of oral clefts, approximately 11 times their background rate, and a high rate of hypospadias. Oral cleft risk with topiramate has been replicated in other studies.28
Data from the National Birth Defects Prevention Study confirmed increased risks for valproate and neural tube defects (odds ratio [OR] 9.7; 95% CI, 3.4–27.5), oral clefts (OR 4.4; 95% CI, 1.6–12.2), heart defects (OR 2.0; 95% CI, 0.78–5.3), and hypospadias (OR 2.4; 95% CI, 0.62–9.0).29 Increased risks were also observed for carbamazepine and neural tube defects (OR 5.0; 95% CI, 1.9–12.7). Similarly, the European Surveillance of Congenital Anomalies (EUROCAT) antiepileptic-study database, which is derived from population-based congenital anomaly registries, also reported significantly increased risks for valproate monotherapy and spina bifida, atrial septal defect, cleft palate, hypospadias, polydactyly, and craniosynostosis. Spina bifida was the only specific major congenital malformation associated with carbamazepine monotherapy compared with no AED use during pregnancy (OR 2.6; 95% CI, 1.2–5.3), but the risk was smaller than for valproate.30
The North American AED Pregnancy Registry released findings comparing the risk of major congenital malformations among infants exposed to different AED monotherapies during the first trimester, compared to an unexposed reference group.31 The lamotrigine monotherapy group was chosen as the exposed reference group for the other AEDs because of a low rate of major congenital malformations and tight CIs (2.0% [95% CI, 1.4–2.8]) (Table 7-331). This table may serve as a particularly instructive tool during preconception counseling for women with epilepsy, demonstrating information for each AED along with sample size and CIs for the risk estimates. Additional analyses confirmed prior reports of a dose-related risk for valproate and major congenital malformations (Figure 7-531). The lower valproate dosage group still had CIs reaching over 7%, and valproate doses less than 500 mg/d are an uncommon dose to maintain seizure control. Valproate was also associated with an increased risk of hypospadias, neural tube defects, and cardiovascular malformations. Concerning analyses of specific major congenital malformations with other drugs, phenobarbital was associated with a higher risk of cardiovascular malformations, and the risk of oral clefts was higher among infants exposed to phenobarbital, valproate, and topiramate, consistent with previous reports.31
Table 7-3.
Risk of Major Congenital Malformations Identified Among Infants Who Had Been Exposed to a Specific Antiepileptic Drug Monotherapy Regimen During the First Trimester and Relative Risk of Major Congenital Malformations Compared to Both Unexposed and to Lamotrigine Groups: North America Pregnancy Registry 1997–2011a
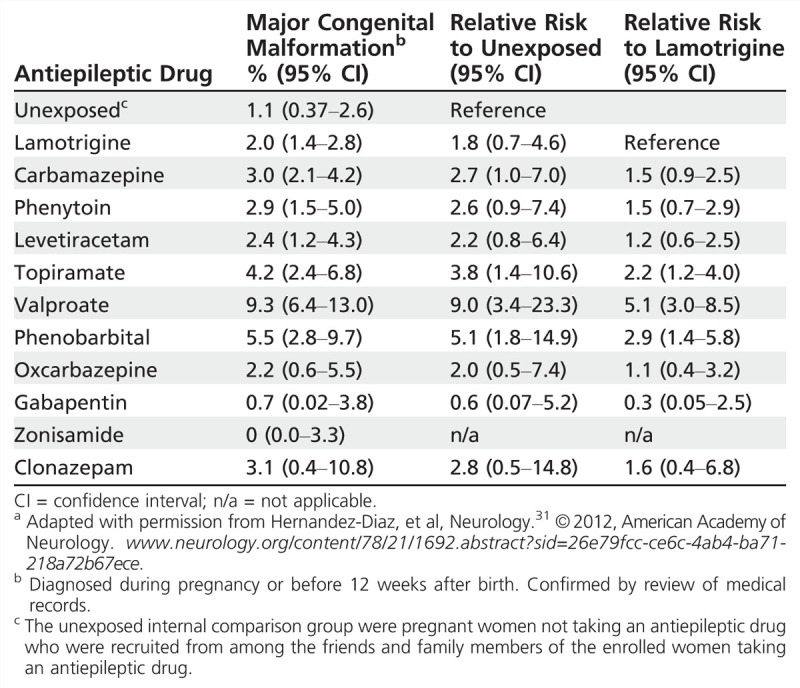
Figure 7-5.
