Abstract
Purpose of Review:
Thrombosis of the dural sinus and/or cerebral veins (CVT) is a rare but potentially devastating type of stroke that tends to occur in young adults, especially women. In this article, the abbreviation CVT refers to either cerebral venous thrombosis or dural sinus thrombosis. The purpose of this review is to review the most up-to-date literature on the epidemiology, diagnosis, management, and prognosis of CVT. In addition, illustrative cases that represent the spectrum of CVT are provided.
Recent Findings:
CVT represents about 0.5% of all strokes and can be challenging to diagnose because headache, rather than focal neurologic symptoms, is the prominent feature. The diagnosis is confirmed with MRI and magnetic resonance venogram (MRV). The mainstay of acute management is anticoagulation, although, in the cases of severe hemorrhagic conversion of a venous infarction, endovascular mechanical thrombectomy may be potentially lifesaving. The evaluation of underlying causes from transient triggers, eg, pregnancy, oral contraceptives, or infection, versus chronic triggers, eg, cancer and thrombophilia, will often influence the duration of anticoagulation. The outcomes after CVT are generally favorable, and the risk of recurrence is low.
Summary:
CVT is an important diagnosis to keep in mind when evaluating patients with headache in the emergency department, and it is important that it not be overlooked because it is treatable.
EPIDEMIOLOGY AND PATHOGENESIS
Thrombosis of the dural sinus and/or cerebral veins (CVT) is relatively rare, accounting for only about 0.5% of patients with stroke and with a prevalence of only 5 per 1 million.1 CVT is also primarily a stroke type that affects individuals younger than the age of 50. In the 624 cases included in the International Study of Cerebral Venous Thrombosis (ISCVT), the median age was 37 years.2 The incidence of CVT in children is 0.67 per 100,000 per year, with 43% occurring in neonates.3 In addition, women make up about 75% of those with CVT, likely a result of the sex-specific risk factors that occur in women, including oral contraceptives, pregnancy, and hormone therapy use.
CVT causes dysfunction by two mechanisms. (1) Thrombosis of cerebral veins causes localized edema of the brain and venous infarction. (2) Thrombosis of the major sinuses leads to intracranial hypertension as a result of increased venous pressure and impaired absorption of CSF.4 Generally, some underlying trigger or predisposing risk factor that increases the risk of thrombosis is the reason for the timing and occurrence of CVT. In the ISCVT, 85% of patients with CVT had at least one identifiable risk factor for thrombosis and 44% had multiple predisposing factors.2 These predisposing factors are discussed in detail below.
CLINICAL PRESENTATION
The presentation of patients with CVT may be variable. It can be categorized into four clinical syndromes: (1) isolated intracranial hypertension (eg, patients may present with headache, diplopia, visual impairment [severe papilledema], decreased level of consciousness, or sixth nerve palsy), (2) focal neurologic deficit (eg, motor weakness, sensory deficit, aphasia), (3) encephalopathy, and (4) seizures (accompanied or not by a focal neurologic deficit).1,4
Headache is the most common symptom, affecting approximately 90% of patients with CVT. Isolated headaches are less common, but may constitute a clinical challenge (Table 5-1).2,5 The presentation of headache associated with focal symptoms or seizures may suggest the presence of a venous infarction.6 Hemorrhagic conversion was reported in 35% to 39% of patients.2 Demographic characteristics associated with hemorrhagic conversion include older age, female sex, and an acute onset (48 hours).6
Table 5-1.
Clinical Manifestations of Cerebral Venous Thrombosis
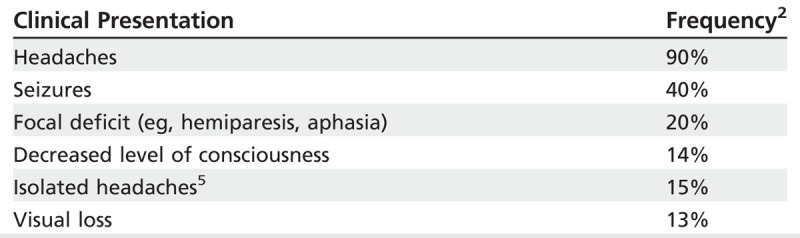
The most commonly affected venous sinus is the superior sagittal (62%) followed by the transverse sinus (41% to 45%; Figure 5-17).2 Patients less commonly present with deep cerebral venous occlusion of the internal cerebral vein or vein of Galen (11%), or straight sinus (18%), but they are at a nearly threefold higher risk of death and dependency during follow-up.2 These patients may present with bilateral thalamic or basal ganglia infarction, and will often have decreased level of consciousness and rapid neurologic deterioration.2 Some symptoms may guide the localization or extension of the CVT. For example, patients with deep cerebral venous occlusion or extensive CVT (involving the superior sagittal plus other sinuses) may present with decreased level of consciousness with bilateral papilledema.7,8 The development of headaches and fever in the context of an underlying otitis or sinusitis may raise suspicion about CVT involving the transverse or sigmoid sinuses.7,9 Delays in diagnosis are common, likely related to an insidious course in up to two-thirds of patients.2
Figure 5-1.
Cerebral venous thrombosis: most commonly affected sinuses. The sagittal sinus is the most commonly affected, followed by the transverse sinuses.
Modified from Saposnik G, et al, Stroke.7 © 2011 American Heart Association, Inc. stroke.ahajournals.org/content/42/4/1158.long.
DIAGNOSIS
The diagnosis of CVT requires a high level of clinical suspicion and confirmation by neuroimaging. The differential diagnosis of CVT is summarized in Table 5-2. D-Dimer, a product of fibrin degradation, has been studied in the context of CVT. Most patients with an acute and extensive CVT have a D-dimer level greater than 500 μg/L.7 A recent meta-analysis of 14 studies reported similar findings, with a weighted mean sensitivity of 93.9% and specificity of 89.7%.10 However, a low D-dimer level does not rule out CVT in patients with high suspicion or in the subacute/chronic phase of the disease, since the D-dimer levels decline with time from onset of symptoms.7,10,11 In addition, those with a lesser clot burden may have false negative D-dimer levels. Therefore, a strong clinical suspicion of CVT should not prohibit further evaluation if the D-dimer level is normal.
Table 5-2.
Differential Diagnosis of Cerebral Venous Thrombosis

Recent advances in brain imaging have played an important role in improving the diagnosis of CVT. CT brain, CT venogram, MRI brain, magnetic resonance venogram (MRV), and conventional angiogram are the common investigations used in the diagnosis of CVT.12 The American Heart Association (AHA) and the European Federation of Neurological Societies (EFNS) guidelines recommend MRI/MRV as the preferred brain image, whereas CT/CT venogram is an acceptable option when MRI is not available.7,13 One of the advantages of MR is the use of novel sequences, eg, T2* susceptibility-weighted imaging, that improve the detection of isolated cortical venous thrombosis identified as a hypointense area.14,15
CT
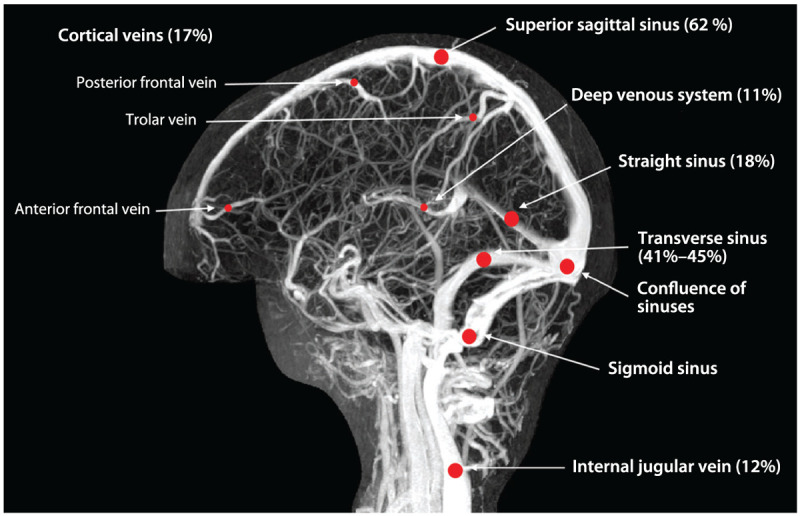
Spontaneous density (dense cord sign) represents the thrombosed cortical vein or sinus that can be seen in noncontrast CT (Figure 5-2A).14,16 The presence of bilateral intracerebral hemorrhages, hemorrhagic infarction, or an infarction outside the boundaries for an arterial territory are useful signs to suspect cerebral venous sinus thrombosis.17 With contrasted CT, the delta sign represents the thrombosis of the posterior portion of the superior sagittal sinus or transverse sinus (Figure 5-2B).14 CT venogram may reveal the thrombosis of major sinuses up to the jugular vein (Figure 5-2B). Three-dimensional reconstitution of the CT venogram may facilitate visualization of the thrombosed sinus (Figure 5-2C). The advantage of CT/CT venogram includes its wide availability and prompt assessment of major sinuses. Radiation, exposure to the risk of IV contrast, and poor visualization of deep and cortical venous thrombosis are known limitations.7
Figure 5-2.
