Abstract
Purpose of Review:
Large artery atherosclerosis is an important cause of ischemic stroke. Recent randomized clinical trials have helped clarify the treatment options for conditions such as carotid stenosis and intracranial atherosclerosis. This review outlines the primary findings of these trials and provides current recommendations for treatment.
Recent Findings:
Carotid revascularization is preferred in patients with severe symptomatic carotid stenosis. Carotid endarterectomy achieves lower rates of stroke or death than carotid artery stenting. The risk of stroke or death with stenting is higher among older patients and women. Intensive medical therapy achieves low stroke and death rates in asymptomatic stenosis. Medical therapy and treatment of atherosclerotic risk factors are the mainstay of therapy for intracranial atherosclerosis, and medical therapy is recommended for patients with vertebral artery origin atherosclerosis.
Summary:
Contemporary medical therapy is paramount in large artery atherosclerosis. Patient demographics, comorbidities, and the periprocedural risks of stroke and death should be carefully weighed while choosing a revascularization procedure for carotid stenosis.
Atherosclerosis of the large arteries is responsible for about 15% of all ischemic strokes. Within the last decade, there has been significant progress in the medical management of atherosclerosis. Blood pressure lowering and control of dyslipidemia have improved, resulting in enhanced secondary stroke prevention. Even with the availability of surgical and endovascular therapies for some large artery atherosclerotic lesions, specifically carotid disease, the importance of intensive medical management cannot be overemphasized. In this article, we will discuss contemporary management principles of three conditions: cervical carotid atherosclerosis, intracranial atherosclerosis, and vertebral artery origin atherosclerosis.
CERVICAL CAROTID ATHEROSCLEROSIS
Carotid atherosclerosis accounts for about 7% of ischemic strokes. In the Framingham Heart study, the degree of stenosis was predicted by common baseline atherosclerotic risk factors such as older age, cigarette smoking, systolic blood pressure, and total cholesterol.1 In studies from the prestatin era, patients with an asymptomatic carotid stenosis less than 75% had an annual stroke risk of 1.3%; with a stenosis greater than 75% the annual risk of stroke was 2.0% to 2.5%. On the other hand, symptomatic carotid stenosis greater than 70% carries an annual stroke risk of 10% to 15%. Intensive medical therapy and carotid revascularization procedures reduce these risks. Clinical trials in carotid stenosis are geared toward answering two questions: Which patients should opt for revascularization procedures (versus intensive medical therapy alone), and which is the appropriate revascularization procedure (carotid endarterectomy [CEA] versus carotid artery stenting [CAS])?
Our understanding of the contemporary management of carotid stenosis is primarily shaped by the findings of the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST). After 4 years of follow-up, the primary outcome (stroke, myocardial infarction [MI], or death in the periprocedural period, or any ipsilateral stroke within 4 years) occurred in 7.2% of the 1262 patients in the CAS group and 6.8% of the 1240 CEA patients (P=.51).2 However, upon review of the individual components of the outcome, certain significant differences were observed. In comparison with the CEA group, patients in the CAS group had significantly higher perioperative strokes (4.1% CAS versus 2.3% CEA [P=.01]) and perioperative minor ipsilateral strokes (2.9% CAS versus 1.4% CEA [P=.009]).2 Perioperative MI was significantly higher among the CEA patients (1.1% CAS versus 2.3% CEA [P=.03]). The significant increase in strokes among the CAS patients was noted up to 4 years (6.2% CAS versus 4.7% CEA [P=.049]).2
The traditionally accepted outcome of stroke and death in the perioperative period, and stroke up to 4 years of follow-up (excluding MI) was significantly higher in the CAS arm (6.4% with CAS and 4.7% with CEA [P=.03]).2 A noteworthy feature of the assessment of outcomes in CREST was the regular screening for MIs with ECGs and cardiac enzymes before and after the procedure.2 Some critics question the inclusion of MI as a primary outcome while evaluating procedures intended for stroke prevention.3 As outcomes go, does a stroke or an MI have greater impact? The physical component of the Short Form-36 questionnaire for health-related quality of life was significantly worse at 1 year among stroke patients, but showed an uncertain effect among MI patients. The mental component in CREST was also significantly worse among stroke patients at 1 year.2 On the other hand, long-term mortality rates were higher among patients who had an MI in the perioperative period, even after adjustment of baseline comorbid factors.4 Whether the perioperative MI event is causally linked with later mortality or is a marker of patients with a greater atherosclerotic disease burden is unclear.
