Abstract
Background
For optimal functionality, immune cells require a robust and adaptable metabolic program that is fueled by dynamic mitochondrial activity. In this study, we investigate the metabolic alterations occurring in immune cells during HIV infection and antiretroviral therapy by analyzing the uptake of metabolic substrates and mitochondrial phenotypes. By delineating changes in immune cell metabolic programming during HIV, we may identify novel potential therapeutic targets to improve anti-viral immune responses.
Methods
After consent and voluntary participation was confirmed, whole blood was drawn from HIV uninfected women and women with chronic HIV infection on long-term combination antiretroviral therapy (HIV/cART). Peripheral blood mononuclear cells-derived immune cells were directly incubated with different fluorescently tagged metabolites and markers of mitochondrial activity: FITC-2-NBDG (2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino]-2-deoxy-D-glucose), FITC-BODIPY (4,4-Difluoro-5,7-Dimethyl-4-Bora-3a,4a-Diaza-s-Indacene-3-Hexadecanoic Acid), FITC-MitoTracker Green and APC-MitoTracker Deep Red. The uptake of glucose and fats and the mitochondrial mass and potential were measured using flow cytometry. All values are reported quantitatively as geometric means of fluorescence intensity.
Results
During chronic HIV infection, cellular uptake of glucose increases in HIV+ dendritic cells in particular. CD4+ T cells had the lowest uptake of glucose and fats compared to all other cells regardless of HIV status, while CD8+ T cells took up more fatty acids. Interestingly, despite the lower utilization of glucose and fats in CD4+ T cells, mitochondrial mass increased in HIV+ CD4+ T cells compared to HIV negative CD4+ T-cells. HIV+ CD4+ T cells also had the highest mitochondrial potential.
Conclusions
Significant disparities in the utilization of substrates by leukocytes during chronic HIV/cART exist. Innate immune cells increased utilization of sugars and fats while adaptive immune cells displayed lower glucose and fat utilization despite having a higher mitochondrial activity. Our findings suggest that cART treated HIV-infected CD4+ T cells be dysfunctional or may prefer alternative fuel sources not included in these studies. This underscores the importance of understanding the metabolic effects of HIV treatment on immune function.
Introduction
Globally, more than 35 million people live with HIV infection. Due to testing and treatment practices and the efficacy of combination antiretroviral therapy (cART), HIV is now considered a chronic disease [1]. To mount robust and effective responses to infections, immune cells actively reprogram their metabolism by modulating sugar and fat utilization. Energy from these substrates increases the biosynthesis of effector cells and molecules and aids in generating immune memory to manage chronic and recurrent infections [2]. The study of the process of metabolic reprogramming in immune cells is known as immunometabolism. The highly flexible metabolic demands of immune cells during HIV infection have made HIV immunometabolism an area of great interest for future anti-HIV therapy. Immunometabolic processes are highly variable and dependent on the cell type and infectious agent.
The metabolic response of dendritic cells (DCs) following chronic viral infections such as HIV are not well understood; however, in response to acute viral infections such as influenza, DCs become highly glycolytic, glutaminolytic, and lipolytic, increasing their metabolic flexibility. DCs utilize several different substrates to fuel aerobic glycolysis and metabolic reprogramming has been postulated to play a major role in the efficacy of DC effector functions [3]. Glucose and fats are used more by educated natural killer (NK) cells than by uneducated NK cells. Interestingly, the mode of NK cell education influences the level of metabolic activity of educated NK cells [4]. Following acute retroviral infection, NK cells increase their uptake of amino acids and iron and reprogram their metabolic machinery by increasing both glycolysis and mitochondrial metabolism [5]. CD4+ T-cells from HIV+ individuals exhibit a highly glycolytic phenotype for sustained cellular activation, proliferation, and effector cytokine production [6, 7]. Indeed, depleting glucose from the CD4+ T-cell culture media and inhibiting hexokinase blocks HIV infection [8, 9]. Interestingly, the source of sugar fueling glycolysis influenced the survival fitness of infected CD4+ T-cells and the glycolytic rate was thought to be controlled by allosteric regulation or post-translational modification of glycolytic enzymes [10]. Fatty acid uptake critically regulates metabolic reprogramming in T-cell receptor-stimulated CD4+ T cells; however, the role of lipid uptake and metabolism in CD4+ T-cell activation during HIV infection remains undefined [11]. For CD8+ T-cells, fatty acid oxidation and oxidative phosphorylation (OXPHOS) fuel the development and optimal function of memory T cells (Tmem) cells, while glycolysis fuels T-effector (Teff) cells [2]. HIV-specific CD8+ T-cells show delayed maturation and limited effector potential [12, 13] and this is associated with a predominant reliance on glucose and glycolysis as the primary mode of energy production [2]. However, a genetically distinct and rare population of CD8+ Tmem cells, termed HIV controllers, that can spontaneously limit viremia below the detection limit by eliminating infected CD4+ T cells without ART, exhibit metabolic flexibility and can utilize both glucose and fatty acids for fuel metabolism [14].
The modulation of mitochondrial phenotypes plays an essential role in the functionality of immune cells with high energy requirements. The effect of HIV and cART (HIV/cART) on mitochondrial mass and mitochondrial membrane potential in innate immune cells has not been studied, and the correlations between these markers of mitochondrial activity and substrate utilization are undefined. The effects of HIV infection on T-cell mitochondrial mass and mitochondrial membrane potential are highly discordant, with some studies reporting no impact of HIV on mitochondrial mass and mitochondrial membrane potential [15] while others have found significant correlations [8, 14, 16]. In one analysis of HIV patients on perennial cART (longer than 24 months on ARTs), the highest mitochondrial density was observed in central CD4+ Tmem cells. However, there were no differences in mitochondrial mass based on HIV status [15]. Another study found that CD4+ T cells with a higher mitochondrial mass had more HIV-1 virus and higher reactive oxygen species (ROS) production [8, 17]. Increased mitochondrial mass was associated with a pro-apoptotic signature, uncontrolled infection, and a survival defect in CD8+ T cells [18]. An increased viral load has also been associated with dysfunctional mitochondria, reduced mitochondrial membrane potential, and higher ROS production in infected and untreated CD8+ T cells [16]. Pharmacological lowering of the mitochondrial membrane potential in CD8+ T-cells from HIV controllers reduced cytokine production and overall responsiveness, but this effect was not observed in HIV+/cART CD8+ T cells [14].
