Abstract
Purpose of Review:
This article aims to provide a broad overview of pediatric arterial ischemic stroke, from recognition and diagnosis to the short-term and long-term management based on current available literature.
Recent Findings:
Arterial ischemic stroke in children represents a significant disorder with a concerning high rate of adverse outcomes, including potentially preventable recurrent stroke. Although awareness of pediatric stroke is increasing, diagnosis is still commonly delayed or missed altogether, particularly in younger children. Current vascular imaging techniques have limitations in accurate diagnosis of arteriopathies that are now recognized as an important cause of childhood stroke. Significant variability exists in treatment of pediatric stroke. Management is based on published consensus guidelines; however, individual children require an individualized approach.
Summary:
As pediatric stroke specialists become increasingly available, the collaboration of such experts on individual management is crucial. Definitive evidence-based treatment for pediatric stroke awaits the development of randomized controlled trials.
INTRODUCTION
Awareness about childhood stroke is increasing. Stroke is among the top 10 causes of death and a significant cause of long-term morbidity in childhood.1 Stroke is traditionally classified into ischemic and hemorrhagic subtypes. This review focuses on pediatric arterial ischemic stroke (AIS) only.
AIS occurs with a frequency of 2 to 3/100,000 children per year.2 Many cases occur during the perinatal or neonatal period, during which the incidence is approximately 1 in 4000 live births.3 Boys are affected more frequently.4 Pediatric AIS differs from adult AIS in several ways. First, AIS is rarer and has a subtler clinical presentation and a wider differential diagnosis in children, which results in delayed and sometimes missed diagnosis.5 Second, the coagulation, vascular, and adaptive components of the neurologic systems differ between children and adults. Third, risk factors for pediatric AIS are frequently multiple, age-dependent across childhood, and distinct from those in adults, necessitating a complex set of etiologic investigations. Fourth, there is still a paucity of robust data on the rates of outcomes in children, including stroke volume, recurrent stroke, and chronic disability. Consequently, evidence supporting specific treatments is lacking, leading to variability in management. Consensus-based pediatric stroke treatment guidelines differ from each other in some aspects—for example, initial aspirin versus anticoagulation for noncardiac stroke.6,7 Finally, the burden of pediatric AIS is longer lasting because a child who survives AIS is expected to live much longer than an adult stroke survivor.
RECOGNITION OF ARTERIAL ISCHEMIC STROKE
Recognition of AIS in neonates and infants is challenging because acute focal neurologic deficits, typically evident in older children and adults, are uncommon. Neonates usually present with seizures and other nonspecific symptoms.8 AIS should be suspected in any newborn with seizures, particularly when seizures begin more than 12 hours after birth.9 Neonates may also present with encephalopathy (irritability, lethargy, and poor feeding) or recurrent apnea. Focal deficits occur in less than 25% of all newborns with AIS. Sometimes acute neonatal AIS is not diagnosed until later infancy or childhood when hemiparesis becomes gradually evident with maturation. In such children, imaging shows remote AIS, which is presumed to have occurred in the prenatal or perinatal period and is called “presumed perinatal ischemic stroke.” The typical history includes motor delay, early hand dominance (before the first birthday), and focal seizures evolving into a classical hemiplegic cerebral palsy.
With increasing age, acute AIS manifests with a clinical presentation similar to that in adults, namely abrupt onset of focal neurologic deficits. However, pediatric health care providers are still not always attuned to the possibility of stroke in childhood, and delayed diagnosis is a consequence. The most common manifestation is hemiparesis; however, depending on the vascular territory involved, aphasia, ataxia, dysarthria, visual deficits, and cranial nerve palsies can occur. Seizures remain an important manifestation even in older children. Some children report headache. A sudden-onset thunderclap headache or neck, shoulder, or occipital pain should arouse suspicion of cervical artery dissection. A typical mode of presentation seen in children is a stuttering/fluctuating course of neurologic deficits.10 This should alert the clinician to the possibility of an underlying vasculopathy involving large vessels of the circle of Willis. Recurrent TIAs also raise the possibility of a vasculopathy or a thromboembolic source. A feature unique to children is a history of paroxysmal episodes of focal neurologic dysfunction precipitated by hyperventilation, which should raise the suspicion of moyamoya. Exercise, strenuous bursts of physical activity, or excessive crying are the usual causes for hyperventilation. The mechanism underlying this phenomenon is ischemia secondary to cerebral vasoconstriction produced by carbon dioxide reduction during hyperventilation.
Based on the patient’s age, awareness of stroke mimics such as focal seizures, hemiplegic migraine, demyelination, tumor with hemorrhage, hypoglycemia, and conversion disorder is important in any child with acute focal deficits.11 Children with progressive vasculopathies such as moyamoya or medium/small vessel CNS vasculitis can present with progressive neurocognitive decline due to subtle recurrent AIS events.
CLINICAL AND HISTORICAL SEARCH FOR ETIOLOGY AND RISK FACTORS
In addition to the history pertaining to the acute presentation, a meticulous history of risk factors should also be obtained.12 Risk factor identification informs three key management questions regarding antithrombotic therapy strategies: (1) Are any predisposing risk factors present? (2) Does the patient have an acute trigger for thrombosis? (3) What is the stroke recurrence risk in the immediate and distant future?
In neonatal AIS, details of maternal, antenatal, perinatal, intrapartum, and immediate postnatal history are critical. Risk factors associated with AIS include maternal (gestational diabetes mellitus, hypertension), perinatal (perinatal depression, perinatal asphyxia, complicated delivery, instrumentation), and neonatal (congenital heart disease, dehydration, and infection). In the absence of any risk factors, a placental source of thrombosis may be considered. Placental vasculopathy may be an important but overlooked cause of perinatal AIS.13 Placental examination can be helpful and can demonstrate placental infarction or infection, which predispose the patient to neonatal stroke.
In older children with AIS, infection, dehydration, trauma, head/neck manipulation, congenital heart disease, systemic diseases (hepatic, renal, gastrointestinal, hematologic, and rheumatologic conditions), iron deficiency anemia, and medication ingestion or substance abuse must be ruled out. Dysmorphic features, neurocutaneous markers, and connective tissue disorders may suggest syndromic genetic vasculopathy.
Risk factors common to both neonates and older children include congenital abnormalities of the coagulation/fibrinolytic system (protein C, protein S, antithrombin, and genetic abnormalities including factor V Leiden mutation, prothrombin gene G20210A, and homozygous thermolabile methylenetetrahydrofolate [MTHFR] mutations). A family history of thrombophilia includes myocardial infarction or stroke, deep vein thrombosis, pulmonary embolism, recurrent spontaneous miscarriages, and any family member on “blood thinners.”
In general, perinatal/neonatal AIS tends to be a one-time event unless the patient has a risk factor such as congenital heart disease or thrombophilia.14 In older children, recurrence risk ranges from 10% to 60% and is determined by the etiology and mechanism of stroke.15 Some children (10% to 15%) never have a clear cause identified despite exhaustive investigations.
