INTRODUCTION
The epidural space is the space between the dura mater and the surrounding bony spinal canal, which extends from the foramen magnum to the posterior sacrococcygeal ligament caudally. The epidural space usually contains loose areolar and adipose tissue, blood vessels, lymphatics and exiting nerve roots. Epidural space pathologies may arise primarily from the space itself or, more commonly, as an extension from the surrounding structures. Epidural space lesions are frequently encountered on magnetic resonance imaging (MRI) and may pose a diagnostic challenge. Awareness of the various radiological findings of these conditions can help narrow down the wide list of conditions, obviate excessive imaging and facilitate management. The MRI signs that are indicative of pathology originating from the epidural space include epidural space expansion, thecal sac displacement, epidural fat replacement and disease extension to the neural foramina. The pathologies depicted in this article are classified according to degenerative, infective, inflammatory, traumatic, vascular, congenital and neoplastic causes.
DEGENERATIVE
Disc sequestration
Disc sequestration is a subtype of disc extrusion in which there is a disc fragment discontinuous with the parent disc and is displaced from the site of extrusion, commonly into the epidural space. The disc fragment usually migrates cranially, caudally or laterally, but seldom to the posterior epidural space.[1] Approximately 80% of cases exhibit higher signal intensity on T2-weighted images and lower signal intensity on T1-weighted images when compared to the degenerate disc of origin [Figure 1], and this is postulated to be either related to the herniated material having a higher water content than the degenerate disc or a reparative process leading to a transient water gain.[2] After gadolinium administration, the sequestered disc typically shows peripheral contrast enhancement. This could be attributed to an inflammatory response with granulation tissue and neovascularisation around the sequestered disc.[2] Commonly misdiagnosed conditions for sequestered discs include epidural abscess, epidural haematoma, synovial cyst, meningioma and neurogenic tumours.[3]
Figure 1.
A 77-year-old man presented with chronic left L5 radiculopathy and neurogenic claudication. (a) Sagittal TIRM and (b) axial T2-W MR images show an extradural heterogeneous intermediate signal intensity lesion posterior to the L5 vertebral body (arrow) compressing the left descending L5 nerve root. (c) On the axial postcontrast, fat saturated T1-W MR image, the lesion also demonstrates peripheral enhancement (arrow).
Facet joint osteoarthritis and synovial cysts
Facet joint osteoarthritis is among the major causes of back pain in adults, together with intervertebral disc degeneration. Synovial cysts may present as a focal outpouching from degenerate facet joints into the posterior epidural space of the spinal canal.[4] Magnetic resonance imaging typically shows variable internal T1- and T2-weighted signal intensity depending on the cyst contents, with possible raised T1-weighted signal due to haemorrhage. The cysts may also show hypointense rim secondary to calcification or haemosiderin deposition.[4] Synovial cysts may also exhibit rim enhancement.[5] Synovial cysts arise from and are typically seen adjacent to a facet joint, which may allow them to be differentiated from ligamentum flavum cysts that are thought to arise from chronic repetitive microtrauma and are usually centred upon the ligamentum flavum itself[6] [Figure 2]. A posterior epidural space location centred around the facet joint helps to discern synovial cyst from disc sequestration, as disc sequestration is rarely posteriorly located.
Figure 2.

A 79-year-old woman presented after a fall. (a) Axial T2-W MR image shows a well-defined hyperintense right-sided extradural synovial cyst (white arrow) decompressing from the right L3–L4 facet joint and causing narrowing of the right lateral recess and spinal canal, with displacement of the thecal sac and cauda equina to the left. (b) Corresponding axial CT image shows rim calcification of the synovial cyst (black arrow).
Ossification of the posterior longitudinal ligament
Ossification of the posterior longitudinal ligament (OPLL) most commonly involves the cervical spine and is more prevalent among patients of Asian descent, with a reported male-to-female ratio of 2:1.[7] Conventional radiography is the simplest method for detecting OPLL, but it has limitations in the early stages of the disease, especially when the thickness of OPLL is less than 3 mm.[8] On MRI, OPLL shows either bone marrow signal intensity or hypointensity on both T1- and T2-weighted sequences; it may be difficult to distinguish OPLL from ligament hypertrophy if the latter signal changes are present. Computed tomography (CT) is the recommended modality for confirming the presence of suspected OPLL on MRI, as well as for detection of the segmental and localised types of OPLL, which may be difficult to appreciate on plain radiographs.[8] Diffuse idiopathic skeletal hyperostosis is a recognised association of OPLL and is characterised by the presence of flowing ossification of the anterior longitudinal ligament, most commonly in the thoracic spine, enthesopathy (such as at the iliac crest, ischial tuberosities and greater trochanters) and by the absence of involvement of the spinal facet joints or sacroiliac joints[9] [Figure 3]. Some other differential diagnoses for epidural lesions with low T1 signal include calcified herniated disc, calcified meningioma and osteochondroma. In these cases, sagittal images would be helpful to differentiate them from OPLL as multilevel involvement is not common in these entities.[10]
Figure 3.