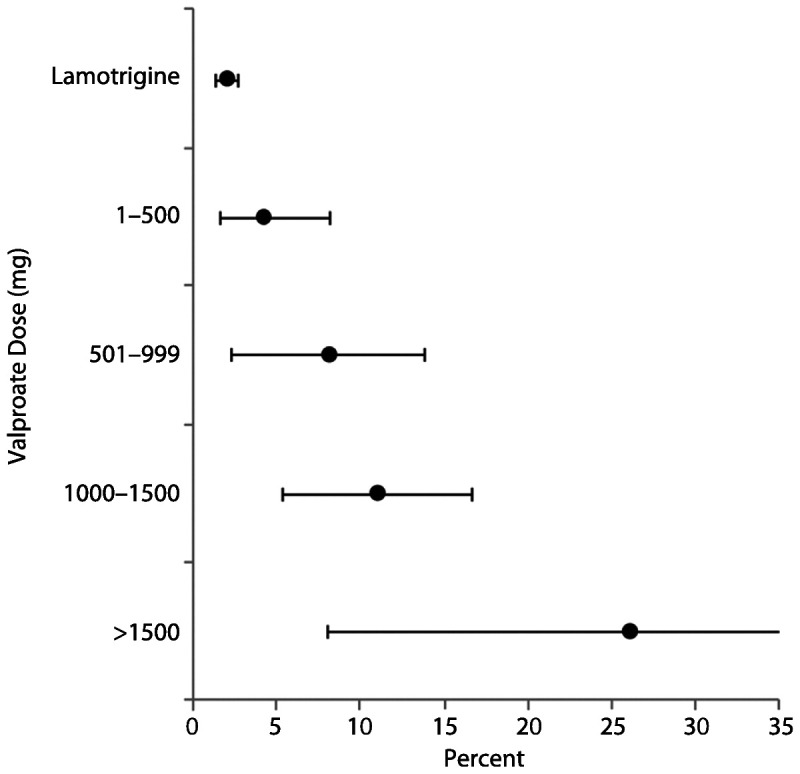
Risk of major malformations by average valproate dose (mg) during the first trimester. Reprinted with permission from Hernández-Díaz S, et al, Neurology.31 © 2012, American Academy of Neurology. www.neurology.org/content/78/21/1692.abstract?sid=26e79fcc-ce6c-4ab4-ba71-218a72b67ece.
Antiepileptic Drug Polytherapy—New Data
Major congenital malformation rates have been reported as consistently higher for AED polytherapy compared to monotherapy regimens.21 These results led to the AAN-AES Practice Parameter recommendation that AED monotherapy is preferred to polytherapy during pregnancy, and that monotherapy should be achieved during the preconception planning phase.21 However, data from the North American AED Pregnancy Registry suggest that not all AED polytherapy combinations are alike.32 Both lamotrigine and carbamazepine had relatively modest major congenital malformation rates in polytherapy regimens that did not contain valproate. The major congenital malformation rate for lamotrigine was 2.9% with any AED other than valproate, but 9.1% for lamotrigine combined with valproate. Similarly, the major congenital malformation rate for carbamazepine was 2.5% with any AED other than valproate, but 15.4% for carbamazepine combined with valproate polytherapy.
Neurodevelopmental Outcomes
Studies investigating cognitive outcome in children of women with epilepsy report an increased risk of mental deficiency.33 Verbal scores on neuropsychometric measures may be selectively more involved. While AEDs appear to play the major role, a variety of other factors contribute to the cognitive problems of children of women with epilepsy, including seizures, maternal focal seizure disorder, minor and major malformations, lower maternal education, and impaired maternal-child relations.33 These risk factors may be additive or even synergistic.