Trained immunity facilitates a faster and enhanced response to recurrent and chronic infections, and is partially mediated by metabolic reprogramming and changes to mitochondrial function [19, 20]. This background provides ample evidence that manipulating metabolic and mitochondrial pathways can rheostatically fine-tune leukocyte function during HIV. However, a better understanding of how HIV affects substrate utilization and mitochondrial phenotypes across different immune cell types in specific patient cohorts and the effect of cART on these parameters is lacking and was the main question of this study. Understanding these mechanisms may be key to strategizing effective HIV therapeutics [21]. Here, we assessed the metabolic functionality of circulating immune cells in HIV+/cART women. Peripheral blood mononuclear cell (PBMC)-derived DCs, NK cells, and CD4+ and CD8+ T cells were assessed for substrate uptake and mitochondrial mass and potential using fluorescent markers of various cellular substrates.
Methods
Study participants
Ethical approval was obtained from the Kenyatta National Hospital-University of Nairobi Ethics and Research Committee (KNH-UoN ERC) (protocol number P817/09/2019) and was reviewed and renewed annually. Both HIV-infected and HIV-uninfected women aged between 18 and 60 years, able and willing to provide informed verbal and written consent, willing to receive HIV counseling, testing, and test results, and willing to comply with the study protocol were recruited for the study from September 2018 to May 2021. Consent was obtained with at least one other clinical staff serving as a signed witness and consent forms were documented and archived on site. The volunteers were screened at the KAVI-Institute of Clinical Research (KAVI-ICR) clinics, where a medical history was obtained, a complete physical examination was conducted, and an HIV rapid test was performed. The authors had no access to information that could identify individual participants during or after data collection. Human samples were collected from September 2018 to May 2021 and the study was conducted from August 2022 to January 2023. Approximately 15 ml of anticoagulated blood was collected in a BD ethylenediaminetetraacetic acid (EDTA) tube and 9 ml of blood was collected in BD serum separator tubes for complete blood count with differential and platelet counts using the Beckman Coulter’s full hemogram machine (Coulter AcT 5diff AL). Liver function tests (aspartate aminotransferase (AST) and alanine aminotransferase (ALT)) and creatinine levels were conducted using the ILAB Aries. For HIV-infected volunteers, CD4/CD8 counts were performed using a BD FACS count machine, and plasma viral load was measured using a GeneXpert machine (Dx system, Cepheid). Only volunteers with hemoglobin levels ≥ 10 g/dL were enrolled, and study samples were collected within 2 to 4 weeks. Volunteers who were malnourished, had acute or chronic illness, were in their 3rd trimester of pregnancy, had a body mass index (BMI) <17kg/m2 or had any condition that would interfere with achieving the study objectives were excluded.
Blood collection and processing
Approximately 20 ml of whole blood was collected in a BD acid citrate dextrose (ACD)-containing tube, and PBMCs were isolated by density gradient centrifugation on Histopaque at 400xg for 40 min with brakes off. The isolated PBMCs were washed twice with Hanks balanced salt solution and once with Roswell Park Memorial Institute (RPMI) –1640 supplemented with 10% fetal bovine serum (FBS), 1% sodium pyruvate solution 100mM, 1% HEPES buffer solution 1M, pH 7.0–7.6, 1% Penicillin (10000 units) -Streptomycin (10 mg/ml) solution, and 1% L-glutamine solution 200mM, here referred to as R10 media. The cells were then counted and stored in 1.2 ml cryovials at a final concentration of 10 million cells per ml of freezing medium (FBS with 10% dimethyl sulfoxide [DMSO]) in liquid nitrogen until use. All reagents were purchased from Sigma–Aldrich (UK).
Flow cytometry
PBMCs were thawed in a 37°C water bath and immediately placed in 9 ml of R20 ((RPMI with 20% FBS, 1% sodium pyruvate solution 100mM, 1% HEPES solution 1M, pH 7.0–7.6, 1% penicillin (10000 units) -streptomycin (10 mg/ml) solution, and 1% L-glutamine solution (200mM). The cells were pelleted at 250xg for 10 min, resuspended in 4 ml of R20, and left to rest overnight (16–24 hours) in a humidified 37°C incubator with 5% CO2. Cells were then pelleted at 2000rpm for 5 min at room temperature, resuspended in 1 ml of phosphate-buffered saline (PBS), and counted [21]. One million PBMCs were plated in 3 different wells of a 96-round bottom well plate. In one well, the cells were incubated with 50μM Fluorescein isothiocyanate (FITC) conjugated 2-NBDG (Cayman Chemical Company), in the second well, the PBMCs were directly incubated with 0.0625μM FITC conjugated BODIPY (Invitrogen), and in the third well, the PBMCs were incubated with 100nM FITC conjugated MTG (Invitrogen), and 12.5nM Allophycocyanin (APC) conjugated MTDR (Invitrogen). The cells were then incubated for 30 min in a dark humidified 37°C incubator with 5% CO2. Cells were then pelleted and washed twice in PBS supplemented with 2% FBS (FACS buffer), before being resuspended in 100μl of fixable viability stain 780 (FVS780) diluted at 1:1000 in PBS. The cells were incubated on ice in the dark for 20 min. After a wash in FACS buffer, cells were stained with 2μl of each of the following fluorochrome-conjugated antibodies in a total of 50μl of FACS buffer: Peridinin chlorophyll cyanine 5.5 (PerCP Cy5.5) mouse anti-human CD3 (BD Pharmingen), Phycoerythrin CF594 (PE-CF594) mouse anti-human CD4 (BD horizon), Brilliant violet 421 (BV421) mouse anti-human CD8 (BD horizon), BV510 mouse anti-human CD56 (BD horizon), Alexa Fluor 700 (AF700) mouse anti-human CD16 (BD Pharmingen), and Phycoerythrin Cyanine 7 (PE-Cy7) mouse anti-human CD11c (BioLegend). The cells were then incubated in the dark on ice for 20 min. Cells were washed twice. Fluorescent signals from the stained cells were acquired immediately on a BD LSR II cytometer.