Symptomatic Carotid Stenosis
The North American Symptomatic Carotid Endarterectomy Trial (NASCET) has set the paradigm for revascularization of patients with moderate (50% to 69%) to severe (70% to 99%) symptomatic carotid stenosis. In this trial, patients with symptomatic carotid stenosis greater than or equal to 30% were randomized to medical therapy versus CEA.5
After a mean follow-up of 2 years, patients with severe stenosis showed a dramatic risk reduction of any ipsilateral stroke from 26% in the medical arm to 9% in the CEA arm. The absolute risk reduction was 17% (P<.001), which translated to a number needed to treat of six.6 Among patients with moderate stenosis, CEA showed a lower, but significant, risk reduction from 22.2% in the medical arm to 15.7% in the CEA arm. The absolute risk reduction was 6.5% (P=.045), resulting in a number needed to treat of 15.5 Patients with mild stenosis of 30% to 49% did not achieve a significant risk reduction of any ipsilateral stroke following CEA.5
The highest risk for a recurrent stroke is during the first month; the risk decreases with time because of plaque stabilization and the development of collaterals.7 Therefore, the benefit obtained from carotid revascularization declines with time from the initial event. However, very early revascularization procedures could potentially lead to hemodynamic changes within an acutely necrotic area of infarcted brain, resulting in intracerebral hemorrhage.7 To determine the ideal time for revascularization surgery, data from 5893 patients with 33,000 patient-years of follow-up from NASCET and the European Carotid Surgery Study were analyzed. Patients with moderate and severe stenosis showed significant benefits from CEA if the procedure was performed within 2 weeks of the index event (Case 4-1). The benefit declined thereafter and was no longer statistically significant beyond 2 weeks in the 50% to 69% group.8 Based on these data, the American Heart Association recommends that it is reasonable to perform CEA when indicated for patients with stroke or TIA within 2 weeks, rather than delaying surgery.9 Surgery within 2 weeks may be contraindicated for patients who have very large infarctions at high risk of hemorrhagic conversion, or already have hemorrhagic conversion of their infarctions.
In CREST, about 53% of the study population had symptomatic carotid stenosis. The periprocedural stroke, death, and MI rates in the symptomatic group were not significantly different between the CAS and CEA groups (6.7% with CAS versus 5.4% with CEA [P=.30]).10 The periprocedural rate of stroke and death was higher in CAS versus CEA (6.0% with CAS versus 3.2% with CEA [P=.02]).10 The rates of MI and cranial nerve palsies on the other hand were higher with CEA compared with CAS.
Case 4-1
An 81-year-old woman presented to the emergency department because of a 1-hour episode of transient expressive speech difficulty and right hand weakness. The patient was on aspirin 81 mg/d but not on a statin. MRI of the brain showed a 1 cm infarct in the left precentral gyrus on diffusion-weighted imaging. Magnetic resonance angiography (MRA) showed severe left internal carotid stenosis. Carotid duplex ultrasonography confirmed severe left internal carotid artery stenosis (80% to 99%) and less than 30% stenosis on the right side. An uneventful carotid endarterectomy (CEA) was performed 4 days after admission.
Comment. Given the patient’s age and symptomatic status, it was felt that she should undergo carotid revascularization, with CEA preferred. CEA is preferred over carotid artery stenting (CAS) given the higher complication rate with CAS in patients aged 70 years or older. One might suggest that a trial of aggressive medical therapy is warranted because she was not on a statin at the time of presentation.
Similar trends were noted in a preplanned meta-analysis of patient data from three randomized controlled European trials: Endarterectomy versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial, the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) trial, and the International Carotid Stenting Study (ICSS).11 The data included 3433 patients with symptomatic carotid stenosis, randomly assigned to CAS versus CEA. In the first 120 days after randomization, significantly more strokes and deaths occurred among the CAS group (8.9%) compared with the CEA group (5.8%; P=.0006).11 In aggregate, these data show that, for patients with symptomatic carotid stenosis, the short-term outcomes for stroke and death are better with CEA compared with CAS. Table 4-1 provides a list of indications where CAS may be a preferred option over CEA.
Table 4-1.