The 2009 AAN-AES Practice Parameter updates reported the following conclusions about in utero exposure throughout the entire pregnancy and risk for poor cognitive outcomes: (1) cognition is not reduced in children of untreated women with epilepsy (probable); (2) carbamazepine does not increase poor cognitive outcomes compared to unexposed controls (probable); (3) monotherapy exposure to valproate reduces cognitive outcomes (probable); (4) monotherapy exposure to phenytoin or phenobarbital reduces cognitive outcomes (possible); and (5) AED polytherapy exposure reduces cognitive outcomes as compared to AED monotherapy (probable).21
Since the 2009 AAN-AES Practice Parameter update, a few notable reports have also contributed to our understanding of factors affecting adverse neurodevelopmental outcomes. The Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study was a prospective, observational, multicenter study in the United States and United Kingdom and assessed the neurodevelopmental effects of in utero exposure to four monotherapy groups (carbamazepine, valproate, phenytoin, and lamotrigine).34 The primary outcome was IQ at 6 years old, adjusted for maternal IQ, AED type, AED standardized dose, gestational age at birth, and use of periconceptional folate. Primary analysis included 305 mothers and 311 children, with 224 children completing the 6-year follow-up. Multivariate analysis demonstrated that valproate-exposed children had lower IQ at age 6 compared to those exposed to carbamazepine, lamotrigine, or phenytoin. High doses of valproate were negatively correlated with IQ, verbal ability, nonverbal ability, memory, and executive function, while the other AEDs did not have a dose effect. Interestingly, mean IQs were higher in the children of mothers who took periconceptional folate (Figure 7-634). In addition, the neurodevelopment research group from Liverpool and Manchester reported preliminary findings that valproate may specifically be associated with autism spectrum disorder. In their small sample size, 6.3% of the children exposed to valproate monotherapy had clinically diagnosed autism spectrum disorder, sevenfold higher than the control group and over tenfold higher than the reported incidence in the general population.35
Figure 7-6.
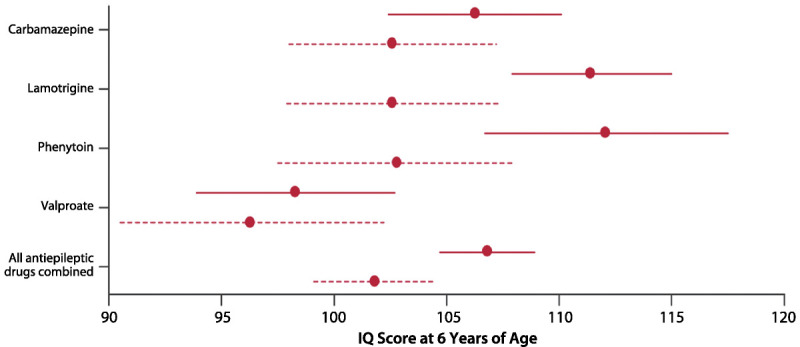
Child IQ at 6 years of age, by exposure to maternal antiepileptic drug use and periconceptional folate. Mean (95% confidence intervals) are shown for folate (solid lines) and no folate (dashed lines).IQ = intelligence quotient.Reprinted from Meador KJ, et al, Lancet Neurol.33 link.springer.com/article/10.1007/s11910-002-0013-6.
Neonatal Complications
Recent reports suggest that there may be increased risk for other neonatal complications for offspring of women with epilepsy on AEDs. Findings from the 2009 AAN-AES Practice Parameter update concluded the following: (1) neonates of women with epilepsy taking AEDs have an increased risk of being small for gestational age of about twice the expected rate (probable); and (2) neonates of women with epilepsy have an increased risk of a 1-minute Apgar score of less than 7 of about twice the expected rate (possible).21
Since this parameter was released, a study from Taiwan reported that seizures in women with epilepsy during pregnancy were independently associated with an approximate 1.5-fold increased risk for preterm delivery or infants being born small for gestational age.36 A recent secondary analysis of the neonatal outcomes from the NEAD cohort reported that adverse neonatal outcome risks may differ between the AEDs; the OR for infants being born small for gestational age was higher for the valproate and carbamazepine groups, and reduced 1-minute Apgar scores occurred more frequently in the phenytoin and valproate groups.37
Seizures During Pregnancy
The effect of pregnancy on seizure frequency is variable. Approximately 20% to 33% of pregnant women with epilepsy experience an increase in seizure frequency, 7% to 25% experience a decrease, and 50% to 83% have no significant change.38 The physiologic changes and psychosocial adjustments that accompany pregnancy can alter seizure frequency, including changes in sex-hormone concentrations, changes in AED metabolism, sleep deprivation, and new stresses. Noncompliance with medications is common during pregnancy and is frequently due to the perception that AEDs during pregnancy are harmful to the fetus. Teratogenic effects of AEDs, while not uncommon, are often exaggerated or misrepresented. Proper education about the risks of AEDs versus the risks of seizures can be very helpful in assuring compliance during pregnancy.