A young woman presenting with headache in the emergency department. A, Plain CT shows increased attenuation involving the superior sagittal sinus, straight sinus, the confluence, and right transverse sinus (yellow arrows). B, CT venogram reveals absence of contrast in the superior sagittal sinus, straight sinus, right transverse sinus (green arrow), right sigmoid sinus, and right internal jugular vein (yellow arrow). C, Three-dimensional reconstruction of the CT venogram shows thrombosis of the superior sagittal sinus (yellow arrows).
MRI
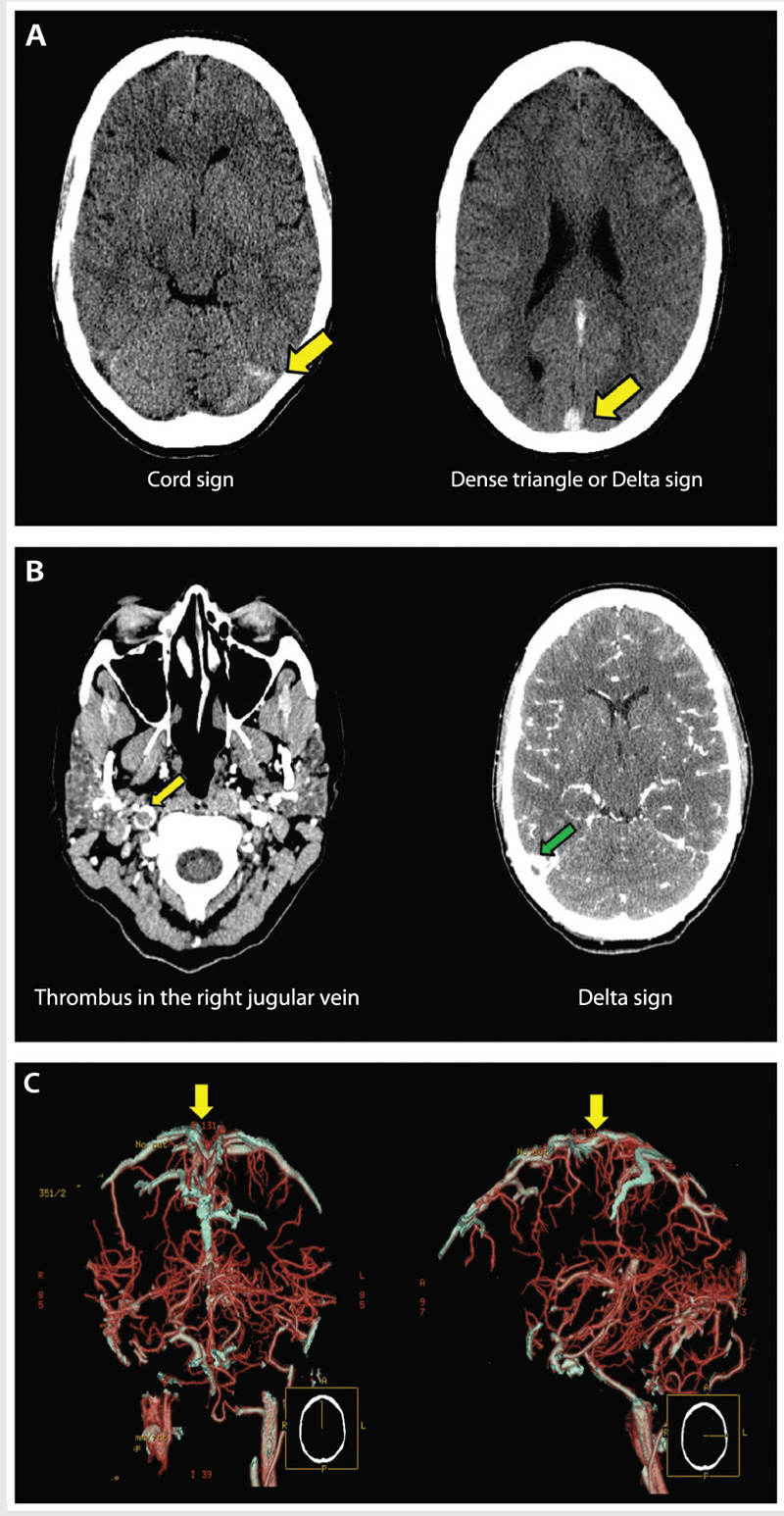
The most common findings using MRI include visualization of the thrombus in T1-weighted images or loss of signal in the venous system on MRV (Case 5-1, Figure 5-3A). Changes of blood products using MRI constitute a limitation for visualization of CVT in the acute phase. For example, in the first 5 days after CVT, the thrombus is isointense on T1-weighted images and hypointense on T2-weighted images because of increased deoxyhemoglobin.7,14 In the subacute stage (5 to 15 days), the thrombus becomes hyperintense on T1-weighted and T2-weighted images. After 15 days, the thrombus will become homogeneous and hypointense in all image sequences.7,14 Contrast-enhanced MRV is more sensitive than time-of-flight MRV in demonstrating the thrombus within small veins.7,18
Figure 5-3.
A young woman with headache. A, Magnetic resonance venogram (MRV) showing an extensive thrombosis throughout the posterior two-thirds of the superior sagittal sinus, involving the torcular herophili, straight sinus, the right transverse and sigmoid sinuses, and extending inferiorly into the right internal jugular vein (arrows). The left transverse and sigmoid sinuses and left internal jugular veins are spared. B, MRV (sagittal view) showing the thrombosis of two-thirds of the superior sagittal sinus, involving the torcular herophili and the straight sinus (arrow). C, MRV at 1 year showing complete recanalization of the previously thrombosed sinuses.
Conventional angiography is less commonly used to assess for CVT and is typically reserved for selected situations when the diagnosis is uncertain or other brain imaging techniques are unavailable.7,13,14
Case 5-1
A 26-year-old right-handed woman with a history of ulcerative colitis and anemia presented to the emergency department with a new onset of headaches, somnolence, and numbness in the fingertips of both hands. She described progressive worsening of sharp headaches within 72 hours accompanied by nausea and vomiting. Current medications included oral contraceptives that were started 2 years ago. On presentation, her blood pressure was 154/84 mm Hg, and her heart rate was 62 beats per minute. She was afebrile. On neurologic examination, she was somnolent, opening her eyes to verbal stimuli and oriented to place and time. Funduscopic examination revealed bilateral papilledema. Cranial nerve examination was otherwise normal, and the remainder of her neurologic examination was unremarkable. Her d-dimer level was greater than 3000 μg/L. A plain CT head scan demonstrated increased attenuation of the superior sagittal and transverse sinuses (Figure 5-2A). CT venography revealed occlusion of the superior sagittal sinus, straight sinus, the confluence of sinuses, and right transverse sinus extending to the right jugular vein (Figure 5-2B and C). Brain MRI and brain magnetic resonance venogram confirmed the observed CT findings with no evidence of venous infarction (Figure 5-3A and B).