A 58-year-old man presented with chronic neck pain for several years. (a) Sagittal T2-W MR image shows a thickened, hypointense posterior longitudinal ligament at the C2–C3 level (arrows). (b) Bone window setting sagittal CT image confirms the presence of ossification of the posterior longitudinal ligament (arrows).
INFECTION/INFLAMMATION
Spinal epidural abscess
Spinal epidural abscesses are relatively uncommon, and peak incidence is in the fifth to seventh decades of life with a male predominance. Most of the cases are caused by haematogenous spread through the epidural vasculature or direct spread from spondylodiscitis/vertebral osteomyelitis extending into the adjacent spinal epidural space. The key to diagnosing a liquid abscess amenable for surgical drainage is the presence of a localised focus of high T2-weighted signal, low T1-weighted signal and a rim of peripheral enhancement. Diffusion weighted imaging/apparent diffusion coefficient commonly demonstrates restricted diffusion of the abscess content[11] [Figure 4]. On unenhanced MRI, the presence of paraspinal oedema is a highly sensitive feature for epidural abscess.
Figure 4.

A 56-year-old woman presented with bilateral upper and lower limb weakness, numbness and lax anal tone. (a) Sagittal TIRM and (b) sagittal T1-W, fat-saturated, postcontrast MR images show a T2-W hyperintense, rim-enhancing collection in the posterior epidural space from the C5–T1 levels (white arrows), which compresses the cervical spinal cord. TIRM signal hyperintensity in the C6 and C7 vertebral bodies and the C6–C7 intervertebral disc (short black arrows in 4a) associated with a rim-enhancing prevertebral rim-enhancing collection (broken white arrow in b) is suspicious for acute spondylodiscitis.
Tuberculous spondylitis
Around 2% of the spinal epidural abscess is contributed by tuberculosis.[12] Also known as ‘Pott’s disease’, tuberculous spondylitis represents the most common form of extrapulmonary tuberculosis. It often involves two or more contiguous vertebral bodies, with spread of infection to other vertebral levels deep to the longitudinal ligaments. Unlike pyogenic infections, the intervertebral discs in tuberculous spondylitis may be preserved. Gadolinium is usually required to differentiate a paraspinal, extradural or subligamentous abscess from an infective phlegmon; an abscess will typically demonstrate rim enhancement, whereas an infective phlegmon is usually diffusely enhancing. The paraspinal abscesses for cases involving the thoracolumbar spine can spread along the iliopsoas muscles to reach the pelvis or thigh and may show calcifications[11] [Figure 5]. Previous or active pulmonary tuberculosis is present in approximately half of the patients with musculoskeletal tuberculosis, and the presence of pulmonary or disseminated tuberculosis, as well as predisposing factors is a valuable clue to diagnosing tuberculous spondylitis. Although both epidural abscesses and disc sequestration can show rim enhancement and present with back pain and neurological deficits, a clinical presentation suggestive of infection, such as fever or raised inflammatory markers, demonstration of restricted diffusion, presence of adjacent spondylodiscitis and extension of the lesion across more than one vertebral level, would favour epidural abscess.
Figure 5.
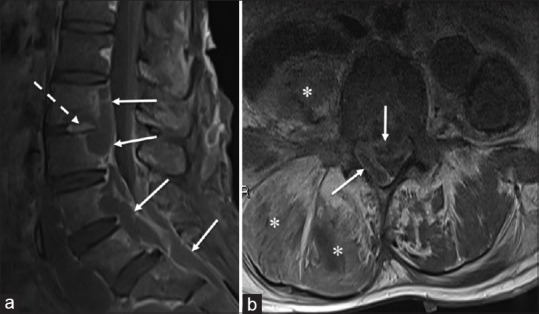
A 37-year-old woman presented with right lower back and leg pain for a week and progressive weight loss of 5–6 kg over 1 year. (a) Sagittal fat-saturated, postcontrast T1-W MR image shows subligamentous rim-enhancing collections, which extend from the level of L3 to the sacrum (arrows), and involves the L2–L3 intervertebral disc (broken arrow). (b) Axial postcontrast T1-W MR image shows a prominent rim-enhancing epidural abscess (arrows) compressing the thecal sac at its anterior and right lateral aspects, with resultant severe narrowing of the spinal canal. Concurrently, there is oedema and smaller collections of fluid insinuating into the right psoas and right posterior paravertebral muscles (*). These findings are compatible with Pott’s disease.
Immunoglobulin G4-related disease
Immunoglobulin G4-related disease (IgG4-RD) is a group of distinct autoimmune disorders that can potentially affect nearly every organ system in the body. Involvement of the central nervous system is uncommon, and there are only a few case reports describing IgG4 involvement of the epidural space. Such cases may invariably entail histological correlation to establish the diagnosis of IgG4-RD and differentiate this entity from malignancy.[13] The coexistent imaging findings of an autoimmune pancreatitis, which may be seen as diffuse inflammation giving rise to a ‘sausage-shaped pancreas’, as well as extrapancreatic manifestations of enlargement, mural inflammation or mass-like lesions involving the central nervous system, orbits, lacrimal or salivary glands, lungs, blood vessels, kidneys, gastrointestinal tract and lymph nodes, may help to suggest a specific diagnosis of IgG4-RD[14] [Figure 6].
Figure 6.

