This article has supplementary material on the web site: www.jdbp.org.
Index terms: precision medicine, personalized medicine, pharmacogenetics, autism spectrum disorder
ABSTRACT:
Objective:
This study investigated outcomes of pharmacogenetic testing of youth with autism spectrum disorder (ASD) referred to a precision medicine clinic and explored associations between patient characteristics and pharmacogenomic testing results.
Methods:
Records for patients diagnosed with ASD and subsequently referred to a pediatric hospital's precision medicine clinic between July 1, 2010, and June 30, 2020, were reviewed. Pharmacogenetic testing results were abstracted focusing on CYP2D6 and CYP2C19. In addition, we compiled counts of patients' co-occurring diagnoses, histories of adverse drug reactions (ADRs), previously trialed ineffective medications, and previous psychiatric medication changes. Logistic regression models were fit to examine CYP2C19 and CYP2D6 metabolizer status as functions of patient demographics and prereferral medication histories.
Results:
Of 202 patients (mean age = 12.18 yrs), 66% were referred to precision medicine because of poor medication response. Among patients with pharmacogenomic testing results for CYP2D6, 9% were classified as poor metabolizers; among patients with results for CYP2C19, 10% were classified as rapid/ultrarapid metabolizers. Patient demographics and medication response history did not predict pharmacogenomic results. However, the number of co-occurring diagnoses positively predicted the number of nonpsychiatric ADRs and a higher probability of CYP2D6 poor metabolizer status; moreover, nonpsychiatric ADRs positively predicted CYP2C19 rapid/ultrarapid metabolizer status.
Conclusion:
In one of the largest reported samples of youth with ASD clinically referred for pharmacogenetic testing, we observed high variability in medication response and yield for actionable results. Our findings suggest potential clinical utility for pharmacogenetic testing and introduce possible clinical profiles associated with metabolizer status.
Autism spectrum disorder (ASD) is present in approximately 1 in 36 children in the United States.1 Children with ASD are more likely to be diagnosed with co-occurring mental health concerns, and best practice guidelines for these conditions include consideration of medication management.2–4 This makes polypharmacy more common in youth with ASD than in neurotypical children, including for youth in the 3- to 5-year-old age range.5,6 Drawing from a national registry of youth with ASD, Rosenberg et al.7 reported that more than 35% of patients were prescribed at least 1 psychotropic medication, and around 10% were prescribed 3 or more. Despite the more frequent use of medications for this population, individuals with ASD are also less likely than neurotypical peers to benefit from medication use, and they are also more likely to experience adverse reactions.3,8,9 Medication management for youth with ASD is further complicated by significant variability in clinical presentation and heterogenous genetic and etiological pathways.
Pharmacogenetic testing is the analysis of genes involved in drug metabolism and response, which can affect drug efficacy and the likelihood of adverse reactions. Results can assist with drug and dose selection, with the goal of improving outcomes.3 For instance, patients identified as poor or rapid metabolizers for a drug's relevant substrate might require lower or higher doses to achieve therapeutic benefit and have an increased risk of drug-drug interactions or higher risk for toxicity.10 Pharmacogenetic testing is a tool often used in personalized or precision medicine, and this approach holds particular promise for individuals with neurodevelopmental disabilities because of the opportunity to investigate genetic and clinical predictors of drug efficacy and adverse reactions.3,11,12 Such predictors could be incorporated into a standardized evaluation process to increase the likelihood of medication benefit and enable ASD-specific therapeutic guidance to be developed.13
Autism spectrum disorder–specific pharmacogenomic research is limited, but broader studies have examined medications commonly prescribed to patients with ASD, targeting behavioral and mental health symptoms. Variations in the metabolism of CYP2D6 and CYP2C19 are of particular interest, given their known relevance for drugs such as atomoxetine, risperidone, sertraline, and escitalopram.14–16 Less evidence exists for the application of pharmacodynamic genetic results, but investigations continue evaluating associations with dopaminergic and serotonergic variants.17 The application of pharmacogenetic information is included on Food and Drug Administration (FDA) labels for some drugs, and multidisciplinary pharmacogenetic guidelines are available for some selective serotonin reuptake inhibitors (SSRIs) and atypical antipsychotics; however, this guidance is not specific to ASD and is commonly based on studies of neurotypical adults.18 Some research has demonstrated the benefits of pharmacogenetic testing in children with epilepsy, which may have relevance for youth with ASD given the elevated rates of epilepsy in this population.19–21
Pharmacogenomic profiles of youth with ASD are nearly absent from the published literature, including descriptions of the outcomes for youth referred to precision medicine clinics seeking guidance on the use of specific medications (risperidone, atomoxetine, and SSRIs).18 Patel et al.8 examined the outcomes after pharmacogenetic testing in youth with developmental and/or behavioral diagnoses (including ASD) in a recent single-site retrospective review. This study identified actionable genes and reported on the frequency of medication changes after testing but did not directly examine the predictors of actionable results or other drivers of prescribing practices in this patient population, such as polypharmacy and adverse drug reactions (ADRs). However, these results do highlight the potential benefits of pharmacogenetic testing after multiple drug failures and/or ADRs, although the heterogeneous sample limits conclusions about youth with ASD specifically.
