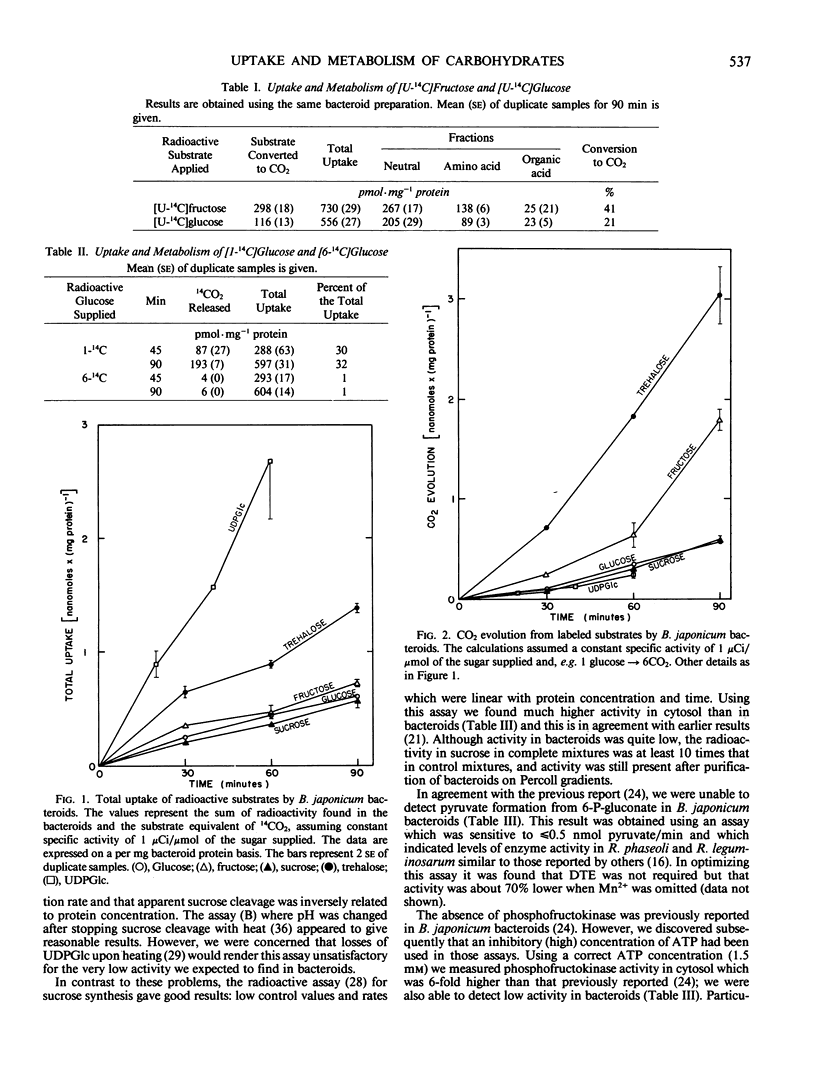
Abstract
Bradyrhizobium japonicum bacteroids were isolated anaerobically and were supplied with 14C-labeled trehalose, sucrose, UDP-glucose, glucose, or fructose under low O2 (2% in the gas phase). Uptake and conversion of 14C to CO2 were measured at intervals up to 90 minutes. Of the five compounds studied, UDP-glucose was most rapidly absorbed but it was very slowly metabolized. Trehalose was the sugar most rapidly converted to CO2, and fructose was respired at a rate at least double that of glucose. Sucrose and glucose were converted to CO2 at a very low but measurable rate (<0.1 nanomoles per milligram protein per hour). Carbon Number 1 of glucose appeared in CO2 at a rate 30 times greater than the conversion of carbon Number 6 to CO2, indicating high activity of the pentose phosphate pathway. Enzymes of the Entner-Doudoroff pathway were not detected in bacteroids, but very low activities of sucrose synthase and phosphofructokinase were demonstrated. Although metabolism of sugars by B. japonicum bacteroids was clearly demonstrated, the rate of sugar uptake was only 1/30 to 1/50 the rate of succinate uptake. The overall results support the view that, although bacteroids metabolize sugars, the rates are very low and are inadequate to support nitrogenase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. L., Sabularse D. C. Inorganic pyrophosphate: D-fructose-6-phosphate 1-phosphotransferase from mung bean. Methods Enzymol. 1982;90(Pt E):91–97. doi: 10.1016/s0076-6879(82)90112-4. [DOI] [PubMed] [Google Scholar]
- Appleby C. A. Electron transport systems of Rhizobium japonicum. I. Haemoprotein P-450, other CO-reactive pigments, cytochromes and oxidases in bacteroids from N2-fixing root nodules. Biochim Biophys Acta. 1969 Jan 14;172(1):71–87. doi: 10.1016/0005-2728(69)90093-0. [DOI] [PubMed] [Google Scholar]
- COOPERSTEIN S. J., LAZAROW A. A microspectrophotometric method for the determination of cytochrome oxidase. J Biol Chem. 1951 Apr;189(2):665–670. [PubMed] [Google Scholar]
- Cséke C., Weeden N. F., Buchanan B. B., Uyeda K. A special fructose bisphosphate functions as a cytoplasmic regulatory metabolite in green leaves. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4322–4326. doi: 10.1073/pnas.79.14.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karr D. B., Waters J. K., Suzuki F., Emerich D. W. Enzymes of the Poly-beta-Hydroxybutyrate and Citric Acid Cycles of Rhizobium japonicum Bacteroids. Plant Physiol. 1984 Aug;75(4):1158–1162. doi: 10.1104/pp.75.4.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele B. B., Jr, Hamilton P. B., Elkan G. H. Glucose catabolism in Rhizobium japonicum. J Bacteriol. 1969 Mar;97(3):1184–1191. doi: 10.1128/jb.97.3.1184-1191.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lobo Z., Maitra P. K. A particulate phosphofructokinase from yeast. FEBS Lett. 1982 Jan 25;137(2):279–282. doi: 10.1016/0014-5793(82)80367-0. [DOI] [PubMed] [Google Scholar]