CONFIRMATION OF DIAGNOSIS: NEUROIMAGING
Initial imaging by CT frequently (in 40% to 80% of cases) misses evidence that would allow for AIS diagnosis.16 Hence, MRI of the brain and magnetic resonance angiography (MRA) of the intracranial (and ideally neck) arteries is indicated for any child with suspected acute AIS. Perfusion-weighted imaging, which may be useful in determining the ischemic penumbra in adults, has not been systematically studied in children. MRA has technical limitations in newborns that may result in undercalling or overcalling of vascular abnormalities. Sedation remains a major barrier to acute MRI in young or noncooperative children, delaying the diagnosis. Collaborative institutional protocols with neuroradiology and anesthesia for emergent MRI under sedation can be helpful. CT remains an option in those who cannot get an MRI even though radiation exposure is an issue. CT angiography (CTA) in the same setting can rule out major circle of Willis abnormalities. Although MRA and CTA have increased the diagnostic yield in pediatric AIS, invasive catheter cerebral angiography still plays an important role either when MRA or CTA is unremarkable or when MRA or CTA has detected a vascular abnormality of unclear etiology. In the authors’ experience, catheter cerebral angiography has been of added value in cervical artery dissection17 and CNS medium-vessel vasculitis (unpublished observations). It has a clear role in presurgical planning of revascularization procedures for moyamoya by demonstrating existing and potential external carotid artery collaterals. Arterial wall imaging, which is increasingly used in adults for detection of active atherosclerotic plaques in intracranial and neck arteries and for diagnosis of CNS vasculitis and cervical artery dissection,18 appears to have a potential role in arteriopathy-related pediatric AIS, where gadolinium enhancement of the arterial wall at the site of stenosis may suggest inflammatory etiologies.19
The role of cranial ultrasound in AIS diagnosis is limited to infants with an open anterior fontanelle. It can be used only as a broad screening tool to detect large areas of ischemia. It remains a good bedside noninvasive modality but is heavily operator dependent, thereby precluding its use as a regular tool for AIS diagnosis. The role of transcranial Doppler in sickle cell disease is well established; for more on sickle cell disease and stroke, see “Genetic Stroke Syndromes” by Drs Kevin Barrett and James Meschia in this issue of CONTINUUM.
ACUTE MANAGEMENT AFTER CONFIRMATION OF DIAGNOSIS
After confirmation of AIS, neuroprotection, specific antithrombotic therapy, and concurrent search for etiology and risk factors during the initial phase are the cornerstones of acute management. A multidisciplinary involvement of a variety of pediatric subspecialty teams including neurology, general pediatrics, neuroradiology, intensive care, thrombosis/hematology, and rehabilitation is generally essential for most stroke cases. Additional involvement from disciplines such as cardiology, interventional neuroradiology, neurosurgery, infectious diseases, and rheumatology can be beneficial depending upon individual cases.
Neuroprotection
Although clear evidence-based data are lacking in children, neuroprotection is logical based on the presumed beneficial effect on adult stroke outcomes. The main aim of neuroprotection is to prevent recruitment of the ischemic penumbra and avoidance of malignant cerebral edema. The American Heart Association (AHA) guidelines have reviewed the role of neuroprotection.6 Simple measures such as avoidance of hyperthermia, hypotension/hypertension, hypovolemia/hypervolemia, and hypoglycemia/hyperglycemia are suggested. Early detection and prompt treatment of seizures (particularly status epilepticus) are recommended to prevent infarct expansion. The authors generally prefer to manage acute stroke patients in intensive care for about 48 to 72 hours, particularly those with higher perceived complication risk (large middle cerebral artery [MCA] or posterior fossa strokes).
Antithrombotic Therapy
Antithrombotic therapy (anticoagulant or antiplatelet therapy) is applicable to vaso-occlusive territorial thromboembolic AIS only; therefore, a determination of whether the AIS pattern is vaso-occlusive or watershed/borderzone on neuroimaging is critical. Antithrombotic therapy might not be an option for watershed infarction because hypoperfusion (and not thromboembolism) is the underlying mechanism. However, the two can coexist within the same child in the setting of congenital heart disease. When considering antithrombotic therapy in any child with AIS, it is critical to continually balance the risk (hemorrhagic complication) of antithrombotic therapy with the risk of nontreatment (extension of preexisting infarction and recurrence of stroke). Because of the lack of clinical trials, antithrombotic therapy varies considerably across centers based on physician preference. The AHA6 and American College of Chest Physicians7 guidelines have extensively reviewed antithrombotic therapy in pediatric AIS.
Thrombolytic therapy. IV tissue plasminogen activator (tPA) is the standard of care in adults with acute AIS and improves outcomes. In children, dose, safety, and efficacy of tPA (IV or local intra-arterial) in AIS have not been established. The risk-benefit ratio of tPA in pediatric AIS is unknown. Use of IV tPA in children for systemic thrombolysis has been associated with a higher complication rate than in adults,20 and children with AIS rarely present within the 4.5-hour (for IV tPA) or 6-hour (intra-arterial tPA) window. Given the fact that outcome from AIS in children is more favorable21 in general than in adults, the drive to initiate IV or intra-arterial tPA even in a child presenting within the appropriate time window should be carefully scrutinized. Individual case reports that have documented successful use of tPA in children are not generalizable.22 The American College of Chest Physicians guidelines7 currently recommend against the use of tPA in childhood AIS outside of clinical trials. The AHA guidelines6 take the same stance, although consensus is lacking regarding the use of tPA in older adolescents who otherwise meet the adult tPA eligibility criteria. The paucity of data with which to make any recommendations regarding mechanical thrombectomy is even greater.
Anticoagulant therapy and antiplatelet therapy. After the diagnosis of acute AIS, antiplatelet and anticoagulant therapies in children are usually selected based on the perceived mechanism for AIS. The rationale of initial therapy is to limit the extension of occlusive thrombosis and early recurrent thrombotic stroke, whereas subsequent maintenance therapy aims to prevent longer-term recurrence. To date, no randomized controlled trials of anticoagulant or antiplatelet therapy have been conducted in pediatric AIS. Some cohort studies have assessed safety and failure rates for anticoagulant and antiplatelet therapy23,24,25; the largest nonrandomized, multicenter, observational cohort study of more than 600 children was conducted via the International Pediatric Stroke Study (IPSS) group.24 The IPSS is a growing body of pediatric stroke investigators that collects comprehensive information on pediatric stroke cases at participating centers across the world through standardized data collection forms with a central database housed in Toronto. In that IPSS cohort, initial acute therapy included anticoagulant therapy (27%), aspirin (28%), anticoagulant therapy and aspirin combination (16%), and no treatment (30%). In that study, subtypes associated with any use of anticoagulant therapy were dissection and congenital heart disease. Factors associated with nonuse of anticoagulant therapy included sickle cell disease and location of the enrollment center in the United States. Antiplatelet therapy use was more frequent in moyamoya, whereas nonuse was more frequent in dissection (anticoagulant therapy being the preferred medication), altered consciousness, and bilateral ischemia.
From a practical standpoint, consensus exists among pediatric stroke experts that some level of antithrombotic therapy is beneficial in preventing acute progression of thrombus and stroke recurrence. The authors of this article generally tend to initiate anticoagulant therapy, regardless of perceived mechanism, unless contraindications are present. Either unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH) without a loading dose is preferred. UFH is chosen when the perceived risk of hemorrhagic complications is higher and a need for rapid reversal is anticipated or when invasive procedures (catheter cerebral angiography, lumbar puncture, etc) are planned. We perform a screening head CT 3 days after starting heparin to rule out subclinical significant intracranial hemorrhage; if this is unremarkable, UFH is switched to LMWH. In moyamoya and sickle cell disease, we prefer to start aspirin over anticoagulant therapy because of perceived higher risk of hemorrhage with anticoagulant therapy. After risk factor screening is complete, antithrombotic therapy duration and type are reviewed. When either aspirin or anticoagulant therapy fails (failure defined as radiologic or clinical TIA/stroke recurrence), the authors of this article have given dual therapy with aspirin and anticoagulant therapy in selected cases. In children older than 12 years of age whose treatment with aspirin fails, the authors have also switched over to clopidogrel. If hemorrhagic complications (4% symptomatic risk on anticoagulant therapy26) occur on antithrombotic therapy, a careful review of the need to prevent stroke recurrence and avoid hemorrhagic complications is undertaken.
Concurrent Search for Risk Factors and Etiology of Arterial Ischemic Stroke
A thorough search for stroke risk factors should be concurrently undertaken while neuroprotection and antithrombotic therapy is being administered. Dedicated neck vessel imaging (if not performed initially) by either MRA or CTA can be helpful. Catheter cerebral angiography may need consideration if cervical artery dissection17 or CNS vasculitis are suspected. In moyamoya, early catheter cerebral angiography and cerebrovascular-reactivity-functional MRI (fMRI) may be helpful if surgical revascularization is indicated urgently. All cases merit cardiac echo with bubbled saline study to rule out a cardiac source of thromboembolism. Blood work that merits immediate completion on confirmation of stroke includes complete blood cell count, platelet count, international normalized ratio, partial thromboplastin time, D-dimer, electrolyte and glucose levels, and renal or liver function tests. Investigations that may be helpful in individual cases include iron profile, inflammatory markers (erythrocyte sedimentation rate, C-reactive protein, complement, von Willebrand antigen, and CSF analysis for opening pressure, cell count, protein, and cultures), serology for infectious agents, and genetic studies.