As the availability of pediatric precision medicine using pharmacogenetic testing increases, youth with ASD appear to be well represented in referred populations.8,22 Additional data on both the clinical use and outcomes of this emerging science are needed to build toward evidence-based dosing guidelines specific to youth with ASD. Therefore, this study had the following objectives: (1) add to the published precision medicine literature by describing the medication histories and metabolism results for youth with ASD referred to a precision medicine service and (2) identify relationships between demographic/clinical patient characteristics and pharmacogenomic testing results.
METHODS
This is a retrospective, descriptive study using records from pediatric patients who received care in the precision medicine clinic at a midwestern pediatric hospital. The clinic functions as a consultation service for a wide variety of patients referred for concerns related to poor drug response and adverse events.22 Local institutional review board approval was obtained before data collection and analysis. Patients were included in this study if they were seen in the precision medicine clinic between July 1, 2010, and June 30, 2020, and had a clinical diagnosis of ASD based on ICD-9/10 codes in the available medical records (including historical diagnoses of autistic disorder, pervasive developmental disorder, and Asperger syndrome). Patient demographics, medical and psychiatric comorbidities, medication history, and reasons for seeking personalized medicine were abstracted from clinic intake records; available pharmacogenomic testing results (from commercial third-party laboratories) and subsequent medication recommendations were extracted from clinic follow-up data and transferred from the electronic medical record to the clinic's secure REDCap database. We focused on CYP2D6 and CYP2C19 results, given the relevance of these drug-metabolizing enzymes for commonly prescribed psychotropic medications (atypical antipsychotics and SSRIs). Updated functional phenotype predictions (i.e., ultrarapid, rapid, intermediate, normal, and poor metabolizer) were determined based on the most recent Clinical Pharmacogenetics Implementation Consortium consensus guidelines to allow for uniform classifications across the study period.23
We computed counts of medical and psychiatric diagnoses, ADRs resulting from the use of psychiatric and nonpsychiatric medications, ineffective medications, and psychiatric medication changes. Using negative binomial regression, we modeled counts of psychiatric and nonpsychiatric ADRs as a function of demographic variables and patients' count of diagnoses. Negative binomial regression was also used to model the count of psychiatric medication changes as a function of patient age, sex, race, reason for the clinic visit, subspecialty referral (yes/no), and total numbers of psychiatric and nonpsychiatric medications reported at baseline. An analogous logistic regression model was fit to model odds of any psychiatric medication change as a function of the same explanatory variables.
In exploratory analyses, we fit logistic regression models to predict metabolizer status for CYP2C19 and CYP2D6 using diagnosis count, count of ineffective medications, count of psychiatric ADRs, and count of nonpsychiatric ADRs. Given the distribution of profiles in the sample, we examined predictors for CYP2C19 rapid or ultrarapid (vs other) status and CYP2D6 poor (vs other) status. We also examined similar models with any ineffective medication (yes/no) replacing the count of ineffective medications and the total count of side effects or any side effect (yes/no) replacing separate count variables for psychiatric and nonpsychiatric ADRs, but these models yielded similar or poorer predictive performance.