The risk of seizures to the fetus should be discussed thoroughly with the patient and other family members. Generalized tonic-clonic seizures can cause maternal and fetal hypoxia and acidosis, fetal heart-rate decelerations, and possibly miscarriages and stillbirths. Nonconvulsive seizures can cause trauma, which can result in ruptured fetal membranes with an increased risk of infection, premature labor, and even fetal death. In addition to the physical risks of seizures to the developing fetus, reemergence of seizures in a woman who had previously experienced seizure control can be devastating. Besides the immediate risk to herself and the fetus, loss of driving privileges may have remarkable psychosocial impact.
Antiepileptic Drug Management in Light of Pharmacokinetic Changes Intrapartum and Postpartum
Maintaining seizure stability during pregnancy depends on maintaining therapeutic AED concentrations. The target concentration should be individually determined in the preconception phase with respect to epilepsy history and previous benchmark AED concentrations. During pregnancy, AED dosing becomes complex and requires an intensive approach. Clearance of most AEDs increases during pregnancy, resulting in decreased serum concentrations (Table 7-4).22,39 Several physiologic factors contribute to declining AED levels during pregnancy, including hepatic enzymatic induction via increased female sex steroid hormones, increased volume of distribution, decreased concentration of albumin and α1-acid glycoproteins, increased renal blood flow, and alterations in drug absorption (see Case 7-1).
Table 7-4.
Alterations of Antiepileptic Drug Clearance and/or Concentrations During Pregnancy: Summary of Class I, II, and III Studiesa
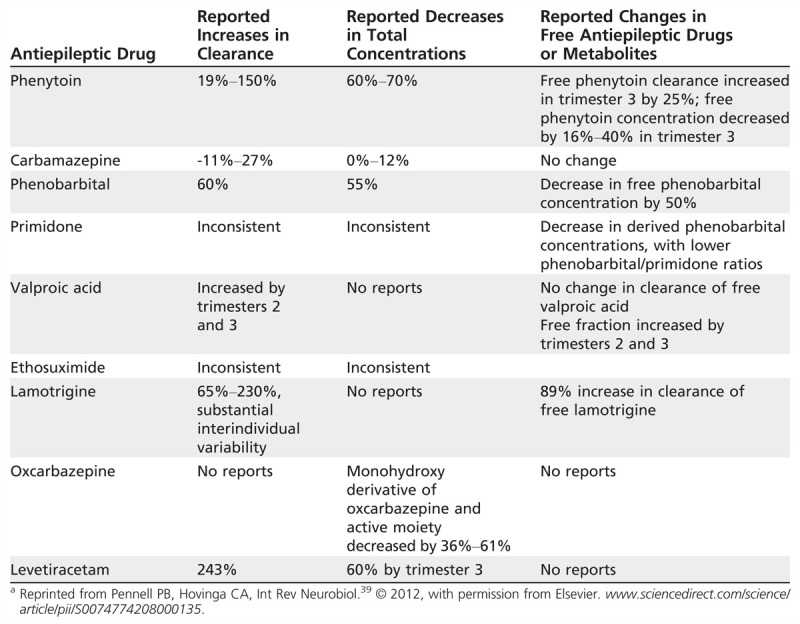
The 2009 AAN-AES Practice Parameter update concluded that pregnancy causes an increase in the clearance and a decrease in the concentration of lamotrigine, phenytoin, and to a lesser extent carbamazepine (probable), and decreases the level of levetiracetam and the active oxcarbazepine metabolite, the monohydroxy derivative (possible).22
The magnitude of enhanced lamotrigine clearance during pregnancy exceeds the clearance described for most older AEDs because it is primarily eliminated via hepatic glucuronidation, which is particularly susceptible to activation during pregnancy because of direct effects of rising female sex steroid hormones levels. Both lamotrigine free and total clearance were increased during all 3 trimesters, with peaks of 94% (total) and 89% (free) in the third trimester in a class I prospective observational study of 53 pregnancies in 53 women, using 305 samples throughout preconception baseline, pregnancy, and postpartum.40 Seizure frequency significantly increased when lamotrigine levels decreased to 65% of the preconceptional individualized target lamotrigine concentration, supporting the recommendation to monitor the levels of AEDs, which decrease duringpregnancy.40 Previous studies of lamotrigine during pregnancy noted a rapid decrease in lamotrigine clearance during the early postpartum period with reports of symptomatic toxicity. Nonadherence to a standard lamotrigine taper schedule has been shown to be associated with a significantly higher risk for postpartum toxicity.40 Most other AED levels gradually increase after delivery and plateau by 10 weeks postpartum. The exact time course is not well established for other AEDs, but AED doses should be adjusted and levels should be followed during the postpartum period.