High Surgical Risk Factors for Carotid Endarterectomy Where Carotid Artery Stenting May Be Considered as an Option
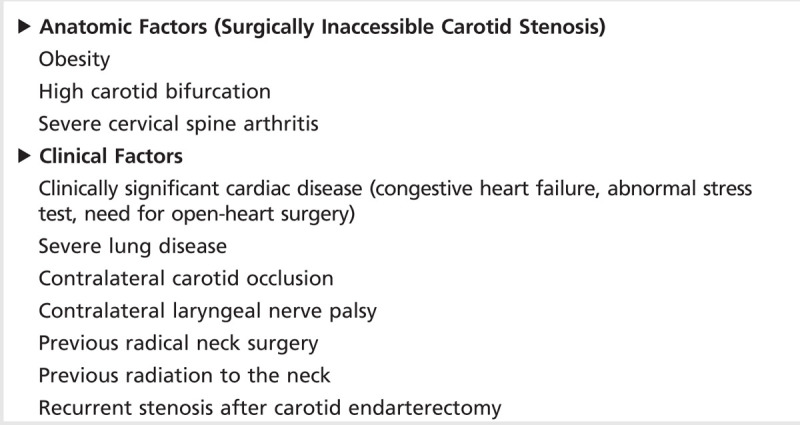
Data regarding intensive medical therapy for symptomatic carotid stenosis are lacking. Only about 15% of patients in NASCET were on lipid-lowering medication. New clinical trials should be undertaken for symptomatic patients with extracranial carotid stenosis utilizing the aggressive medical therapy regimen from the Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial (described in the section on intracranial atherosclerosis).
Asymptomatic Carotid Stenosis
The risks of stroke in patients with asymptomatic stenosis are lower than in those with symptomatic stenosis. Increasing evidence shows that intensive medical therapy only, without revascularization, can reduce the ischemic stroke risk dramatically. Over the past decade, the intensity of medical therapy has improved significantly. In NASCET, only 16% of patients assigned to the medical arm and 13% of patients assigned to the CEA arm were on lipid-lowering medications.5 In the Asymptomatic Carotid Surgery Trial-1 (ACST-1), use of lipid-lowering medications improved from 7% to 11% in the early 1990s at the start of the trial, to 80% to 82% in the late 2000s toward the end of long-term follow-up.12 The use of antihypertensive drugs similarly improved, corresponding to a drop in mean diastolic blood pressures over time.12
Abbott analyzed 11 asymptomatic carotid intervention studies between 1985 and 2007.13 Raw data from these trials were used to calculate rates of ipsilateral stroke, ipsilateral stroke/TIA, any territory stroke, and any territory stroke/TIA in the medical therapy arm; the rate of each outcome decreased during this time period.13 The outcome rates from medical therapy in the more contemporary studies were quite similar, if not better, than in the CEA arm in the ACAS trial.13 In conclusion, as seen in Case 4-2, extremely low stroke rates (1% per year or less) can be achieved by intensive medical therapy among patients with asymptomatic carotid stenosis. Asymptomatic patients with ongoing microemboli14 and men younger than 75 years merit consideration for CEA. Ongoing microemboli can be detected using transcranial Doppler studies, but these are not in widespread clinical use. Men below 75 years of age are considered candidates more frequently than women as a result of uncertainty regarding whether asymptomatic women benefit.
Special Considerations
Carotid revascularization in women. Across most carotid revascularization trials, women have carried more perioperative risk than men. One potential mechanism of this phenomenon is the smaller diameter of the carotid artery in women.15 Sex differences in perioperative risk from carotid revascularization in CREST have been published. The rate of 30-day stroke, death, or MI in the CAS arm was 4.3% among men and 6.8% among women.16 Among women, the 30-day stroke and death rate in the CAS arm was 5.5%, and 2.2% in the CEA arm (P=.013).16 Among symptomatic women, CAS resulted in a higher 30-day stroke and death rate compared with CEA (7.5% versus 2.7%, P=.03). In summary, women appear to have higher periprocedural stroke risk compared with men, with risks being potentially greater with CAS compared with CEA.
Carotid revascularization in older adults. An important finding in the CREST study was an effect modification of the primary outcome by age. Patients older than 70 years appeared to do better with CEA, whereas patients younger than 70 years fared better with CAS (P=.02 for interaction).2 The rate of primary outcome with CEA was 6.1% for subjects younger than 65 years, 6.8% for subjects between 65 and 74 years, and 7.4% for subjects 75 years and older.17 In contrast, the rate of primary outcome rose much more steeply with age in the CAS arm: 3.9% in patients younger than 65 years, 6.3% in the 65 to 74 years age group, and 12.7% in patients 75 years and older.17 This age-treatment interaction was found in symptomatic and asymptomatic subjects.17 A similar interaction was also found when the stroke end point (periprocedural stroke and postprocedure ipsilateral stroke) was examined in CREST.