A 64 year-old man with IgG4-related disease presented with bilateral lower limb weakness. (a) Axial and (b) sagittal postcontrast T1-W fat-saturated MR image shows enhancing epidural soft tissue extending from the C6 to T7 vertebral levels (arrows) causing severe spinal canal narrowing and spinal cord compression (*).
TRAUMA
Epidural haematoma
Spinal epidural haematomas can be caused by trauma, vascular malformations, iatrogenic interventions or coagulopathy, but they are idiopathic in most cases. The imaging modality of choice is MRI, with early and later subacute haematomas appearing T1-weighted hyperintense. Susceptibility may be present on T2-weighted or gradient echo-recalled sequences, and the haematoma may demonstrate peripheral enhancement if intravenous contrast is given. If there is continuous contrast extravasation, central enhancement can potentially be seen.[15] In cases of trauma, other associated acute bony injuries, such as vertebral fractures and marrow oedema, and soft tissue injuries, such as disruption of the anterior longitudinal ligament and posterior ligamentous complex detected on MRI help to differentiate this condition from degenerative process such as the aforementioned disc sequestration [Figure 7]. In addition, the high T1-weighted signal in epidural haematoma can distinguish it from disc sequestration.
Figure 7.

A 39-year-old male cyclist involved in a road traffic accident. (a) Sagittal T1-W MR image shows a T1-W isointense anterior epidural lesion extending from the level of C5–C6 causing moderate spinal canal stenosis (white arrows). (b) On sagittal T2-W MR image, the epidural lesion appears heterogeneous with mild susceptibility (white arrows), and there is associated disruption of the posterior annulus and the posterior longitudinal ligament (black arrowhead). Also, there is disruption of the ligamentum flavum (white arrowhead) and interspinous ligament at the C5–C6 level, with widening of the interspinous space (broken white arrow).
Iatrogenic
Postprocedure epidural granulation tissue
Reactive postprocedure granulation tissue may form in the epidural space following spinal surgery or epidural spinal injections, and can be associated with mass effect and compression of the adjacent thecal sac or nerve roots. The granulation soft tissue is usually T1-weighted hypointense with variable T2-weighted hyperintense signal, and demonstrates postcontrast enhancement[16] [Figure 8]. The lesion (in the case mentioned in Figure 8) resolved on subsequent follow-up MRI (not shown), thereby supporting the diagnosis of postintervention granulation tissue.
Figure 8.

