Summary.
What is already known about this topic?
Respiratory syncytial virus (RSV) causes substantial morbidity and mortality in older adults. In June 2023, CDC recommended RSV vaccination for adults aged ≥60 years, using shared clinical decision-making and prioritizing those at highest risk for severe disease.
What is added by this report?
Among 1,634 patients aged ≥60 years hospitalized with RSV, 54% were aged ≥75 years, and 17% resided in long-term care facilities (LTCFs). Obesity, chronic obstructive pulmonary disease (COPD), and congestive heart failure (CHF) were common underlying conditions.
What are the implications for public health practice?
Clinicians and patients should consider age (particularly age ≥75 years), LTCF residence, and underlying medical conditions, including COPD and CHF, in shared decision-making regarding RSV vaccination to prevent severe RSV-associated outcomes.
Abstract
Respiratory syncytial virus (RSV) causes substantial morbidity and mortality in older adults. In May 2023, two RSV vaccines were approved for prevention of RSV lower respiratory tract disease in adults aged ≥60 years. In June 2023, CDC recommended RSV vaccination for adults aged ≥60 years, using shared clinical decision-making. Using data from the Respiratory Syncytial Virus–Associated Hospitalization Surveillance Network, a population-based hospitalization surveillance system operating in 12 states, this analysis examined characteristics (including age, underlying medical conditions, and clinical outcomes) of 3,218 adults aged ≥60 years who were hospitalized with laboratory-confirmed RSV infection during July 2022–June 2023. Among a random sample of 1,634 older adult patients with RSV-associated hospitalization, 54.1% were aged ≥75 years, and the most common underlying medical conditions were obesity, chronic obstructive pulmonary disease, congestive heart failure, and diabetes. Severe outcomes occurred in 18.5% (95% CI = 15.9%–21.2%) of hospitalized patients aged ≥60 years. Overall, 17.0% (95% CI = 14.5%–19.7%) of patients with RSV infection were admitted to an intensive care unit, 4.8% (95% CI = 3.5%–6.3%) required mechanical ventilation, and 4.7% (95% CI = 3.6%–6.1%) died; 17.2% (95% CI = 14.9%–19.8%) of all cases occurred in long-term care facility residents. These data highlight the importance of prioritizing those at highest risk for severe RSV disease and suggest that clinicians and patients consider age (particularly age ≥75 years), long-term care facility residence, and underlying medical conditions, including chronic obstructive pulmonary disease and congestive heart failure, in shared clinical decision-making when offering RSV vaccine to adults aged ≥60 years.
Introduction
Respiratory syncytial virus (RSV) causes substantial morbidity and mortality in older adults, resulting in approximately 60,000–160,000 hospitalizations and 6,000–10,000 deaths annually among adults aged ≥65 years (1). In May 2023, the Food and Drug Administration approved two RSV vaccines for prevention of RSV lower respiratory tract disease in adults aged ≥60 years.* In June 2023, CDC recommended RSV vaccination for adults aged ≥60 years using shared clinical decision-making between patient and clinicians;† adults at highest risk for severe RSV disease are most likely to benefit and should be prioritized for vaccination (1). To describe persons who experienced severe RSV disease requiring hospitalization, data from a large, geographically diverse surveillance system were analyzed to identify characteristics of adults aged ≥60 years hospitalized with laboratory-confirmed RSV infection during the 2022–23 respiratory virus season.
Methods
The Respiratory Syncytial Virus–Associated Hospitalization Surveillance Network (RSV-NET)§ conducts population-based surveillance for RSV-associated hospitalizations in approximately 300 hospitals in 58 counties across 12 states,¶ covering approximately 9% of the U.S. population. RSV-NET identifies residents within the network catchment area who are hospitalized with positive RSV tests results for provider-ordered reverse transcription–polymerase chain reaction (RT-PCR) or rapid antigen detection tests during their hospitalization or during the 14 days preceding admission.