Gating strategy
Lymphocytes were gated from all events on side scatter area (SSC-A) and forward scatter area (FSC-A) plots, after which singlets were double-gated on an FSC height (FSC-H) and FSC-A plot and SSC-H and SSC-A plot, respectively. Viable cells (FVS780 negative) were gated on the SSC-A and FVS780 plots. T cells (CD3 positive) and non-T cells (CD3 negative) were gated on an SSC-A and PerCP Cy5.5 CD3-A plot. From the T cells, CD4 positive and CD8 positive cells were gated on PE-CF594 CD4-A and BV421 CD8-A plots, respectively, and the expression levels of the different fluorochrome-conjugated substrates (as indicated above) in either the CD4+ or CD8+ T-cell populations were analyzed. For non-T cells, total natural killer (NK) cells were gated on BV510 CD56-A and AF700 CD16-A plots (including CD56brightCD16-, CD56dimCD16+, and CD56-CD16+), while dendritic cells (DCs) were gated on SSC-A and PE-Cy7 CD11c–A plots. Similarly, the expression levels of different substrates (as indicated above) were analyzed in both NK cells and DCs. The gating strategy is shown in S1 & S2 Figs.
Data and statistical analyses
FCS files obtained from the BD LSR II cytometer were analyzed using FlowJo software version 10.8.1 (FlowJo, LLC, OR, USA) to determine geometric mean fluorescence intensities (MFIs). The gating strategies are described above and shown in S1 Fig. The geometric mean of the MFIs of different samples was tabulated using Graph Pad Prism software version 8.0.1 (Graph Pad Software, San Diego, CA, USA). Two-way analysis of variance (ANOVA) with Tukey’s multiple comparison test was performed to compare the MFIs between groups. A non-parametric Spearman rank correlation test was performed for correlation analyses. Statistical significance was set at p < 0.05.
Results
Patient characteristics
A total of 31 women volunteers, 16 HIV-positive individuals on cART and 15 HIV-negative individuals, were recruited (Table 1). There was a significant difference in age between the HIV+/cART (median age of 40 years) and HIV seronegative volunteers (median age of 30 years) (p<0.0001). Absolute CD4+ T cell counts and the ratio of CD4+ T cells and CD8+ T cells were not evaluated in HIV-negative individuals. As expected, the percentage of CD3+CD4+ T cells was significantly lower in HIV+/cART than in HIV-negative individuals (p<0.0427) according to flow cytometric analyses. There were no differences in DCs and NKs between HIV+/cART and HIV negatives. A detailed description of patient characteristics and type of ART are shown in S1 Table. We then compared differences in substrate uptake and mitochondrial phenotypes between HIV-negative vs HIV-positive immune cells and compared the same outputs between different types of immune cells from HIV-positive participants.
Table 1. Study participant characteristics.
| Variable | HIV positive/cART | HIV negative | p-value |
|---|---|---|---|
| N | 16 | 15 | |
| Gender | Female | Female | N/A |
| Age (years) median (25th– 75th quartile) |
40 (34–46) | 30 (24–35) | <0.0001 |
| CD4+ T cell counts median (25th– 75th quartile) |
529 (308–1175) | N/A | N/A |
| %CD4 T cells median (25th– 75th quartile) |
34.35(26.95–47.53) | 43.20(39.40–47.20) | 0.0427 |
| %CD8 T cells median (25th– 75th quartile) |
23.50(19.20–33.88) | 22.60(14.40–24.80) | 0.0801 |
| %DCs cells median (25th– 75th quartile) |
17.55(13.73–30.68) | 14.80(10.30–23.00) | 0.1110 |
| %NKs cells median (25th– 75th quartile) |
4.94(3.64–8.59) | 4.23(2.67–6.30) | 0.1931 |
| CD4+/CD8+ T cell ratio median (25th– 75th quartile) |
0.72(0.44–1.32) | N/A | N/A |
| Viral load (copies/ml) (GeneXpert machine viral load detection limit was <20 copies/ml) |
Undetected N = 11 Detected N = 4 (50, 420, 600, 142360) Missing data N = 1 |
N/A | N/A |
| Time on cART in months median (25th– 75th quartile) |
108 (75–120) | N/A | N/A |
Substrate uptake, mitochondrial phenotypes and activity between HIV- vs HIV+ leukocytes
HIV viremia increases mitochondrial mass in CD4 T-cells
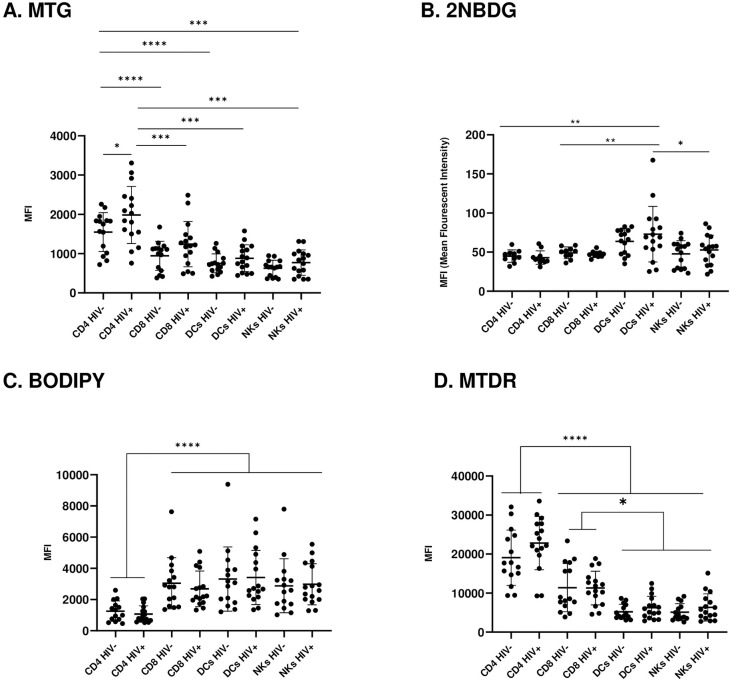
MTG was used to measure mitochondrial mass/density (Fig 1A). MTG is a fluorescent mitochondria-specific dye that accumulates in the mitochondrial matrix and binds to mitochondrial proteins by reacting with free thiol groups of cysteine residues. Generally, adaptive immune cells had a higher mitochondrial mass compared to innate immune cells. Even without HIV infection, CD4+ T cells have the highest mitochondrial mass, mirroring data from a subset of CD4+ T cells from previous studies [15]. HIV increased mitochondrial mass in all leukocyte populations, but the difference due to HIV status was significant only in HIV/cART CD4+ T cells, which had the highest mitochondrial mass of all leukocytes. The increase in mitochondrial mass in CD4+ T cells may be an adaptive measure to increase substrate utilization, which is severely hampered in these cells.