A 68-year-old man underwent a recent spinal procedure. (a) Axial T2-W and (b) axial fat-saturated, postcontrast T1-W MR images show intermediate signal intensity soft tissue in the right aspect of the epidural space at the level of L5, which compresses the thecal sac and demonstrates postcontrast enhancement (arrows).
Cerebrospinal fluid leakage
Other postoperative complications with epidural lesions include postsurgical cerebrospinal fluid (CSF) leakage, a well-recognised complication of spinal surgery most commonly caused by an inadvertent dural tear during surgery[16] [Figure 9]. The incidence of dural tears increases with greater extent and complexity of the spinal surgery, and with revision surgeries. A pseudomeningocoele is a CSF-filled herniation of an arachnoid lined cyst through a small rent in the dura. In the assessment for postsurgical CSF leaks, MRI is the imaging modality of choice, while slow leaks may require dedicated CT, MR or radionuclide myelography for detection.[16] In contrast to epidural granulation tissue, MRI of postsurgical CSF leakage shows an epidural collection extending from the posterior extradural space into the subcutaneous plane. On axial and sagittal T2-weighted sequences, thecal sac communication can be delineated. This lesion typically shows no restricted diffusion with similar signal characteristics as CSF, and may show a thin peripheral enhancement within 1 year of surgery.[17]
Figure 9.

A 72-year-old man with recent posterior decompression at the L4–L5 levels presented with back pain. (a) Axial T2-W and (b) axial fat-saturated, postcontrast T1-W MR images show a pseudomeningocoele with a large T2-W hyperintense epidural collection showing mild rim enhancement (arrowheads) and extending from the posterior extradural space to the subcutaneous plane. There is consequent severe spinal canal narrowing and cauda equina compression.
VASCULAR
Spinal arteriovenous shunts
Spinal arteriovenous shunts are abnormal connections between the arteries and veins of the spine bypassing the capillary network. Spinal arteriovenous shunts can be classified into five groups: Type I: dural arteriovenous fistulas (AVFs), Type II: intramedullary glomus arteriovenous malformation (AVM), Type III: juvenile AVM, Type IV: perimedullary AVF and Type V: extradural AVF.[18] Spinal extradural AVFs (Type V) are rare and distinct from the more common and potentially more symptomatic dural AVFs that are located within the dura, and they may be associated with cord ischaemia or haemorrhage. These extradural lesions may be an incidental finding, but can be symptomatic due to mass effect of the distended vessels on the spinal cord or nerve roots.[18] On MRI, the classical appearance of tortuous and enlarged vessels displaying flow voids is suggestive of spinal AVFs. There may also be associated spinal cord enlargement and oedema, a reflection of venous congestion. However, digital subtraction angiography is the gold standard imaging modality for spinal AVFs, as it depicts mapping of the feeding and draining vessels of the fistulas [Figure 10].
Figure 10.

A 19-year-old man presented with thoracic back pain. (a) Sagittal T1-W MR image shows curvilinear signal voids in the posterior epidural space spanning the T2–T4 vertebral levels (arrows). (b) Axial T2-W MR image at the T4–T5 level shows the aberrant vessels (arrowheads) traversing the right neural exit foramen and abutting the exiting right T4 nerve root (arrow). These vessels were noted to drain into the azygos vein (not shown). No cord oedema is seen.
Spinal epidural lipomatosis
Spinal epidural lipomatosis is a rare disorder characterised by excessive proliferation of the epidural fat, resulting in narrowing of the spinal canal. This disorder occurs predominantly in obese patients and is associated with corticosteroid usage and endogenous overproduction of glucocorticoid.[19] The disorder has a non-specific symptomatology and is commonly an incidental finding. On MRI, an increase in excess fat in the epidural space is demonstrated, with hyperintensity on T1- and T2-weighted sequences and signal suppression on fat-saturated, T2-weighted images. There is resultant narrowing of the spinal canal, which may resemble a stellate or ‘Y’ configuration [Figure 11].
Figure 11.

