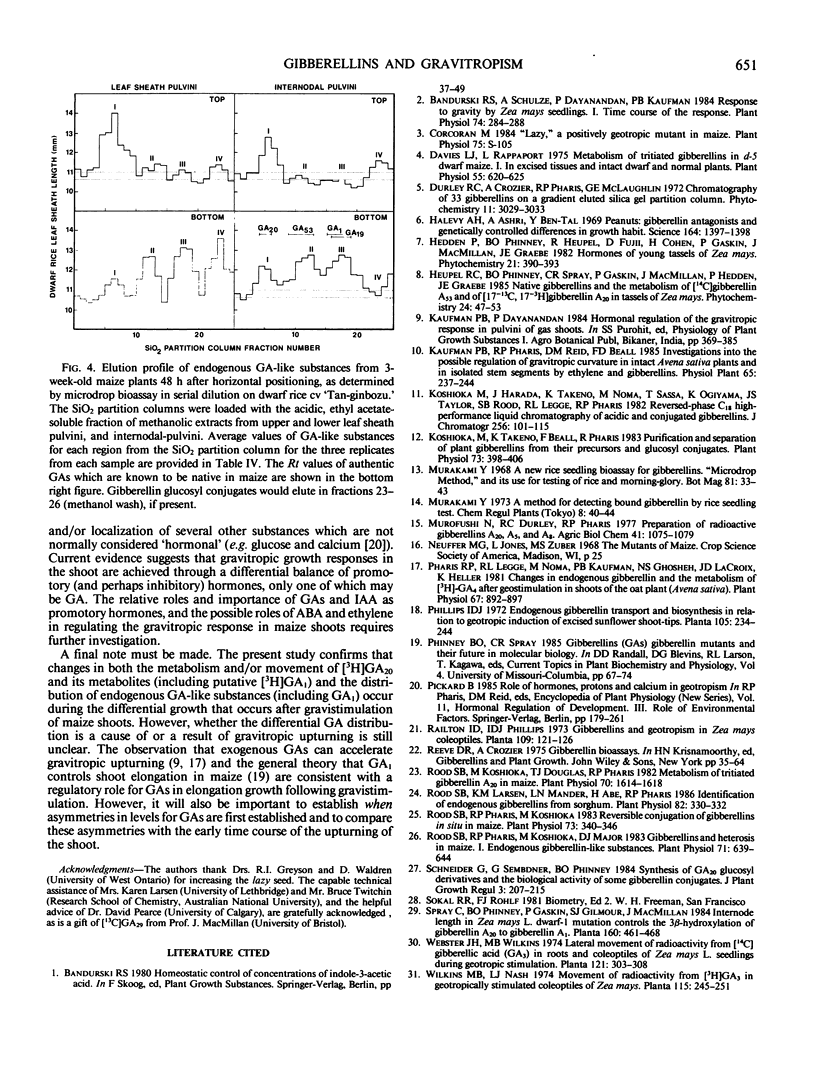
Abstract
[3H]Gibberellin A20 (GA20) of high specific radioactivity (49.9 gigabecquerel per millimole) was applied equilaterally in a ring of microdrops to the internodal pulvinus of shoots of 3-week-old gravistimulated and vertical normal maize (Zea mays L.), and to a pleiogravitropic (prostrate) maize mutant, lazy (la). All plants converted the [3H]GA20 to [3H]GA1− and [3H]GA29-like metabolites as well as to several metabolites with the partitioning and chromatographic behavior of glucosyl conjugates of [3H]GA1, [3H]GA29, and [3H]GA8. The tentative identification of these putative [3H]GA glucosyl conjugates was further supported by the release of the free [3H]GA moiety after cleavage with cellulase. Within 12 hours of the [3H]GA20 feed, there was a significantly higher proportion of total radioactivity in lower than in upper halves of internode and leaf sheath pulvini in gravistimulated normal maize. Further, there was a significantly higher proportion of putative free GA metabolites of [3H]GA20, especially [3H]GA1, in the lower halves of normal maize relative to upper halves. The differential localization of the metabolites between upper and lower halves was not apparent in the pleiogravitropic mutant, la. Endogenous GA-like substances were also examined in gravistimulated maize shoots. Forty-eight hours after gravistimulation of 3-week-old maize seedlings, endogenous free GA-like substances in upper and lower leaf sheath and internode pulvini halves were extracted, chromatographed, and bioassayed using the `Tanginbozu' dwarf rice microdrop assay. Lower halves contained consistently higher total levels of GA-like activity. The qualitative elution profile of GA-like substances differed consistently, upper halves containing principally a GA20-like substance and lower halves containing mainly GA1-like and GA19-like substances. Gibberellins A1 (10 nanograms per gram) and A20 (5 nanograms per gram) were identified from these lower leaf sheath pulvini by capillary gas chromatography-selected ion monitoring. Results from all of these experiments are consistent with a role for GAs in the differential shoot growth that follows gravitropism, although the results do not eliminate the possibility that the redistribution of GAs results from the gravitropic response.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandurski R. S., Schulze A., Dayanandan P., Kaufman P. B. Response to gravity by Zea mays seedlings. I. Time course of the response. Plant Physiol. 1984;74:284–288. doi: 10.1104/pp.74.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies L. J., Rappaport L. Metabolism of Tritiated Gibberellins in d-5 Dwarf Maize: I. In Excised Tissues and Intact Dwarf and Normal Plants. Plant Physiol. 1975 Apr;55(4):620–625. doi: 10.1104/pp.55.4.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevy A. H., Ashri A., Ben-Tal Y. Peanuts: gibberellin antagonists and genetically controlled differences in growth habit. Science. 1969 Jun 20;164(3886):1397–1398. doi: 10.1126/science.164.3886.1397. [DOI] [PubMed] [Google Scholar]
- Kaufman P., Pharis R. P., Reid D. M., Beall F. D. Investigations into the possible regulation of negative gravitropic curvature in intact Avena sativa plants and in isolated stem segments by ethylene and gibberellins. Physiol Plant. 1985;65:237–244. doi: 10.1111/j.1399-3054.1985.tb02389.x. [DOI] [PubMed] [Google Scholar]
- Koshioka M., Takeno K., Beall F. D., Pharis R. P. Purification and separation of plant gibberellins from their precursors and glucosyl conjugates. Plant Physiol. 1983 Oct;73(2):398–406. doi: 10.1104/pp.73.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pharis R. P., Legge R. L., Noma M. Changes in Endogenous Gibberellins and the Metabolism of [H]GA(4) after Geostimulation in Shoots of the Oat Plant (Avena sativa). Plant Physiol. 1981 May;67(5):892–897. doi: 10.1104/pp.67.5.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood S. B., Koshioka M., Douglas T. J., Pharis R. P. Metabolism of tritiated gibberellin a(20) in maize. Plant Physiol. 1982 Dec;70(6):1614–1618. doi: 10.1104/pp.70.6.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood S. B., Larsen K. M., Mander L. N., Abe H., Pharis R. P. Identification of endogenous gibberellins from sorghum. Plant Physiol. 1986 Sep;82(1):330–332. doi: 10.1104/pp.82.1.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood S. B., Pharis R. P., Koshioka M., Major D. J. Gibberellins and heterosis in maize : I. Endogenous gibberellin-like substances. Plant Physiol. 1983 Mar;71(3):639–644. doi: 10.1104/pp.71.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood S. B., Pharis R. P., Koshioka M. Reversible conjugation of gibberellins in situ in maize. Plant Physiol. 1983 Oct;73(2):340–346. doi: 10.1104/pp.73.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]