Because the 2022–23 RSV season started earlier than did seasons preceding the COVID-19 pandemic (2), this description of demographic characteristics of hospitalized RSV-NET patients includes those hospitalized during July 1, 2022–June 30, 2023. Using previously described methods (3), clinical data were collected by trained surveillance officers from a random sample of medical charts for adults hospitalized during October 1, 2022–April 30, 2023, and stratified by age and site. Sampled data are presented as unweighted case counts and weighted percentages that were weighted for the probability of selection and adjusted to better represent the hospitalized population of the catchment area (3). Age distributions of patients aged ≥60 years who were hospitalized and experienced severe outcomes, defined as intensive care unit (ICU) admission, mechanical ventilation, and in-hospital death, were compared with the overall age distribution of persons ≥60 years in the RSV-NET catchment area. Underlying medical conditions among hospitalized patients and those with severe outcomes were assessed and described. Data were analyzed using SAS survey procedures (version 9.4; SAS Institute). Differences were assessed using chi-square tests; p-values <0.05 were considered statistically significant. This activity was reviewed by CDC, deemed not research, and was conducted consistent with applicable federal law and CDC policy.**
Results
Among 3,218 adults aged ≥60 years with an identified RSV-associated hospitalization during July 2022–June 2023, a total of 1,738 (54.0%) were aged ≥75 years (this group constituted 29.0% of the catchment population of adults aged ≥60 years); 434 (13.5%) and 1,208 (37.5%) of RSV-associated hospitalizations occurred in persons aged 60–64 and ≥80 years, respectively. Overall, 222 (6.9%) patients were Hispanic or Latino (Hispanic), 2,159 (67.2%) patients were non-Hispanic White (White), 496 (15.4%) non-Hispanic Black or African American (Black), 228 (7.1%) non-Hispanic Asian or Pacific Islander (A/PI), 13 (0.4%) non-Hispanic American Indian or Alaska Native (AI/AN) persons, and 100 (3.2%) persons were of other or unknown race. The median patient age was 75 years (IQR = 68–84 years). The median age of White patients (77 years; IQR = 69–85 years) was significantly higher than that of patients who were Black (70 years; IQR = 65–77), Hispanic (74 years; IQR = 66–83 years), or AI/AN (72 years; IQR = 71–75 years) and was lower than that among A/PI (79 years; IQR = 71–87 years) patients (p-value <0.01 for all) (Supplementary Table 1; https://stacks.cdc.gov/view/cdc/133296). The proportion of hospitalized patients whose race was reported as Hispanic or Black decreased with increasing age (p-value <0.01); Black patients accounted for 28.2% of hospitalized patients aged 60–64 years and 8.2% of those aged ≥80 years (Supplementary Table 2; https://stacks.cdc.gov/view/cdc/133297).
Among a random sample of 1,634 adults aged ≥60 years hospitalized during October 2022–April 2023 whose medical charts were reviewed, 54.1% were aged ≥75 years, and 290 (17.2%) were long-term care facility (LTCF) residents, including 175 (26.9%) of those aged ≥80 years (Table). Nearly all patients (1,553 [95%]) had SARS-CoV-2 test results available, among which 39 (2.4%) were positive; 1,587 (97.1%) had influenza testing results, among which 23 (2.2%) were positive.†† Prevalence of severe outcomes was not higher among patients with viral codetections compared with those with RSV alone detected (p>0.5). The median length of hospitalization was 4.1 days (IQR = 2.2–7.6 days). A substantial proportion (332 [18.5%; 95% CI = 15.9%–21.2%]) of patients had at least one severe outcome, including 297 (17.0%) who required ICU admission, 94 (4.8%) who required mechanical ventilation, and 98 (4.7%) who died while hospitalized.
TABLE. Characteristics of a random sample of patients aged ≥60 years hospitalized with laboratory-confirmed respiratory syncytial virus infection* (N = 1,634), stratified by age and site — Respiratory Syncytial Virus–Associated Hospitalization Surveillance Network, 12 states,† October 2022–April 2023.
