Abstract
Background:
Infants with cystic fibrosis (CF) develop structural lung disease early in life, and viral infections are associated with progressive lung disease. We hypothesized that the presence of respiratory viruses would be associated with structural lung disease on computed tomography (CT) of the chest in infants with CF.
Methods:
Infants with CF were enrolled before 4 months of age. Multiplex PCR assays were performed on nasal swabs to detect respiratory viruses during routine visits and when symptomatic. Participants underwent CT imaging at approximately 12 months of age. Associations between Perth–Rotterdam Annotated Grid Morphometric Analysis for CF (PRAGMA-CF) CT scores and respiratory viruses and symptoms were assessed with Spearman correlation coefficients.
Results:
Sixty infants were included for analysis. Human rhinovirus was the most common virus detected, on 28% of tested nasal swabs and in 85% of participants. The median (IQR) extent of lung fields that was healthy based on PRAGMA-CF was 98.7 (0.8)%. There were no associations between PRAGMA-CF and age at first virus, or detection of any virus, including rhinovirus, respiratory syncytial virus, or parainfluenza. The extent of airway wall thickening was associated with ever having wheezed (ρ = 0.31, p = 0.02) and number of encounters with cough (ρ = 0.25, p = 0.0495).
Conclusions:
Infants with CF had minimal structural lung disease. We did not find an association between respiratory viruses and CT abnormalities. Wheezing and frequency of cough were associated with early structural changes.
Keywords: Cystic fibrosis, viruses, respiratory infection, Chest computed tomography, newborn screening
Introduction
Infants with cystic fibrosis (CF) can develop trapped air and bronchiectasis early in life, even before 6 months of age, and in the absence of detectable CF respiratory pathogens (1). Viral infections are associated with progressive clinical deterioration and significant morbidity in people with CF (2-5). However, there is insufficient information regarding the role of respiratory viruses in the pathogenesis of early CF lung disease. Viruses may alter the airway environment to permit bacterial colonization and interfere with bacterial clearance from the lungs (6-10). We have previously shown that respiratory viruses were present in 52% of acute courses of oral antibiotics in infants with CF, and a history of detection of respiratory viruses is associated with elevated neutrophil concentrations and bacterial isolates in bronchoalveolar (BAL) fluid (11).
We hypothesized that early viral infections accelerate progression of structural lung disease in infants with CF. In this manuscript, we describe the frequency of detection of respiratory viruses and respiratory symptoms over the first year of life in infants with CF who were evaluated prospectively at four US and Australian CF centers. In addition, using computerized tomographic (CT) imaging of the chest, we describe lung and airway abnormalities and their association with respiratory viruses and respiratory symptoms, building on our previous work (11).
Materials and Methods
Participants
Participants were enrolled at four sites, Riley Hospital for Children (Indianapolis, USA); St. Louis Children's Hospital (St. Louis, USA); Princess Margaret Hospital for Children (Perth, Australia); and Royal Children's Hospital (Melbourne, Australia). Study approval was obtained from the regulatory boards at each institution. Written informed consent was obtained from parents prior to enrollment.
All participants were identified following newborn screening and enrolled by 4 months of age. Inclusion criteria included a diagnosis of CF based on sweat chloride levels ≥60 mEq/L or two pathogenic cystic fibrosis transmembrane conductance regulator (CFTR) variants. Exclusion criteria included the inability to successfully or safely perform study visits.
Study visits were scheduled at 1- to 3-month intervals, coordinating with routine clinic appointments. A history and physical examination were performed at visits. Parents were contacted weekly via telephone to assess for new respiratory symptoms using a standardized questionnaire (Online supplement). Specifically, parents were asked if their infant wheezed, had a cough, nasal symptoms, fever, altered breathing, or reduced activity. The same symptoms may have been reported on weekly phone calls and at clinic visits.
Chest imaging
Chest CT imaging was performed with sedation or anesthesia at 12 to 18 months of age. Volumetric images were obtained after recruitment inflations at a standardized transpulmonary pressure, 25 cm H2O, and repeated after exhalation at a standardized transpulmonary pressure, 0 cm H2O (12, 13). At Erasmus Medical Center (Rotterdam, Netherlands), deidentified chest CT scans were scored in random order by an independent, highly experienced, certified observer using the quantitative Perth-Rotterdam Annotated Grid Morphometric Analysis for CF (PRAGMA-CF) scoring system (14). The extent (represented as volume proportions of the lung) of bronchiectasis (% Bronchiectasis), mucus plugging (% Mucus plugging), airway wall thickening (% Airway wall thickening), atelectasis (% Atelectasis), and total airways disease (% Total airway disease, equivalent to the sum of the three subscores) were determined after adjusting for the extent of atelectasis. On expiratory scans, trapped air (% Trapped air) was determined. A second independent certified observer scored a subset of the chest CT scans to assess inter-observer agreement in PRAGMA-CF subscores.