The yield and influence of prothrombotic workup on stroke management and recurrence have been recently reviewed.27 The presence of a these disorders appears to be an important predisposing cause for thrombotic stroke, frequently acting in concert with other triggers for the stroke. A typical thrombophilia panel includes protein C, protein S, antithrombin, lupus anticoagulant, anticardiolipin antibody, factor VIII, plasma homocysteine, factor V Leiden mutation and activated protein C resistance, prothrombin gene G20210A mutation, MTHFR mutation, and lipoprotein-a. Interpretation of abnormalities in a prothrombotic workup is challenging; any abnormality on the prothrombotic workup except genetic tests should be repeated after at least 12 weeks of initial testing and off anticoagulant therapy to determine whether the abnormality was transient or persistent, as the latter may influence duration of antithrombotic therapy.
MANAGEMENT BASED ON ETIOLOGIES OF PEDIATRIC ARTERIAL ISCHEMIC STROKE
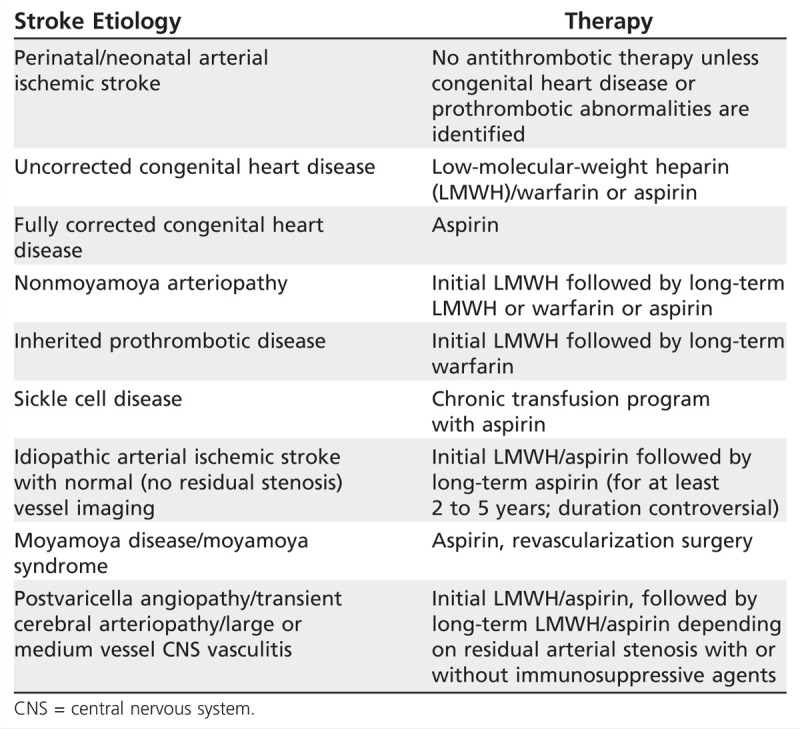
Most pediatric AIS cases fall into one of the following broad categories: perinatal/neonatal, cardiogenic, arteriopathic (vasculopathic), prothrombotic, and idiopathic/cryptogenic. This categorization is helpful in tailoring management and estimating prognosis. Strategies for antithrombotic therapy are summarized in Table 7-1.
Table 7-1.
Long-Term Antithrombotic Therapy Based on Arterial Ischemic Stroke Etiology
Perinatal/Neonatal Arterial Ischemic Stroke
Antithrombotic therapy is rarely indicated in most cases because of negligible recurrence risk except in the presence of congenital heart disease28 and abnormal prothrombotic workup. The focus of acute management is neuroprotection. Long-term management involves rehabilitation, including physiotherapy, occupational therapy, and speech therapy. Because deficits may only emerge with maturation, longitudinal observation is necessary even in apparently healthy infants. It is possible to select neonates at risk of hemiparesis for early interventions based on the presence of acute restricted diffusion signal in the corticospinal tract emanating from the zone of primary infarction and best seen in the ipsilesional cerebral peduncle on the acute MRI. This signal represents acute Wallerian degeneration29 and reliably predicts future hemiplegia (Case 7-1).30 Recently, constraint-induced therapy has been demonstrated to be effective in infants and children with hemiparesis due to AIS. Neuropsychological testing can be helpful during early school age.
Case 7-1
A 4-day-old newborn boy presented with apneic episodes, reduced feeding, and recurrent twitching of the right upper and lower limb beginning on the third day of life. Antenatal history and pregnancy were unremarkable except the mother’s history of three spontaneous miscarriages before this pregnancy, for which no cause was found. Birth was unremarkable without any complications or need for assisted delivery. Apgar scores were 7 and 9 at 1 minute and 5 minutes, respectively. The baby was well on the first 2 days, without any neurologic or systemic concerns, but he began having seizures as described above soon after he was discharged home on day 3. Neonatal neurologic examination did not reveal any focal findings. MRI of the brain revealed a large left middle cerebral artery territory stroke; magnetic resonance angiogram appeared normal. Diffusion-weighted imaging also showed the presence of restricted diffusion along the corticospinal tracts (Figure 7-1). Seizures were well controlled with phenobarbital. Despite the presence of stroke, the baby was otherwise well and nonencephalopathic. Septic and prothrombotic workup was normal. Placenta was available for histopathologic examination and showed widespread thrombosis and infarction, suggesting placental vasculopathy, which was presumed to be the cause of the baby’s stroke. Follow-up at 2 years of age revealed moderate-severe right-sided hemiplegia and mild language and cognitive delay.
Figure 7-1.
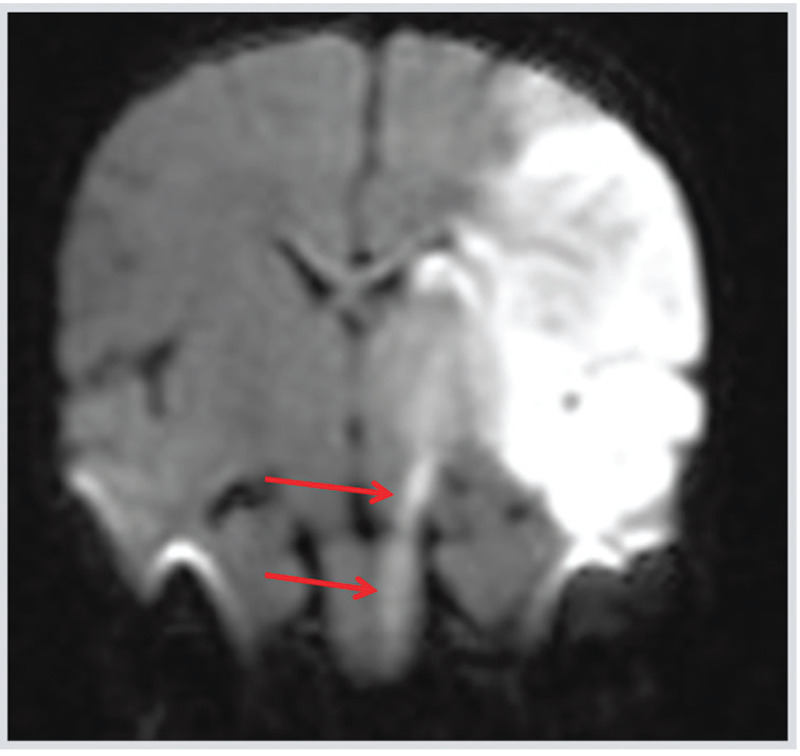
Coronal diffusion-weighted image (DWI) of the patient in Case 7-1 showing a neonatal acute left middle cerebral artery arterial ischemic stroke showing acute Wallerian degeneration along the descending corticospinal tract from the infarcted area to the midbrain (cerebral peduncle) (arrows).