The interaction with age was also seen in a meta-analysis of three carotid intervention trials: EVA-3S, SPACE, and ICSS.11 The event rate for any stroke or death in the CEA arm was 5.7% in subjects younger than 70 years and 5.9% in subjects 70 years and older. On the other hand, any stroke or death occurred in 5.8% of subjects younger than 70 years in the CAS arm and 12% of subjects 70 years and older.11
While the above lines of evidence suggest that CAS in older adults has high rates of 30-day stroke and death, very few clinical trial data of complication rates from CEA or medical therapy in this subgroup are available. The ACST-1 study had 30 of 1662 patients in the CEA arm who were over the age of 75 years, and 38 of 1701 subjects in the medical arm over the age of 75 years.12 Within this subgroup of elderly patients in ACST 1, medical therapy achieved equivalent results to revascularization by CEA (odds ratio: 0.81, 95% CI 0.43 to 1.51).12 In view of the uncertain benefit of CEA in asymptomatic, elderly patients, the American Academy of Neurology guidelines recommend consideration of CEA only in asymptomatic patients younger than 75 years.18
Case 4-2
An 80-year-old man was referred for left carotid stenosis detected by his internist after a carotid bruit was heard. He had a history of hypertension and dyslipidemia. He was on aspirin 325 mg/d, atorvastatin 80 mg/d, ramipril 10 mg/d, and a diuretic. Blood pressure was 136/78 mm Hg and heart rate was 64 beats/min and regular. Neurologic examination was normal, except for the presence of a left carotid bruit. Carotid duplex ultrasonography revealed 80% to 99% stenosis on the left and 30% to 49% on the right. CT angiography (CTA) was interpreted as showing 70% to 80% stenosis on the left and 40% on the right. Low-density lipoprotein was 51 mg/dL. The patient was counseled regarding the uncertain benefit of revascularization in his age group.
Comment. This patient was continued on aggressive medical therapy and has been symptom-free for 3 years. This type of patient could be considered for enrollment in a clinical trial such as CREST-2, which is comparing outcomes with aggressive medical therapy alone versus aggressive medical therapy plus carotid endarterectomy or carotid artery stenting.
In the CREST study, the rate of the primary outcome among asymptomatic patients was similar: 5.6% with CAS and 4.9% with CEA.10 However, the stroke and death rate by 4 years was 4.5% in the CAS arm and 2.7% in the CEA arm (P=.07).10 The study methods did not provide enough power to identify significant differences in the asymptomatic subgroup. A similar trial designed with sufficient power in the asymptomatic subgroup could plausibly have found the above difference in stroke and death rate to be significant.3
INTRACRANIAL ATHEROSCLEROSIS
Intracranial atherosclerotic disease is an important cause of ischemic stroke. An impressive racial difference exists, such that intracranial atherosclerotic disease–related strokes comprise 9%, 17%, and 15% of all ischemic strokes among white, African American, and Hispanic patients, respectively.19 The proportion is even higher in the Asian population. Patients with 50% to 99% stenosis of intracranial vessels who develop symptoms are at a 12% to 14% risk for a recurrent stroke during a 2-year follow-up, in spite of antiplatelet therapy or anticoagulation therapy. The annual risk may exceed 20% in high-risk groups.20
In the Warfarin-Aspirin Symptomatic Intracranial Disease Trial (WASID), subjects with stroke or TIA attributed to stenosis of an intracranial artery (intracranial carotid, middle cerebral, intracranial vertebral, or basilar) were randomized to receive warfarin (titrated to an international normalized ratio [INR] of 2 to 3) versus aspirin 1300 mg/d.21 The arterial stenosis was verified at 50% to 99% by cerebral angiogram. After a mean follow-up of 1.8 years, the primary end point (ischemic stroke, brain hemorrhage, or vascular death other than from stroke) occurred in 22.1% in the aspirin group and 21.8% in the warfarin group (P=.83). While not providing any efficacy benefit, the warfarin group had a significantly higher rate of death, major hemorrhage, and MIs,21 and the study was stopped early for safety concerns. Thus, in patients with symptomatic intracranial atherosclerotic disease, antiplatelet drugs are the mainstay of secondary prevention.9
The WASID investigators described the importance of risk factor control in prevention of recurrent ischemic stroke in patients with intracranial atherosclerotic disease.22 Over the 2-year follow-up period, the patients in WASID showed no improvement in blood pressure control. They did show improvements in the proportion of patients with total cholesterol less than 200 mg/dL (54.6% to 79.2%, P<.001) and low-density lipoprotein (LDL) cholesterol less than 100 mg/dL (28.7% to 55.9%, P<.001). In a multivariate analysis, systolic blood pressure greater than 140 mm Hg, no alcohol consumption, and total cholesterol greater than 200 mg/dL increased the risk of recurrent stroke, MI, or vascular death.22 This study laid the foundation of intensive medical management in contemporary studies of intracranial atherosclerosis.