Specimens
Nasal swabs were obtained at research clinic visits. When participants were symptomatic, the parents were instructed to collect a swab at home (FLOQSwabsTM and UTMTM Viral Transport Media, COPAN Diagnostics, Murrieta, CA). Parents were trained in collection techniques by research personnel in clinic. Viral analysis was performed at the Special Projects Laboratory at Washington University. Respiratory virus polymerase chain reaction (PCR) was performed using the GenMark eSensor Respiratory Viral Panel using a research protocol that allowed identification of the following viruses: influenza A, influenza A 2009 H1N1, influenza B, respiratory syncytial virus A and B, parainfluenza viruses 1-4, human rhinovirus, human metapneumovirus, coronaviruses OC43, 229E, NL63, and HKU1, adenovirus B/E, adenovirus C (GenMark Diagnostics Inc. Carlsbad, CA).
Statistical analysis
Comparisons were performed using Student's t-tests for continuous variables, with log-transformations or Wilcoxon-Kruskal-Wallis tests when the data were skewed, and Chi-Square tests for categorical variables, with Fisher's Exact tests when cell counts were small. The intraclass correlation coefficient (ICC) was used to measure inter-observer agreement for PRAGMA-CF subscores. ICC values between 0<0.4, 0.4 and <0.6, 0.6 and <0.8, or ≥0.8 were considered to indicate poor, moderate, good, and excellent agreement, respectively.
Associations between the detection of respiratory viruses and PRAGMA-CF scores were tested with Spearman correlation coefficients. Participants were classified as “ever” or “never” having any detected respiratory virus, as well as ever/never for specific viral species, and the frequency of positive viral swabs. Comparisons were repeated, removing any potential confounding effect of continent by computing partial correlation coefficients. Associations between symptom reporting and PRAGMA-CF scores were assessed similarly. Reported respiratory symptoms were further categorized according to Korten et al:(15) nasal symptoms only, upper respiratory tract symptoms (cough without wheezing or altered breathing), and lower respiratory tract symptoms (wheezing and/or altered breathing). Based on the initial associations of interest between respiratory viruses, respiratory symptoms, and PRAGMA-CF scores, we performed two sensitivity analysis: (1) to determine if the subset of detected respiratory viruses that occurred within ±7 days of lower respiratory tract symptoms were correlated with PRAGMA-CF scores, and (2) to determine if the subset of respiratory symptoms that occurred within ±7 days of detected respiratory viruses were correlated with PRAGMA-CF scores.
In order to examine variables as possible confounders, we performed bivariate analyses using correlation or Wilcoxon tests, depending on the distribution of the data, including continent, age, sex, genotype, smoke exposure (in utero or secondhand), daycare, breast feeding, and the presence of classical CF pathogens, defined as: Haemophilus influenzae, methicillin-resistant (MRSA) and -sensitive (MSSA) Staphylococcus aureus, Pseudomonas aeruginosa, and Stenotrophomonas maltophilia, from oropharyngeal swabs obtained clinically or in bronchoalveolar lavage (BAL) fluid. In the event the initial analyses were significant, confounding variables were to be used in multivariate models to determine if the initial result would be attenuated. Analyses were performed using SAS v9.4 (SAS Institute, Cary, NC) with p-values < 0.05 considered statistically significant.
Results
Study cohort
A total of 109 infants were screened from May 2013 to December 2016. Seventy-eight infants were enrolled and 73 were included for analysis (21 from the US and 52 from Australia) (Table 1). Five participants were excluded from this analysis because of insufficient follow-up data to describe detection of respiratory viruses. Thirty-four participants also participated in a randomized trial of azithromycin; these participants could have received azithromycin or placebo. Mean (SD) age at enrollment was 3.1 (0.8) months. Participants remained in the study for a mean (SD) duration of 8.7 (1.5) months. All but one participant from Australia received daily amoxicillin-clavulanate as respiratory prophylaxis.
Table 1. Cohort Characteristics (N = 73).