Fig 1. Substrate uptake and assessment of mitochondrial phenotype and activity in HIV/cART leukocytes.
(A) Assessment of mitochondrial mass (via MTG staining) in uninfected and chronically infected HIV adaptive and innate immune cells. (B) Glucose uptake (via 2NBDG staining) in uninfected and chronically infected HIV+ adaptive and innate immune cells. (C) Fat uptake (via BODIPY staining) in uninfected and chronically infected HIV adaptive and innate immune cells. (D) Assessment of mitochondrial potential (via MTDR staining) in uninfected and chronically infected HIV adaptive and innate immune cells (* p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001).
Substrate uptake, mitochondrial phenotypes and activity within HIV+ leukocytes
High glucose metabolism in HIV+ dendritic cells
Overall, glucose uptake, as measured by 2-NBDG staining (Fig 1B), was unexpectedly low based on previous reports of high glycolytic rates, particularly in HIV-infected adaptive T-cells [7]. Surprisingly, the highest glucose utilization was observed in HIV+ DCs, compared to the lowest glucose uptake in CD4+ and CD8+ T cells. Contrary to previous reports suggesting higher glucose utilization with HIV [2], HIV viremia did not affect glucose utilization in adaptive T cells. HIV+ DC glucose uptake significantly exceeded that of HIV+ NK cells and CD4+ and CD8+ T-cells. To the best of our knowledge, no studies have compared glucose utilization in DCs with other immune cells in HIV+/cART individuals. NK cells displayed low/background glucose utilization levels, analogous to those of CD4+ and CD8+ T cells. Taken together, NK cells in chronic HIV/cART do not rely on glucose as the substrate. These results suggest a significant role for DCs glucose metabolism in overall glucose utilization in HIV-infected leukocytes.
Low fatty acid utilization in HIV+ CD4+ T-cells
We assessed fatty acid uptake by measuring BODIPY staining (Fig 1C). Compared to all other profiled leukocytes, fatty acid utilization was significantly lower in CD4+ T cells. CD8+ T cells took up as many lipids as DCs and NK cells, suggesting a higher reliance of chronically HIV-infected CD8+ T cells on fatty acids than glucose usage which was null. The ramifications of this preference for fatty acid metabolism in CD8+ T cells during chronic HIV infection remains unknown. Our findings suggest that when presented with a choice of sugar or fats CD8+ T-cells would prefer to use fatty acids to fuel metabolism.
The mitochondrial potential is highest in adaptive T-cells
MTDR, a dye that stains mitochondria based on charge polarization across the membrane, was used to measure mitochondrial membrane potential (Fig 1D). We found that the mitochondrial membrane potential was significantly higher in adaptive T-cells than in innate immune cells. Interestingly, CD4+ T-cell mitochondrial membrane potential was significantly higher than that of CD8+ T-cells, which contradicts previous studies that reported no differences between the two adaptive T-cell types or a higher mitochondrial membrane potential in CD8+ T-cells [15, 17]. We postulate that the increased mitochondrial membrane potential in adaptive T cells is an adaptive measure to facilitate substrate utilization and energy production.
