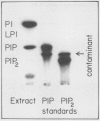
Abstract
The major metabolites of the phosphatidylinositol cycle from extracts of [32PO4]- and [3H]-inositol-labeled Samanea saman pulvini were separated. The membrane localized phosphoinositides were separated by thin layer chromatography, identified by comparison with purified lipid standards, and quantitated based on incorporation of radiolabel. The ratio of radioactivity in phosphatidylinositol:phosphatidylinositol 4-phosphate:phosphatidylinositol 4,5-bisphosphate is about 32:8:1. The aqueous inositol phosphates were separated by anion exchange chromatography using conventional liquid chromatography and by high performance liquid chromatography (HPLC) and were identified by comparison with standards. Analysis by HPLC reveals that 32P-labeled pulvini have inositol 1-phosphate, inositol 1,4-bisphosphate, and inositol 1,4,5-trisphosphate that co-migrate with red blood cell inositol phosphates, but 3H-inositol-labeled pulvini appear to have a variant profile.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Boss W. F., Massel M. O. Polyphosphoinositides are present in plant tissue culture cells. Biochem Biophys Res Commun. 1985 Nov 15;132(3):1018–1023. doi: 10.1016/0006-291x(85)91908-4. [DOI] [PubMed] [Google Scholar]
- Crain R. C., Zilversmit D. B. Two nonspecific phospholipid exchange proteins from beef liver. I. Purification and characterization. Biochemistry. 1980 Apr 1;19(7):1433–1439. doi: 10.1021/bi00548a026. [DOI] [PubMed] [Google Scholar]
- Das R., Sopory S. K. Evidence of regulation of calcium uptake by phytochrome in maize protoplasts. Biochem Biophys Res Commun. 1985 May 16;128(3):1455–1460. doi: 10.1016/0006-291x(85)91103-9. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The polyphosphoinositide phosphodiesterase of erythrocyte membranes. Biochem J. 1981 Jul 15;198(1):133–140. doi: 10.1042/bj1980133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drøbak B. K., Ferguson I. B. Release of Ca2+ from plant hypocotyl microsomes by inositol-1,4,5-trisphosphate. Biochem Biophys Res Commun. 1985 Aug 15;130(3):1241–1246. doi: 10.1016/0006-291x(85)91747-4. [DOI] [PubMed] [Google Scholar]
- Fein A., Payne R., Corson D. W., Berridge M. J., Irvine R. F. Photoreceptor excitation and adaptation by inositol 1,4,5-trisphosphate. Nature. 1984 Sep 13;311(5982):157–160. doi: 10.1038/311157a0. [DOI] [PubMed] [Google Scholar]
- Goto K., Laval-Martin D. L., Edmunds L. N., Jr Biochemical modeling of an autonomously oscillatory circadian clock in Euglena. Science. 1985 Jun 14;228(4705):1284–1288. doi: 10.1126/science.2988128. [DOI] [PubMed] [Google Scholar]
- Heslop J. P., Irvine R. F., Tashjian A. H., Jr, Berridge M. J. Inositol tetrakis- and pentakisphosphates in GH4 cells. J Exp Biol. 1985 Nov;119:395–401. doi: 10.1242/jeb.119.1.395. [DOI] [PubMed] [Google Scholar]
- Hollander J., Hallenbeck J. M., Agranoff B. W. Rapidly-labelled, acidic phospholipids of the goldfish brain. J Neurochem. 1970 Aug;17(8):1247–1261. doi: 10.1111/j.1471-4159.1970.tb03374.x. [DOI] [PubMed] [Google Scholar]
- Jolles J., Wirtz K. W., Schotman P., Gispen W. H. Pituitary hormones influence polyphosphoinositide metabolism in rat brain. FEBS Lett. 1979 Sep 1;105(1):110–114. doi: 10.1016/0014-5793(79)80897-2. [DOI] [PubMed] [Google Scholar]
- Roux S. J. Ca2+ and phytochrome action in plants. Bioscience. 1984 Jan;34(1):25–29. [PubMed] [Google Scholar]
- Rubin R. P., Godfrey P. P., Chapman D. A., Putney J. W., Jr Secretagogue-induced formation of inositol phosphates in rat exocrine pancreas. Implications for a messenger role for inositol trisphosphate. Biochem J. 1984 Apr 15;219(2):655–659. doi: 10.1042/bj2190655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satter R. L., Guggino S. E., Lonergan T. A., Galston A. W. The effects of blue and far red light on rhythmic leaflet movements in samanea and albizzia. Plant Physiol. 1981 May;67(5):965–968. doi: 10.1104/pp.67.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon E., Satter R. L., Galston A. W. Circadian Rhythmicity in Excised Samanea Pulvini: II. Resetting the Clock by Phytochrome Conversion. Plant Physiol. 1976 Sep;58(3):421–425. doi: 10.1104/pp.58.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]