BONE HEALTH
Both men and women with epilepsy have elevated rates of fractures (two- to sixfold higher) compared to the general population.41 Although seizure-related injuries may contribute to these elevated rates, AEDs independently contribute to this risk, especially the enzyme-inducing AEDs.41,42
Dual-energy x-ray absorptiometry (DXA) can assess bone mineral density. Depressed bone mineral density is the most significant predictor of fracture. Bone mineral density measurements reveal osteopenia or osteoporosis in 38% to 60% of people with epilepsy receiving AEDs in specialty clinics, with longer duration of AED therapy associated with further decreased bone mineral density. Enzyme-inducing AEDs independently reduce bone mineral density.41
The effects of enzyme-inducing AEDs are thought to be related to their cytochrome P450 (CYP) enzyme induction leading to accelerated metabolism of vitamin D to inactive metabolites and/or lower (free) estradiol levels.43 However, some enzyme-inducing AEDs are more consistently associated with increased fracture, decreased bone mineral density, and alterations in bone metabolism. For example, chronic phenobarbital is associated with decreased active vitamin D levels, and carbamazepine is not consistently associated with decreased bone mineral density.41 On the other hand, phenytoin use has more consistently demonstrated properties of increased bone turnover in premenopausal women with epilepsy compared to other AEDs (valproate, lamotrigine, and even carbamazepine).44 In another study, premenopausal women received one of these four AEDs for 1 year.45 In the phenytoin group, the biochemical pattern differed and demonstrated a pattern consistent with hyperparathyroidism and increased remodeling. In just 1 year, significant bone mineral density loss (2.6%) was seen at the femoral neck in the phenytoin group (Case 7-2).
Case 7-2
A 60-year-old, right-handed woman was referred to reassess phenytoin use for epilepsy in the setting of a recent osteoporosis diagnosis. Seizure onset was at 19 years old. Characteristic habitual clinical seizures began with a visual aura, followed by a generalized tonic-clonic seizure. Initial antiepileptic drugs were phenobarbital, then phenytoin, which eventually produced seizure freedom, although complications included gingival hyperplasia requiring surgical treatment, and the patient recently learned she had osteoporosis despite an intensive fitness regimen. Recent brain MRI also revealed moderate cerebellar atrophy, and a left frontal cavernous malformation. Neurologic examination was notable for difficulty with tandem gait and findings of a mild distal polyneuropathy; 25-OH vitamin D levels were within normal limits. The patient was transitioned to lamotrigine and remained seizure free. Her primary care physician was contacted to continue to follow and manage her osteoporosis.
Comment. A common practice is to “leave well enough alone” when a patient has been seizure free. However, neurologists should be aware of increased risks for osteopenia and osteoporosis with chronic use of enzyme-inducing antiepileptic drugs, especially phenytoin, and consider substitution to prevent bone fracture.