| Characteristic | Age group, yrs |
|||||||
|---|---|---|---|---|---|---|---|---|
| Overall |
60–69 |
70–79 |
≥80 |
|||||
| No. | Weighted % (95% CI) | No. | Weighted % (95% CI) | No. | Weighted % (95% CI) | No. | Weighted % (95% CI) | |
|
Total, row %
|
1,634
|
100
|
523
|
32
|
554
|
34
|
557
|
34
|
|
Sex
| ||||||||
| Female |
975 |
60.5 (57.0–63.8) |
311 |
60.7 (54.8–66.4) |
317 |
57.5 (51.7–63.1) |
347 |
62.8 (56.7–68.7) |
| Male |
659 |
39.5 (36.2–43.0) |
212 |
39.3 (33.6–45.2) |
237 |
42.5 (36.9–48.3) |
210 |
37.2 (31.3–43.3) |
|
Race and ethnicity
§
| ||||||||
| AI/AN |
7 |
0.3 (0.1–0.7) |
3 |
0.5 (0.1–1.5) |
4 |
0.5 (0.1–1.5) |
0 |
— |
| A/PI, NH |
95 |
7.1 (5.2–9.5) |
31 |
7.3 (3.6–12.8) |
23 |
3.9 (2.3–6.2) |
41 |
9.8 (6.1–14.6) |
| Black or African American, NH |
213 |
13.0 (11.0–15.2) |
111 |
22.4 (18.0–27.4) |
69 |
13.0 (9.6–17.0) |
33 |
5.7 (3.5–8.7) |
| White, NH |
1,181 |
70.2 (67.0–73.3) |
333 |
60.6 (54.6–66.4) |
404 |
70.2 (64.7–75.4) |
444 |
77.6 (72.1–82.4) |
| Hispanic or Latino |
92 |
6.7 (5.0–8.7) |
33 |
7.2 (4.4–11.0) |
33 |
9.1 (5.5–13.9) |
26 |
4.2 (2.4–6.7) |
| All other races¶ |
5 |
0.4 (0.1–1.3) |
1 |
0.1 (0.0–0.9) |
2 |
0.3 (0.0–1.2) |
2 |
0.7 (0.0–3.3) |
| Unknown |
41 |
2.3 (1.6–3.3 |
11 |
1.9 (0.8–3.6) |
19 |
3.0 (1.7–4.9) |
11 |
2.0 (0.9–3.9) |
|
LTCF residence** |
290 |
17.2 (14.9–19.8) |
36 |
5.8 (3.8–8.5) |
79 |
16.1 (12.0–20.9) |
175 |
26.9 (22.2–32.0) |
|
Viral codetection
††
| ||||||||
| SARS-CoV-2 |
39 |
2.4 (1.5–3.6) |
11 |
1.6 (0.7–3.1) |
19 |
3.4 (1.7–5.9) |
9 |
2.2 (0.8–4.9) |
| Influenza |
23 |
2.2 (1.2–3.8) |
7 |
1.9 (0.4–5.0) |
9 |
2.3 (0.6–5.7) |
7 |
2.4 (0.8–5.5) |
|
Hospitalization outcome§§
| ||||||||
| Hospital stay, days, median (IQR) |
4.1 (2.2–7.6) |
— |
4.0 (2.0–7.4) |
— |
4.1 (2.3–7.7) |
— |
4.2 (2.2–7.7) |
— |
| BiPAP/CPAP |
339 |
19.8 (17.3–22.6) |
116 |
23.3 (18.3–28.9) |
131 |
22.6 (18.1–27.6) |
92 |
14.8 (11.2–19.2) |
| High-flow nasal cannula |
80 |
4.3 (3.2–5.7) |
22 |
3.9 (2.1–6.7) |
31 |
5.4 (3.3–8.2) |
27 |
3.7 (2.2–5.8) |
| ≥1 severe outcome¶¶ |
332 |
18.5 (15.9–21.2) |
112 |
20.5 (16.3–25.3) |
124 |
22.3 (17.2–28.1) |
96 |
13.7 (10.2–17.8) |
| ICU admission |
297 |
17.0 (14.5–19.7) |
111 |
20.5 (16.2–25.2) |
110 |
20.6 (15.5–26.4) |
76 |
11.3 (8.0–15.4) |
| Invasive mechanical ventilation |
94 |
4.8 (3.5–6.3) |
42 |
6.4 (4.4–9.0) |
33 |
4.9 (2.9–7.7) |
19 |
3.5 (1.4–6.9) |
| In-hospital death |
98 |
4.7 (3.6–6.1) |
22 |
3.0 (1.7–4.8) |
39 |
5.8 (3.7–8.5) |
37 |
5.2 (3.2–7.9) |
|
Underlying medical condition
| ||||||||
| ≥1 underlying medical condition*** |
1,584 |
95.5 (93.2–97.2) |
501 |
96.3 (94.0–97.9) |
540 |
97.2 (95.1–98.6) |
543 |
93.5 (87.3–97.2) |
| Chronic lung disease |
813 |
49.2 (45.7–52.7) |
290 |
54.4 (48.2–60.4) |
292 |
53.9 (48.0–59.7) |
231 |
41.2 (35.3–47.3) |
| COPD |
552 |
33.7 (30.5–37.0) |
197 |
38.9 (33.1–44.8) |
189 |
34.4 (28.9–40.4) |
166 |
29.1 (24.0–34.6) |
| Asthma |
332 |
19.1 (16.6–21.8) |
134 |
25.4 (20.4–31.0) |
108 |
16.5 (12.9–20.7) |
90 |
16.4 (12.3–21.2) |
| Other††† |
72 |
5.4 (3.8–7.3) |
17 |
3.0 (1.6–5.1) |
34 |
8.4 (5.0–13.1) |
21 |
4.6 (2.4–8.0) |
| Cardiovascular disease |