A 58-year-old man with multilevel degenerative disc disease who presented with lower back pain. Axial T1-W MR image shows incidental finding of prominent fat in the epidural space (arrows), which results in the thecal sac (T) resembling a ‘stellate’ configuration.
SPINAL EPIDURAL NEOPLASMS
Spinal epidural neoplasms, primary or secondary, can arise as either direct extension from a vertebral neoplasm or by tumour depositing directly into the spinal canal. Metastases are the most common spinal lesions [Figure 12]. Spinal epidural metastasis is a common site of spread for cancer, occurring in 5%–10% of all patients with cancer, with prostate, breast, lung, renal and thyroid cancers contributing the majority of such cases.[20] When the epidural lesions affect multiple sites, considerations include metastasis, multiple myeloma and lymphoma.[21] Apart from primary malignant vertebral tumours showing epidural extension, such as osteosarcoma, chondrosarcoma and chordoma, aggressive benign tumours such as osteoblastoma and haemangioma can also demonstrate epidural soft tissue extension[21] [Figure 13].
Figure 12.

A 52-year-old woman presented with sudden onset of paraparesis on a background of progressively worsening back pain and bilateral lower limb weakness over the past 6 months. (a) Sagittal T1-W MR sequence shows two posterior epidural T1-W hyperintense lesions at the T7–T8 and T9–T10 levels (arrows). (b) Sagittal T1-W fat-saturated, postcontrast MR image shows avid contrast enhancement of the lesions.
Figure 13.

A 67-year-old woman with T5 cavernous haemangioma presented with upper back pain. (a) Sagittal STIR and (b) axial postcontrast T1-W fat-suppressed MR images show a STIR-hyperintense and avidly enhancing lesion with lobulated margins replacing the posterior elements, pedicles and posterior aspect of the vertebral body (arrows). There is extraosseous extension of the lesion into the posterior epidural space (*), with narrowing of the spinal canal and compression of the spinal cord. STIR: short tau inversion recovery
Conventional radiography has limited capabilities in the evaluation. Computed tomography can assess cortical integrity and its relationship with the epidural lesion as well as presence of matrix calcification/ossification. Magnetic resonance imaging is the modality of choice for evaluating osseous and extraosseous extent of the lesion and involvement of the nerve roots and spinal cord. On T1-weighted sequences, vertebral metastasis and epidural soft tissue commonly demonstrate low signal relative to muscle. Metastases arising from a primary malignant melanoma may, however, be T1-weighted hyperintense due to their paramagnetic melanin content. On T2-weighted sequences, metastatic lesions are usually much brighter than bone marrow due to their high water content. Metastasis often demonstrates a rim of bright T2 signal (halo sign).[22] After gadolinium administration, the lesions demonstrate enhancement, which would be further accentuated with application of fat saturation technique [Figures 14 and 15].
Figure 14.

A 55-year-old man with known renal cell carcinoma presented with back pain. (a) Axial precontrast T1-W and (b) axial postcontrast T1-W fat-suppressed MR images show bony destruction and replacement of the left posterior vertebral body, left pedicle and left pedicle of the L3 vertebra, with an irregular T1-W hypointense mass demonstrating postcontrast enhancement (*) and left-sided extradural extension (arrows), with the latter causing narrowing of the spinal canal. These are compatible with osseous metastasis with extradural extension.
Figure 15.