| N (%) | |
|---|---|
| Australia | 52 (71%) |
| Sex (Male) | 32 (44%) |
| Race (Caucasian) | 71 (97%) |
| Ethnicity (Hispanic) | 2 (3%) |
| CFTR Genotype | |
| F508del homozygous | 40 (55%) |
| F508del heterozygous | 31 (42%) |
| Other | 2 (3%) |
| In-utero Smoke Exposure, n=70 | 8 (11%) |
| Secondhand Smoke Exposure, n=69 | 7 (10%) |
| Any Breast Feeding, n=65 | 38 (58%) |
| Attending Day Care at enrollment, n=70 | 1 (1%) |
| Dornase alfa (ever) | 15 (21%) |
| Hypertonic saline (ever) | 3 (4%) |
| Inhaled tobramycin (ever) | 15 (21%) |
| Amoxicillin clavulanate prophylaxis | 51 (70%) |
| Amoxicillin clavulanate acute treatment (ever) | 23 (32%) |
| Trimethoprim-sulfamethoxasole (ever) | 21 (29%) |
| Cephalosporin (ever) | 14 (19%) |
| Intravenous (IV) antibiotics (ever) | 24 (33%) |
| Mean (SD) | |
| Weight for age at enrollment (z-score); n=71 | −0.4 (1.1) |
| Length for age at enrollment (z-score); n=69 | −0.4 (1.7) |
Respiratory viruses
A total of 820 nasal swabs were tested, a median of 10 swabs collected per participant (range 7-14) (Table 2). Overall, 304 (37%) nasal swabs were positive for at least one virus, with at least one positive swab in 65 (89%) participants. Among these 65 infants, the mean (SD) number of positive swabs was 4.7 (3.5). Human rhinovirus was the most common virus detected, found in 28% of analyzed nasal swabs and in 85% of participants. Adenovirus and influenza were detected infrequently and not tested for associations with PRAGMA-CF scores.
Table 2. Summary of Virus Detection.
| Nasal swabs (N=820) n (%) |
Infants (N=73) n (%) |
|
|---|---|---|
| Any positive | 304 (37%) | 65 (89%) |
| Human rhinovirus | 228 (28%) | 62 (85%) |
| Human metapneumovirus | 9 (1%) | 5 (7%) |
| Respiratory syncytial virus | 20 (2%) | 11 (15%) |
| Coronavirus | 35 (4%) | 21 (29%) |
| Parainfluenza virus | 36 (4%) | 19 (26%) |
| Adenovirus | 7 (1%) | 3 (4%) |
| Influenza | 3 (0.4%) | 3 (4%) |
| 2 viruses detected simultaneously | 25 (3%) | 14 (19%) |
| 3+ viruses detected simultaneously | 4 (0.5%) | 2 (3%) |
PRAGMA-CF Scores
Sixty of the 73 participants had a chest CT scan scored using the quantitative PRAGMA-CF scoring system at a mean (SD) age of 13.0 (1.8) months (Table 3 and Figure E1). Expiratory images were evaluable for 53 participants. Overall, of the lung fields evaluated in these participants, the median (IQR) percent that was healthy was 98.7 (0.8)%. The inter-observer ICC was excellent for % Bronchiectasis and % Atelectasis, good for % Total airways disease, moderate for % Trapped air, and poor for % Mucus plugging and % Airway wall thickening.
Table 3. PRAGMA-CF scores.
| PRAGMA-CF Subscores | Total N = 60 |
Australia N = 47 |
US N = 13 |
P-value |
|---|---|---|---|---|
| % Bronchiectasis | 0 (0, 0) | 0 (0, 0) | 0 (0, 0.04) | 0.62 |
| % Mucus plugging | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0.78 |
| % Airway wall thickening | 1.1 (0.8, 1.5) | 1.0 (0.7, 1.2) | 1.7 (1.3, 2.0) | 0.0023 |
| % Total airways disease* | 1.1 (0.8, 1.8) | 1.0 (0.8, 1.2) | 1.9 (1.4, 2.1) | 0.0051 |
| % Trapped air** | 3.5 (2.2, 6.0) | 4.2 (2.5, 6.6) | 2.8 (2.0, 3.8) | 0.12 |
| % Atelectasis | 0.2 (0, 0.9) | 0.1 (0, 1.1) | 0.3 (0, 0.3) | 0.76 |
Values are medians (IQRs) with p-values from Wilcoxon non-parametric rank-sum tests.
Sum of % Bronchiectasis, % Mucus plugging, % Airway wall thickening
Expiratory scans available for 53 total, 41 in Australia and 12 in the US.
Correlations between any virus detected, age of first virus detection, or presence of rhinovirus, respiratory syncytial virus (RSV), or parainfluenza with any of the PRAGMA-CF subscores were weak and no differences were found (Table E1). Similarly, there were also no significant differences in mean PRAGMA-CF scores when comparing participants who were ever/never positive for any virus (Figure 1), rhinovirus, RSV, or parainfluenza, or by total number of positive viral swabs (Figure E2) or total number of positive rhinovirus swabs (Figure E3). Adjusting for any potential confounding effect of continent using partial correlations did not substantially change correlations (Table E2). Correlations between PRAGMA-CF subscores and detection of parainfluenza, coronavirus, or human metapneumovirus were also weak (data not shown).
Figure 1.