1,108 |
67.1 (63.7–70.5) |
304 |
55.0 (48.8–61.0) |
371 |
67.5 (61.8–72.8) |
433 |
76.3 (70.0–81.8) |
| CHF§§§ |
545 |
33.2 (30.0–36.5) |
165 |
31.5 (26.1–37.2) |
165 |
29.8 (24.4–35.7) |
215 |
37.4 (31.7–43.4) |
| CAD¶¶¶ |
435 |
26.4 (23.5–29.5) |
109 |
20.9 (16.3–26.3) |
151 |
28.8 (23.7–34.4) |
175 |
28.6 (23.6–34.1) |
| CVA**** |
253 |
13.7 (11.7–15.9) |
55 |
9.6 (6.9–13.0) |
90 |
14.0 (10.7–17.8) |
108 |
16.7 (12.8–21.1) |
| Immunocompromising condition |
292 |
18.6 (16.0–21.4) |
101 |
19.0 (14.5–24.1) |
121 |
22.8 (18.0–28.1) |
70 |
14.8 (10.8–19.6) |
| Diabetes mellitus |
553 |
32.6 (29.5–35.8) |
200 |
38.0 (32.4–43.9) |
195 |
32.7 (27.6–38.1) |
158 |
28.4 (23.1–34.2) |
| Neurologic condition |
439 |
27.3 (24.3–30.5) |
96 |
17.3 (13.4–21.7) |
135 |
25.2 (20.3–30.6) |
208 |
36.8 (31.0–42.9) |
| Dementia†††† |
183 |
12.4 (10.1–15.0) |
7 |
1.0 (0.4–2.4) |
40 |
8.5 (5.5–12.5) |
136 |
24.5 (19.4–30.1) |
| Other |
256 |
14.9 (12.6–17.4) |
89 |
16.2 (12.5–20.6) |
95 |
16.7 (12.6–21.4) |
72 |
12.3 (8.8–16.6) |
| Kidney disorder |
477 |
29.3 (26.3–32.5) |
134 |
24.7 (19.7–30.1) |
156 |
30.0 (24.8–35.5) |
187 |
32.3 (26.9–38.0) |
| Obesity | 572 | 37.8 (34.3–41.4) | 230 | 46.4 (40.3–52.5) | 213 | 42.4 (36.5–48.6) | 129 | 27.1 (21.3–33.6) |
Abbreviations: AI/AN = American Indian or Alaska Native; A/PI = Asian or other Pacific Islander; BiPAP/CPAP = bilevel positive airway pressure/continuous positive airway pressure; CAD = coronary artery disease; CHF = congestive heart failure; COPD = chronic obstructive pulmonary disease; CVA = cerebrovascular accident; ICU = intensive care unit; LTCF = long-term care facility; NH = non-Hispanic.
* Data are from a weighted sample of hospitalized adults with completed medical record abstractions. Sample sizes presented are unweighted with weighted percentages.
† Includes persons admitted to a hospital with an admission date during October 1, 2022–April 30, 2023. Selected counties in California, Colorado, Connecticut, Georgia, Maryland, Michigan, Minnesota, New Mexico, New York, Oregon, Tennessee, and Utah.
§ If ethnicity was unknown, NH ethnicity was assumed.
¶ Includes NH persons reported as other or multiple races.
** LTCF residents include hospitalized adults who were identified as residents of a nursing home or skilled nursing facility, rehabilitation facility, assisted living or residential care, long-term acute care hospital, group or retirement home, or other LTCF upon hospital admission. A free-text field for other types of residences was examined; patients with an LTCF-type residence were also categorized as LTCF residents.
†† Results reported among adults who received testing (as opposed to all hospitalized adults). Because of testing practices, denominators differed among the viral respiratory pathogens based on type of test results available: SARS-CoV-2 = 95% (1,553) and influenza (influenza A, influenza B, and flu [not subtyped]) = 97% (1,587). Among 375 (24.2%) patients who received testing for other viruses, 15 additional viruses were detected: nine rhinoviruses, four seasonal coronaviruses, and two parainfluenza viruses.