Comment. This case highlights the lack of identifiable focal deficits on neurologic examination in neonatal stroke, although in this case focal seizures were a clue. The etiology of “idiopathic” neonatal stroke was likely placental vasculopathy. The case also illustrates well the value of restricted diffusion in the corticospinal tract as a marker of pre-Wallerian degeneration in acute neonatal ischemic stroke and as a predictor of future hemiplegia.
Arterial Ischemic Stroke With Congenital Heart Disease
Multiple mechanisms interact to produce AIS in congenital heart disease: thromboembolism from valvular or septal defects, cardiac catheterization, valvular/septal devices, intracardiac shunts, cardiopulmonary bypass, and direct surgical procedures; polycythemia in cyanotic congenital heart disease; and hypotension. Additional factors include endocarditis, cardiomyopathy, cardiac arrhythmias, and cardiac arrest. Children with cardiac disorders may be prothrombotic as a result of chronic illness, infection, iron deficiency anemia, persistent low-grade inflammation, and multiple surgical or circulatory support interventions.28
Antithrombotic therapy is strongly recommended for all children with congenital heart disease and AIS. Anticoagulant therapy is preferred until risk factors causing stroke are fully corrected. Antithrombotic therapy duration and type depends on the child’s age, congenital heart disease, and procedure type. Details of antithrombotic therapy recommendations in congenital heart disease are available.6,7 Although congenital heart disease correction should theoretically eliminate the cardiogenic stroke risk, recent data demonstrate that recurrence risk remains increased for extended periods even after full congenital heart disease correction.28
Arterial Ischemic Stroke With Arteriopathy
Beyond the newborn period, arteriopathy (vasculopathy) accounts for approximately 60% of all childhood AIS and predicts stroke recurrence.15 Steno-occlusive arteriopathies cause stroke by local in situ thrombosis, artery-to-artery embolism, or hypoperfusion. Classification has been attempted based on vessel size, distribution, the perceived pathology, temporal evolution, and genetic or nongenetic basis.31,32 Recognition of a radiologic pattern of a vasculopathy phenotype is beneficial in guiding management. This is feasible with judicious use of vascular imaging techniques such as MRA, CTA, catheter cerebral angiography, and arterial wall imaging. This discussion focuses on the three commonly encountered arteriopathies associated with pediatric AIS: cervical artery dissection, transient cerebral arteriopathy, and moyamoya.33
Cervical arterial dissection. Dissection involving the intracranial or extracranial portion of the internal carotid artery (ICA) and vertebral artery (VA) causes approximately 14% of pediatric AIS cases.34 These can occur spontaneously or in association with trivial mechanical injury or major head or neck trauma. The intradural-extradural junction of the ICA and C1-C2 portion of the VA (Case 7-2) are common sites for dissection. Stroke occurs as a result of artery-artery embolism from intimal disruption, arterial occlusion causing hypoperfusion, or distal extension of dissection along the artery wall, to disrupt the origin of branch arteries. Anticoagulant therapy is usually favored over aspirin as the first line of antithrombotic therapy in most centers, although recent adult data have suggested no difference between the two approaches.35 Published guidelines recommend initial anticoagulant therapy for pediatric AIS with cervical artery dissection. Anticoagulant therapy duration ranges from 6 weeks to 6 months after diagnosis. Anticoagulant therapy is followed by aspirin for several years. Stroke recurrence can occur even several years after the initial event, longer than the commonly believed 6- to 12-month period. The authors consider degree of residual vessel stenosis, stroke location (brainstem at risk), and associated risk factors in deciding antithrombotic therapy duration. Anticoagulant therapy remains controversial for intracranial arterial dissection; management needs to be tailored to the individual situation.
Transient cerebral arteriopathy. Transient cerebral arteriopathy of childhood is a well-described unilateral focal arteriopathy of presumed inflammatory origin. Transient cerebral arteriopathy is characterized by basal ganglia infarction with ipsilateral irregular stenosis involving the carotid T-junction represented by the distal ICA, proximal anterior cerebral artery (ACA), and proximal MCA (Case 7-3, Figure 7-5). Focal cerebral arteriopathy is a recently coined term for this same pattern of radiologic abnormalities.36 An inflammatory basis is presumed because postvaricella angiopathy presents with an identical clinicoradiologic syndrome following varicella infection within the preceding year. In postvaricella angiopathy, varicella virus has been demonstrated in the arterial wall at the carotid T-junction on histopathology.37 Following that description of postvaricella angiopathy, other infectious agents such as enteroviruses, Borrelia, and Bartonella have been associated with this syndrome. In addition to acute antithrombotic therapy, some children have been treated with a short course of anti-inflammatory therapy, such as high-dose corticosteroids (IV methylprednisone) followed by tapering oral doses for 6 to 12 weeks (unpublished observations). Such treatment remains controversial pending published data supporting its safety and efficacy. Acyclovir coverage can also be considered until varicella is ruled out if the patient has a history of chickenpox within a year preceding the stroke event. Follow-up imaging is helpful to decide on maintenance antithrombotic treatment, either anticoagulation or aspirin. In transient cerebral arteriopathy, the vasculopathy stabilizes by 6 months with no further worsening of the arterial findings; progression makes transient cerebral arteriopathy unlikely, raising the suspicion of a progressive angiitis of the CNS. The radiologic outcome of transient cerebral arteriopathy is either full recanalization of the affected arterial segments or some degree of residual stenosis that eventually may dictate the need for long-term antithrombotic therapy. Although an “inflammatory” basis has been presumed for this syndrome, it is important to consider other possibilities such as intracranial arterial dissection, which may have a very similar clinical and radiologic presentation.38 Last, the possibility of unilateral moyamoya may need consideration, although if initial angiography does not demonstrate the classical hypertrophied collateral vessels, evolution to that entity appears rare.36
Figure 7-5.
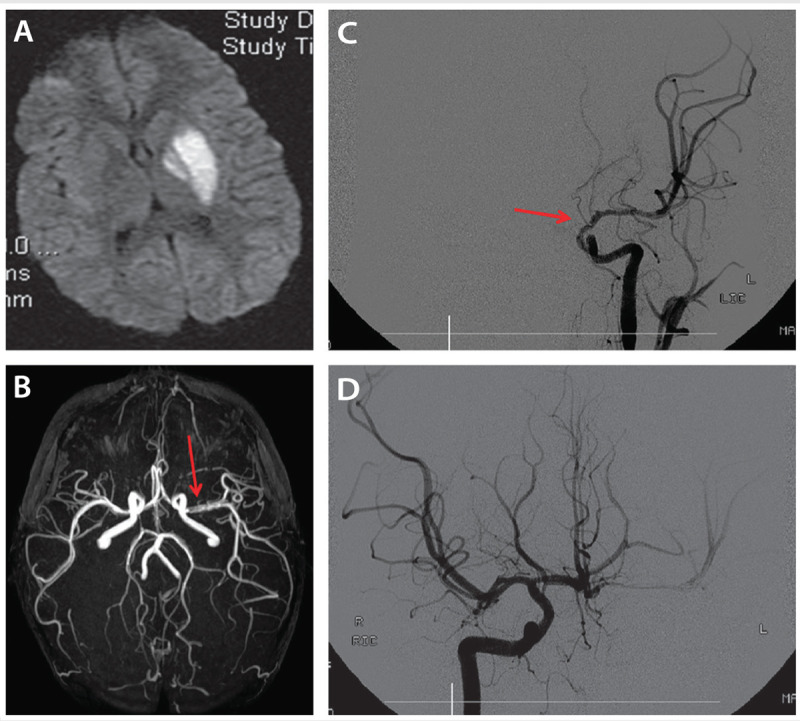
A 4-year-old boy with transient cerebral arteriopathy. Diffusion-weighted image (DWI) (A) shows acute left globus pallidus and putamen infarct. Magnetic resonance angiography (B) shows irregular and reduced flow in the proximal left middle cerebral artery (arrow). Catheter cerebral angiography (C) reveals significant caliber reduction and irregularity (arrow) of the distal left internal carotid artery, proximal middle cerebral artery, and absent flow in the anterior cerebral artery with cross filling from the opposite side (D).