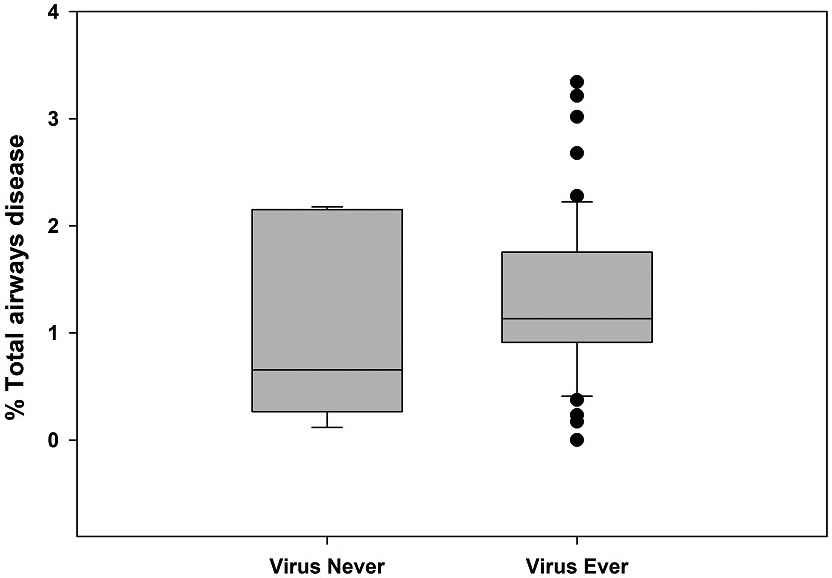
Box and whisker plot for Ever/Never virus positive and (A) % Total airways disease and (B) % Airway wall thickening.
We explored cohort characteristics listed in Table 1 for potential confounders. Median (IQR) % Airway wall thickening was higher in the US, [1.7 (1.3, 2.0)%], than in Australia [1.0 (0.7, 1.2)%], p = 0.0023 (Table 3). This difference also drove a signficiant difference between the US and Australia in % Total airways disease. The median (IQR) percent of the lung fields that was healthy did not differ by continent, p = 0.21. Male sex was associated with a higher mean (SD) number of total positive swabs, compared to female sex [6.0 (5.2) vs 3.6 (3.7), p = 0.036]. Older age was associated with a greater extent of % Airway wall thickening on chest CT, r = 0.26, p = 0.048. There were no associations between PRAGMA-CF subscores and sex or genotype. No associations were identified with detection of respiratory viruses or PRAGMA-CF subscores and smoke exposure (in utero or secondhand), daycare, or breast feeding. Given small differences, we did not further assess any of these characteristics using multivariate regression models. There were some associations between respiratory medications and more frequent respiratory symptoms and higher (i.e., worse) PRAGMA-CF subscores, likely a result of indication bias (data not shown). Sixty-eight participants had respiratory culture results available from BAL fluid obtained at a mean (SD) age of 13.1 (1.9) months. P. aeruginosa and S. aureus (MRSA or MSSA) were only present in 4 participants each. Any classical CF pathogen was present in 11 (16%) participants. There were no associations between BAL respiratory culture results and detection of respiratory viruses.
Respiratory symptoms
The presence of symptoms was reported commonly by parents and physicians: the median (IQR) number of positive reports of symptoms during the 9-12 month study period by parents and/or physicians was 11 (7, 15) (Table 4); resolution of symptoms was not recorded, so the same episode of symptoms may have been reported more than once. Viruses were detected on nasal swabs in a median (IQR) of 2 (0, 4) reports of symptoms, but nasal swabs were collected for less than half of the reports of symptoms. There was a similar median (IQR) number of reports of symptoms in which the nasal swab was negative for any virus, 3 (1, 5), or no swab was obtained, 3 (1, 5).
Table 4. Symptom reporting per participant during the study.
Reports of symptoms include physician documentation at clinic visits as well as parental reports via weekly phone calls. Resolution of symptoms was not recorded; the same episode of symptoms may have been reported more than once.