Correlations between substrate utilization and mitochondrial phenotype
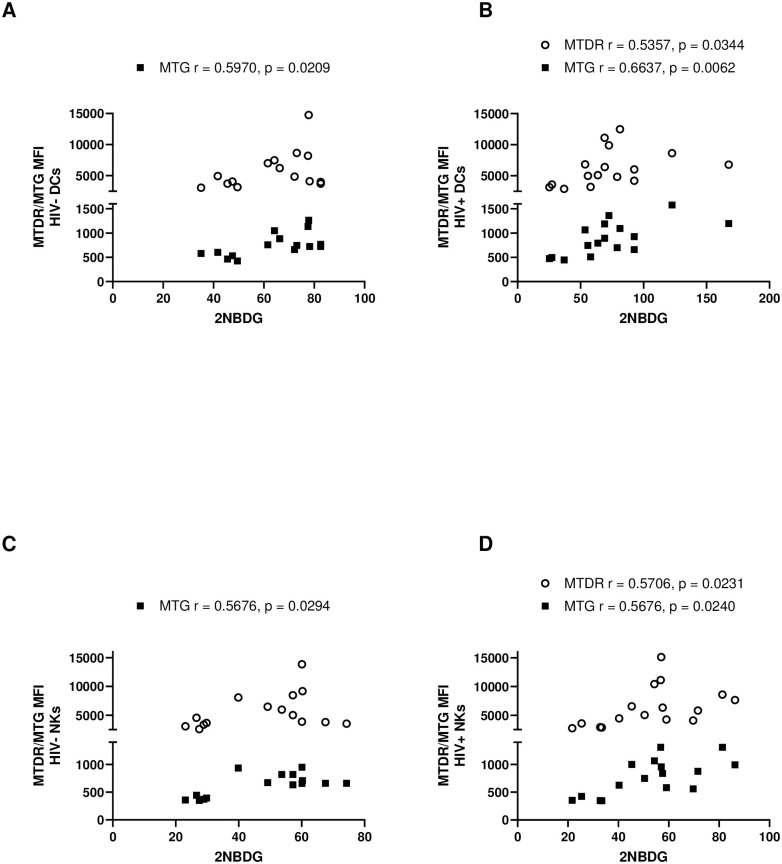
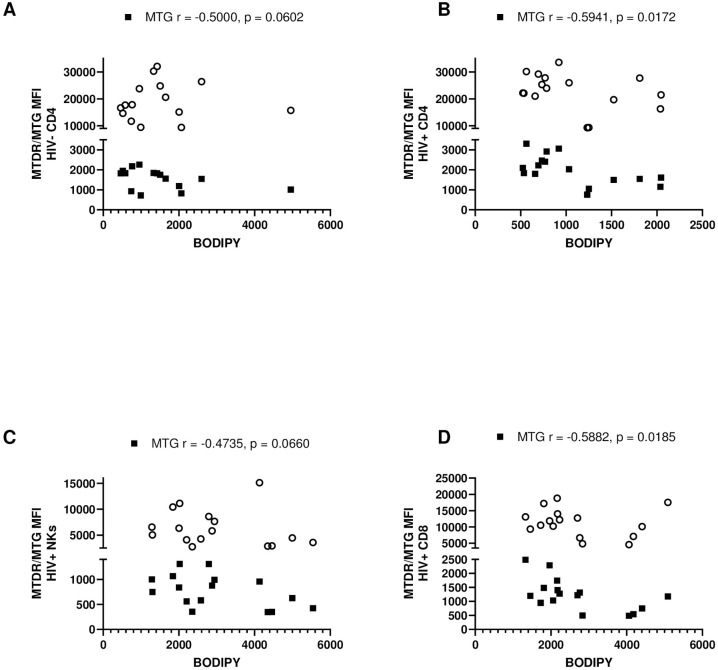
The substrates measured in this study were the primary fuel sources for glycolysis and OXPHOS for production of ATP. ATP generation is a key function of the mitochondria and requires a membrane potential to be generated across the outer and inner mitochondrial membranes. Therefore, we sought to correlate the utilization of substrates in different leukocyte populations with the mitochondrial membrane potential and mitochondrial mass (Figs 2A–2D and 3A–3D). Once again, we assessed correlative differences due to HIV-status and differences between various HIV+ immune cells.
Fig 2. Correlation of mitochondrial mass ■ (via MTG staining) and mitochondrial membrane potential ○ (via MTDR staining) with glucose uptake (via 2NBDG staining) in uninfected and chronically infected HIV adaptive and innate immune cells.
(A) Correlations in uninfected DCs. (B) Correlations in HIV-infected DCs. (C) Correlations in uninfected NK cells. (D) Correlations in HIV infected NK cells. Statistical significance is shown in charts.
Fig 3. Correlation of mitochondrial mass ■ (via MTG staining) with lipid uptake (via BODIPY staining) in uninfected and chronically infected HIV adaptive and innate immune cells.
(A) Correlations in uninfected CD4+ T-cells. (B) Correlations in HIV CD4+ T-cells. (C) Correlations in HIV infected NK cells. (D) Correlations in HIV infected CD8+ T-cells. Statistical significance is shown in charts.
Correlations in HIV- vs HIV+ leukocytes
Correlations with glucose uptake: In innate immune cells, a positive and significant correlation exists between mitochondrial mass and glucose uptake persists with HIV infection. The significance of this correlation increased exponentially in HIV+ DCs. HIV viremia revealed a positive and significant correlation between mitochondrial membrane potential and glucose uptake in innate immune cells (Fig 2A–2D).
Correlations with lipid uptake: In CD4+ T cells, a negative correlation between mitochondrial mass and fat uptake became significant with HIV infection (Fig 3A and 3B).
Correlations in different HIV+ leukocytes
Correlations with lipid uptake: Regarding fatty acid uptake, in HIV+ NK and CD8+ T cells, there was trending and significant negative correlation with mitochondrial mass respectively (Fig 3C and 3D). Therefore, the higher the mitochondrial mass, the lower the fatty acid intake by these cells. Taken together, these results suggest that a high mitochondrial membrane potential and high mitochondrial mass may be disadvantageous to HIV-infected cells.
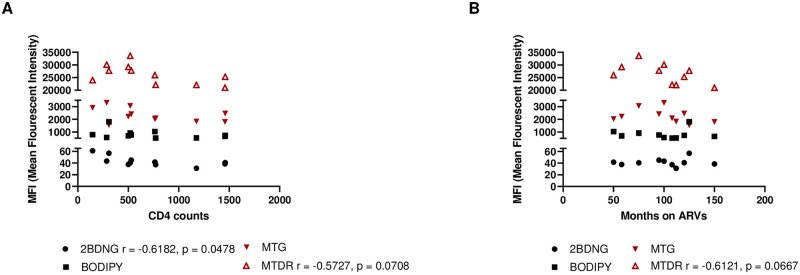
Correlations between substrate utilization and CD4+ T-cell counts and time on ARVs
Finally, to determine whether substrate utilization is associated with HIV infection, we correlated substrate uptake in HIV/cART patients with CD4+ T-cell count (Fig 4A). cART therapy interrupts the HIV life cycle and can cause an increase in CD4+ T-cell counts. We also correlated substrate utilization with the duration of various cART regimens in our HIV-infected cohort (Fig 4B). CD4 counts were negatively associated with glucose uptake in HIV-infected patients, suggesting that the higher the CD4 count, the lower was the glucose uptake. A trending negative correlation existed between mitochondrial membrane potential and CD4 counts, suggesting a higher mitochondrial membrane potential with low CD4+ T-cell counts. The mitochondrial membrane potential was negatively correlated with patient time on ARV treatment, but with trending significance. Our results highlight that substrate utilization and mitochondrial phenotypes in chronic HIV/cART are complex and differ significantly among immune cell types. Therefore, for future immunometabolism-based therapeutic strategies, it would be beneficial to consider these two arms of immunity as distinct entities and to target therapies accordingly.