§§ Hospitalization outcomes are not mutually exclusive categories, and patients can be included in more than one category.
¶¶ Severe outcome is defined as requiring ICU admission or mechanical ventilation or experiencing in-hospital death.
*** Defined as one or more of the following: chronic lung disease, including asthma; chronic metabolic disease including diabetes mellitus; blood disorder or hemoglobinopathy; cardiovascular disease; neurologic disorder; immunocompromising condition; renal disease; gastrointestinal or liver disease; rheumatologic, autoimmune, or inflammatory condition; obesity; feeding tube dependency; and wheelchair dependency.
††† Other chronic lung diseases include interstitial lung disease, pulmonary fibrosis, restrictive lung disease, sarcoidosis, asbestosis, and chronic respiratory failure including oxygen dependence.
§§§ CHF includes cardiomyopathy, heart failure with preserved ejection fraction, and heart failure with reduced ejection fraction.
¶¶¶ CAD includes history of coronary artery bypass graft and myocardial infarction.
**** CVA includes history of stroke or transient ischemic attack.
†††† Dementia includes Alzheimer disease and other types of dementia.
Almost all sampled patients (1,584; 95.5%) had at least one underlying medical condition, most commonly obesity (37.8%), chronic obstructive pulmonary disease (COPD) (33.7%), congestive heart failure (CHF) (33.2%), and diabetes mellitus (32.6%); 18.6% had an immunocompromising condition (Table) (Figure 1). The following underlying conditions were significantly more prevalent in patients with severe outcomes than in those without severe outcomes: COPD (40.0% versus 32.0%; p = 0.047), other chronic lung diseases excluding COPD and asthma (9.1% versus 4.4%; p = 0.04), and CHF (41.2% versus 31.4%; p = 0.01).
FIGURE 1.
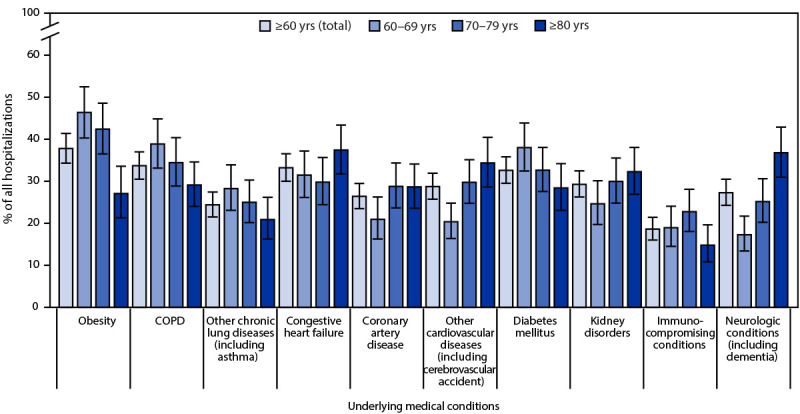
Underlying medical conditions*,† among patients hospitalized with laboratory-confirmed respiratory syncytial virus infection§ — Respiratory Syncytial Virus–Associated Hospitalization Surveillance Network, 12 states,¶ October 2022–April 2023
Abbreviation: COPD = chronic obstructive pulmonary disease.
* With 95% CIs indicated by error bars.
† Congestive heart failure includes cardiomyopathy; coronary artery disease includes history of coronary artery bypass graft and myocardial infarction; cerebrovascular accident includes history of stroke or transient ischemic attack; dementia includes Alzheimer disease and other types of dementia.
§ Data are from a weighted sample of hospitalized adults with completed medical record abstractions. Sample sizes presented are unweighted with weighted percentages.
¶ Select counties in California, Colorado, Connecticut, Georgia, Maryland, Michigan, Minnesota, New Mexico, New York, Oregon, Tennessee, and Utah.
Whereas adults aged 75–79 years and ≥80 years accounted for 12.4% and 16.2% of the catchment area populations, respectively (Figure 2), they accounted for 16.0% (95% CI = 13.5%–18.8%) and 38.1% (95% CI = 34.7%–41.7%) of hospitalizations, 21.2% (95% CI = 13.2%–31.3%) and 25.5% (95% CI = 18.6%–33.5%) of ICU admissions, and 25.6% (95% CI = 14.8%–39%) and 42.1% (95% CI = 29.1%–55.9%) of in-hospital deaths, respectively. Orders to not resuscitate or intubate were in place for 321 (20%) patients, including 211 (35%) patients aged ≥80 years.