RESULTS
All 202 patients with clinical diagnoses of ASD seen in the precision medicine clinic had known co-occurring medical and/or psychiatric conditions, the most common of which included attention-deficit/hyperactivity disorder (72%), anxiety (54%), developmental delay (26%), a known genetic disorder (19%), seizure disorder (16%), mood disorder (13%), tic disorder/Tourette syndrome (11%), and depression (11%). Most patients were referred from subspecialty providers (Table 1). The most common reasons for referral and subsequent pharmacogenetic testing were poor medication response (66%) and adverse drug reactions (ADRs) (49%). The mean age at the time of the first visit was 12.2 years (SD 4.2). On initial presentation to the clinic, patients were already receiving a mean of 6.2 medications (SD of 4.4). The most prescribed categories of medication included medications used for sleep (52%), medications for gastrointestinal disorders (50%), alpha-2 adrenergic agonists (49%), anticonvulsants (44%), stimulants (41%), second-generation atypical antipsychotics (40%), and selective serotonin reuptake inhibitors (30%). Of all the medications prescribed, only 11% had prescribing guidelines from either Clinical Pharmacogenetics Implementation Consortium (CPIC) or the Dutch Pharmacogenetics Working Group.23,24
Table 1.
Sample Demographics, Referral, and Metabolism Characteristics
| Variable | Mean (SD) | Range |
| Age (yr) | 12.18 (4.2) | 2.9–22.6 |
| Variable | Count | % |
| Sex | ||
| Female | 63 | 31 |
| Male | 139 | 69 |
| Race | ||
| Black/African American | 18 | 9 |
| White | 168 | 83 |
| Others | 10 | 5 |
| Ethnicity | ||
| Hispanic/Latino | 4 | 2 |
| Non-Hispanic/Latino | 198 | 98 |
| Referral reason | ||
| ADR | 98 | 49 |
| Poor medication response | 131 | 66 |
| Genotype result review | 5 | 2 |
| Genotyping requested by the physician | 29 | 14 |
| Genotyping requested by the family | 25 | 12 |
| Others | 44 | 22 |
| Referral source | ||
| Primary care provider | 34 | 16 |
| Subspecialist | 158 | 78 |
| Self-referral | 10 | 5 |
| CYP2C19 statusa | ||
| Poor | 4 | 3 |
| Intermediate | 39 | 29 |
| Normal | 76 | 57 |
| Rapid/ultrarapid | 15 | 10 |
| Missing | 68 | — |
| CYP2D6 statusa | ||
| Poor | 13 | 9 |
| Intermediate | 75 | 56 |
| Normal | 89 | 66 |
| Rapid/ultrarapid | 1 | 0.7 |
| Missing | 24 | — |
Percentages were calculated based on the available number of genotype results for each enzyme.
Pharmacogenomic testing results for CYP2D6 were available for 88% of patients. Of these patients, 9% were classified as poor CYP2D6 metabolizers under current CPIC guidelines. Results for CYP2C19 were available for 66% of patients, of whom 10% had results classifying them as rapid or ultrarapid CYP2C19 metabolizers. Roughly two-thirds (66%) of the overall sample had results available for both CYP2D6 and CYP2C19. The distributions of metabolism status are presented visually in Figure 1 (CYP2D6) and Figure 2 (CYP2C19).
Figure 1.
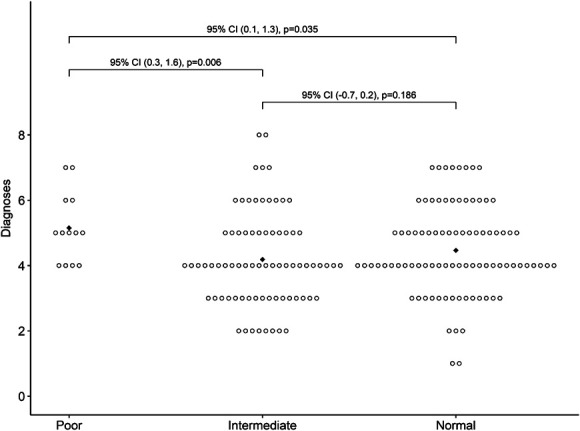
Count of medical and psychiatric co-occurring diagnoses by CYP2D6 metabolizer status with bootstrap 95% CIs and p values for pairwise mean differences. Diamonds indicate mean values. CI, confidence interval.