The SAMMPRIS trial investigated the safety and efficacy of percutaneous transluminal angioplasty and stenting (PTAS, self-expanding Wingspan stent) with aggressive medical management versus aggressive medical management only, in patients with recent TIA or stroke within 30 days, attributed to 70% to 99% stenosis of a major intracranial artery.23 The various components of aggressive medical management are outlined in Table 4-2. The primary end point was stroke or death within 30 days or following a revascularization procedure of the target lesion during follow-up; or ischemic stroke during the long-term follow-up. The trial was stopped early for reasons of safety and futility.23
Table 4-2.
Intensive Medical Therapy for Patients With Intracranial Atherosclerosis, Used in the Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) Trial

SAMMPRIS stopped enrolling after 451 patients were randomized, when the primary end point at 30 days (stroke or death) occurred in 14.7% in the PTAS group and 5.8% in the medical management group (P=.002) (Case 4-3).23 A third of the strokes in the PTAS arm were brain hemorrhages. All strokes in the PTAS arm occurred within the first week after the procedure; most occurred within the first 24 hours. Periprocedural ischemic strokes were associated with older age, diabetes mellitus, basilar stenosis, and nonsmoking.24 Five (2.2%) stroke-related deaths occurred in the PTAS arm and one (0.4%) non–stroke-related death occurred with medical management. The 1-year rate of the primary end point was significantly higher in the PTAS arm (20.0%) versus the medical arm (12.2%; P=.009).23 The differences in 1-year event rates were primarily driven by the increased 30-day events in the PTAS arm. In SAMMPRIS, the event rates in the PTAS arm (14.7%) were significantly higher than anticipated from the Wingspan stent registry (4.5%).25 The medical arm, on the other hand, fared much better than historically expected from event rates in WASID at 1 month (5.8% observed rate in SAMMPRIS versus 10.7% expected based on WASID) and at 1 year (12.2% observed versus 25% expected).23 Differences in the intensity of medical management, including dual antiplatelet therapy for the first 3 months and aggressive blood pressure and LDL cholesterol reduction were probably responsible for the improvement in event rates.
Case 4-3
A 64-year-old woman presented with multiple episodes of dizziness, diplopia, and transient visual loss affecting both eyes over a 1-week period. Each episode lasted 5 minutes. She had a history of uncontrolled hypertension, dyslipidemia, diabetes mellitus, and smoking. CTA showed severe stenosis in the proximal third of the basilar artery. She was placed on aspirin, clopidogrel, and 40 mg rosuvastatin, and her blood pressure medication was intensified. After 3 months of dual antiplatelet therapy, clopidogrel was discontinued, and she continued on aspirin.
Comment. This patient should be placed on a SAMMPRIS-style regimen (Table 4-2). At present, there is no proven role for endovascular intervention in this type of patient. The importance of medication compliance and lifestyle modifications should be reinforced.
A subsequent detailed analysis of the 30-day events in the PTAS arm revealed that a large number of the ischemic strokes occurred from occlusion of perforators (more commonly the basilar perforators to the pons, or the lenticulostriate perforators from the middle cerebral artery). Stenting can result in a “snowplowing” effect in which atheromatous debris is pushed into the perforators and occludes them.26 A few wire perforations resulted in periprocedural subarachnoid hemorrhages. A significant number of intraparenchymal hemorrhages were also noted. They were associated with a higher degree of stenosis, a high activated clotting time (a laboratory test that measures intraprocedural anticoagulation) maintained during the procedure, along with a loading dose of clopidogrel 600 mg.24 Increased perfusion in the territory supplied by a previously stenosed vessel (hyperperfusion syndrome) is also a proposed mechanism.24
Balloon angioplasty alone, without stenting, has been proposed as a method to reduce perforator strokes. No randomized trials have compared angioplasty against intensive medical management. In a retrospective case series of 74 patients, technical success (less than 50% residual stenosis) was achieved in 92%, and periprocedural success (no event within 72 hours) was achieved in 88%. The 30-day stroke and death rate was 5%.27 While this complication rate is lower than seen with SAMMPRIS, there is a concern about the long-term durability of angioplasty and whether long-term restenosis rates will be favorable.
The high rate of recurrent events in patients with intracranial atherosclerotic disease has led to a search for novel approaches to reduce stroke risk. In a proof-of-concept study, 68 patients with symptomatic intracranial atherosclerotic disease were randomized to either standard medical management or medical management with brief, repetitive, bilateral arm ischemic preconditioning within 30 days of symptoms.28 Ischemic preconditioning was applied using a blood pressure tourniquet. The pressure was raised to 200 mm Hg and maintained for 5 minutes followed by 5 minutes of reperfusion. Five such cycles were performed in each arm, and this treatment was continued daily for 300 days. A marked reduction was seen in the incidence of recurrent ischemic stroke in the ischemic preconditioning group compared with standard medical treatment at 90 days (5% versus 23.3%, P<.01) and 300 days (7.9% versus 26.7%, P<.01).28 Upon single-photon emission CT imaging, ischemic preconditioning reduced the perfusion to metabolism mismatch in the ischemic areas of the brain.28 The ischemic preconditioning concept originated from the study of cardiac disease, in which brief episodes of myocardial ischemia were associated with reduced MI size, suggesting that brief, reversible ischemia could trigger vasculoprotective factors such as improved collateral flow. Further studies are needed to assess the preconditioning paradigm for the brain.