Moyamoya vasculopathy. Moyamoya is a progressive occlusion of arteries of the circle of Willis, mainly the distal ICA, proximal MCA, proximal ACA, and sometimes posterior cerebral artery (PCA). The presence of hypertrophied, friable collateral vessels in the basal ganglia region (“puff of smoke”) detected by prominent flow voids on T1 MRI and the “ivy sign” (leptomeningeal high signal resembling creeping ivy) on fluid-attenuated inversion recovery (FLAIR) images representing prominent leptomeningeal collaterals is typical (Case 7-4). The idiopathic variety is called moyamoya disease, while that occurring in the presence of conditions such as sickle cell disease, neurofibromatosis (NF-1), and other systemic medical and genetic conditions is termed moyamoya syndrome. The condition is typically bilateral, although it can begin unilaterally and progress to the other side. In some conditions, moyamoya tends to stay unilateral, as in NF-1. Pediatric moyamoya mainly presents with ischemic stroke, whereas adult moyamoya often presents with hemorrhagic stroke. The hallmarks of moyamoya are recurrent TIAs, recurrent clinical or radiologic infarcts (mainly in central white matter), and sometimes territorial hypoperfusion-related or vaso-occlusive ischemic strokes. Presentation with recurrent headaches and progressive cognitive decline is also seen. Aspirin is the favored antithrombotic therapy for stroke prophylaxis, but long-term data regarding the efficacy and safety of aspirin are lacking. Surgical revascularization seems to have a beneficial effect in reducing the stroke risk and is generally advocated in children with clear vascular progression, recurrent stroke/TIA, and progressive cognitive decline. Surgical outcome in experienced centers is encouraging.39 The main aim of surgery is to provide an alternative blood supply to the brain via either a direct (superficial temporal artery [STA]–MCA anastomosis) or indirect (encephaloduroarteriosynangiosis [EDAS]) external carotid–internal carotid (EC-IC) bypass procedure. The latter involves laying of an STA branch over the cerebral surface without direct anastomosis in the hope that new vessels will eventually grow from the overlaid branch. EDAS is preferred over STA-MCA bypass in young children because the small size of the arteries makes surgery challenging.
Case 7-2
A previously well 17-year-old girl presented with progressive neck pain, occipital headache, nausea, and vomiting for 8 to 10 hours, which then developed into left face and tongue numbness, dysarthria, and ataxia over the next 2 to 3 hours. She also noted episodic diplopia with visual blurring on lateral neck movements. Over the past month, she had experienced episodic vertigo, diplopia, visual blurring, and bifrontal headache 2 to 3 times a week, as well as photophobia and phonophobia. Neurologic examination revealed horizontal and vertical gaze-evoked bilateral nystagmus, a left sixth nerve palsy, left internuclear ophthalmoplegia, mild right arm and leg hypotonia with pyramidal type weakness, severe bilateral appendicular ataxia, and dysarthria. Noncontrast head CT revealed a hyperdense basilar artery (Figure 7-2A). Diffusion-weighted imaging revealed ischemia in the left inferior cerebellar peduncle and left pons (Figure 7-2B, Figure 7-2C). Magnetic resonance angiography (MRA) showed greatly reduced flow in the entire left vertebral artery throughout its course (Figure 7-2D). Unfractionated heparin was initiated as a left vertebral artery dissection was suspected. Echocardiogram, lumbar puncture, inflammatory markers, and prothrombotic blood work results were normal. Catheter cerebral angiography revealed an intimal flap and double lumen, confirming a left vertebral artery dissection at the C1-C2 vertebral level, and occlusion of the distal third of the basilar artery (Figure 7-2E). Unfractionated heparin was switched to low-molecular-weight heparin (LMWH). One week later, the patient had new headache, nausea, and vomiting; repeat MRI revealed a new ischemic stroke in the right pons (Figure 7-3A). Aspirin was added; thereafter, she remained stable with no further TIAs or stroke. A 3-month follow-up catheter cerebral angiography revealed a pseudoaneurysm at the dissection site with persistent basilar artery occlusion (Figure 7-3B). LMWH and aspirin were continued for a further 3 months. Repeat catheter cerebral angiography at 6 months was unchanged; LMWH was stopped, and aspirin was continued. Follow-up MRA at 1 year and 2 years showed a stable pseudoaneurysm. The patient remained on aspirin and made a full neurologic recovery except for a mild residual left gaze palsy.
Figure 7-2.
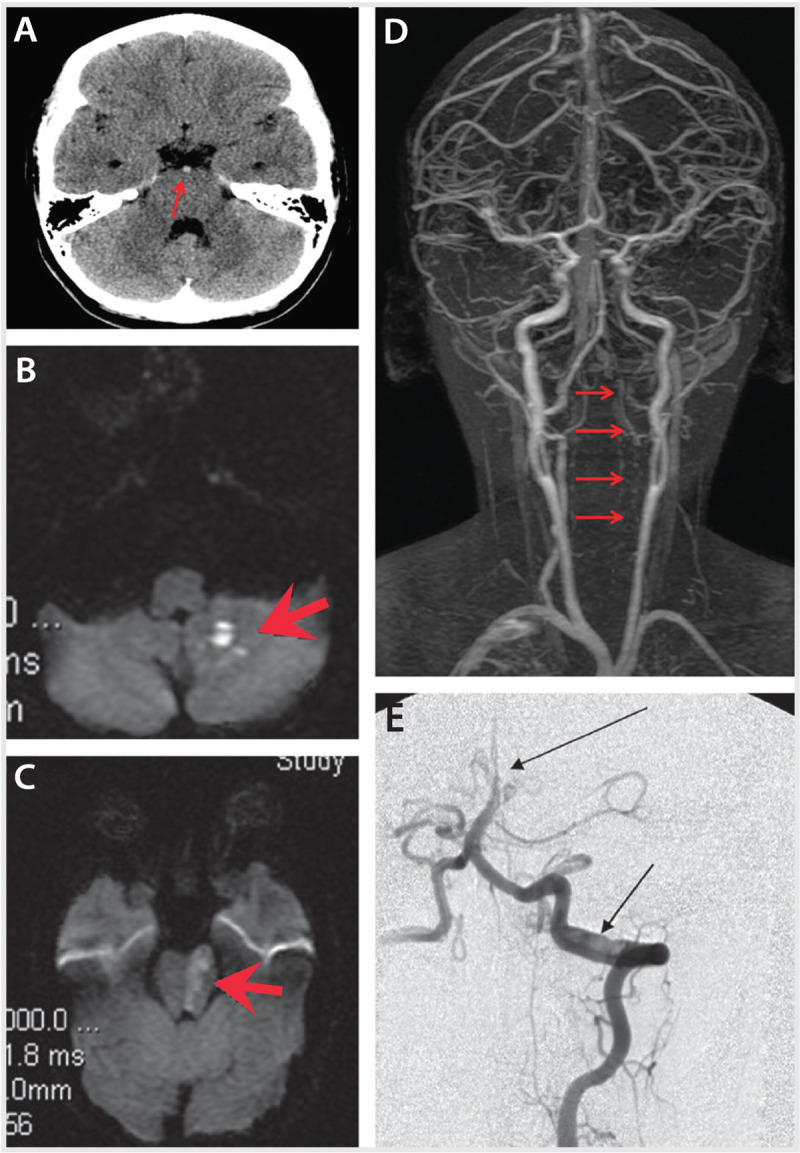
Imaging studies at presentation of the patient in Case 7-2. A, Noncontrast head CT reveals hyperdense basilar artery (arrow). B and C, Diffusion-weighted image (DWI) reveals ischemia (arrows) in the left inferior cerebellar peduncle and left pons. D, Magnetic resonance angiography reveals greatly reduced flow in the entire left vertebral artery throughout its course (arrows). E, Catheter cerebral angiography reveals an intimal flap and double lumen (lower arrow) confirming left vertebral artery dissection at the C1-C2 vertebral level and occlusion of the distal one-third of the basilar artery (upper arrow).
Figure 7-3.