Figure 2.
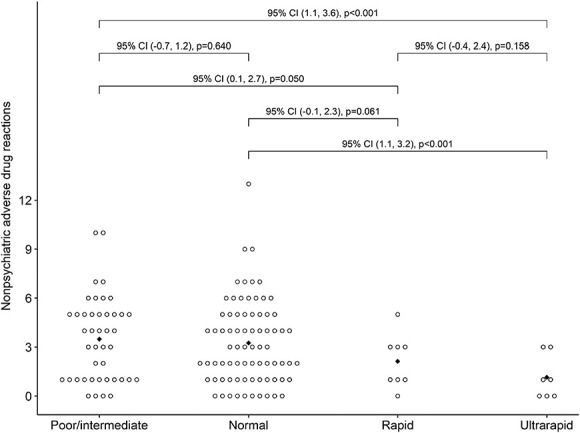
Count of nonpsychiatric ADRs by CYP2C19 metabolizer status with bootstrap 95% CIs and p values for pairwise mean differences. Diamonds indicate mean values. ADRs, adverse drug reactions; CI, confidence interval.
Results from negative binomial regression modeling are displayed in Table 2. Age at visit was a significant predictor of nonpsychiatric (but not psychiatric) ADRs, although this effect was small (Exp(B) 1.06, 95% confidence interval [CI] 1.03–1.09, p < 0.001). The number of co-occurring mental health diagnoses was positively associated with the number of ADRs, with an estimated average increase of 9% in the count of psychiatric ADRs (Exp(B) 1.09, 95% CI 0.99–1.20, p = 0.069) and 13% increase in the count of nonpsychiatric ADRs (Exp(B) 1.13, 95% CI 1.03–1.24, p = 0.013) per additional diagnosis, adjusting for the other model variables. The estimated average count of psychiatric medication changes increased by 15% (Exp(B) 1.15, 95% CI 0.97–1.36, p = 0.107) per additional psychiatric medication and decreased by 10% (Exp(B) 0.90, 95% CI 0.84–0.98, p = 0.010) per additional nonpsychiatric medication, again after adjustment for all other model variables.
Table 2.
Results of Negative Binomial Models for Psychiatric and Nonpsychiatric ADRs as Functions of Demographic Variables and Diagnoses
| Variable | Psychiatric ADRsa | Nonpsychiatric ADRsa | ||||||
| Exp(B) | Low95 | Upp95 | p | Exp(B) | Low95 | Upp95 | p | |
| Age at visit | 1.02 | 0.99 | 1.06 | 0.133 | 1.06 | 1.03 | 1.09 | <0.001 |
| Male | 1.18 | 0.90 | 1.56 | 0.229 | 1.20 | 0.90 | 1.58 | 0.210 |
| Diagnosis count | 1.09 | 0.99 | 1.20 | 0.069 | 1.13 | 1.03 | 1.24 | 0.013 |
| Race/ethnicity | ||||||||
| Black | 0.97 | 0.63 | 1.49 | 0.874 | 0.88 | 0.56 | 1.37 | 0.562 |
| Others | 0.82 | 0.48 | 1.40 | 0.469 | 0.82 | 0.48 | 1.40 | 0.464 |
| White | Referent | |||||||
ADRs, adverse drug reactions.
(Supplemental Digital Content 1, http://links.lww.com/JDBP/A434). Odds of at least 1 psychiatric medication change were higher for patients with an ADR or poor medication response listed as the reason for their clinic visit, but 95% CIs were also too wide to rule out effects in the opposite direction.