SUMMARY
Optimal treatment of women with epilepsy includes consideration of the complex interactions of female sex steroid hormones with epilepsy and AEDs, and the potential risks of any AED prescribed during pregnancy. Understanding these key factors enhances the ability to make informed treatment recommendations that will provide improved seizure control with fewer adverse health effects for women with epilepsy during their reproductive years, and maximize chances for optimal maternal and child outcomes after AED treatment during pregnancy.
KEY POINTS
Criteria for catamenial epilepsy have become more consistent and formalized.
For patients having a threefold or greater increase in seizure frequency in a C1 pattern, cyclic progesterone lozenges are an adjunctive treatment option.
Long-acting reversible contraceptives (including the progestin implant) and intrauterine devices are excellent choices for women with epilepsy receiving enzyme-inducing antiepileptic drugs.
Determining the individual target concentration of an antiepileptic drug in a woman with epilepsy before conception can be a valuable tool for therapeutic drug monitoring during pregnancy.
Recent data support the concern that the amount of fetal exposure to an antiepileptic drug is important, as well as the type of antiepileptic drug. Therefore, reduction of the dose before conception while maintaining seizure control may reduce the risk of structural teratogenicity.
Children born to women with epilepsy receiving valproate during pregnancy are at a fivefold higher risk of having a major congenital malformation, lower IQ, and possibly autism spectrum disorder.
Therapeutic drug monitoring of antiepileptic drug concentrations during pregnancy is recommended. Establishing the individualized ideal antiepileptic drug concentration is important as a target to maintain during pregnancy. Dosages of the antiepileptic drug will need to be readjusted in the postpartum period.
Although more research is needed to understand underlying mechanisms for bone density loss associated with antiepileptic drugs, awareness of risk for osteopenia or osteoporosis should be a consideration in antiepileptic drug selection and maintenance.
Footnotes
Relationship Disclosure: Dr Pennell reports no disclosure.
Unlabeled Use of Products/Investigational Use Disclosure: Dr Pennell discusses the unlabeled use of progesterone lozenges for the treatment of catamenial epilepsy.
REFERENCES
- 1.Harden CL,, Pennell PB. Neuroendocrine considerations in the treatment of men and women with epilepsy. Lancet Neurol 2013; 12 (1): 72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herzog AG,, Klein P,, Ransil BJ. Three patterns of catamenial epilepsy. Epilepsia 1997; 38 (10): 1082–1088. [DOI] [PubMed] [Google Scholar]
- 3.Mattson RH,, Cramer JA,, Caldwell BV,, Siconolfi BC. Treatment of seizures with medroxyprogesterone acetate: preliminary report. Neurology 1984; 34 (9): 1255–1258. [DOI] [PubMed] [Google Scholar]
- 4.Herzog AG,, Fowler KM,, Smithson SD, et al. Progesterone vs placebo therapy for women with epilepsy: a randomized clinical trial. Neurology 2012; 78 (24): 1959–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein P,, Serje A,, Pezzullo JC. Premature ovarian failure in women with epilepsy. Epilepsia 2001; 42 (12): 1584–1589. [DOI] [PubMed] [Google Scholar]
- 6.Harden CL,, Koppel BS,, Herzog AG, et al. Seizure frequency is associated with age of menopause in women with epilepsy. Neurology 2003; 61 (4): 451–455. [DOI] [PubMed] [Google Scholar]
- 7.Harden CL,, Pulver MC,, Ravdin L,, Jacobs AR. The effect of menopause and perimenopause on the course of epilepsy. Epilepsia 1999; 40 (10): 1402–1407. [DOI] [PubMed] [Google Scholar]
- 8.Harden CL,, Herzog AG,, Nikolov BG, et al. Hormone replacement therapy in women with epilepsy: a randomized, double-blind, placebo-controlled study. Epilepsia 2006; 47 (9): 1447–1451. [DOI] [PubMed] [Google Scholar]