EXTRACRANIAL VERTEBRAL ARTERY DISEASE
Vertebral artery atherosclerosis, commonly affecting the V1 segment (origin of the artery from the subclavian artery), is found in about 20% of ischemic strokes in the vertebrobasilar territory, but is often associated with other causes of ischemic stroke.29 Many of these patients often have stenosis of bilateral vertebral artery origins and, less commonly, coexist with subclavian artery stenosis, making it difficult to identify which lesion is truly symptomatic.29 Patients with symptomatic vertebrobasilar atherosclerosis of 50% or more have a higher rate of recurrent events compared with patients with symptomatic carotid stenosis.30
Various operative approaches are described for vertebral origin stenosis, including transposition of the vertebral artery to the common carotid artery. However, these methods are not systematically studied and are not commonly performed. In a large series of 369 extracranial vertebral artery reconstructions from the 1990s, perioperative morbidity and mortality were low. Long-term patency of the vessel was 80%, and stroke-free survival was 97%.31 In a literature review of 300 endovascular interventions in symptomatic vertebral artery origin stenosis, periprocedural neurologic complications occurred in 5.5% and the restenosis rate was 26%.32 Nevertheless, at long-term follow-up (mean 14.2 months), the risk of death was 0.3%, and the risk for posterior stroke was 0.7%. The risks of adverse events are generally higher with distal vertebral or basilar interventions and when interventions are performed for urgent revascularization.32
In the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS), 16 patients with symptomatic vertebral artery stenosis were randomized to receive best medical therapy versus angioplasty or stenting.33 Over a mean follow-up period of 4.7 years, no vertebrobasilar territory strokes occurred in either arm of the study. In both arms of the study, three patients died of either MIs or carotid territory strokes. Thus, in this small group of patients, optimal medical treatment had equivalent outcomes to endovascular stenting.33
In the Oxford Vascular Study (OXVASC), a population-based study of 141 patients with vertebrobasilar strokes or TIAs, 37 (26.2%) had 50% or greater stenosis in the extracranial vertebral (n = 23) or intracranial vertebral/basilar arteries (n = 14).30 Medical therapy was determined by the patient’s general practitioners. The 90-day risk of recurrent vertebrobasilar events (strokes or TIAs) was high (46%) in the patients with 50% or greater stenosis compared with patients who did not have 50% or greater stenosis (21%). The incidence of recurrent vertebrobasilar strokes in the OXVASC population (excluding TIAs) was 22% within 90 days.30 This is higher than the recurrence rates of events in patients with carotid stenosis, although the medical therapy was not standardized in this study.
In the absence of clinical trial evidence of the superiority of revascularization methods, optimal medical therapy should be the default treatment modality for symptomatic vertebral artery stenosis. It is generally recommended that medical therapy should follow the principles and targets of the medical treatment of carotid atherosclerosis.34 Based on expert consensus, patients with acute vertebrobasilar ischemic syndromes who have angiographic evidence of a thrombus in the extracranial vertebral artery may benefit from 3 months of anticoagulation.34 Endovascular interventions may be considered on an individual basis, such as in the patient with recurrent vertebrobasilar symptoms in spite of maximal medical therapy.
CONCLUSION
Large artery atherosclerosis is an important, medically treatable, cause of ischemic stroke. Strict control of atherosclerotic risk factors is essential. Surgical and endovascular options benefit patients with symptomatic, moderate to severe carotid stenosis, although the benefit is not as robust in women. Endovascular or surgical interventions for asymptomatic cervical carotid stenosis must be held to stringent safety standards, given the low stroke rates achieved by contemporary intensive medical therapy. Aggressive medical therapy is also beneficial for symptomatic intracranial atherosclerosis and will serve as a benchmark for any future comparisons of endovascular treatment.
KEY POINTS
Symptomatic carotid stenosis has a markedly increased risk of stroke compared with asymptomatic stenosis.
In the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) study, periprocedural stroke was higher with carotid artery stenting, whereas periprocedural myocardial infarction was higher with carotid endarterectomy.
In the CREST study, periprocedural stroke had a greater impact on patients’ quality of life than periprocedural myocardial infarction.
Carotid revascularization within 2 weeks of a stroke or TIA is preferred over delaying revascularization for 6 weeks or more.