FIGURE 2.
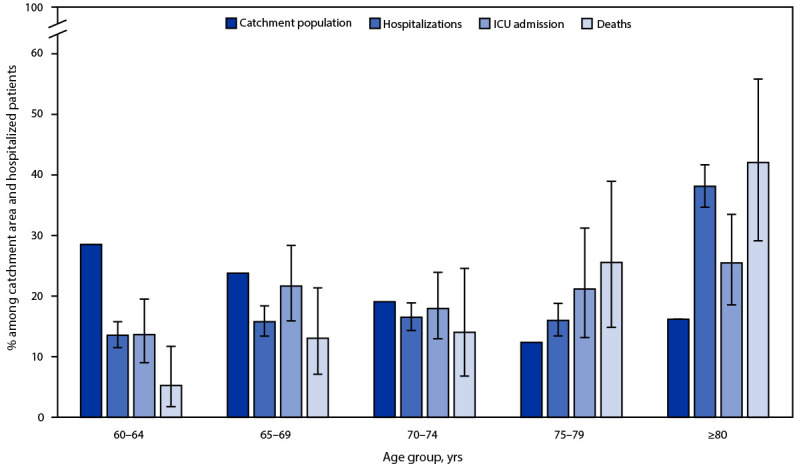
Age distribution* among persons aged ≥60 years residing in the surveillance network catchment area† and among laboratory-confirmed respiratory syncytial virus–associated hospitalizations, intensive care unit admissions, and in-hospital deaths — Respiratory Syncytial Virus–Associated Hospitalization Surveillance Network, 12 states, October 2022–April 2023
Abbreviations: ICU = intensive care unit; RSV = respiratory syncytial virus; RSV-NET = Respiratory Syncytial Virus–Associated Hospitalization Surveillance Network.
* With 95% CIs indicated by error bars.
† The RSV catchment area includes select counties in California, Colorado, Connecticut, Georgia, Maryland, Michigan, Minnesota, New Mexico, New York, Oregon, Tennessee, and Utah. RSV-associated hospitalizations among RSV-NET catchment area residents have hospital admission dates from October 1, 2022 through April 30, 2023. Those with severe RSV disease might be more likely to receive RSV testing; therefore, these data could potentially overestimate the proportion of severe outcomes among hospitalized patients.
Discussion
During July 2022–June 2023, RSV-associated hospitalizations among adults aged ≥60 years in a large population-based surveillance system occurred predominantly among those aged ≥75 years (54%); many (17.2%) of these patients resided in long-term care facilities. The median age of hospitalized AI/AN, Black, and Hispanic patients was lower than that of hospitalized White patients. Viral coinfections reported in RSV-NET were infrequent, despite comprehensive testing for SARS-CoV-2 and influenza, indicating that RSV alone caused substantial morbidity and mortality in this population. Most patients hospitalized with RSV had underlying medical conditions, notably CHF and COPD, which were associated with severe outcomes. Severe outcomes were common, with 17.0% of hospitalized patients requiring ICU admission and nearly 5% dying during their hospitalization.
CDC recommends RSV vaccination for adults aged ≥60 years using shared clinical decision-making, which may consider a patient’s individual risk for severe disease (1). Adults aged ≥75 years were overrepresented among older adult RSV-NET hospitalizations, consistent with previous studies demonstrating increased RSV hospitalization rates with increasing age (4,5). However, the median age of hospitalized older adults who were AI/AN, Black, and Hispanic patients was lower than that for White patients, such that persons in these three groups accounted for a larger proportion of RSV-NET hospitalizations among the younger age groups. This finding likely reflects different age distributions, as well as life expectancy, within the catchment population, as well as potentially higher risk for hospitalization at younger ages resulting from racial and ethnic disparities in underlying medical conditions, access to medical care, and socioeconomic status (6–8).
The prevalence of underlying medical conditions among hospitalized patients was high, including CHF and COPD, both of which were disproportionately associated with severe outcomes in this analysis. Both CHF and COPD have been previously associated with increased RSV hospitalization rates (4,5). One study indicated that older adults with COPD (aged ≥65 years) and CHF (aged 60–79 years) had RSV hospitalization rates that were 3.5–13.4 times and 5.9–7.6 times higher, respectively, than rates among those without those conditions (5). The large proportion of LTCF residents among RSV-NET hospitalizations is also consistent with published literature demonstrating this population’s vulnerability to institutional outbreaks and hospitalization (9).