In logistic regression models, we used counts of co-occurring medical and psychiatric diagnoses, ineffective medications, psychiatric ADRs, and nonpsychiatric ADRs to predict metabolism status for CYP2D6 and CYP2C19 (Supplemental Digital Content 2, http://links.lww.com/JDBP/A434). For CYP2D6, higher diagnostic counts were associated with a higher probability for CYP2D6 poor (vs. intermediate or normal) metabolizer status, with an adjusted odds ratio (aOR) of 1.55 (95% CI 1.03−2.38, p = 0.036; Fig. 1). For CYP2C19, higher counts of nonpsychiatric ADRs were predictive of a lower probability for CYP2C19 rapid or ultrarapid (vs nonrapid) metabolizer status (aOR 0.70, 95% CI 0.48–0.95, p = 0.036). As shown in Figure 2, the average number of nonpsychiatric ADRs decreased from its high (3.5) in the poor/intermediate metabolizer group to its low (1.1) in the ultrarapid group.
DISCUSSION
In our sample of youth with ASD referred for precision medicine, we saw high rates of poor medication response and medication adverse reactions consistent with prior reports. Youth with ASD in this sample had notably high rates of poor CYP2D6 metabolism relative to rates found in large population-based samples (9% vs 0.4%–5.4%, depending on ethnicity).25 We also observed lower rates of CYP2C19 rapid/ultrarapid metabolism compared with population-based samples (10% vs 31.5%).26 Larger comparisons are needed to establish whether these differences are replicable, and whether the ASD phenotype itself is related to the pharmacogenomic profile. If future studies do continue to identify base-rate differences in metabolizer status for individuals with ASD, this awareness might inform dosing recommendations and help explain why this population appears to benefit less from medication use and experience more adverse reactions.3,8,9
Although the metabolism profile was not associated with demographics in our sample, we did observe that the number of co-occurring diagnoses was positively associated with the number of psychiatric and nonpsychiatric adverse drug reactions (ADRs) experienced by patients. Based on the extremely high rates of both medical/psychiatric complexity and medication use seen throughout our sample, we question whether this finding can be attributed to polypharmacy alone; it may be better explained by the relationship between phenotypic complexity and the underlying genotype. The American Academy of Pediatrics already encourages providers to consider pharmacogenetic testing in the course of medication management for youth with ASD, and the FDA recommends testing after repeated medication failures.2 Although more research would be needed to support a recommendation for proactive pharmacogenomic testing (i.e., before initiating medication management) for youth with ASD, the consistently high rates of polypharmacy and poor medication outcomes in this population invite this future consideration. In a cluster-randomized crossover implementation trial of prospective pharmacogenomic testing, Swen et al.27 recently demonstrated a 30% reduction of severe ADRs in a sample of adults prescribed drugs with actionable dosing guidelines; notably, CYP2D6 accounted for the highest proportion of patients with actionable variants. Prospective pharmacogenomic testing would also mirror the movement toward “genotype-first” approaches to understanding etiologic heterogeneity in ASD, potentially yielding both clinical benefits and support bottom-up investigation of metabolism profiles and medication response for this population.28,29 Alternatively, our demonstration of differential clinical predictors for CYP2D6 and CYP2C19 metabolizer status suggests the possibility that future guidance on when to initiate pharmacogenetic testing (and what specific drug-metabolizing enzymes should be examined) might be derived from a patient's clinical profile. A third of the patients in our sample did not receive pharmacogenetic testing that addressed both CY2D6 and CYP2C19 status, likely reflecting historical practices of ordering single-gene studies; our findings underscore the value of testing focused on relevant genes with actionable implications.
This study is one of the largest to date that focuses specifically on youth with ASD referred to clinical precision medicine. Future studies should include comparison samples of typically developing youth and youth with developmental differences other than ASD. Given the significant heterogeneity in the genotype and phenotype within the ASD population, generalizability of our findings may be limited. Future studies should also integrate more detailed medication histories and clinical data to support the exploration of whether the phenotypic heterogeneity observed in ASD might be leveraged to identify youth most likely to receive actionable precision medicine results. Larger samples and more nuanced characterization should increase the potential to identify specific medication guidelines in which significant dosage adjustments are needed to optimize care based on pharmacogenetic results within the pediatric ASD population.