A 61-year-old man with chondrosarcoma presented with chronic upper back pain. (a) Axial T2-W MR image shows an expansile hyperintense lesion arising from the posterior elements of the T8 vertebra (arrowheads), which is associated with soft tissue extension to the right lateral and posterior epidural space (arrows). (b) Sagittal postcontrast T1-W MR image shows heterogeneous enhancement of the lesion (*), with soft tissue extension to the posterior epidural space (arrows).
CONCLUSION
Spinal epidural space lesions are frequently encountered on MRI and can sometimes pose a diagnostic challenge. Careful consideration of the various clinicoradiological findings may aid the clinician and reporting radiologist to significantly narrow the list of differential diagnoses, reduce the need for further imaging investigation and expedite appropriate management.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
SMC CATEGORY 3B CME PROGRAMME
Online Quiz: https://www.sma.org.sg/cme-programme
Deadline for submission: 6 pm, 13 October 2023
| Question | True | False |
|---|---|---|
| 1. Regarding ossification of the posterior longitudinal ligament (OPLL): | ||
|
| ||
| (a) Computed tomography (CT) is the recommended modality for confirming the presence of suspected OPLL on magnetic resonance imaging (MRI). | ||
|
| ||
| (b) Diffuse idiopathic skeletal hyperostosis is a recognised association of OPLL. | ||
|
| ||
| (c) It is more prevalent among patients of Asian descent. | ||
|
| ||
| (d) On MRI, OPLL may show bone marrow signal intensity. | ||
|
| ||
| 2. Regarding spinal epidural haematoma: | ||
|
| ||
| (a) It is best evaluated on CT. | ||
|
| ||
| (b) It demonstrates susceptibility and peripheral enhancement on MRI. | ||
|
| ||
| (c) It can show hyperintense signal on T1-weighted sequences. | ||
|
| ||
| (d) It most commonly involves the lumbar spine. | ||
|
| ||
| 3. Regarding tuberculous spondylitis: | ||
|
| ||
| (a) It often involves two or more contiguous vertebral bodies. | ||
|
| ||
| (b) It may spare the intervertebral discs. | ||
|
| ||
| (c) It is the least common extrapulmonary manifestation. | ||
|
| ||
| (d) Spread of infection to other vertebral levels deep to the longitudinal ligaments may occur. | ||
|
| ||
| 4. Regarding spinal epidural neoplasms: | ||
|
| ||
| (a) Computed tomography can assess cortical integrity and its relationship with the epidural lesion as well as presence of matrix calcification/ossification. | ||
|
| ||
| (b) The most common cause of multiple spinal epidural neoplasms is metastases. | ||
|
| ||
| (c) MRI is the modality of choice in assessing for the presence of compression or involvement of the spinal cord and nerve roots. | ||
|
| ||
| (d) Osteoblastomas and cavernous haemangiomas are examples of aggressively behaving malignant vertebral tumours. | ||
|
| ||
| 5. The following statements are true/false: | ||
|
| ||
| (a) Most spinal epidural abscesses are caused by haematogenous spread through the epidural vasculature or are due to direct spread from spondylodiscitis or vertebral osteomyelitis. | ||
|
| ||
| (b) Digital subtraction angiography is the gold standard imaging modality for spinal arteriovenous fistulas. | ||
|
| ||
| (c) Epidural lipomatosis occurs predominantly in underweight patients. | ||
|
| ||
| (d) Spinal epidural space involvement is common in IgG4-related disease. | ||
REFERENCES
- 1.Chen CY, Chuang YL, Yao MS, Chiu WT, Chen CL, Chan WP. Posterior epidural migration of a sequestrated lumbar disk fragment: MR imaging findings. AJNR Am J Neuroradiol. 2006;27:1592–4. [PMC free article] [PubMed] [Google Scholar]
- 2.Masarynk TJ, Ross JS, Modic MT, Boumphrey F, Bohlman H, Wilber G. High-resolution MR imaging of sequestered lumbar intervertebral disks. AJR Am J Neuroradiol. 1988;150:1155–62. doi: 10.2214/ajr.150.5.1155. [DOI] [PubMed] [Google Scholar]
- 3.Afonso AM, Sierra OM, Corral OLG de S del, López AJV, González-Quarante LH, Vendrell ES, et al. Misdiagnosis of posterior sequestered lumbar disc herniation: Report of three cases and review of the literature. Spinal Cord Ser Cases. 2018;4:61. doi: 10.1038/s41394-018-0100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu SS, Williams KD, Drayerr BP, Spetzler RF, Sonntag VKH. Synovial cysts of the lumbosacral spine: Diagnosis by MR imaging. AJR Am J Roentgenol. 1990;154 doi: 10.2214/ajr.154.1.2104702. doi:10.2214/ajr. 154.1.210470. [DOI] [PubMed] [Google Scholar]