Comment. This case illustrates the common clinical presentation with signs and symptoms suggestive of intracranial hypertension, elevated d-dimer level, and imaging finding consistent with CVT involving multiple sinuses extending to the right jugular vein.
RISK FACTORS AND TRANSIENT VERSUS CHRONIC TRIGGERS
Once a CVT has been diagnosed, it is important to search for the potential causes and predisposing factors. Female sex is one of the major risk factors for CVT, as cases in women outnumber cases in men 3 to 1. In sex-specific analysis of those enrolled in the ISCVT, women were younger, had a shorter time from onset of symptoms to admission, and more often had headache at presentation.19 Sixty-five percent of identifiable causes of CVT in women were related to a risk factor unique to women, including oral contraceptives, pregnancy (Case 5-2), the puerperium, and hormone therapy.19
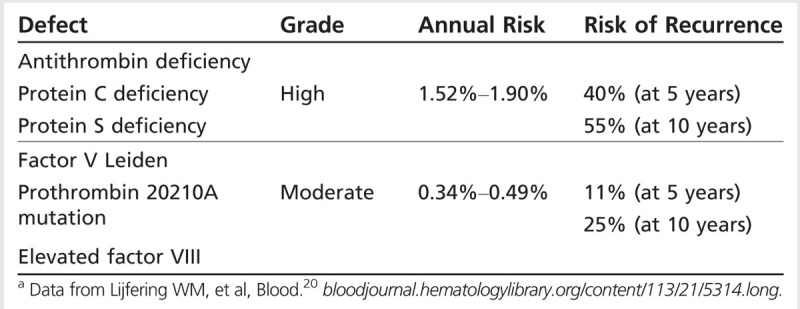
Transient risks and triggers are frequently considered to be related to a temporary condition, such as pregnancy/puerperium, CNS or ear/sinus/mouth/face infections, exposure to drugs (eg, oral contraceptives, steroids, oncology treatments), head trauma, or procedures (eg, lumbar puncture, jugular catheter placement; Table 5-3). Chronic triggers include hereditary or acquired thrombophilias (disorders that increase the likelihood of blood clotting) that are established causes of venous thromboembolism. Specific types include deficiencies of protein S, protein C, or antithrombin III, as well as antiphospholipid antibody syndrome, dysfibrinogenemia, increased factor VIII levels, factor V Leiden mutation (causing resistance to protein C), and prothrombin 20210A mutation. Each thrombophilia has been associated with CVT, with varying annual risk of recurrence of venous thromboembolism (Table 5-420).7 In one study of 145 patients with CVT, the prothrombin mutation was found in 19%,21 although a separate study reported antiphospholipid antibodies as the most prevalent thrombophilia in that group of patients (5.9%).2 It is important to note that multiple predisposing factors are often found in these patients. For example, in the study of 145 patients with CVT, the combination of oral contraceptives and thrombophilia was present in 37% of women.21
Table 5-3.
Common Underlying Causes of Cerebral Venous Thrombosis or Dural Sinus Thrombosis Designated by Transient Versus Chronic/High-Risk Triggers
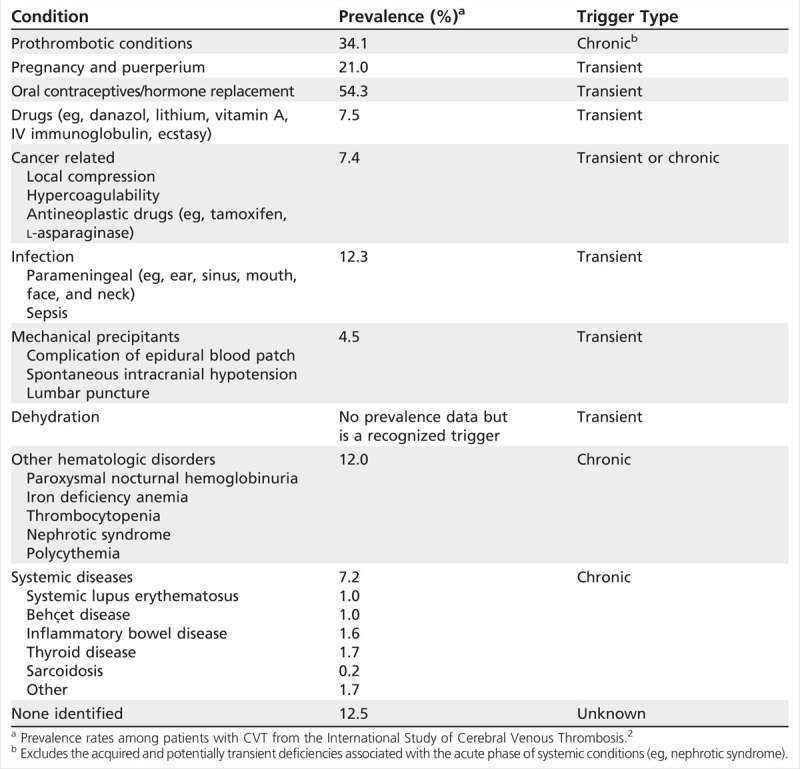
Table 5-4.
Thrombophilias: Annual Risk and Recurrence Rate of Venous Thromboembolism Among Families With Hereditary Disordersa
Case 5-2
A 27-year-old woman, 19 weeks pregnant with a history of migraine headaches, developed severe occipital headache that increased in severity over the course of 5 days, unlike her usual migraine. Her neurologic examination was normal. Magnetic resonance venogram (MRV) showed acute thrombosis of the transverse and sigmoid sinuses on the left, and MRI showed adjacent left mastoiditis (Figure 5-4A and B). She was treated with antibiotics and unfractionated heparin subcutaneously until delivery, and then switched to adjusted-dose warfarin daily after delivery with a targeted international normalized ratio of 2 to 3. The repeat MRI/MRV on follow-up 1 year later revealed complete recanalization of the left transverse and sigmoid sinuses, and the mastoiditis had resolved (Figure 5-4C). Warfarin was discontinued.
Figure 5-4.
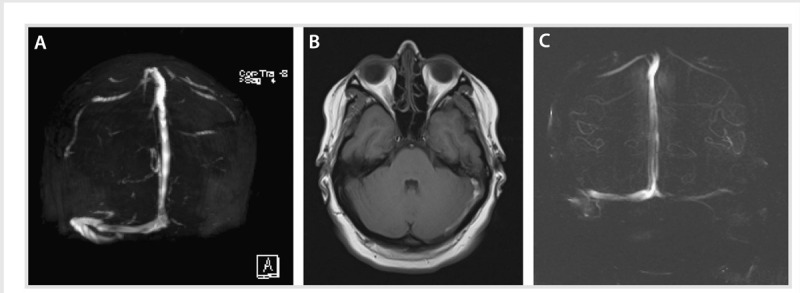
A young pregnant woman with mastoiditis. A, Magnetic resonance venogram showing acute thrombosis of the left transverse and sigmoid sinuses. B, T1-weighted MRI of the brain showing left mastoid effusion and adjacent thrombosis that include the cortical veins of the tentorium. C, Resolution of the left transverse and sigmoid thrombosis 20 months later.
Comment. This case illustrates the transient trigger of both pregnancy and mastoiditis in a young woman who later had complete resolution of the thromboses as well as the mastoiditis.
ACUTE MANAGEMENT
The acute management of CVT involves strategies aimed at the recanalization of the thrombosed sinus or sinuses (Figure 5-5) and the prevention of medical complications. The treatment of the underlying causes is discussed in another section.