Our sample had limited racial/ethnic diversity, highlighting the need for proactive efforts to increase access to this service to all youth and to increase diverse representation in research reference samples. The demographic representation of youth with ASD in our precision medicine clinic (83% White, 9% Black, and 8% multiracial/other) was starkly different from the demographic characteristics of our hospital's autism clinic (66% White, 14% Black/African American, and 20% multiracial/other) and the hospital's overall patient population (61% White, 16% Black/African American, and 24% multiracial/other), raising questions about referral biases and other systemic factors limiting access to precision medicine beyond the known barriers to ASD subspecialty care.30 Our team is currently investigating modifiable barriers and facilitators for accessing pharmacogenetic testing through a survey supported by Simons Foundation Powering Autism Research for Knowledge, with the overarching goal of identifying evidence-based approaches for increasing equitable access to this service. Moving forward, we encourage precision medicine clinics to monitor the representation of referred patients in relation to the communities they serve.
In one of the largest reported samples of youth with ASD clinically referred for pharmacogenetic testing, we observed high variability in medication response and yield for actionable results. Our exploratory analyses also demonstrate the possibility of pursuing clinical and phenotypic predictors of metabolizer status. Overall, these findings add to the emerging literature on the utility of pharmacologic testing, including the need for additional research on how to address health disparities in precision medicine access and when pharmacologic testing should be incorporated into clinical care.
Supplementary Material
Footnotes
Disclosure: The authors declare no conflict of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jdbp.org).
See the Video Abstract at www.jdbp.org
Contributor Information
Jennifer Wagner, Email: jawagner@cmh.edu.
Tracy Sandritter, Email: tlsandritter@cmh.edu.
Vincent S. Staggs, Email: vstaggs@cmh.edu.
Sarah Soden, Email: ssoden@cmh.edu.
Cy Nadler, Email: cnadler@cmh.edu.
REFERENCES
- 1.Maenner MJ, Warren Z, Williams AR, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2020. MMWR Surveill Summ. 2023;72:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hyman SL, Levy SE, Myers SM, et al. Identification, evaluation, and management of children with autism spectrum disorder. Pediatrics. 2020;145:e20193447. [DOI] [PubMed] [Google Scholar]
- 3.Bose-Brill S, Xing J, Barnette D, et al. Pharmacogenomic testing: aiding in the management of psychotropic therapy for adolescents with autism spectrum disorders. Pharmacogenomics Pers Med. 2017;10:247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbaresi WJ, Campbell L, Diekroger EA, et al. Society for developmental and behavioral pediatrics clinical practice guideline for the assessment and treatment of children and adolescents with complex attention-deficit/hyperactivity disorder. J Dev Behav Pediatr. 2020;41:S35–S57. [DOI] [PubMed] [Google Scholar]
- 5.Ziskind D, Bennett A, Jawad A, et al. Therapy and psychotropic medication use in young children with autism spectrum disorder. Pediatrics. 2020;145:S99–S107. [DOI] [PubMed] [Google Scholar]
- 6.Wiggins LD, Nadler C, Rosenberg S, et al. Many young children with autism who use psychotropic medication do not receive behavior therapy: a multisite case-control study. J Pediatr. 2021;232:264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg RE, Mandell DS, Farmer JE, et al. Psychotropic medication use among children with autism spectrum disorders enrolled in a national registry, 2007–2008. J Autism Dev Disord. 2010;40:342–351. [DOI] [PubMed] [Google Scholar]
- 8.Patel JN, Mueller MK, Guffey WJ, et al. Drug prescribing and outcomes after pharmacogenomic testing in a developmental and behavioral health pediatric clinic. J Dev Behav Pediatr. 2020;41:65–70. [DOI] [PubMed] [Google Scholar]
- 9.Bowers K, Lin PI, Erickson C. Pharmacogenomic medicine in autism: challenges and opportunities. Pediatr Drugs. 2014;17:115–124. [DOI] [PubMed] [Google Scholar]