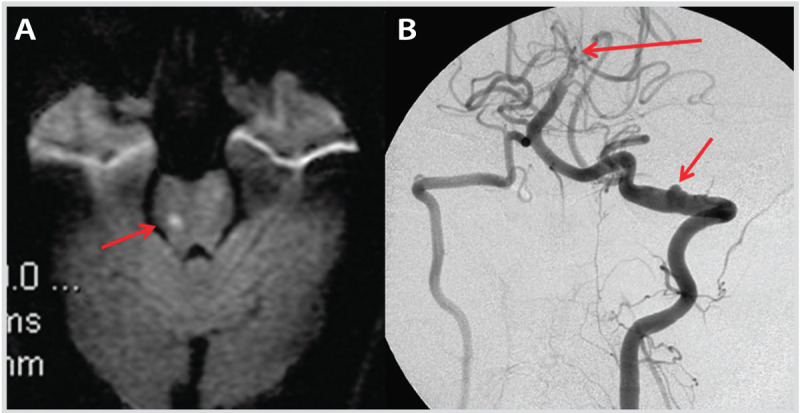
Imaging studies at follow-up of the patient in Case 7-2. A, Diffusion-weighted image (DWI) shows a new ischemic stroke in the right pons (arrow) 1 week after initial presentation. B, Catheter cerebral angiography 3 months after the initial presentation reveals pseudoaneurysm (lower arrow) at the site of dissection and persistent basilar artery occlusion (upper arrow).
Comment. This case illustrates the risk of recurrent stroke in the few days immediately after diagnosis of pediatric arterial ischemic stroke, particularly in the presence of vasculopathies (in this case arterial dissection), and highlights the need for close clinical and radiologic surveillance.
Case 7-3
An 11-year-old right-handed girl who had chickenpox 7 months before but was otherwise healthy presented with stuttering right arm, leg, and facial weakness over 2 hours. Brain MRI demonstrated diffusion restriction in the left internal capsule and globus pallidus (Figure 7-4A), with narrowing of the distal left internal carotid artery on magnetic resonance angiography (MRA) (Figure 7-4B). MRA pattern was consistent with transient cerebral arteriopathy. High-resolution coronal T1-weighted two-dimensional fluid-attenuated inversion recovery (FLAIR) sequence demonstrated concentric wall enhancement of the distal left internal carotid artery, proximal middle cerebral artery, and proximal anterior cerebral artery, suggestive of inflammation (Figure 7-4C), compared with the opposite side, which did not enhance. Serology was positive for varicella IgG; CSF white blood cell count was 2/ml, and CSF varicella PCR was negative. She was treated with IV methylprednisolone 30 mg/kg/d for 5 days, followed by a 3-month weaning course of oral prednisone starting at 2 mg/kg, in addition to 3 weeks of oral acyclovir. On follow-up imaging, the concentric wall enhancement was less intense by 1 month and had completely resolved by 6 months, with normalization of vessel wall caliber. Clinical recovery was complete. She remained on aspirin for secondary stroke prophylaxis.
Figure 7-4.
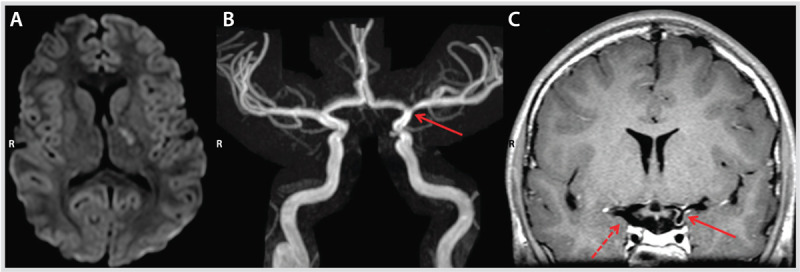
Imaging studies at presentation of the patient in Case 7-3. Diffusion-weighted image (DWI) demonstrating diffusion restriction in the left internal capsule and globus pallidus (A), with narrowing of the distal left internal carotid artery (arrow) on magnetic resonance angiography (B). Arterial wall imaging (C) shows concentric wall enhancement of the distal left internal carotid artery, proximal middle cerebral artery, and proximal anterior cerebral artery (solid arrow) suggestive of inflammation compared with the opposite side (dashed arrow) that did not enhance.
Comment. This case illustrates the “stuttering presentation” of pediatric arterial ischemic stroke in the context of an arteriopathy such as postvaricella angiitis, as well as the potential utility of newer vascular imaging techniques such as high-resolution arterial wall imaging. The typical unilateral involvement (stenosis, occlusion, irregularity) of the region of bifurcation of the internal carotid artery into anterior and middle cerebral artery should also raise the suspicion of transient cerebral arteriopathy in a child with ischemic stroke.
Case 7-4
A 6-year-old boy had a history of recurrent alternating left- and right-sided face, arm, and leg weakness lasting several minutes for 3 to 4 weeks before presentation. Some of these episodes were provoked by running on the playground. He had four to five episodes of migraine-type headaches for a few months before presentation. He presented with the sudden onset of a left-sided hemiparesis that lasted longer than the previous episodes. On examination, he had persistent mild left-sided weakness. MRI showed acute patchy restricted diffusion in the right corona radiata and centrum semiovale in the deep middle cerebral artery, middle cerebral artery/anterior cerebral artery, and middle cerebral artery/posterior cerebral artery watershed zones (Figure 7-6A). T1-weighted MRI sequence showed prominent flow voids in the basal ganglia region (Figure 7-6B). Fluid-attenuated inversion recovery (FLAIR) sequence revealed bilateral asymmetric (right greater than left) prominent leptomeningeal high signal, consistent with an “ivy sign” (Figure 7-6C). Magnetic resonance angiography (Figure 7-6D) revealed significant stenosis of the bilateral distal internal carotid arteries and proximal middle cerebral arteries, and absent flow through both anterior cerebral arteries with bilateral moyamoya collaterals. Aspirin was started. He underwent catheter cerebral angiography, which confirmed bilateral moyamoya vasculopathy (right greater than left). Surgical revascularization (indirect bypass with bilateral pial synangiosis) was successfully performed. He remained symptom-free at the time of his last follow-up after 4 years on aspirin.
Figure 7-6.
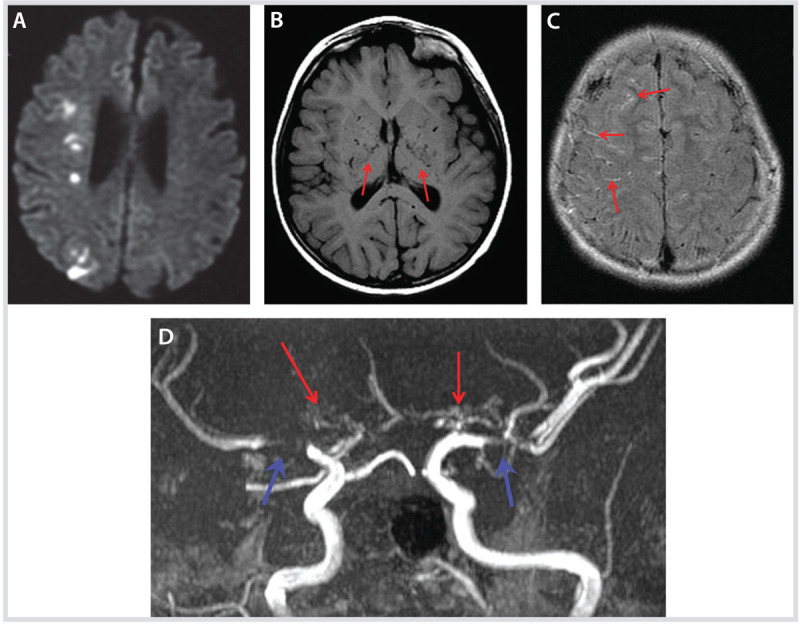
Imaging studies at presentation of the patient in Case 7-4 A, Diffusion-weighted image (DWI) shows acute patchy restricted diffusion in the right corona radiata and centrum semiovale in the deep middle cerebral artery/anterior cerebral artery, and middle cerebral artery/posterior cerebral artery watershed zones. B, Axial T1-weighted sequence shows prominent flow voids in the basal ganglia region (arrows). C, Axial fluid-attenuated inversion recovery (FLAIR) sequence reveals bilateral asymmetric (right > left) prominent leptomeningeal high signal, consistent with an “ivy sign” (arrows). D, Magnetic resonance angiography revealed significant stenosis (blue arrows) of bilateral distal internal carotid arteries and proximal middle cerebral arteries, and absent flow through both anterior cerebral arteries with bilateral “moyamoya” collaterals (red arrows).