| Number of Reports of Symptoms Median (IQR) |
Number of Reports of Symptoms Virus Positive Median (IQR) |
Number of Reports of Symptoms Virus Negative Median (IQR) |
Number of Reports of Symptoms with no Nasal Swabs obtained Median (IQR) |
|
|---|---|---|---|---|
| Any symptoms (Parental report + physician document) | 11 (7, 15) | 2 (0, 4) | 3 (1, 5) | 3 (0, 5) |
| Any nasal symptoms | 7 (3, 10) | 2 (0, 3) | 2 (0, 3) | 2 (0, 4) |
| Any cough | 6 (3, 9) | 1 (0, 3) | 2 (0, 3) | 2 (0, 4) |
| Wheeze | 1 (0, 2) | 0 (0, 1) | 0 (0, 1) | 0 (0, 1) |
| Altered breathing | 0 (0, 2) | 0 (0, 1) | 0 (0, 0) | 0 (0, 0) |
| Fever | 0 (0, 1) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| Reduced activity | 1 (0, 2) | 0 (0, 1) | 0 (0, 0) | 0 (0, 0) |
| Nasal symptoms only (no cough, wheeze, or altered breathing) | 4 (2, 6) | 0 (0, 1) | 1 (0, 2) | 2 (0, 4) |
| Upper respiratory tract symptoms (cough, no wheeze or altered breathing) | 5 (3, 7) | 1 (0, 2) | 2 (0, 4) | 4 (2, 6) |
| Lower respiratory tract symptoms (wheezing and/or altered breathing) | 1 (0, 4) | 0 (0, 1) | 0 (0, 1) | 0 (0, 1) |
Correlations between symptoms and PRAGMA-CF subscores were assessed using Spearman correlation coefficients (Table 5), as well as visually (Figure E4). There were no correlations between any symptoms and % Mucus plugging or % Trapped air (data not shown), or between any PRAGMA-CF subscore and fever, parental report of altered breathing, reduced physical activity, or nasal symptoms only. There was a statistically significant correlation between ever having wheezed and % Airway wall thickening. This correlation accounted for a similar correlation between ever having wheezed and % Total airways disease. More frequent encounters with cough was also associated with % Airway wall thickening. More frequent encounters with cough and lower respiratory tract symptoms (wheezing and/or altered breathing) were correlated with % Atelectasis. Though differences existed in the correlations between symptoms and PRAGMA-CF subscores between continents, these comparisons were limited by the relatively small number (n = 13) of US participants.
Table 5. Correlations between reported symptoms and PRAGMA-CF subscores.
| PRAGMA-CF Subscores |
Wheeze – ever |
Cough – per number of encounters |
Any nasal symptoms – per number of encounters |
Lower respiratory tract symptoms – per number of encounters |
|---|---|---|---|---|
| % Bronchiectasis | 0.18 (0.16) | 0.18 (0.18) | 0.21 (0.11) | 0.22 (0.09) |
| % Airway wall thickening | 0.31 (0.02) | 0.25 (0.0495) | 0.11 (0.39) | 0.18 (0.18) |
| % Total airways disease* | 0.29 (0.03) | 0.24 (0.07) | 0.12 (0.35) | 0.21 (0.11) |
| % Atelectasis | 0.23 (0.08) | 0.28 (0.03) | 0.11 (0.41) | 0.30 (0.02) |
Values are Spearman correlation coefficients (p-values). Correlations with p-values <0.05 are in bold
Sum of % Bronchiectasis, % Mucus plugging, % Airway wall thickening
We examined correlations between detected viruses occurring within two weeks of any respiratory symptoms and PRAGMA-CF subscores (Figure E5 and Figure E6) and performed two sensitivity analyses. Correlations between the subset of detected respiratory viruses occurring within two weeks of lower respiratory tract symptoms and PRAGMA-CF subscores were assessed using Spearman correlation coefficients (Table E3). There were statistically significant correlations between having any respiratory virus detected within two weeks of an episode of lower respiratory tract symptoms and % Airway wall thickening, and between having rhinovirus detected within two weeks of an episode of lower respiratory tract symptoms, % Bronchiectasis, and % Airway wall thickening. There was no correlation between the subset of cough, wheeze, or nasal symptoms reported within two weeks of detected respiratory viruses (Table E3). These data were also visualized using a heat map, which did not reveal additional associations (Figure E7).
Discussion
This prospective evaluation of infants with CF failed to show that the detection of respiratory viruses significantly contribute to the presence of structural lung disease assessed at approximately one year of age. Overall, we found minimal structural lung disease in these children. However, we did observe frequent respiratory symptoms and an association with early airway wall thickening (wheeze and cough) and atelectasis (cough and lower respiratory tract symptoms). These findings indicate that respiratory symptoms in infants with CF deserve attention as potential indicators of emerging structural lung disease, and a window of opportunity exists to address respiratory symptoms; thereby potentially preventing the development of structural lung disease.
There are conflicting reports in the literature. In one study, Australian infants born between 2005 and 2012 with CF had a significant burden of structural lung disease (1) , while another cohort born in Great Britain between 2009 and 2012 had only minimal disease burden (16). These differences may be related to changes of care over the past 15 years, including implementation of newborn screening. In addition, 34 of the infants in the current study also participated in a randomized study of early intervention with azithromycin (NCT01270074), which may have contributed to improvements in lung disease compared to historical reports. It is also possible that the current results are due to differences in chest CT scoring systems. Efforts are underway to evaluate chest CT scans from earlier reports of structural lung disease in infants with CF using PRAGMA-CF. Finally, current imaging analysis systems may not be sufficiently sensitive to detect early, subtle signs of CF lung disease. More sensitive and automated scoring systems are in development (17, 18).