Limitations
The findings in this report are subject to at least three limitations. First, RSV-associated hospitalizations might have been missed because of test availability or clinician testing practices that limit RSV testing among hospitalized adults. Second, and conversely, severely ill patients might have been more likely to undergo RSV testing, potentially overestimating the proportion of severe outcomes among hospitalized patients. Finally, because RSV-NET covers 9% of the U.S. population, these findings might not be nationally generalizable.
Implications for Public Health Practice
RSV causes substantial morbidity and mortality in adults aged ≥60 years; these findings suggest that advanced age (particularly ≥75 years), LTCF residence, and the presence of underlying medical conditions, including COPD and CHF, might be risk factors for clinicians and patients to consider in shared decision-making regarding RSV vaccination. It is important that special attention be paid to equitable access to vaccines for AI/AN, Black, and Hispanic adults, who were hospitalized for RSV at younger ages than were White adults.
Acknowledgments
Ashley Coates, Brenna Hall, Monica Napoles, Jeremy Roland, Gretchen Rothrock, California Emerging Infections Program; Isaac Armistead, Nina Strayhorn, Colorado Department of Public Health & Environment; Julia Desiato, Noelle Labazzo, Hazhia Sorosindi, Melanie Szajai, Kimberly Yousey-Hindes, Emily Zmek, Connecticut Emerging Infections Program, Yale School of Public Health; Emily Bacon, Meghann Cantey, Rayna Ceaser, Alyssa Clausen, Emily Fawcett, Sydney Hagley-Alexander, Sabrina Hendrick, Johanna Hernandez, Asmith Joseph, Annabel Patterson, Allison Roebling, MaCayla Servais, Emma Grace Turner, Hope Wilson, School of Medicine, Emory University, Georgia Emerging Infections Program, Georgia Department of Public Health, Veterans Affairs Medical Center; Alicia Brooks, Maryland Department of Health; Chloe Brown, Jim Collins, Anna Falkowski, Justin Henderson, Shannon Johnson, Lindsay Leigh, Sanchitha Meda, Elizabeth McCormick, Alyanna Melicor, Val Tellez Nunez, Libby Reeg, Michigan Department of Health & Human Services; Sumaya Alfath, Erica Bye, Kathy Como-Sabetti, Angela Hershberger, Jennifer Zipprich, Minnesota Department of Health; Mark Montoya, Kelly Plymesser, Susan Ropp, Chad Smelser, Daniel Sosin, New Mexico Department of Health; Nancy Eisenberg, Sarah Khanlian, Francesca Pacheco, Yadira Salazar-Sanchez, New Mexico Emerging Infections Program; Bridget Anderson, Kerianne Engesser, Suzanne McGuire, Jemma Rowlands, Nancy Spina, New York State Department of Health; Sophrena Bushey, Christina Felsen, Maria Gaitan, Erin Licherdell, Kevin Popham, Katherine St George, University of Rochester School of Medicine and Dentistry; Kathy Billings, Katie Dyer, Karen Leib, Tiffanie Markus, Terri McMinn, Danielle Ndi, Emmanuel Sackey, Vanderbilt University Medical Center; Ashton Bruno, Amanda Carter, Ryan Chatelain, Melanie Crossland, Andrea George, Rosie Gonzalez, Andrew Haraghey, Mary Hill, Emma Mendez, Kristen P. Olsen, Andrea Price, Isabella Reyes, Courtney H. Sacco, Holly Staten, Ashley Swain, Hafsa Zahid, Salt Lake County Health Department.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Brenda L. Tesini reports honoraria from Merck, unrelated to the current work. No other potential conflicts of interest were disclosed.