Symptomatic patients appear to have a lower complication rate with carotid endarterectomy than with carotid artery stenting.
A new clinical trial, CREST-2, will evaluate whether optimal medical therapy alone is the preferred treatment for asymptomatic carotid stenosis.
Intensified medical therapy has reduced the stroke rate for patients with asymptomatic carotid stenosis.
In CREST, women had a higher complication rate with carotid artery stenting, compared with carotid endarterectomy.
Patients older than 70 years have a higher complication rate with carotid artery stenting than with carotid endarterectomy.
Ethnic differences exist with regard to the frequency of intracranial atherosclerosis.
Antiplatelet therapy, rather than anticoagulation, is preferred for intracranial atherosclerosis.
Control of hyperlipidemia and blood pressure is critical in patients with intracranial atherosclerosis.
In the Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial, medical therapy was superior to intracranial stenting for stroke prevention.
Procedural strokes following intracranial stenting can be due to diverse mechanisms.
No high-quality studies have been done to evaluate treatment methods for vertebral artery origin stenosis.
Aggressive medical therapy is preferred as initial treatment for vertebral artery origin stenosis.
Footnotes
Relationship Disclosure: Dr Chaturvedi has received compensation for expert witness testimony and research support from AstraZeneca, Daiichi Sankyo, and Johnson & Johnson Services, Inc. Dr Chaturvedi serves as a consultant for Abbott Vascular and W. L. Gore & Associates, Inc; on the executive committee of the Asymptomatic Carotid Trial (ACT)–1 and Carotid Revascularization Endarterectomy versus Stenting Trial (CREST)–2 studies; and as a contributing editor to NEJM Journal Watch Neurology. Dr Bhattacharya has received a research grant from the Ethel & James Flinn Foundation to study poststroke depression in stroke patients.
Unlabeled Use of Products/Investigational Use Disclosure: Drs Chaturvedi and Bhattacharya report no disclosures.
REFERENCES
- 1.Fine-Edelstein JS,, Wolf PA,, O’Leary DH, et al Precursors of extracranial carotid atherosclerosis in the Framingham Study. Neurology 1994; 44 (6): 1046–1050. [DOI] [PubMed] [Google Scholar]
- 2.Brott TG,, Hobson RW, 2nd,, Howard G, et al Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 2010; 363 (1): 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaturvedi S,, Wechsler LR. Carotid revascularization strategies: the need for more data. Stroke 2012; 43 (4): 929–930. [DOI] [PubMed] [Google Scholar]
- 4.Blackshear JL,, Cutlip DE,, Roubin GS, et al Myocardial infarction after carotid stenting and endarterectomy: results from the carotid revascularization endarterectomy versus stenting trial. Circulation 2011; 123 (22): 2571–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnett HJ,, Taylor DW,, Eliasziw M, et al Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1998; 339 (20): 1415–1425. [DOI] [PubMed] [Google Scholar]
- 6.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991; 325 (7): 445–453. [DOI] [PubMed] [Google Scholar]
- 7.Rajamani K,, Chaturvedi S. Surgery insight: carotid endarterectomy—which patients to treat and when? Nat Clin Pract Cardiovasc Med 2007; 4 (11): 621–629. [DOI] [PubMed] [Google Scholar]
- 8.Rothwell PM,, Eliasziw M,, Gutnikov SA, et al Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet 2004; 363 (9413): 915–924. [DOI] [PubMed] [Google Scholar]
- 9.Furie KL,, Kasner SE,, Adams RJ, et al Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42 (1): 227–276. [DOI] [PubMed] [Google Scholar]
- 10.Silver FL,, Mackey A,, Clark WM, et al Safety of stenting and endarterectomy by symptomatic status in the Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST). Stroke 2011; 42 (3): 675–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carotid Stenting Trialists’ Collaboration, Bonati LH,, Dobson J,, Algra A, et al Short-term outcome after stenting versus endarterectomy for symptomatic carotid stenosis: a preplanned meta-analysis of individual patient data. Lancet 2010; 376 (9746): 1062–1073. [DOI] [PubMed] [Google Scholar]
- 12.Halliday A,, Harrison M,, Hayter E, et al 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet 2010; 376 (9746): 1074–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbott AL. Medical (nonsurgical) intervention alone is now best for prevention of stroke associated with asymptomatic severe carotid stenosis: results of a systematic review and analysis. Stroke 2009; 40 (10): e573–e583. [DOI] [PubMed] [Google Scholar]