- 10.Leeder JS. Who believes they are “just average”: informing the treatment of individual patients using population data. Clin Pharmacol Ther. 2019;106:939–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H. Pharmacogenomics: a promising approach towards treatment of autism. J Pharmacogenomics Pharmacoproteomics. 2012;3:e110. [Google Scholar]
- 12.Hongkaew Y, Gaedigk A, Wilffert B, et al. Pharmacogenomics factors influencing the effect of risperidone on prolactin levels in Thai pediatric patients with autism spectrum disorder. Front Pharmacol. 2021;12:743494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kostic A, Buxbaum JD. The promise of precision medicine in autism. Neuron. 2021;109:2212–2215. [DOI] [PubMed] [Google Scholar]
- 14.Brown JT, Abdel-Rahman SM, van Haandel L, et al. Single dose, CYP2D6 genotype-stratified pharmacokinetic study of atomoxetine in children with ADHD. Clin Pharmacol Ther. 2016;99:642–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L, Brown SJ, Shan Y, et al. CYP2D6 genetic polymorphisms and risperidone pharmacokinetics: a systematic review and meta-analysis. Pharmacotherapy. 2020;40:632–647. [DOI] [PubMed] [Google Scholar]
- 16.Rossow KM, Aka IT, Maxwell-Horn AC, et al. Pharmacogenetics to predict adverse events associated with antidepressants. Pediatrics. 2020;146:e20200957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hervas A, Serra-LLovich A, Rueda I, et al. Pharmacogenetic influences on the response to pharmacological treatment in autism spectrum disorders. J Translational Genet Genomics. 2021;5:278–287. [Google Scholar]
- 18.Lapato DM, Moore AA, Findling R, et al. An update on precision medicine advances in neurodevelopmental disorders. Psychiatr Ann. 2021;51:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watkins L, O'Dwyer M, Shankar R. A review of the pharmacotherapeutic considerations for managing epilepsy in people with autism. Expert Opin Pharmacother. 2022;23:841–851. [DOI] [PubMed] [Google Scholar]
- 20.Specchio N, Di Micco V, Trivisano M, et al. The epilepsy–autism spectrum disorder phenotype in the era of molecular genetics and precision therapy. Epilepsia. 2022;63:6–21. [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Sun X, Sun C, et al. Prevalence of epilepsy in autism spectrum disorders: a systematic review and meta-analysis. Autism. 2022;26:33–50. [DOI] [PubMed] [Google Scholar]
- 22.Sandritter TL, Dinh JC, Wagner JA, et al. Description of an innovative pediatric individualized therapeutics clinic: working toward precision drug therapy. Children. 2019;6:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caudle KE, Sangkuhl K, Whirl-Carrillo M, et al. Standardizing CYP2D6 genotype to phenotype translation: consensus recommendations from the Clinical Pharmacogenetics Implementation Consortium and Dutch Pharmacogenetics Working Group. Clin Translational Sci. 2020;13:116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swen JJ, Nijenhuis M, de Boer A, et al. Pharmacogenetics: from bench to byte—an update of guidelines. Clin Pharmacol Ther. 2011;89:662–673. [DOI] [PubMed] [Google Scholar]
- 25.Gaedigk A, Sangkuhl K, Whirl-Carrillo M, et al. Prediction of CYP2D6 phenotype from genotype across world populations. Genet Med. 2017;19:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campos AI, Byrne EM, Mitchell BL, et al. Impact of CYP2C19 metaboliser status on SSRI response: a retrospective study of 9500 participants of the Australian Genetics of Depression Study. Pharmacogenomics J. 2022;22:130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swen JJ, van der Wouden CH, Manson LE, et al. A 12-gene pharmacogenetic panel to prevent adverse drug reactions: an open-label, multicentre, controlled, cluster-randomised crossover implementation study. Lancet. 2023;401:347–356. [DOI] [PubMed] [Google Scholar]
- 28.Arnett AB, Trinh S, Bernier RA. The state of research on the genetics of autism spectrum disorder: methodological, clinical and conceptual progress. Curr Opin Psychol. 2019;27:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lombardo MV, Lai MC, Baron-Cohen S. Big data approaches to decomposing heterogeneity across the autism spectrum. Mol Psychiatry. 2019;24:1435–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wallis KE, Guthrie W, Bennett AE, et al. Adherence to screening and referral guidelines for autism spectrum disorder in toddlers in pediatric primary care. PLoS One. 2020;15:e0232335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