Comment. This case illustrates the peculiar presenting symptom of hyperventilation-induced (in this case, from running hard on the playground) paroxysmal episodes of focal neurologic deficits unique to moyamoya. The case also demonstrates the success of revascularization surgery in moyamoya.
OUTCOMES FROM PEDIATRIC ARTERIAL ISCHEMIC STROKE
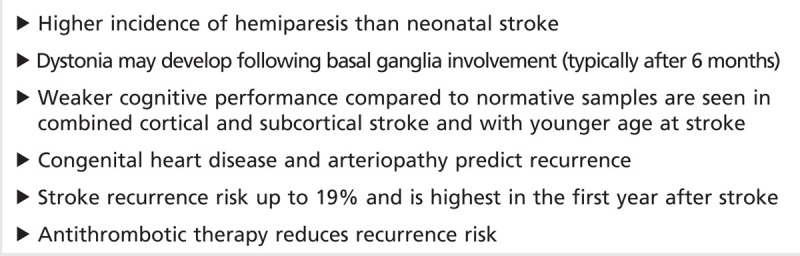
Approximately 50% of neonates diagnosed with AIS will be left with a cognitive, language, or motor deficit, typically hemiparesis40 as summarized in Table 7-2. Death is rare. Usually, the cognitive deficits range from mild to moderate, but boys seem to do worse.41 However, in presumed perinatal ischemic stroke, because such children come to diagnosis only because of clinical deficits (mainly hemiparesis), the outcome is of a more severe neurologic deficit—for example, moderate to severe hemiparesis. Epilepsy occurs in 15% to 40% of patients and can occasionally be refractory.41
Table 7-2.
Outcome From Perinatal/Neonatal Arterial Ischemic Stroke

Outcome from childhood AIS is summarized in Table 7-3. In older infants and children, death due to stroke occurs in 5%, frequently from malignant MCA syndrome. The outcome in survivors ranges from 38% normal, 42% mild disability, 8% moderate disability, to 12% severe handicap. Outcome at 1 year post stroke predicted final functional outcome, and arteriopathy predicted recurrence in one study.20
Table 7-3.
Outcome From Childhood Arterial Ischemic Stroke
CONCLUSIONS
Pediatric AIS, which is probably more common than previously thought, presents unique diagnostic and management challenges that are distinct from adult AIS. A multidisciplinary approach is essential to optimize short-term and long-term management.
KEY POINTS
Arterial ischemic stroke is rarer and has a subtler clinical presentation and a wider differential diagnosis in children than in adults, which results in delayed and sometimes missed diagnosis.
Arterial ischemic stroke should be suspected in any newborn with seizures, particularly when seizures begin more than 12 hours after birth.
A feature of TIAs unique to children is a history of paroxysmal episodes of neurologic dysfunction precipitated by hyperventilation, which should raise the suspicion of moyamoya.
When considering antithrombotic therapy in any child with arterial ischemic stroke, it is critical to continually balance the risk (hemorrhagic complication) of antithrombotic therapy with the risk of nontreatment (extension of preexisting infarction and recurrence of stroke).
The risk-benefit ratio of tissue plasminogen activator in pediatric arterial ischemic stroke is unknown.
After the diagnosis of acute arterial ischemic stroke, antiplatelet and anticoagulant therapies in children are usually selected based on the perceived mechanism for arterial ischemic stroke.
From a practical standpoint, consensus exists among pediatric stroke experts that some level of antithrombotic therapy is beneficial in preventing acute progression of thrombus and stroke recurrence.
Antithrombotic therapy is rarely indicated in most cases of perinatal or neonatal arterial ischemic stroke because of negligible recurrence risk except in the presence of congenital heart disease and abnormal prothrombotic workup.
Because deficits from perinatal or neonatal arterial ischemic stroke may only emerge with maturation, longitudinal observation is necessary even in apparently healthy infants.
Although congenital heart disease correction should theoretically eliminate the cardiogenic stroke risk, recent data demonstrate that recurrence risk remains increased for extended periods even after full congenital heart disease correction.
Beyond the newborn period, arteriopathy (vasculopathy) accounts for approximately 60% of all childhood arterial ischemic stroke and predicts stroke recurrence.
Transient cerebral arteriopathy of childhood is a well-described unilateral focal arteriopathy of presumed inflammatory origin. Transient cerebral arteriopathy is characterized by basal ganglia infarction with ipsilateral irregular stenosis involving the carotid T-junction represented by the distal internal carotid artery, proximal anterior cerebral artery, and proximal middle cerebral artery.
Pediatric moyamoya mainly presents with ischemic stroke whereas adult moyamoya often presents with hemorrhagic stroke.
Encephaloduroarteriosynangiosis is preferred over superficial temporal artery–middle cerebral artery bypass in young children because the small size of the arteries makes surgery challenging.
Footnotes
Relationship Disclosure: Dr Moharir reports no disclosure. Dr deVeber’s spouse holds an investment of more than $10,000 in Thornhill Research Inc.
Unlabeled Use of Products/Investigational Use Disclosure: Drs Moharir and deVeber report no disclosures.
REFERENCES
- 1.National center for health statistics. Deaths, percentage of total deaths, and death rates for the 10 leading causes of death in selected age groups, by race and sex: United States, 2002. In: National Vital Statistics Reports Vol. 53, No. 17. CDC website [online]. Available at www.cdc.gov/nchs/data/nvsr/nvsr53/nvsr53_17.pdf. Published March 7, 2005. Accessed February 10, 2014. [Google Scholar]
- 2.Fullerton HJ,, Wu YW,, Zhao S,, Johnston SC. Risk of stroke in children: ethnic and gender disparities. Neurology 2003; 61 (2): 189–194. [DOI] [PubMed] [Google Scholar]
- 3.Nelson KB,, Lynch JK. Stroke in newborn infants. Lancet Neurol 2004; 3 (3): 150–158. [DOI] [PubMed] [Google Scholar]
- 4.Golomb MR,, Fullerton HJ,, Nowak-Gottl U,, Deveber G; International Pediatric Stroke Study Group. Male predominance in childhood ischemic stroke: findings from the international pediatric stroke study. Stroke 2009; 40 (1): 52–57. [DOI] [PubMed] [Google Scholar]
- 5.Rafay MF,, Pontigon AM,, Chiang J, et al Delay to diagnosis in acute pediatric arterial ischemic stroke. Stroke 2009; 40 (1): 58–64. [DOI] [PubMed] [Google Scholar]
- 6.Roach ES,, Golomb MR,, Adams R, et al Management of stroke in infants and children: a scientific statement from a Special Writing Group of the American Heart Association Stroke Council and the Council on Cardiovascular Disease in the Young. Stroke 2008; 39 (9): 2644–2691. [DOI] [PubMed] [Google Scholar]
- 7.Monagle P,, Chan AK,, Goldenberg NA, et al; American College of Chest Physicians. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141 (2 suppl): e737S–e801S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirton A,, Armstrong-Wells J,, Chang T, et al Symptomatic neonatal arterial ischemic stroke: the International Pediatric Stroke Study. International Pediatric Stroke Study Investigators. Pediatrics 2011; 128 (6): e1402–e1410. [DOI] [PubMed] [Google Scholar]
- 9.Rafay MF,, Cortez MA,, de Veber GA, et al Predictive value of clinical and EEG features in the diagnosis of stroke and hypoxic ischemic encephalopathy in neonates with seizures. Stroke 2009; 40 (7): 2402–2407. [DOI] [PubMed] [Google Scholar]