Fig 4. Correlations of substrate uptake with CD4 counts and duration of treatment in HIV+ participants.
(A) Correlation of substrate uptake with CD4 counts in chronically infected HIV/cART participants and (B) Correlation of substrate uptake with time on ARVs. Statistical significance shown in charts.
Discussion
Our data point to a variable pattern of sugar and fat uptake in different circulating leukocyte populations in chronic HIV/cART. DCs showed the highest uptake of both sugars and fats, and HIV+ DCs had a significantly higher glucose uptake than all other HIV+ leukocytes. DCs play a pivotal role in activating the adaptive immune system; therefore, the fact that HIV+ DCs are more metabolically responsive may point to increased activation and antigen-presenting capacity in the context of HIV. In fact, recent studies affirm that DCs possess the machinery to recognize HIV replication products and could therefore play a role in humoral, mucosal, and cellular immunity against HIV [22]. NK cells showed little variation in sugar and fat uptake in chronic HIV/cART. This suggests that NK cells are metabolically quiescent in this context and that their level of education and activation may be inherently impaired [4].
Interestingly, the primary cellular target of HIV infection, CD4+ T cells, had a remarkably muted immunometabolic profile in comparison to DCs with low glucose and fat uptake. Additionally, CD4 counts in HIV-infected patients were negatively correlated with glucose utilization, implying that glucose is barely utilized as a substrate. However, mitochondrial mass was greatest in HIV+ CD4+ T-cells, and mitochondrial membrane potential was augmented in CD4+ T cells, while DCs and NK cells had lower mitochondrial mass and mitochondrial membrane potential overall. The hypometabolic profile of CD4+ T cells may be an innate feature of circulating CD4+ T cells from women, or it may be that CD4+ T cells use other fuel sources, such as amino acids. However, this possibility was not assessed in the present study. It is possible that the CD4+ T-cells in these patients are exhausted and therefore do not actively take up the substrates administered in this study or use alternative pathways. Even in aviremic patients undergoing long-term cART, studies have found that T-cells remain irreversibly defective and continue to show markers of exhaustion [23]. Therefore, the robust mitochondrial phenotypes observed in CD4+ T cells could be a failing attempt to restore metabolic function in T cells, aided by the effects of long-term cART. Another possibility not evaluated in this study is mitochondrial turnover. As mitochondria become obsolete, they are normally cleared by mitophagy, a homeostastic process of cellular waste management where the damaged contents are sequestered in a vesicle for lysosomal elimination. In HIV, mitophagy plays an integral role in maintaining mitochondrial mass and controlling mitochondrial health. When this process is impaired, mitochondrial density is increased [24]. Therefore, it is possible that chronic HIV/cART impaired mitophagy in CD4+ T-cells which would be consistent with the mitochondrial phenotypes observed in these cells.
It is also possible that the increased mitochondrial mass and mitochondrial membrane potential in CD4+ T cells signals further dysfunction rather than a marker of an attempt to improve function. This hypothesis is supported by our own findings; the cells with the lowest mitochondrial mass and mitochondrial membrane potential (DCs and NK cells) had a more robust metabolic profile and that mitochondrial mass and mitochondrial membrane potential in these cells were positively correlated specifically with glucose utilization. Unlike the rapid switch to glucose use and glycolysis observed in acutely activated T-cells, chronic antigen stimulation and exhaustion suppress glycolysis, with reduced cellular glucose uptake and dysregulated mitochondrial function [25]. The same study found that increased mitochondrial mass and membrane potential in exhausted T-cells were coupled with increased ROS production [25]. Byproducts of normal mitochondrial metabolism can produce a buildup of damaging ROS and initiate a regenerative feedforward cycle of ROS production in a phenomenon known as ROS-induced ROS release (RIRR) [26]. We did not measure ROS concentrations in this study; however, this could be a possible mechanism for continued mitochondrial dysfunction in HIV+ CD4+ T cells. Our study also did not measure T-cell exhaustion, but previous results suggest that a similarly distorted metabolic phenotype is an upstream indicator of T-cell exhaustion [25]. While these results were observed specifically in CD8+ Teff-cells, we contend that similar mechanisms ensue in CD4+ T-cells.
As in CD4+ T cells, CD8+ T cells had low levels of glucose utilization; however, fatty acid uptake was significantly higher in CD8+ T-cells cells than in CD4+ T cells, irrespective of HIV status. The use of fatty acid metabolism to fuel OXPHOS in CD8+ T cells is associated with memory formation [2]. Indeed, once viral loads are reduced, as is the case in this patient cohort on chronic cART, Tmem cells revert to a quiescent state that uses fatty acids for OXPHOS [27]. Therefore, these results suggest that overall, there is little CD8+ Teff function present, but a restoration of CD8+ Tmem functionality with cART since the level of fatty acid uptake is the same as that of HIV-uninfected patients. Fatty acid uptake was negatively correlated with mitochondrial mass in CD8+ T cells, suggesting that an increase in mitochondrial mass does not correlate with better metabolic fitness in these cells, and reinforces the observation that fatty acid utilization in these cells is associated with cellular quiescence.