Other factors may also contribute to the lack of association between the detection of respiratory viruses and structural lung disease. Although our study encompassed the entire first year of life, it is possible that the effects of viral infections on structural lung disease are cumulative and will only become evident over time.The epidemiology and frequency of respiratory viral infection among people with CF does not differ from that of the general population, but infections tend to be more severe and have a longer duration than in people without CF (2, 3, 19, 20). Our sensitivity analysis may indicate that respiratory viruses that are detected concurrently with lower respiratory tract symptoms are associated with the development of structural lung disease. Viral infections of the respiratory tract alter the inflammatory response, and prospective studies have shown that these infections influence early immune development in the lung (4, 21-24). We have previously shown that a history of detection of respiratory viruses is associated with elevated neutrophil concentrations and bacterial isolates in BAL fluid in early infancy (11). Given that neutrophilic inflammation is a risk factor for structural lung disease in children with CF, (1) it may be that serial CT imaging may eventually show progression of lung disease.
Our findings suggest that respiratory symptoms in infants with CF deserve attention as potential indicators of early structural lung disease, and a window of opportunity exists to address these respiratory symptoms; thereby potentially preventing the development of structural lung disease. The presence of respiratory symptoms may reflect undetected viral infections, but there are other contributors to lung disease, such as airway inflammation, gastroesophageal reflux, cigarette smoke exposure, and air pollution (13, 25-27). More frequent treatment of respiratory symptoms with oral antibiotics in older children with CF is associated with improved outcomes (28). Our study design, which included weekly phone calls in addition to the standard frequency of CF clinic visits, allowed for the detection of these important clinical symptoms, which may have otherwise been missed.
Improvements in lung disease in infants with CF is a positive development for patients, but does limit our ability to assess the impact of respiratory viruses. For example, although PRAGMA-CF subscores have high inter-observer agreement, (14) the ICC for % Mucus plugging and % Airway wall thickening in our young, healthier, study cohort were poor. This is because % Mucus plugging is a rare event in young children, and differentiating between normal airway wall thickness and a thickened airway wall in young children is difficult. In contrast, the ICC for % Atelectasis was excellent. While atelectasis may occur as a result of sedation or anesthesia used during imaging, it is also more common in children with CF with more severe lung disease (29). Despite these limitations, we did observe associations between respiratory symptoms and early airway wall thickening and atelectasis.
The small number of participants and low frequency of structural lung disease precluded the use of more sophisticated regression models and limited our ability to adjust for important confounders. For example, infants followed in Australia were nearly all treated with daily oral antibiotics as respiratory prophylaxis (30). Infants treated with prophylactic antibiotics had reduced bacterial diversity of the upper and lower airways, as well as lower inflammation on bronchoalvolar lavage (30). In the present report, infants from Australia had less % Airway wall thickening compared to the US, had more encounters with abnormal breath sounds, and were slightly younger at the time of the chest CT. Our ability to sort out all of these associations is limited. Similarly, we could not overcome indication bias to assess the potential impact of medication use on preventing early CF lung disease. Resolution of symptoms were not recorded, so it is possible that a single episode of symptoms was reported more than once, i.e., by parents and physicians, or across multiple weekly parental surveillance reports. However, more frequent reports of symptoms may serve as an indicator of severity or persistence, and thus may be a useful indicator of symptoms that warrant additional investigations or treatments. We did not collect information on siblings, a known risk factor for more frequent viral infections.
Our study is further limited by incomplete collection of nasal swabs during symptomatic episodes despite our frequent surveillance methods. Our ability to identify a difference in PRAGMA-CF CT scores based on detection of specific viruses was limited. Detection of rhinovirus occurred in 85% of study participants; this is higher than the 20% of infants who had rhinovirus detected in another recent study (15). It is difficult to determine the clinical significance of rhinovirus detected by sensitive molecular tests, since it may be a non-commensal organism that is present but does not incite clinical manifestations (31-33). Furthermore, viruses typically associated with more severe illnesses, e.g., influenza, were detected infrequently. Using a recently reported symptom classification scheme,(15) we attempted to differentiate between upper and lower respiratory tract symptoms, though our ability to accurately examine any differential effects on progression of lung disease may be limited due to overall mild disease. Past reports have suggested worsening lung disease in infants with CF with lower respiratory tract infections, but not in those with upper respiratory tract infections (2).
In conclusion, our multicenter, binational cohort of infants with CF have minimal structural lung disease at one year of age. We did not find an association between the detection of respiratory viruses and structural lung disease at the end of the first year of life, but frequent respiratory symptoms may be an indication that structural lung disease is progressing.
Supplementary Material
Acknowledgments
The authors would like to thank the families who participated and the research coordinators who collected data and cared for the infants including Miriam Davis, Jane Quante, Nadeene Clarke, Rachel Foong, and Alana Harper. They would like to thank the personnel from the Washington University Special Projects Laboratory for their expertise and contributions.