Figure 5-5.
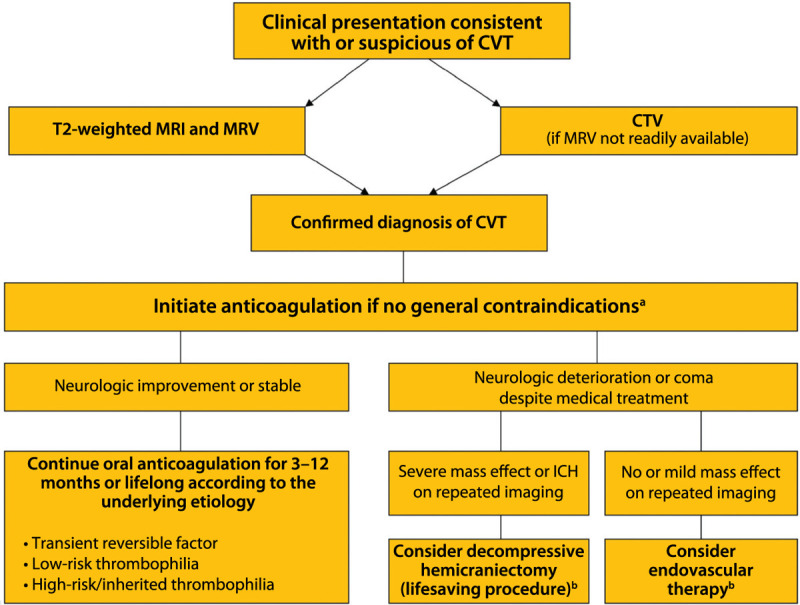
Algorithm for the management of cerebral venous thrombosis.
CVT = cerebral venous thrombosis; MRI = magnetic resonance imaging; MRV = magnetic resonance venography; CTV = computed tomography venography; ICH = intracerebral hemorrhage.
a Anticoagulation remains the principal therapy and is aimed at preventing thrombus propagation and increasing recanalization. This algorithm is not comprehensive, nor is it applicable to all clinical scenarios; patient management must be individualized.
b Limited evidence is available on the benefits of decompressive hemicraniectomy and endovascular therapy for the management of cerebral venous thrombosis. Modified from Saposnik G, et al, Stroke.7 © 2011 American Heart Association, Inc. stroke.ahajournals.org/content/42/4/1158.long.
Anticoagulation
Despite the limited evidence from large randomized clinical trials, initial anticoagulation is the standard treatment for patients with CVT. Anticoagulation is used to prevent thrombus growth, to facilitate recanalization, and to prevent other thrombotic events (eg, deep venous thrombosis and pulmonary embolism). A recent meta-analysis of two trials22,23 including 79 patients revealed that anticoagulation therapy was associated with a lower relative risk of death (0.33, 95% confidence interval [CI], 0.08 to 1.21) and of death or dependency (0.46, 95% CI, 0.16 to 1.31).24 A sensitivity analysis, in which two of the excluded (one reported as an abstract, and another one not having vascular imaging) randomized studies (n = 176) were added, revealed a significant reduction in the odds of death (odds ratio [OR] 0.33; 95% CI, 0.14 to 0.78; P<.006) with anticoagulation therapy.24 Since intracerebral hemorrhage (ICH) may be a consequence of venous hypertension caused by the thrombosed vein or sinus, guidelines recommend anticoagulation therapy even in the presence of ICH or hemorrhagic transformation (Table 5-5).7,13,25
Table 5-5.
Comparison Among Guidelines for the Acute Management Options of Cerebral Venous Thrombosis
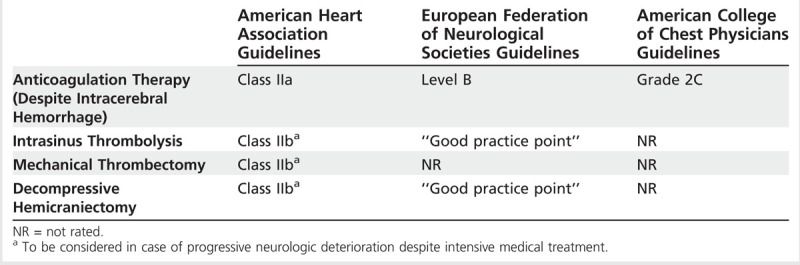
Data from the International Study on Cerebral Vein and Dural Sinus Thrombosis suggest low-molecular-weight heparin (LMWH) might be safer and perhaps more efficacious than unfractionated heparin. For example, among CVT participants in the study, 119 (28%) patients received LMWH and 302 (72%) received unfractionated heparin.26 CVT patients receiving LMWH were more likely to be functionally independent at 6 months compared with patients receiving unfractionated heparin (OR = 2.4; 95% CI, 1.0 to 5.7). Post hoc analysis also revealed a nonsignificant lower chance of ICH (adjusted OR = 0.29; 95% CI, 0.07 to 1.3) for patients receiving LMWH than for those receiving unfractionated heparin.26
Case 5-3
A 59-year-old woman with a history of psoriatic arthritis and migraine headaches had the onset of severe occipital headaches over the course of several days and presented to an outside emergency department multiple times during the week before admission. Brain CT scans from these emergency department visits had been read as normal. On the day of admission, she presented with worsening headache, visual disturbances, and left-sided weakness. Her neurologic examination showed a normal mental status, but she had a gaze deviation to the right, left homonymous hemianopia, left facial droop, and moderate left-sided weakness. Brain CT revealed a right temporal ICH, and MRI brain showed a right sigmoid and transverse sinus thrombosis, extending into the right jugular vein (Figure 5-6A and B). Because of the size of her hemorrhage, the extension of the venous sinus thrombosis, and progressive neurologic deterioration, she underwent endovascular mechanical thrombectomy and balloon venoplasty of the right mid-sigmoid sinus, which resulted in successful recanalization and antegrade flow through the sigmoid and transverse sinuses and right internal jugular vein (Figure 5-6C). This procedure was followed by an unfractionated heparin drip. She had immediate improvement of her headache, but it returned the next day. Her left-sided visual field deficits and weakness resolved during the next 3 weeks.
Figure 5-6.
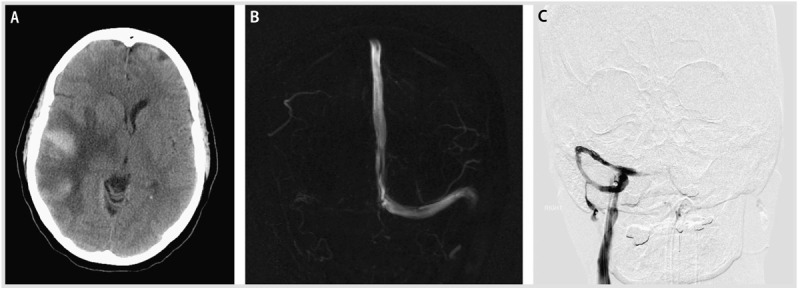
Hemorrhagic venous infarction and endovascular therapy. A, Head CT at presentation showing the large right temporal venous infarct with hemorrhagic conversion and mass effect. B, Magnetic resonance venogram (MRV) showing thrombosis of the right transverse and sigmoid sinus. C, Interventional angiogram with catheter in the right transverse sinus, status post thrombolysis and balloon angioplasty, and resolution of the thrombus.
Comment. This case illustrates the progression of CVT and the difficulty with recognition of this condition by CT scan until venous infarction with hemorrhagic conversion leads to progression of neurologic deficits. In addition, it illustrates the dilemma of treatment in the setting of severe thrombosis and significant hemorrhage causing mass effect and edema. Aggressive anticoagulation in the setting of this much bleeding was thought to be contraindicated acutely, leading to the decision to perform endovascular mechanical thrombectomy.