- 14.Spence JD,, Coates V,, Li H, et al Effects of intensive medical therapy on microemboli and cardiovascular risk in asymptomatic carotid stenosis. Arch Neurol 2010; 67 (2): 180–186. [DOI] [PubMed] [Google Scholar]
- 15.Rothwell PM,, Slattery J,, Warlow CP. Clinical and angiographic predictors of stroke and death from carotid endarterectomy: systematic review. BMJ 1997; 315 (7122): 1571–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howard VJ,, Lutsep HL,, Mackey A, et al Influence of sex on outcomes of stenting versus endarterectomy: a subgroup analysis of the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST). Lancet Neurol 2011; 10 (6): 530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voeks JH,, Howard G,, Roubin GS, et al Age and outcomes after carotid stenting and endarterectomy: the carotid revascularization endarterectomy versus stenting trial. Stroke 2011; 42 (12): 3484–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaturvedi S,, Bruno A,, Feasby T, et al Carotid endarterectomy—an evidence-based review: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2005; 65 (6): 794–801. [DOI] [PubMed] [Google Scholar]
- 19.White H,, Boden-Albala B,, Wang C, et al Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation 2005; 111 (10): 1327–1331. [DOI] [PubMed] [Google Scholar]
- 20.Qureshi AI,, Feldmann E,, Gomez CR, et al Intracranial atherosclerotic disease: an update. Ann Neurol 2009; 66 (6): 730–738. [DOI] [PubMed] [Google Scholar]
- 21.Chimowitz MI,, Lynn MJ,, Howlett-Smith H, et al Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med 2005; 352 (13): 1305–1316. [DOI] [PubMed] [Google Scholar]
- 22.Chaturvedi S,, Turan TN,, Lynn MJ, et al Risk factor status and vascular events in patients with symptomatic intracranial stenosis. Neurology 2007; 69 (22): 2063–2068. [DOI] [PubMed] [Google Scholar]
- 23.Chimowitz MI,, Lynn MJ,, Derdeyn CP, et al Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 2011; 365 (11): 993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiorella D,, Derdeyn CP,, Lynn MJ, et al Detailed analysis of periprocedural strokes in patients undergoing intracranial stenting in Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS). Stroke 2012; 43 (10): 2682–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bose A,, Hartmann M,, Henkes H, et al A novel, self-expanding, nitinol stent in medically refractory intracranial atherosclerotic stenoses: the Wingspan study. Stroke 2007; 38 (5): 1531–1537. [DOI] [PubMed] [Google Scholar]
- 26.Derdeyn CP,, Fiorella D,, Lynn MJ, et al Mechanisms of stroke after intracranial angioplasty and stenting in the SAMMPRIS trial. Neurosurgery 2013; 72 (5): 777–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen TN,, Zaidat OO,, Gupta R, et al Balloon angioplasty for intracranial atherosclerotic disease: periprocedural risks and short-term outcomes in a multicenter study. Stroke 2011; 42 (1): 107–111. [DOI] [PubMed] [Google Scholar]
- 28.Meng R,, Asmaro K,, Meng L, et al Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology 2012; 79 (18): 1853–1861. [DOI] [PubMed] [Google Scholar]
- 29.Caplan LR,, Wityk RJ,, Glass TA, et al New England Medical Center Posterior Circulation registry. Ann Neurol 2004; 56 (3): 389–398. [DOI] [PubMed] [Google Scholar]
- 30.Marquardt L,, Kuker W,, Chandratheva A, et al Incidence and prognosis of > or = 50% symptomatic vertebral or basilar artery stenosis: prospective population-based study. Brain 2009; 132 (pt 4): 982–988. [DOI] [PubMed] [Google Scholar]
- 31.Berguer R,, Flynn LM,, Kline RA,, Caplan L. Surgical reconstruction of the extracranial vertebral artery: management and outcome. J Vasc Surg 2000; 31 (1 pt 1): 9–18. [DOI] [PubMed] [Google Scholar]
- 32.Eberhardt O,, Naegele T,, Raygrotzki S, et al Stenting of vertebrobasilar arteries in symptomatic atherosclerotic disease and acute occlusion: case series and review of the literature. J Vasc Surg 2006; 43 (6): 1145–1154. [DOI] [PubMed] [Google Scholar]
- 33.Coward LJ,, McCabe DJ,, Ederle J, et al Long-term outcome after angioplasty and stenting for symptomatic vertebral artery stenosis compared with medical treatment in the Carotid And Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomized trial. Stroke 2007; 38 (5): 1526–1530. [DOI] [PubMed] [Google Scholar]
- 34.Brott TG,, Halperin JL,, Abbara SG, et al 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. Developed in collaboration with the American Academy of Neurology and Society of Cardiovascular Computed Tomography. Catheter Cardiovasc Interv 2013; 81 (1): E76–E123. [DOI] [PubMed] [Google Scholar]