- 9.Herzog AG. A relationship between particular reproductive endocrine disorders and the laterality of epileptiform discharges in women with epilepsy. Neurology 1993; 43 (10): 1907–1910. [DOI] [PubMed] [Google Scholar]
- 10.Azziz R,, Carmina E,, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril 2009; 91 (2): 456–488. [DOI] [PubMed] [Google Scholar]
- 11.Pylvänen V,, Pakarinen A,, Knip M,, Isojärvi J. Characterization of insulin secretion in Valproate-treated patients with epilepsy. Epilepsia 2006; 47 (9): 1460–1464. [DOI] [PubMed] [Google Scholar]
- 12.Morrell MJ,, Hayes FJ,, Sluss PM, et al. Hyperandrogenism, ovulatory dysfunction, and polycystic ovary syndrome with valproate versus lamotrigine. Ann Neurol 2008; 64 (2): 200–211. [DOI] [PubMed] [Google Scholar]
- 13.Morrell MJ,, Flynn KL,, Doñe S,. Sexual dysfunction, sex steroid hormone abnormalities, and depression in women with epilepsy treated with antiepileptic drugs. Epilepsy Behav 2005; 6 (3): 360–365. [DOI] [PubMed] [Google Scholar]
- 14.Sukumaran SC,, Sarma PS,, Thomas SV. Polytherapy increases the risk of infertility in women with epilepsy. Neurology 2010; 75 (15): 1351–1355. [DOI] [PubMed] [Google Scholar]
- 15.Dansky LV,, Andermann E,, Andermann F. Marriage and fertility in epileptic patients. Epilepsia 1980; 21 (3): 261–271. [DOI] [PubMed] [Google Scholar]
- 16.Artama M,, Isojarvi JI,, Raitanen J, et al. Birth rate among patients with epilepsy: a nationwide population-based cohort study in Finland. Am J Epidemiol 2004; 159 (11): 1057–1063. [DOI] [PubMed] [Google Scholar]
- 17.Olafsson E,, Hallgrimsson JT,, Hauser WA, et al. Pregnancies of women with epilepsy: a population-based study in Iceland. Epilepsia 1998; 39 (8): 887–892. [DOI] [PubMed] [Google Scholar]
- 18.Crawford P,, Hudson S. Understanding the information needs of women with epilepsy at different lifestages: results of the ‘Ideal World’ survey. Seizure 2003; 12 (7): 502–507. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC). U S. Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep 2010; 59 (RR-4): 1–86. [PubMed] [Google Scholar]
- 20.Bounds W,, Guillebaud J. Observational series on women using the contraceptive Mirena concurrently with anti-epileptic and other enzyme-inducing drugs. J Fam Plann Reprod Health Care 2002; 28 (2): 78–80. [DOI] [PubMed] [Google Scholar]
- 21.Harden CL,, Meador KJ,, Pennell PB, et al. Practice parameter update: management issues for women with epilepsy—focus on pregnancy (an evidence-based review): teratogenesis and perinatal outcomes: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology 2009; 73 (2): 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harden CL,, Pennell PB,, Koppel BS, et al. Practice parameter update: management issues for women with epilepsy—focus on pregnancy (an evidence-based review): vitamin K, folic acid, blood levels, and breastfeeding: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology 2009; 73 (2): 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harden CL,, Hopp J,, Ting TY, et al. Practice parameter update: management issues for women with epilepsy—focus on pregnancy (an evidence-based review): obstetrical complications and change in seizure frequency: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology 2009; 73 (2): 126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pennell PB. Antiepileptic drugs during pregnancy: what is known and which AEDs seem to be safest? Epilepsia 2008; 49 (suppl 9): 43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomson T,, Battino D,, Bonizzoni E, et al. Dose-dependent risk of malformations with antiepileptic drugs: an analysis of data from the EURAP epilepsy and pregnancy registry. Lancet Neurol 2011; 10 (7): 609–617. [DOI] [PubMed] [Google Scholar]
- 26.Tomson T,, Battino D. Teratogenic effects of antiepileptic drugs. Lancet Neurol 2012; 11 (9): 803–813. [DOI] [PubMed] [Google Scholar]