[end case]
Endovascular Therapy
Different endovascular approaches may be considered for patients with CVT who develop progressive neurologic deterioration despite intensive medical treatment, including anticoagulation. Unfortunately, the evidence is limited by a nonrandomized design and small sample size for most studies.7
Thrombolysis is regarded as a potential treatment to improve the probability of early recanalization for patients who were unsuccessful with anticoagulation. A combination of four studies may suggest a higher recanalization (87%) compared with anticoagulation alone (range 47% to 100%).27,28,29 However, invasive procedures carry some risk of complications, including intracranial bleeding. A randomized trial comparing thrombolysis versus anticoagulation is underway (Thrombolysis or Anticoagulation for Cerebral Venous Thrombosis [TO-ACT] trial).30
Mechanical thrombectomy is another alternative to improve recanalization in patients with CVT (Case 5-3). A recent literature review from 1990 to 2012 included 64 patients who received mechanical thrombectomy.31 The most commonly used device was the AngioJet (46.9%), followed by balloon venoplasty without stenting (18.7%) and the Penumbra system (4.7%). Mortality was reported in nine (16.1%) patients; 40 (62.5%) patients had no disability or minor disability.31 A large randomized multicenter trial is needed before reaching conclusions about the efficacy of these devices and which patients to target.
Decompressive Hemicraniectomy
Rarely, patients with CVT may develop a malignant cerebral infarction (Case 5-4). Limited evidence is available regarding the role of decompressive hemicraniectomy in CVT. In a small case series including 10 patients (median age 41 years) who underwent decompressive hemicraniectomy following CVT, five patients recovered without disability at 12 months (modified Rankin Scale score of 0 to 1) and two patients died of progressive cerebral edema and expansion of the hemorrhagic infarcts.32 In another review including 13 patients with severe CVT who underwent decompressive hemicraniectomy, 11 (84.6%) achieved a favorable outcome (modified Rankin Scale score of 3 or less).33 This procedure should be reserved for young CVT patients who develop a malignant hemispheric stroke with progressive neurologic deterioration despite intensive medical treatment.
Case 5-4
A 37-year-old woman was found by her mother with incoherent speech and reports of headaches. When the paramedics arrived, she developed a generalized tonic-clonic seizure. On arrival to the emergency department, she was lethargic and had an expressive aphasia and right-sided weakness. Medical history was remarkable for menorrhagia and anemia. A noncontrast CT on admission revealed an extensive left frontal hypodensity with mass effect (Figure 5-7). A CT venogram confirmed thrombosis of several left frontal cortical veins with extension into the superior sagittal sinus. MRI confirmed the extensive cortical vein thrombosis and a venous infarction with hemorrhagic conversion (Figure 5-8A–D). The patient underwent hemicraniectomy for a malignant venous infarction secondary to CVT. Despite anticoagulation and intensive medical and surgical treatment of her cerebral edema with osmotic agents, the patient developed neurologic deterioration with an increased intracranial pressure (35 mm Hg) after hemicraniectomy. Brain CT revealed enlargement of the venous infarction with associated multifocal hemorrhagic components. Figure 5-9 shows herniation of the brain through the craniectomy with signs of severe mass effect on the midline structures and basal cisterns. The patient died 24 hours after the increased intracranial pressure was diagnosed.
Figure 5-7.
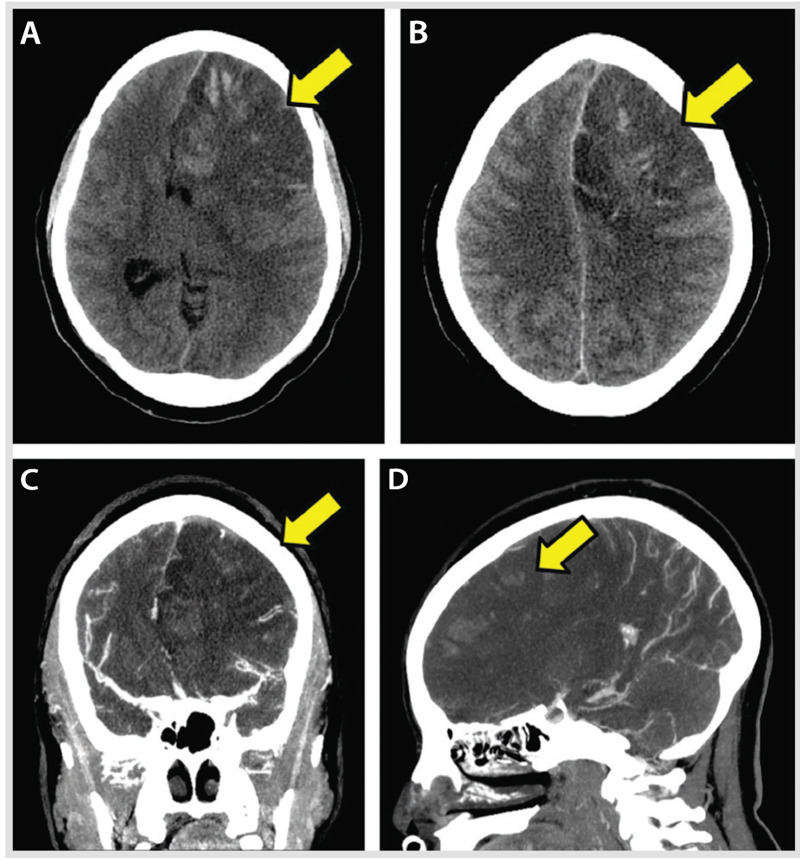
Mass effect from the hemorrhagic conversion of a venous infarction (A–D, arrows) within the left frontal lobe lesion. Signs of brain herniation are evident, as is thrombosis of several left frontal cortical veins with very subtle extension in the superior sagittal sinus. The lack of visualization of residual lumen represents the occluded cortical veins and the associated severe mass effect from the adjacent brain edema (C, D, arrows).
Figure 5-8.
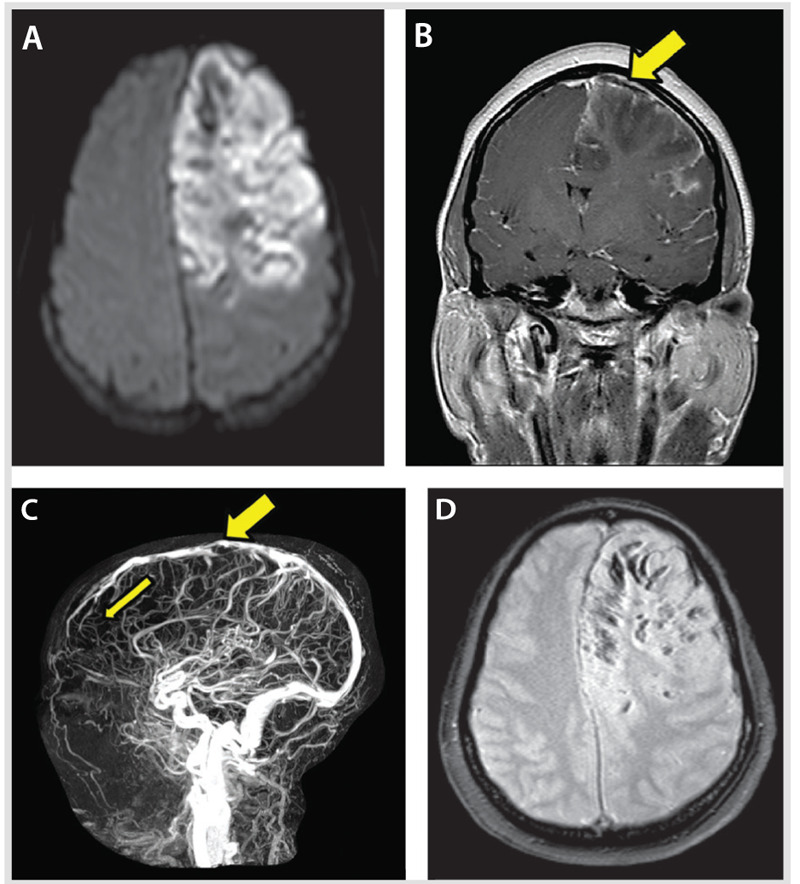
Large area of altered signal and swelling with restricted diffusion (A, B) involving the cortex and underlying white matter of most of the left frontal lobe. Several areas of hypointense signal are present, more obvious on the gradient-echo and susceptibility-weighted imaging sequence in keeping with multiple hemorrhagic foci (D). The magnetic resonance venogram (C) shows few multifocal filling defects involving the superior sagittal sinus. These defects are nonobstructive. A focal severe attenuation of the central lumen of the superior sagittal sinus (B, C, thick arrows) is present, as is a paucity of expected opacified left convexity cortical veins compared with the right frontal lobe (thin arrow).