Footnotes
GlaxoSmithKline vaccine: https://www.fda.gov/media/167806/download; Pfizer vaccine: https://www.fda.gov/media/168890/download
Unlike age- and risk-based recommendations, for which the default decision should be to vaccinate the patient unless vaccination is contraindicated, shared clinical decision-making recommendations have no default. The decision of whether to vaccinate may consider the best available evidence regarding who would benefit from vaccination; the individual patient’s characteristics, values, and preferences; the vaccine characteristics; and the clinician’s discretion. https://www.cdc.gov/vaccines/acip/acip-scdm-faqs.html
RSV-NET is one of three Respiratory Virus Hospitalization Surveillance Network (RESP-NET) platforms that conduct population-based surveillance for hospitalizations for RSV (RSV-NET), COVID-19 (COVID-NET), and influenza (FluSurv-NET). https://www.cdc.gov/surveillance/resp-net/dashboard.html
Selected counties in California, Colorado, Connecticut, Georgia, Maryland, Michigan, Minnesota, New Mexico, New York, Oregon, Tennessee, and Utah. https://www.cdc.gov/rsv/research/rsv-net/overview-methods.html
45 C.F.R. part 46.102(l)(2), 21 C.F.R. part 56; 42 U.S.C. Sect. 241(d); 5 U.S.C. Sect. 552a; 44 U.S.C. Sect. 3501 et seq.
Among 375 (24.2%) patients receiving testing for other viruses, nine rhinovirus, four seasonal coronavirus, and two parainfluenza virus codetections were identified.
Contributor Information
Pam Daily Kirley, California Emerging Infections Program.
Elizabeth Austin, Colorado Department of Public Health & Environment.
Daewi Kim, Connecticut Emerging Infections Program, Yale School of Public Health.
Chandler Surell, Emory University School of Medicine, Georgia Emerging Infections Program, Georgia Department of Public Health, Atlanta Veterans Affairs Medical Center.
Maya Monroe, Maryland Department of Health.
Lauren Leegwater, Michigan Department of Health & Human Services.
Erica Mumm, Minnesota Department of Health.
Molly Bleecker, University of New Mexico Emerging Infections Program.
Adam Rowe, New York State Department of Health.
Kevin Popham, University of Rochester School of Medicine and Dentistry.
Arilene Novak, Public Health Division, Oregon Health Authority.
William Schaffner, Vanderbilt University Medical Center.
Holly Staten, Salt Lake County Health Department..
References
- 1.Melgar M, Britton A, Roper LE, et al. Use of respiratory syncytial virus vaccines in older adults: recommendations of the Advisory Committee on Immunization Practices—United States, 2023. MMWR Morb Mortal Wkly Rep 2023;72:793–801. 10.15585/mmwr.mm7229a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamid S, Winn A, Parikh R, et al. Seasonality of respiratory syncytial virus—United States, 2017–2023. MMWR Morb Mortal Wkly Rep 2023;72:355–61. 10.15585/mmwr.mm7214a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Halloran A, Whitaker M, Patel K, et al. Developing a sampling methodology for timely reporting of population-based COVID-19-associated hospitalization surveillance in the United States, COVID-NET 2020–2021. Influenza Other Respir Viruses 2023;17:e13089. 10.1111/irv.13089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kujawski SA, Whitaker M, Ritchey MD, et al. Rates of respiratory syncytial virus (RSV)–associated hospitalization among adults with congestive heart failure—United States, 2015–2017. PLoS One 2022;17:e0264890. 10.1371/journal.pone.0264890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Branche AR, Saiman L, Walsh EE, et al. Incidence of respiratory syncytial virus infection among hospitalized adults, 2017–2020. Clin Infect Dis 2022;74:1004–11. 10.1093/cid/ciab595 [DOI] [PubMed] [Google Scholar]
- 6.Caraballo C, Herrin J, Mahajan S, et al. Temporal trends in racial and ethnic disparities in multimorbidity prevalence in the United States, 1999–2018. Am J Med 2022;135:1083–1092.e14. 10.1016/j.amjmed.2022.04.010 [DOI] [PubMed] [Google Scholar]
- 7.Arias E, Tejada-Vera T, Kochanek KD, Ahmad FB. Provisional life expectancy estimates for 2021. NVSS vital statistics rapid release; no 23. Hyattsville, MD: US Department of Health and Human Services, CDC, National Center for Health Statistics; 2022. https://www.cdc.gov/nchs/data/vsrr/vsrr023.pdf
- 8.Tsao CW, Aday AW, Almarzooq ZI, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2023 update: a report from the American Heart Association. Circulation 2023;147:e93–621. 10.1161/CIR.0000000000001123 [DOI] [PubMed] [Google Scholar]
- 9.Childs A, Zullo AR, Joyce NR, et al. The burden of respiratory infections among older adults in long-term care: a systematic review. BMC Geriatr 2019;19:210. 10.1186/s12877-019-1236-6 [DOI] [PMC free article] [PubMed] [Google Scholar]