- 27.Hunt S,, Russell A,, Smithson WH, et al. Topiramate in pregnancy: preliminary experience from the UK Epilepsy and Pregnancy Register. Neurology 2008; 71 (4): 272–276. [DOI] [PubMed] [Google Scholar]
- 28.Margulis AV,, Mitchell AA,, Gilboa SM, et al. Use of topiramate in pregnancy and risk of oral clefts. Am J Obstet Gynecol 2012; 207 (5): 405.e1–405.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Werler MM,, Ahrens KA,, Bosco JL, et al. Use of antiepileptic medications in pregnancy in relation to risks of birth defects. Ann Epidemiol 2011; 21 (11): 842–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pennell PB. The devil is in the details: not all AED-associated major congenital malformations are equal. Epilepsy Curr 2011; 11 (3): 79–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hernández-Díaz S,, Smith CR,, Shen A, et al. Comparative safety of antiepileptic drugs during pregnancy. Neurology 2012; 78 (21): 1692–1699. [DOI] [PubMed] [Google Scholar]
- 32.Holmes LB,, Mittendorf R,, Shen A, et al. Fetal effects of anticonvulsant polytherapies: different risks from different drug combinations. Arch Neurol 2011; 68 (10): 1275–1281. [DOI] [PubMed] [Google Scholar]
- 33.Meador KJ. Neurodevelopmental effects of antiepileptic drugs. Curr Neurol Neurosci Rep 2002; 2 (4): 373–378. [DOI] [PubMed] [Google Scholar]
- 34.Meador KJ,, Baker GA,, Browning N, et al. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol 2013; 12 (3): 244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bromley RL,, Mawer GE,, Briggs M, et al. The prevalence of neurodevelopmental disorders in children prenatally exposed to antiepileptic drugs [published online ahead of print January 31, 2013]. J Neurol Neurosurg Psychiatry 2013. doi:10.1136/jnnp-2012-304270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen YH,, Chiou HY,, Lin HC,, Lin HL. Affect of seizures during gestation on pregnancy outcomes in women with epilepsy. Arch Neurol 2009; 66 (8): 979–984. [DOI] [PubMed] [Google Scholar]
- 37.Pennell PB,, Klein AM,, Browning N, et al. Differential effects of antiepileptic drugs on neonatal outcomes. Epilepsy Behav 2012; 24 (4): 449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.EURAP Study Group. Seizure control and treatment in pregnancy: observations from the EURAP epilepsy pregnancy registry. Neurology 2006; 66 (3): 354–360. [DOI] [PubMed] [Google Scholar]
- 39.Pennell PB,, Hovinga CA. Antiepileptic drug therapy in pregnancy I: gestation-induced effects on AED pharmacokinetics. Int Rev Neurobiol 2008; 83: 227–240. [DOI] [PubMed] [Google Scholar]
- 40.Pennell PB,, Peng L,, Newport DJ, et al. Lamotrigine in pregnancy: clearance, therapeutic drug monitoring, and seizure frequency. Neurology 2008; 70 (22 pt 2): 2130–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brodie MJ,, Mintzer S,, Pack AM, et al. Enzyme induction with antiepileptic drugs: cause for concern? Epilepsia 2013; 54 (1): 11–27. [DOI] [PubMed] [Google Scholar]
- 42.Carbone LD,, Johnson KC,, Robbins J, et al. Antiepileptic drug use, falls, fractures, and BMD in postmenopausal women: findings from the women’s health initiative (WHI). J Bone Miner Res 2010; 25 (4): 873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pack AM,, Morrell MJ,, McMahon DJ,, Shane E. Normal vitamin D and low free estradiol levels in women on enzyme-inducing antiepileptic drugs. Epilepsy Behav 2011; 21 (4): 453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pack AM,, Morrell MJ,, Marcus R, et al. Bone mass and turnover in women with epilepsy on antiepileptic drug monotherapy. Ann Neurol 2005; 57 (2): 252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pack AM,, Morrell MJ,, Randall A, et al. Bone health in young women with epilepsy after one year of antiepileptic drug monotherapy. Neurology 2008; 70 (18): 1586–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]