- 10.Braun KP,, Rafay MF,, Uiterwaal CS, et al Mode of onset predicts etiological diagnosis of arterial ischemic stroke in children. Stroke 2007; 38 (2): 298–302. [DOI] [PubMed] [Google Scholar]
- 11.Shellhaas RA,, Smith SE,, O’Tool E, et al Mimics of childhood stroke: characteristics of a prospective cohort. Pediatrics 2006; 118 (2): 704–709. [DOI] [PubMed] [Google Scholar]
- 12.Mackay MT,, Wiznitzer M,, Benedict SL, et al Arterial ischemic stroke risk factors: the International Pediatric Stroke Study. Ann Neurol 2011; 69 (1): 130–140. [DOI] [PubMed] [Google Scholar]
- 13.Elbers J,, Viero S,, MacGregor D, et al Placental pathology in neonatal stroke. Pediatrics 2011; 127 (3): e722–e729. [DOI] [PubMed] [Google Scholar]
- 14.Rodan L,, McCrindle BW,, Manlhiot C, et al Stroke recurrence in children with congenital heart disease. Ann Neurol 2012; 72 (1): 103–111. [DOI] [PubMed] [Google Scholar]
- 15.Fullerton HJ,, Wu YW,, Sidney S,, Johnston SC. Risk of recurrent childhood arterial ischemic stroke in a population-based cohort: the importance of cerebrovascular imaging. Pediatrics 2007; 119 (3): 495–501. [DOI] [PubMed] [Google Scholar]
- 16.deVeber G. Delays in the timely diagnosis of stroke in children. Nat Rev Neurol 2010; 6 (2): 64–66. [DOI] [PubMed] [Google Scholar]
- 17.Tan MA,, DeVeber G,, Kirton A, et al Low detection rate of craniocervical arterial dissection in children using time-of-flight magnetic resonance angiography: causes and strategies to improve diagnosis. J Child Neurol 2009; 24 (10): 1250–1257. [DOI] [PubMed] [Google Scholar]
- 18.Swartz RH,, Bhuta SS,, Farb RI, et al Intracranial arterial wall imaging using high-resolution 3-tesla contrast-enhanced MRI. Neurology 2009; 72 (7): 627–634. [DOI] [PubMed] [Google Scholar]
- 19.Payne ET,, Wei XC,, Kirton A. Reversible wall enhancement in pediatric cerebral arteriopathy. Can J Neurol Sci 2011; 38 (1): 139–140. [DOI] [PubMed] [Google Scholar]
- 20.Elbers J,, Deveber G,, Pontigon AM,, Moharir M. Long-term outcomes of pediatric ischemic stroke in adulthood [published online ahead of print April 15 2013]. J Child Neurol 2013. doi:10.1177/0883073813484358. [DOI] [PubMed] [Google Scholar]
- 21.Gupta AA,, Leaker M,, Andrew M, et al Safety and outcomes of thrombolysis with tissue plasminogen activator for treatment of intravascular thrombosis in children. J Pediatr 2001; 139 (5): 682–688. [DOI] [PubMed] [Google Scholar]
- 22.Amlie-Lefond C,, deVeber G,, Chan AK, et al; International Pediatric Stroke Study. Use of alteplase in childhood arterial ischaemic stroke: a multicentre, observational, cohort study. Lancet Neurol 2009; 8 (6): 530–536. [DOI] [PubMed] [Google Scholar]
- 23.Sträter R,, Kurnik K,, Heller C, et al Aspirin versus low-dose low-molecular-weight heparin: antithrombotic therapy in pediatric ischemic stroke patients: a prospective follow-up study. Stroke 2001; 32 (11): 2554–2558. [DOI] [PubMed] [Google Scholar]
- 24.Goldenberg NA,, Bernard TJ,, Fullerton HJ, et al Antithrombotic treatments, outcomes, and prognostic factors in acute childhood-onset arterial ischaemic stroke: a multicentre, observational, cohort study. Lancet Neurol 2009; 8 (12): 1120–1127. [DOI] [PubMed] [Google Scholar]
- 25.Bernard TJ,, Goldenberg NA,, Tripputi M, et al Anticoagulation in childhood onset arterial ischemic stroke with non-moyamoya arteriopathy: findings from the Colorado and German (COAG) collaboration. Stroke 2009; 40 (8): 2869–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schechter T,, Kirton A,, Laughlin S, et al Safety of anticoagulants in children with arterial ischemic stroke. Blood 2012; 119 (4): 949–956. [DOI] [PubMed] [Google Scholar]
- 27.Kenet G,, Lutkhoff LK,, Albisetti M, et al Impact of thrombophilia on risk of arterial ischemic stroke or cerebral sinovenous thrombosis in neonates and children: a systematic review and meta-analysis of observational studies. Circulation 2010; 121 (16): 1838–1847. [DOI] [PubMed] [Google Scholar]
- 28.Rodan L,, McCrindle BW,, Manlhiot C, et al Stroke recurrence in children with congenital heart disease. Ann Neurol 2012; 72 (1): 103–111. [DOI] [PubMed] [Google Scholar]
- 29.Jones KC,, Hawkins C,, Armstrong D, et al Association between radiographic Wallerian degeneration and neuropathological changespost childhood stroke. Dev Med Child Neurol 2013; 55 (2): 173–177. [DOI] [PubMed] [Google Scholar]
- 30.Domi T,, deVeber G,, Shroff M, et al Corticospinal tract pre-wallerian degeneration: a novel outcome predictor for pediatric stroke on acute MRI. Stroke 2009; 40 (3): 780–787. [DOI] [PubMed] [Google Scholar]
- 31.Sébire G,, Fullerton H,, Riou E,, deVeber G. Toward the definition of cerebral arteriopathies of childhood. Curr Opin Pediatr 2004; 16 (6): 617–622. [DOI] [PubMed] [Google Scholar]
- 32.Bernard TJ,, Manco-Johnson MJ,, Lo W, et al Towards a consensus-based classification of childhood arterial ischemic stroke. Stroke 2012; 43 (2): 371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amlie-Lefond C,, Bernard TJ,, Sebire G, et al Predictors of cerebral arteriopathy in children with arterial ischemic stroke: results of the international pediatric stroke study. Circulation 2009; 119 (10): 1417–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rafay MF,, Armstrong D,, Deveber G, et al Craniocervical arterial dissection in children: clinical and radiographic presentation and outcome. J Child Neurol 2006; 21 (1): 8–16. [DOI] [PubMed] [Google Scholar]
- 35.Arauz A,, Ruiz A,, Pacheco G, et al Aspirin versus anticoagulation in intra- and extra cranial vertebral artery dissection. Eur J Neurol 2013; 20 (1): 167–172. [DOI] [PubMed] [Google Scholar]
- 36.Bulder MM,, Braun KP,, Leeuwis JW, et al The course of unilateral intracranial arteriopathy in young adults with arterial ischemic stroke. Stroke 2012; 43 (7): 1890–1896. [DOI] [PubMed] [Google Scholar]
- 37.Gilden D,, Cohrs RJ,, Mahalingam R,, Nagel MA. Varicella zoster virus vasculopathies: diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol 2009; 8 (8): 731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dlamini N,, Freeman JL,, Mackay MT, et al Intracranial dissection mimicking transient cerebral arteriopathy in childhood arterial ischemic stroke. J Child Neurol 2011; 26 (9): 1203–1206. [DOI] [PubMed] [Google Scholar]
- 39.Kim SK,, Cho BK,, Phi JH, et al Pediatric moyamoya disease: an analysis of 410 consecutive cases. Ann Neurol 2010; 68 (1): 92–101. [DOI] [PubMed] [Google Scholar]
- 40.Golomb MR. Outcomes of perinatal arterial ischemic stroke and cerebral sinovenous thrombosis. Semin Fetal Neonatal Med 2009; 14 (5): 318–322. [DOI] [PubMed] [Google Scholar]
- 41.Westmacott R,, MacGregor D,, Askalan R,, deVeber G. Late emergence of cognitive deficits after unilateral neonatal stroke. Stroke 2009; 40 (6): 2012–2019. [DOI] [PubMed] [Google Scholar]