Figure 5-9.
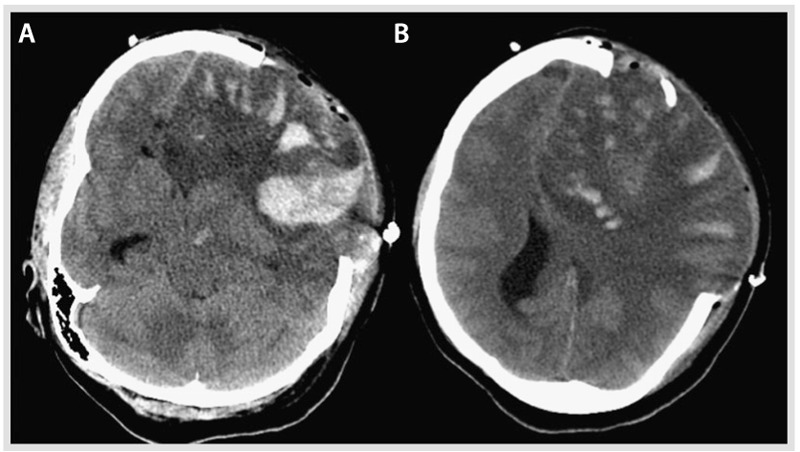
Noncontrast CT post craniectomy showing further enlargement of the large left hemispheric lesion and associated multifocal hemorrhagic components and brain herniating through the craniectomy site with persistent signs of severe mass effect on the midline structures and basal cisterns. A, Inferior section, and B, superior section, both of which illustrate the mass effect and the hemorrhagic conversion.
Comment. This case illustrates the challenges in the management and early complications of malignant edema from CVT, despite aggressive surgical measures.
[end case]
PREVENTION OF MEDICAL COMPLICATIONS
Seizures
Seizures may occur in one-third of adults and nearly one-half of children with CVT (Case 5-5).3 The most common factors associated with the development of seizures include the presence of a venous infarction, hemorrhagic conversion, and intracranial hypertension.
In the ISCVT, 39% of patients presented with seizures and 6.9% experienced an early seizure within 2 weeks after diagnosis. Supratentorial parenchymal lesions on CT/MRI at the time of the diagnosis (present in 58%) were associated with occurrence of early seizures (OR = 3.1; 95% CI, 1.6 to 9.6).34 No benefit has been found for the routine use of antiepileptic drugs for patients with CVT. Guidelines for the management of CVT recommend early initiation of antiepileptic drugs in patients with CVT and a single or multiple seizures.7
Hydrocephalus
The main sites for CSF absorption are the arachnoid granulations, which protrude across the sinuses. The thrombosis of major sinuses (eg, superior sagittal, transverse sinuses) may affect CSF drainage, resulting in communicating hydrocephalus. In a large study of 11,400 patients with CVT, hydrocephalus was observed in 15.0% of patients. Hydrocephalus was associated with 3 times higher risk of death (OR = 3.2; 95% CI, 5.5 to 15.1).35 The appropriate treatment includes measures to decrease the production of CSF, recanalization of the thrombosed sinus, and management of intracranial hypertension. Neurosurgical consultation for evacuation of CSF either with ventriculostomy or ventriculoperitoneal shunt may be necessary.7
Intracranial Hypertension
The development of intracranial hypertension is usually caused by venous outflow obstruction and CSF malabsorption. Intracranial hypertension may be seen in 15% to 40% of patients with CVT.2,7
Symptomatic treatment includes the use of diuretics (ie, acetazolamide) and serial lumbar punctures. Steroids are ineffective.7,36 In refractory cases, lumboperitoneal shunt may be required.37 However, no randomized trials have tested any of these interventions.
Visual Loss
The persistent and prolonged pressure on the optic nerves can result in loss of vision, reported in up to 13% of patients with CVT.2 However, early recognition and the use of advanced imaging suggest this has become a less common phenomenon. Ophthalmic consultation, monitoring for papilledema, and visual field testing are commonly recommended measures. For patients at high risk of blindness, optic nerve fenestration may be considered.7,13
Case 5-5
A 32-year-old woman presented to the emergency department after having a generalized tonic-clonic seizure witnessed by her husband. One week before admission, she delivered a baby via an uneventful cesarean delivery. Her medical history was remarkable for a prior deep venous thrombosis. She had no family history of thrombophilia. On neurologic examination, she was drowsy and had left-sided weakness and an enlarged pupil on the right side. A noncontrast brain CT revealed foci of intraparenchymal hemorrhage in the right parietal subcortical region; CT venography revealed thrombus within a cortical vein. IV heparin was initiated. MRI revealed heterogeneous signal intensity on fluid-attenuated inversion recovery (FLAIR)/T2 and T1 sequences with hyperintense components and susceptibility artifact on the gradient sequence, indicating acute focal bleeding in the right parietal lobe. The right posterior parietal hyperintensity in the FLAIR sequence is consistent with a cortical venous thrombosis (Figure 5-10A). Her level of consciousness decreased (ie, she was able to open her eyes only to painful stimuli) 6 hours after admission, and she underwent hematoma evacuation and hemicraniectomy (Figure 5-10B). The patient was discharged on warfarin and phenytoin 10 days after admission. Neurologic examination 6 months later was unremarkable, and her functional status with activities of daily living was intact (Barthel Index of 100). Blood testing at that time revealed a significantly low protein S level (0.26 mg/L) and free protein S antigen level at 0.38 mg/L. Lifelong anticoagulation was recommended by a hematologist because of a protein S deficiency identified as a severe thrombophilia, with high risk of recurrence of venous thromboembolism (Table 5-4).7,37
Figure 5-10.
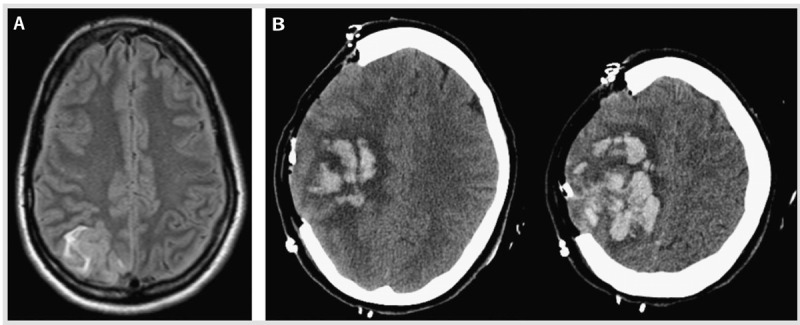
Seizures and intracerebral hemorrhage due to cerebral venous thrombosis in the puerperium. A, Fluid-attenuated inversion recovery (FLAIR) T2 MRI shows hyperintensity in the right posterior parietal region consistent with acute cortical vein thrombosis. B, A head CT post hemicraniectomy demonstrates a large right frontoparietal intraparenchymal hemorrhage with associated mass effect and mild herniation of the brain through the craniectomy defect.