Sources of Support:
The authors were supported by the National Institutes of Health (NIH) grants, HL116211 (SDD, TWF, SR), National Health and Medical Research Council award, NHMRC1043768 (SR), NHMRC1000896 (SMS), NHMRC1025550 (GLH), the Cystic Fibrosis Foundation grants DAVIS08Y2 (SDD), DESCHA15D0 and DESCHA16D0 (ARD), the Indiana Physician Scientist Award (Eli Lilly) (SDD), and the Royal Children's Hospital Cystic Fibrosis Research Trust (SR).
Abbreviations
- CT
Computed tomography
- ICC
Intraclass correlation coefficient
- PRAGMA-CF
Perth–Rotterdam Annotated Grid Morphometric Analysis for CF
Footnotes
Supplementary material associated with this article can be found, in the online version, at
References
- 1.Sly PD, Gangell CL, Chen L, Ware RS, Ranganathan S, Mott LS, et al. Risk factors for bronchiectasis in children with cystic fibrosis. N Engl J Med. 2013;368(21):1963–70. [DOI] [PubMed] [Google Scholar]
- 2.Hiatt PW, Grace SC, Kozinetz CA, Raboudi SH, Treece DG, Taber LH, et al. Effects of viral lower respiratory tract infection on lung function in infants with cystic fibrosis. Pediatrics. 1999;103(3):619–26. [DOI] [PubMed] [Google Scholar]
- 3.van Ewijk BE, van der Zalm MM, Wolfs TF, Fleer A, Kimpen JL, Wilbrink B, et al. Prevalence and impact of respiratory viral infections in young children with cystic fibrosis: prospective cohort study. Pediatrics. 2008;122(6):1171–6. [DOI] [PubMed] [Google Scholar]
- 4.Wat D, Gelder C, Hibbitts S, Cafferty F, Bowler I, Pierrepoint M, et al. The role of respiratory viruses in cystic fibrosis. J Cyst Fibros. 2008;7(4):320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esther CR Jr., Lin FC, Kerr A, Miller MB, Gilligan PH. Respiratory viruses are associated with common respiratory pathogens in cystic fibrosis. Pediatr Pulmonol. 2014;49(9):926–31. [DOI] [PubMed] [Google Scholar]
- 6.Pribble CG, Black PG, Bosso JA, Turner RB. Clinical manifestations of exacerbations of cystic fibrosis associated with nonbacterial infections. J Pediatr. 1990;117(2 Pt 1):200–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smyth AR, Smyth RL, Tong CY, Hart CA, Heaf DP. Effect of respiratory virus infections including rhinovirus on clinical status in cystic fibrosis. Arch Dis Child. 1995;73(2):117–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colasurdo GN, Fullmer JJ, Elidemir O, Atkins C, Khan AM, Stark JM. Respiratory syncytial virus infection in a murine model of cystic fibrosis. J Med Virol. 2006;78(5):651–8. [DOI] [PubMed] [Google Scholar]
- 9.Van Ewijk BE, Wolfs TF, Aerts PC, Van Kessel KP, Fleer A, Kimpen JL, et al. RSV mediates Pseudomonas aeruginosa binding to cystic fibrosis and normal epithelial cells. Pediatr Res. 2007;61(4):398–403. [DOI] [PubMed] [Google Scholar]
- 10.Hendricks MR, Bomberger JM. Digging through the Obstruction: Insight into the Epithelial Cell Response to Respiratory Virus Infection in Patients with Cystic Fibrosis. J Virol. 2016;90(9):4258–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deschamp AR, Hatch JE, Slaven JE, Gebregziabher N, Storch G, Hall GL, et al. Early respiratory viral infections in infants with cystic fibrosis. J Cyst Fibros. 2019;18(6):844–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stick SM, Brennan S, Murray C, Douglas T, von Ungern-Sternberg BS, Garratt LW, et al. Bronchiectasis in infants and preschool children diagnosed with cystic fibrosis after newborn screening. J Pediatr. 2009;155(5):623–8 e1. [DOI] [PubMed] [Google Scholar]
- 13.Sly PD, Brennan S, Gangell C, de Klerk N, Murray C, Mott L, et al. Lung disease at diagnosis in infants with cystic fibrosis detected by newborn screening. Am J Respir Crit Care Med. 2009;180(2):146–52. [DOI] [PubMed] [Google Scholar]
- 14.Rosenow T, Oudraad MC, Murray CP, Turkovic L, Kuo W, de Bruijne M, et al. PRAGMA-CF. A Quantitative Structural Lung Disease Computed Tomography Outcome in Young Children with Cystic Fibrosis. Am J Respir Crit Care Med. 2015;191(10):1158–65. [DOI] [PubMed] [Google Scholar]
- 15.Korten I, Kieninger E, Klenja S, Mack I, Schlapfer N, Barbani MT, et al. Respiratory viruses in healthy infants and infants with cystic fibrosis: a prospective cohort study. Thorax. 2018;73(1):13–20. [DOI] [PubMed] [Google Scholar]