- Martínez-De Drets G., Arias A. Enzymatic basis for differentiation of Rhizobium into fast- and slow-growing groups. J Bacteriol. 1972 Jan;109(1):467–470. doi: 10.1128/jb.109.1.467-470.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Drets G., Gardiol A., Arias A. 6-Phospho-D-gluconate:NAD+ 2-oxidoreductase (decarboxylating) from slow-growing Rhizobia. J Bacteriol. 1977 Jun;130(3):1139–1143. doi: 10.1128/jb.130.3.1139-1143.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell M., Copeland L. Enzymes of sucrose breakdown in soybean nodules: alkaline invertase. Plant Physiol. 1984 Apr;74(4):1030–1034. doi: 10.1104/pp.74.4.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell M., Copeland L. Sucrose synthase of soybean nodules. Plant Physiol. 1985 May;78(1):149–154. doi: 10.1104/pp.78.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulongoy K., Elkan G. H. The role of 6-phosphogluconate dehydrogenase in Rhizobium. Can J Microbiol. 1977 Sep;23(9):1293–1298. doi: 10.1139/m77-193. [DOI] [PubMed] [Google Scholar]
- Reibach P. H., Streeter J. G. Evaluation of active versus passive uptake of metabolites by Rhizobium japonicum bacteroids. J Bacteriol. 1984 Jul;159(1):47–52. doi: 10.1128/jb.159.1.47-52.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reibach P. H., Streeter J. G. Metabolism of C-labeled photosynthate and distribution of enzymes of glucose metabolism in soybean nodules. Plant Physiol. 1983 Jul;72(3):634–640. doi: 10.1104/pp.72.3.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabularse D. C., Anderson R. L. D-Fructose 2,6-bisphosphate: a naturally occurring activator for inorganic pyrophosphate:D-fructose-6-phosphate 1-phosphotransferase in plants. Biochem Biophys Res Commun. 1981 Dec 15;103(3):848–855. doi: 10.1016/0006-291x(81)90888-3. [DOI] [PubMed] [Google Scholar]
- Salerno G. L., Gamundi S. S., Pontis H. G. A procedure for the assay of sucrose synthetase and sucrose phosphate synthetase in plant homogenates. Anal Biochem. 1979 Feb;93(1):196–199. [PubMed] [Google Scholar]
- Salminen S. O., Streeter J. G. Enzymes of alpha,alpha-Trehalose Metabolism in Soybean Nodules. Plant Physiol. 1986 Jun;81(2):538–541. doi: 10.1104/pp.81.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stournaras C., Maurer P., Kurz G. 6-phospho-D-gluconate dehydrogenase from Pseudomonas fluorescens. Properties and subunit structure. Eur J Biochem. 1983 Feb 1;130(2):391–396. doi: 10.1111/j.1432-1033.1983.tb07165.x. [DOI] [PubMed] [Google Scholar]
- Streeter J. G. Accumulation of alpha,alpha-trehalose by Rhizobium bacteria and bacteroids. J Bacteriol. 1985 Oct;164(1):78–84. doi: 10.1128/jb.164.1.78-84.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J. C. Purification and Characterization of Sucrose Synthetase from the Shoot of Bamboo Leleba oldhami. Plant Physiol. 1977 Jul;60(1):17–21. doi: 10.1104/pp.60.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. X., Smyth D. A., Black C. C. Fructose 2,6-bisphosphate and the regulation of pyrophosphate-dependent phosphofructokinase activity in germinating pea seeds. Plant Physiol. 1983 Sep;73(1):188–191. doi: 10.1104/pp.73.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]