Comment. This case illustrates the development of CVT in the puerperium presenting with seizures and an intracerebral hemorrhage. Treatment with phenytoin was initiated after the initial seizures in the presence of intracerebral hemorrhage.
[end case]
PROGNOSIS AND RISK OF RECURRENCE
Outcomes after CVT are generally favorable. Women tend to have a better prognosis than men; in the ISCVT, 81% of women had complete recovery versus 71% of men (P=.01).19 However, women without sex-specific risk factors tended to have a worse outcome than women with these risk factors (OR for poor outcome 3.7; 95% CI, 1.9 to 7.4).19
The recurrence of any thrombotic event after a CVT is about 6.5% per year, including systemic venous thromboembolism.2,38 Patients with CVT have been reported to develop deep venous thrombosis, pulmonary embolism, ischemic stroke, and acute limb ischemia in addition to recurrent CVT, but these events were mostly in those not currently anticoagulated.2
The presence of thrombophilia, and whether this is considered mild or severe, has been used as a decision point for risk of recurrence and duration of treatment. Mild thrombophilias include heterozygous factor V Leiden and prothrombin G20210A mutations, and dysfibrinogenemia, whereas protein C, protein S, and antithrombin III deficiencies; antiphospholipid antibodies; homozygous factor V Leiden mutation; and multiple abnormalities are categorized as severe. A follow-up study of participants with CVT revealed that patients with severe thrombophilia were 4 times more likely to have a deep venous thrombosis or pulmonary embolism (adjusted hazard ratio 4.19; 95% CI, 1.25 to 14.0) than those without thrombophilia. The risk of recurrence was similar in those with mild thrombophilia and those without thrombophilia.21 Among hereditary thrombophilias, the highest risk of recurrence of venous thromboembolism (not specifically CVT) is with homozygous factor V Leiden and antithrombin III, protein C, and protein S deficiencies (40% at 5 years), whereas the risk with heterozygous factor V Leiden, prothrombin G20210A mutations, and elevated factor VIII is much less (11% at 5 years) (Table 5-4).20
CHRONIC ANTICOAGULATION: HOW LONG TO TREAT
Duration of anticoagulation following CVT has not been studied in any randomized controlled trials, and most recommendations are based on observational data.7 Considerations should be based on the risk for recurrence, patient preference, bleeding risk, and the risk of thrombosis without anticoagulation.7,20,21 Guideline recommendations for the duration of anticoagulation in patients with CVT are dependent on the presence of provoked (based on a transient risk factor) versus unprovoked events, as well as the presence of mild versus severe thrombophilia. For those with provoked CVT, warfarin (target international normalized ratio of 2.0 to 3.0) for 3 to 6 months is recommended.7 Patients with unprovoked CVT may be treated with warfarin for 6 to 12 months. In patients with recurrent CVT, venous thromboembolism after CVT, or first CVT (provoked or unprovoked) with severe thrombophilia, indefinite anticoagulation may be considered. It is important to note that these recommendations are based on consensus.7 It is recommended that patients with CVT undergo thrombophilia testing, including functional testing for protein C, protein S, antithrombin III; quantitative measurement of antiphospholipid antibodies (lupus anticoagulant and anticardiolipin antibodies); and genetic testing for factor V Leiden and prothrombin G20210A mutations. Consultation with a physician with expertise in coagulation disorders, such as a hematologist, may also be helpful.7
CONCLUSION
In conclusion, CVT is an uncommon cause of stroke, but it requires accurate diagnosis since the pathophysiology and treatment differ from arterial stroke. Understanding the transient and chronic risk factors is the key to prognosis for recurrent CVT and whether long-term treatment with anticoagulation is needed. In addition, although the outcomes after CVT are generally favorable, they also depend on patient factors, such as sex and the female-specific risk factors.
KEY POINTS
Cerebral venous thrombosis is a cerebrovascular disease of the young, primarily of women.
Headache is the most common symptom of cerebral venous thrombosis.
The most common clinical syndromes in cerebral venous thrombosis include (1) intracranial hypertension, (2) focal neurologic deficits (eg, motor weakness, sensory deficit, aphasia), (3) encephalopathy, and (4) seizure disorder.
MRI/magnetic resonance venography is the recommended imaging for the diagnosis of cerebral venous thrombosis. Specific sequences (eg, T2* susceptibility-weighted imaging) are useful to assist in the diagnosis of isolated cortical venous thrombosis.
Transient risk factors for cerebral venous thrombosis include pregnancy/puerperium, CNS or ear/sinus/mouth/face infections, exposure to drugs (eg, oral contraceptives, steroids, cancer treatments), head trauma, or procedures (eg, lumbar puncture, jugular catheter placement).
Chronic triggers of cerebral venous thrombosis include hereditary or acquired thrombophilias that are established causes of venous thromboembolism.
Anticoagulation is the main treatment for the acute management of cerebral venous thrombosis.
All available guidelines recommend anticoagulation therapy for the acute management of cerebral venous thrombosis.
Endovascular interventions are reserved for patients with progressive neurologic deterioration despite intensive medical treatment.
Seizures are a common manifestation, observed in 30% to 50% of patients with cerebral venous thrombosis.
Antiepileptic drug treatment for cerebral venous thrombosis is only recommended for patients who have had a seizure with or without parenchymal lesions (eg, venous infarction, intracranial hemorrhage). Prophylactic use of antiepileptic drugs is not recommended.
Intracranial hypertension may be seen in 15% to 40% of patients with cerebral venous thrombosis.
The risk of recurrence after an unprovoked cerebral venous thrombosis is low, but severe thrombophilias are associated with a high risk of recurrence of cerebral venous thrombosis as well as systemic venous thromboembolism.
For provoked cerebral venous thrombosis, anticoagulation consisting of adjusted-dose warfarin is recommended for 3 to 6 months. An evaluation for thrombophilia will potentially aid in the decision for the duration of anticoagulation.
Footnotes
Relationship Disclosure: Dr Bushnell has received grants from the Hazel K. Goddess Fund for Stroke Research in Women, the North Carolina Stroke Care Collaborative, the World Federation of Neurology, and the World Stroke Organization. Dr Saposnik is supported by the Distinguished Clinician Scientist Award from the Heart and Stroke Foundation of Canada.
Unlabeled Use of Products/Investigational Use Disclosure: Drs Bushnell and Saposnik report no disclosures.