- 5.Venkatanarasimha N, Priya Suresh S. AJR teaching file: An uncommon cause of spinal canal stenosis. AJR Am J Roentgenol. 2009;193 doi: 10.2214/AJR.07.7110. doi:10.2214/AJR.07.7110. [DOI] [PubMed] [Google Scholar]
- 6.Mahallati H, Wallace CJ, Hunter KM, Bilbao JM, Clark AW. MR imaging of a hemorrhagic and granulomatous cyst of the ligamentum flavum with pathologic correlation. AJNR Am J Neuroradiol. 1999;20:1166. [PMC free article] [PubMed] [Google Scholar]
- 7.Abiola R, Rubery P, Mesfin A. Ossification of the posterior longitudinal ligament: Etiology, diagnosis, and outcomes of nonoperative and operative management. Global Spine J. 2015;6 doi: 10.1055/s-0035-1556580. doi:10.1055/s-0035-1556580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang MS, Lee JW, Zhang HY, Cho YE, Park YM. Diagnosis of cervical OPLL in lateral radiograph and MRI: Is it reliable? Korean J Spine. 2012;9:205–8. doi: 10.14245/kjs.2012.9.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cammisa M, de Serio A, Guglielmi G. Diffuse idiopathic skeletal hyperostosis. Eur J Radiol. 1998;27:7–11. doi: 10.1016/s0720-048x(98)00036-9. [DOI] [PubMed] [Google Scholar]
- 10.Otake S, Matsuo M, Nishizawa S, Sano A, Kuroda Y. Ossification of the Posterior Longitudinal Ligament: MR Evaluation. 1992;13:1059–67. [PMC free article] [PubMed] [Google Scholar]
- 11.Moorthy S, Prabhu NK. Spectrum of MR imaging findings in spinal tuberculosis. Am J Roentgenol. 2002 doi: 10.2214/ajr.179.4.1790979. doi:10.2214/ajr. 179.4.1790979. [DOI] [PubMed] [Google Scholar]
- 12.Hasan GA, Kani SM, Alqatub A. Tuberculous lumbar spinal epidural abscess in a young adult (case report) SICOT J. 2018;4:5. doi: 10.1051/sicotj/2018005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams MM, Mashaly H, Puduvalli VK, Jin M, Mendel E. Immunoglobulin G4-related disease mimicking an epidural spinal cord tumor: Case report. J Neurosurg Spine. 2017 doi: 10.3171/2016.5.SPINE16119. doi:10.3171/2016.5. SPINE16119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan TJ, Ng YL, Tan D, Fong WS, Low ASC. Extrapancreatic findings of IgG4-related disease. 2014 doi: 10.1016/j.crad.2013.09.021. doi:10.1016/j.crad. 2013.09.021. [DOI] [PubMed] [Google Scholar]
- 15.Pierce JL, Donahue JH, Nacey NC, Quirk CR, Perry MT, Faulconer N, et al. Spinal hematomas: What a radiologist needs to know. 2018 doi: 10.1148/rg.2018180099. doi:101148/rg2018180099 38:1516-1535. [DOI] [PubMed] [Google Scholar]
- 16.Malhotra A, Kalra VB, Wu X, Grant R, Bronen RA, Abbed KM. Imaging of lumbar spinal surgery complications. Insights Imaging. 2015;6:579. doi: 10.1007/s13244-015-0435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCormack BM, Zide BM, Kalfas IH. Cerebrospinal fluid fistula and pseudomeningocele after spine surgery. Spine Surg. 2005;2:2033–42. [Google Scholar]
- 18.Rangel-Castilla L, Holman PJ, Krishna C, Trask TW, Klucznik RP, Diaz OM. Spinal extradural arteriovenous fistulas: A clinical and radiological description of different types and their novel treatment with Onyx: Clinical article. J Neurosurg Spine. 2011;15:541–9. doi: 10.3171/2011.6.SPINE10695. [DOI] [PubMed] [Google Scholar]
- 19.Fassett DR, Schmidt MH. Spinal epidural lipomatosis: A review of its causes and recommendations for treatment. Neurosurg Focus. 2004 doi:10.3171/foc. 2004.16.4.12. [PubMed] [Google Scholar]
- 20.Grossman SA, Lossignol D. Diagnosis and treatment of epidural metastases. Oncology (Williston Park) 1990;4:47. [PMC free article] [PubMed] [Google Scholar]
- 21.Rodallec MH, Feydy A, Larousserie F, Anract P, Campagna R, Babinet A, et al. Diagnostic imaging of solitary tumors of the spine: What to do and say. RadioGraphics. 2008;28:1019–41. doi: 10.1148/rg.284075156. [DOI] [PubMed] [Google Scholar]
- 22.Schweitzer ME, Levine C, Mitchell DG, Gannon FH, Gomella LG. Bull's-eyes and halos: Useful MR discriminators of osseous metastases. Radiology. 1993 doi: 10.1148/radiology.188.1.8511306. doi:10.1148/radiology. 188.1.8511306. [DOI] [PubMed] [Google Scholar]