- 16.Thia LP, Calder A, Stocks J, Bush A, Owens CM, Wallis C, et al. Is chest CT useful in newborn screened infants with cystic fibrosis at 1 year of age? Thorax. 2014;69(4):320–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuo W, de Bruijne M, Petersen J, Nasserinejad K, Ozturk H, Chen Y, et al. Diagnosis of bronchiectasis and airway wall thickening in children with cystic fibrosis: Objective airway-artery quantification. European radiology. 2017;27(11):4680–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuo W, Soffers T, Andrinopoulou ER, Rosenow T, Ranganathan S, Turkovic L, et al. Quantitative assessment of airway dimensions in young children with cystic fibrosis lung disease using chest computed tomography. Pediatr Pulmonol. 2017;52(11):1414–23. [DOI] [PubMed] [Google Scholar]
- 19.Lipuma JJ. The changing microbial epidemiology in cystic fibrosis. Clinical microbiology reviews. 2010;23(2):299–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sutanto EN, Kicic A, Foo CJ, Stevens PT, Mullane D, Knight DA, et al. Innate inflammatory responses of pediatric cystic fibrosis airway epithelial cells: effects of nonviral and viral stimulation. Am J Respir Cell Mol Biol. 2011;44(6):761–7. [DOI] [PubMed] [Google Scholar]
- 21.Openshaw PJ, Yamaguchi Y, Tregoning JS. Childhood infections, the developing immune system, and the origins of asthma. J Allergy Clin Immunol. 2004;114(6):1275–7. [DOI] [PubMed] [Google Scholar]
- 22.Culley FJ, Pollott J, Openshaw PJ. Age at first viral infection determines the pattern of T cell-mediated disease during reinfection in adulthood. J Exp Med. 2002;196(10):1381–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller AL, Bowlin TL, Lukacs NW. Respiratory syncytial virus-induced chemokine production: linking viral replication to chemokine production in vitro and in vivo. J Infect Dis. 2004;189(8):1419–30. [DOI] [PubMed] [Google Scholar]
- 24.Copenhaver CC, Gern JE, Li Z, Shult PA, Rosenthal LA, Mikus LD, et al. Cytokine response patterns, exposure to viruses, and respiratory infections in the first year of life. Am J Respir Crit Care Med. 2004;170(2):175–80. [DOI] [PubMed] [Google Scholar]
- 25.Palm K, Sawicki G, Rosen R. The impact of reflux burden on Pseudomonas positivity in children with cystic fibrosis. Pediatr Pulmonol. 2012;47(6):582–7. [DOI] [PubMed] [Google Scholar]
- 26.Ong T, Schechter M, Yang J, Peng L, Emerson J, Gibson RL, et al. Socioeconomic Status, Smoke Exposure, and Health Outcomes in Young Children With Cystic Fibrosis. Pediatrics. 2017;139(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goss CH, Newsom SA, Schildcrout JS, Sheppard L, Kaufman JD. Effect of ambient air pollution on pulmonary exacerbations and lung function in cystic fibrosis. Am J Respir Crit Care Med. 2004;169(7):816–21. [DOI] [PubMed] [Google Scholar]
- 28.Schechter MS, Regelmann WE, Sawicki GS, Rasouliyan L, VanDevanter DR, Rosenfeld M, et al. Antibiotic treatment of signs and symptoms of pulmonary exacerbations: a comparison by care site. Pediatr Pulmonol. 2015;50(5):431–40. [DOI] [PubMed] [Google Scholar]
- 29.Oudraad MCJ, Kuo W, Rosenow T, Andrinopoulou ER, Stick SM, Tiddens H. Assessment of early lung disease in young children with CF: A comparison between pressure-controlled and free-breathing chest computed tomography. Pediatr Pulmonol. 2020;55(5):1161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pittman JE, Wylie KM, Akers K, Storch GA, Hatch J, Quante J, et al. Association of Antibiotics, Airway Microbiome, and Inflammation in Infants with Cystic Fibrosis. Ann Am Thorac Soc. 2017;14(10):1548–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Benten I, Koopman L, Niesters B, Hop W, van Middelkoop B, de Waal L, et al. Predominance of rhinovirus in the nose of symptomatic and asymptomatic infants. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2003;14(5):363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Zalm MM, van Ewijk BE, Wilbrink B, Uiterwaal CS, Wolfs TF, van der Ent CK. Respiratory pathogens in children with and without respiratory symptoms. J Pediatr. 2009;154(3):396–400, .e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rhedin S, Lindstrand A, Rotzén-Östlund M, Tolfvenstam T, Ohrmalm L, Rinder MR, et al. Clinical utility of PCR for common viruses in acute respiratory illness. Pediatrics. 2014;133(3):e538–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