In comparison with similar studies investigating metabolic functionality and mitochondrial phenotypes in HIV infection, subtle similarities and differences exist. The analysis of substrate utilization in DCs and NK cells with HIV/cART and the finding that DCs are major glucose utilizers are novel. Additionally, a comparison of fatty acid uptake in HIV-infected DCs, NKs, CD4, and CD8+ T cells is also unprecedented. Previous studies have described uniformly increased glucose utilization [2] and glucose transporter expression [15] in HIV, while we observed a more nuanced pattern. While others found that mitochondrial mass was the highest in CD4+ T cells [15], they did not observe a significant increase in mitochondrial mass with HIV viremia. All participants in this study ranged in the pre-and postmenopausal stages and had been on different cART regimens for at least 30 months at the time of this study. The effects of sex, hormones, viral loads, and type of chronic ARV treatment may play a role in the differences in phenotypes observed in different cell types and should be considered.
There are several limitations to this study. Firstly, all our participants were adult women, therefore, there may be some differences based on sex, as is the case in other aspects of HIV virulence such as susceptibility to HIV acquisition [28]. Secondly, B-cells and monocytes were not included on our analyses, and therefore, we are unable to comment on how HIV status affects substrate uptake and mitochondrial phenotypes in these cells. We had a finite number of PBMCs that were harvested from the 20ml blood draw. In ethical consideration of the comfort of the participants who had several separate blood volumes being drawn in a single visit for use in other confirmatory tests, we did not seek to increase this volume. Therefore, we could only focus on a subset of cell types to maximize our output. Thirdly, due to the limitations of non-overlapping spectra our flow cytometer, we were unable to accommodate more fluorochromes in our analyses. Fourthly, although there was a significant difference in the ages of HIV- and HIV+ participants with the latter being significantly older, the effect of this age difference was not investigated in this study. Finally, while our results highlight significant differences in consumption of glucose and lipids and mitochondrial phenotypes between HIV- and HIV+ leukocytes and within different HIV+ leukocytes, the consequences of these differences can only be inferred at this time. Whether these differences are enough to change the intrinsic properties of the cells involved and what this means for disease progression is a question of great interest to us and will be addressed in future studies.
Conclusions
The primary mechanism of HIV infection is to target and manipulate immune responses to the advantage of the virus and immune cell metabolism is one of the nodes that are compromised in this goal. It now common practice to initiate ARTs immediately after HIV seropositivity is confirmed. Therefore, understanding how HIV and cARTs interact to either bolster or hinder immune responses is an area of interest. The major finding uncovered by our results was a maladapted metabolic profile of CD4+ T-cells with HIV/cART that suggests quiescence and mitochondrial dysfunction. Additionally, our results demonstrate that leukocyte metabolism in the context of HIV/cART is controlled by several factors, including the duration of infection, efficacy, and duration of cART, and is cell-autonomous. This study highlights the need for further investigations into the mechanisms of the various phenotypes observed: for example whether and how they affect glycolytic and OXPHOS rates and defining regulators of the cellular and mitochondrial responses in different cell populations such as Glut1 and T-cell exhaustion markers such as PD-1, mTOR, PGC1α, and Foxo1, as has been suggested in previous work [23]. The search for an HIV vaccine is elusive, and novel strategies to improve HIV immunity are of great interest. This study outlines fundamental metabolic changes during chronic HIV/cART, broadening our understanding of the intracellular processes underlying HIV infection and cART therapy. This may open new therapeutic possibilities for the modulation of the related immune responses via metabolic reprogramming. Indeed, recent studies suggest that vaccine formulations that induce training and in particular metabolic reprogramming in immune cells may have broad therapeutic benefit [29, 30].
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the manuscript and its Supporting information files.
Funding Statement
Deutsche Forschungsgemeinschaft, BU 3630/2–1, Dr. Marianne Mureithi. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Klein Geltink RI. The metabolic tug of war between HIV and T cells. Nature Metabolism. 2019;1(7):653–5. doi: 10.1038/s42255-019-0091-2 [DOI] [PubMed] [Google Scholar]
- 2.Shehata HM, Murphy AJ, Lee MKS, Gardiner CM, Crowe SM, Sanjabi S, et al. Sugar or Fat?-Metabolic Requirements for Immunity to Viral Infections. Front Immunol. 2017;8:1311. doi: 10.3389/fimmu.2017.01311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rezinciuc S, Bezavada L, Bahadoran A, Duan S, Wang R, Lopez-Ferrer D, et al. Dynamic metabolic reprogramming in dendritic cells: An early response to influenza infection that is essential for effector function. PLOS Pathogens. 2020;16(10):e1008957. doi: 10.1371/journal.ppat.1008957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Highton AJ, Diercks BP, Möckl F, Martrus G, Sauter J, Schmidt AH, et al. High Metabolic Function and Resilience of NKG2A-Educated NK Cells. Front Immunol. 2020;11:559576. doi: 10.3389/fimmu.2020.559576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Littwitz-Salomon E, Moreira D, Frost JN, Choi C, Liou KT, Ahern DK, et al. Metabolic requirements of NK cells during the acute response against retroviral infection. Nature Communications. 