REFERENCES
- 1.Bousser MG,, Ferro JM. Cerebral venous thrombosis: an update. Lancet Neurol 2007; 6 (2): 162–170. [DOI] [PubMed] [Google Scholar]
- 2.Ferro J,, Canhao P,, Stam J, et al Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke 2004; 35 (3): 664–670. [DOI] [PubMed] [Google Scholar]
- 3.deVeber G,, Andrew M,, Adams C, et al Cerebral sinovenous thrombosis in children. N Engl J Med 2001; 345 (6): 417–423. [DOI] [PubMed] [Google Scholar]
- 4.Stam J. Thrombosis of the cerebral veins and sinuses. N Engl J Med 2005; 352 (17): 1791–1798. [DOI] [PubMed] [Google Scholar]
- 5.Crassard I,, Bousser MG. Headache in patients with cerebral venous thrombosis [in French]. Rev Neurol (Paris) 2005; 161 (6–7): 706–708. [DOI] [PubMed] [Google Scholar]
- 6.Girot M,, Ferro JM,, Canha∼o P, et al Predictors of outcome in patients with cerebral venous thrombosis and intracerebral hemorrhage. Stroke 2007; 38 (2): 337–342. [DOI] [PubMed] [Google Scholar]
- 7.Saposnik G,, Barinagarrementeria F,, Brown R, et al Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42 (4): 1158–1192. [DOI] [PubMed] [Google Scholar]
- 8.Biousse V,, Ameri A,, Bousser MG. Isolated intracranial hypertension as the only sign of cerebral venous thrombosis. Neurology 1999; 53 (7): 1537–1542. [DOI] [PubMed] [Google Scholar]
- 9.Roach ES,, Golomb MR,, Adams R, et al Management of stroke in infants and children: a scientific statement from a Special Writing Group of the American Heart Association Stroke Council and the Council on Cardiovascular Disease in the Young. Stroke 2008; 39 (9): 2644–2691. [DOI] [PubMed] [Google Scholar]
- 10.Dentali F,, Squizzato A,, Marchesi C, et al D-dimer testing in the diagnosis of cerebral vein thrombosis: a systematic review and a meta-analysis of the literature. J Thromb Haemost 2012; 10 (4): 582–589. [DOI] [PubMed] [Google Scholar]
- 11.Hiltunen S,, Putaala J,, Haapaniemi E, et al D-dimer and clinicoradiologic features in cerebral venous thrombosis. J Neurol Sci 2013; 327 (1–2): 12–14. [DOI] [PubMed] [Google Scholar]
- 12.Leach MJ,, Gall SL,, Dewey HM, et al Factors associated with quality of life in 7-year survivors of stroke. J Neurol Neurosurg Psychiatry 2011; 82 (12): 1365–1371. [DOI] [PubMed] [Google Scholar]
- 13.Einhäupl K,, Bousser MG,, de Bruijn SF, et al EFNS guideline on the treatment of cerebral venous and sinus thrombosis. Eur J Neurol 2006; 13 (6): 553–559. [DOI] [PubMed] [Google Scholar]
- 14.Leach JL,, Fortuna RB,, Jones BV,, Gaskill-Shipley MF. Imaging of cerebral venous thrombosis: current techniques, spectrum of findings, and diagnostic pitfalls. Radiographics 2006; 26 (suppl 1): S19–S41. [DOI] [PubMed] [Google Scholar]
- 15.Selim M,, Fink J,, Linfante I, et al Diagnosis of cerebral venous thrombosis with echo-planar T2*-weighted magnetic resonance imaging. Arch Neurol 2002; 59 (6): 1021–1026. [DOI] [PubMed] [Google Scholar]
- 16.Rodallec MH,, Krainik A,, Feydy A, et al Cerebral venous thrombosis and multidetector CT angiography: tips and tricks. Radiographics 2006; 26 (suppl 1): S5–S18. [DOI] [PubMed] [Google Scholar]
- 17.Wasay M,, Azeemuddin M. Neuroimaging of cerebral venous thrombosis. J Neuroimaging 2005; 15 (2): 118–128. [DOI] [PubMed] [Google Scholar]
- 18.Ganeshan D,, Narlawar R,, McCann C, et al Cerebral venous thrombosis—a pictorial review. Eur J Radiol 2010; 74 (1): 110–116. [DOI] [PubMed] [Google Scholar]
- 19.Coutinho JM,, Ferro JM,, Canha∼o P, et al Cerebral venous and sinus thrombosis in women. Stroke 2009; 40 (7): 2356–2361. [DOI] [PubMed] [Google Scholar]
- 20.Lijfering WM,, Brouwer JL,, Veeger NJ, et al Selective testing for thrombophilia in patients with first venous thrombosis: results from a retrospective family cohort study on absolute thrombotic risk for currently known thrombophilic defects in 2479 relatives. Blood 2009; 113 (21): 5314–5322. [DOI] [PubMed] [Google Scholar]
- 21.Martinelli I,, Bucciarelli P,, Passamonti SM, et al Long-term evaluation of the risk of recurrence after cerebral sinus-venous thrombosis. Circulation 2010; 121 (25): 2740–2746. [DOI] [PubMed] [Google Scholar]
- 22.Einhäupl KM,, Villringer A,, Meister W, et al Heparin treatment in sinus venous thrombosis. Lancet 1991; 338 (8767): 597–600. [DOI] [PubMed] [Google Scholar]
- 23.de Bruijn S,, Stam J. Randomized, placebo-controlled trial of anticoagulant treatment with low-molecular-weight heparin for cerebral sinus thrombosis. Stroke 1999; 30 (3): 484–488. [DOI] [PubMed] [Google Scholar]
- 24.Coutinho J,, de Bruijn SF,, Deveber G,, Stam J. Anticoagulation for cerebral venous sinus thrombosis. Cochrane Database Syst Rev 2011; (8): CD002005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lansberg MG,, O’Donnell MJ,, Khatri P, et al Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141 (2 suppl): e601S–e636S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coutinho JM,, Ferro JM,, Canha∼o P, et al Unfractionated or low-molecular weight heparin for the treatment of cerebral venous thrombosis. Stroke 2010; 41 (11): 2575–2580. [DOI] [PubMed] [Google Scholar]
- 27.Stolz E,, Trittmacher S,, Rahimi A, et al Influence of recanalization on outcome in dural sinus thrombosis: a prospective study. Stroke 2004; 35 (2): 544–547. [DOI] [PubMed] [Google Scholar]
- 28.Baumgartner RW,, Studer A,, Arnold M,, Georgiadis D. Recanalisation of cerebral venous thrombosis. J Neurol Neurosurg Psychiatry 2003; 74 (4): 459–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strupp M,, Covi M,, Seelos K, et al Cerebral venous thrombosis: correlation between recanalization and clinical outcome—a long-term follow-up of 40 patients. J Neurol 2002; 249 (8): 1123–1124. [DOI] [PubMed] [Google Scholar]
- 30.Coutinho JM,, Ferro JM,, Zuurbier SM, et al Thrombolysis or anticoagulation for cerebral venous thrombosis: rationale and design of the TO-ACT trial. Int J Stroke 2013; 8 (2): 135–140. [DOI] [PubMed] [Google Scholar]
- 31.Borhani Haghighi A,, Mahmoodi M,, Edgell RC, et al Mechanical thrombectomy for cerebral venous sinus thrombosis: a comprehensive literature review [printed online ahead of print January 7, 2013]. Clin Appl Thromb Hemost 2013. doi:10.1177/1076029612470968. [DOI] [PubMed] [Google Scholar]
- 32.Zuurbier SM,, Coutinho JM,, Majoie CB, et al Decompressive hemicraniectomy in severe cerebral venous thrombosis: a prospective case series. J Neurol 2012; 259 (6): 1099–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coutinho JM,, Majoie CB,, Coert BA,, Stam J. Decompressive hemicraniectomy in cerebral sinus thrombosis: consecutive case series and review of the literature. Stroke 2009; 40 (6): 2233–2235. [DOI] [PubMed] [Google Scholar]
- 34.Ferro JM,, Canha∼o P,, Bousser MG, et al; ISCVT Investigators. Early seizures in cerebral vein and dural sinus thrombosis: risk factors and role of antiepileptics. Stroke 2008; 39 (4): 1152–1158. [DOI] [PubMed] [Google Scholar]
- 35.Nasr DM,, Brinjikji W,, Cloft HJ, et al Mortality in cerebral venous thrombosis: results from the national inpatient sample database. Cerebrovasc Dis 2013; 35 (1): 40–44. [DOI] [PubMed] [Google Scholar]
- 36.Canha∼o P,, Cortesa∼o A,, Cabral M, et al Are steroids useful to treat cerebral venous thrombosis? Stroke 2008; 39 (1): 105–110. [DOI] [PubMed] [Google Scholar]
- 37.Hanley DF,, Feldman E,, Borel CO, et al Treatment of sagittal sinus thrombosis associated with cerebral hemorrhage and intracranial hypertension. Stroke 1988; 19 (7): 903–909. [DOI] [PubMed] [Google Scholar]
- 38.Ferro JM,, Lopes MG,, Rosas MJ, et al; Cerebral Venous Thrombosis Portuguese Collaborative Study Group. Long-term prognosis of cerebral vein and dural sinus thrombosis. Results of the VENOPORT study. Cerebrovasc Dis 2002; 13 (4): 272–278. [DOI] [PubMed] [Google Scholar]