2021;12(1):5376. doi: 10.1038/s41467-021-25715-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cretenet G, Clerc I, Matias M, Loisel S, Craveiro M, Oburoglu L, et al. Cell surface Glut1 levels distinguish human CD4 and CD8 T lymphocyte subsets with distinct effector functions. Sci Rep. 2016;6(1):24129. doi: 10.1038/srep24129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmer CS, Ostrowski M, Gouillou M, Tsai L, Yu D, Zhou J, et al. Increased glucose metabolic activity is associated with CD4+ T-cell activation and depletion during chronic HIV infection. Aids. 2014;28(3):297–309. doi: 10.1097/QAD.0000000000000128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clerc I, Abba Moussa D, Vahlas Z, Tardito S, Oburoglu L, Hope TJ, et al. Entry of glucose- and glutamine-derived carbons into the citric acid cycle supports early steps of HIV-1 infection in CD4 T cells. Nature Metabolism. 2019;1(7):717–30. doi: 10.1038/s42255-019-0084-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valle-Casuso JC, Angin M, Volant S, Passaes C, Monceaux V, Mikhailova A, et al. Cellular metabolism is a major determinant of HIV-1 reservoir seeding in CD4+ T cells and offers an opportunity to tackle infection. Cell metabolism. 2019;29(3):611–26.e5. doi: 10.1016/j.cmet.2018.11.015 [DOI] [PubMed] [Google Scholar]
- 10.Hollenbaugh JA, Munger J, Kim B. Metabolite profiles of human immunodeficiency virus infected CD4+ T cells and macrophages using LC-MS/MS analysis. Virology. 2011;415(2):153–9. doi: 10.1016/j.virol.2011.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angela M, Endo Y, Asou HK, Yamamoto T, Tumes DJ, Tokuyama H, et al. Fatty acid metabolic reprogramming via mTOR-mediated inductions of PPARγ directs early activation of T cells. Nature Communications. 2016;7(1):13683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takata H, Buranapraditkun S, Kessing C, Fletcher JL, Muir R, Tardif V, et al. Delayed differentiation of potent effector CD8(+) T cells reducing viremia and reservoir seeding in acute HIV infection. Sci Transl Med. 2017;9(377). doi: 10.1126/scitranslmed.aag1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Appay V, Papagno L, Spina CA, Hansasuta P, King A, Jones L, et al. Dynamics of T Cell Responses in HIV Infection. The Journal of Immunology. 2002;169(1):607-. doi: 10.4049/jimmunol.168.7.3660 [DOI] [PubMed] [Google Scholar]
- 14.Angin M, Volant S, Passaes C, Lecuroux C, Monceaux V, Dillies M-A, et al. Metabolic plasticity of HIV-specific CD8+ T cells is associated with enhanced antiviral potential and natural control of HIV-1 infection. Nature Metabolism. 2019;1(7):704–16. doi: 10.1038/s42255-019-0081-4 [DOI] [PubMed] [Google Scholar]
- 15.Masson JJR, Murphy AJ, Lee MKS, Ostrowski M, Crowe SM, Palmer CS. Assessment of metabolic and mitochondrial dynamics in CD4+ and CD8+ T cells in virologically suppressed HIV-positive individuals on combination antiretroviral therapy. PLoS One. 2017;12(8):e0183931. doi: 10.1371/journal.pone.0183931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perrin S, Cremer J, Roll P, Faucher O, Ménard A, Reynes J, et al. HIV-1 infection and first line ART induced differential responses in mitochondria from blood lymphocytes and monocytes: the ANRS EP45 "Aging" study. PLoS One. 2012;7(7):e41129. doi: 10.1371/journal.pone.0041129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu F, Hao Y, Zhao H, Xiao J, Han N, Zhang Y, et al. Distinct Mitochondrial Disturbance in CD4+T and CD8+T Cells From HIV-Infected Patients. J Acquir Immune Defic Syndr. 2017;74(2):206–12. doi: 10.1097/QAI.0000000000001175 [DOI] [PubMed] [Google Scholar]
- 18.Petrovas C, Mueller YM, Dimitriou ID, Altork SR, Banerjee A, Sklar P, et al. Increased mitochondrial mass characterizes the survival defect of HIV-specific CD8+ T cells. Blood. 2006;109(6):2505–13. doi: 10.1182/blood-2006-05-021626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, et al. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345(6204):1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Domínguez-Andrés J, Joosten LA, Netea MG. Induction of innate immune memory: the role of cellular metabolism. Curr Opin Immunol. 2019;56:10–6. doi: 10.1016/j.coi.2018.09.001 [DOI] [PubMed] [Google Scholar]
- 21.Sperk M. Immunometabolic Regulation in Natural Hiv-1 Control: Karolinska Institutet (Sweden); 2022.
- 22.Martin-Gayo E, Yu XG. Role of Dendritic Cells in Natural Immune Control of HIV-1 Infection. Frontiers in Immunology. 2019;10. doi: 10.3389/fimmu.2019.01306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fenwick C, Joo V, Jacquier P, Noto A, Banga R, Perreau M, Pantaleo G. T-cell exhaustion in HIV infection. Immunol Rev. 2019;292(1):149–63. doi: 10.1111/imr.12823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saha B, Salemi M, Williams GL, Oh S, Paffett ML, Phinney B, Mandell MA. Interactomic analysis reveals a homeostatic role for the HIV restriction factor TRIM5α in mitophagy. Cell Rep. 2022;39(6):110797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bengsch B, Johnson AL, Kurachi M, Odorizzi PM, Pauken KE, Attanasio J, et al. Bioenergetic Insufficiencies Due to Metabolic Alterations Regulated by the Inhibitory Receptor PD-1 Are an Early Driver of CD8(+) T Cell Exhaustion. Immunity. 2016;45(2):358–73. doi: 10.1016/j.immuni.2016.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiological reviews. 2014;94(3):909–50. doi: 10.1152/physrev.00026.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Sullivan D, Pearce EL. Targeting T cell metabolism for therapy. Trends Immunol. 2015;36(2):71–80. doi: 10.1016/j.it.2014.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griesbeck M, Scully E, Altfeld M. Sex and gender differences in HIV-1 infection. Clin Sci (Lond). 2016;130(16):1435–51. doi: 10.1042/CS20160112 [DOI] [PubMed] [Google Scholar]
- 29.Sánchez-Ramón S, Conejero L, Netea MG, Sancho D, Palomares Ó, Subiza JL. Trained Immunity-Based Vaccines: A New Paradigm for the Development of Broad-Spectrum Anti-infectious Formulations. Front Immunol. 2018;9:2936. doi: 10.3389/fimmu.2018.02936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teer E, Mukonowenzou NC, Essop MF. The Role of Immunometabolism in HIV-1 Pathogenicity: Links to Immune Cell Responses. Viruses. 2022;14(8). doi: 10.3390/v14081813 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting information files.