The role of albumin in kidney injury remains controversial. While increased albuminuria is recognized as a marker and risk factor of progressive kidney disease, the exact role of urinary albumin remains unknown. Is it a marker of advancing glomerular injury, a marker of proximal tubule injury and dysfunction, or more likely a combination of both? A recent study, using proximal tubule cells on a chip, examined the role of serum proteins or albumin in cell injury.1 Interestingly, the authors found that isolated human albumin from a commercial source did not induce proximal tubule cell injury, but serum proteins did.1 However, it is difficult to know the composition of the commercial albumin used because the company has several albumins available and likely they are all derived from participants without diseases leading to modifications in albumin. Did this albumin contain bound-free fatty acids, the naturally occurring form of serum albumin responsible for the delivery of proximal tubule-free fatty acids, the major energy source for proximal tubules? Were there albumin derivatives, such as glycated, carbamylated, or oxidized albumin, in the commercial albumin? Given albumin's long serum half-life of 19 days, there is significant time for modifications to occur. Albumin modifications are known to cause systemic disease2 and thus may also be responsible for progressive kidney proximal tubule cell injury. Finally, albumin derivatives are known to have an increased rate of production and accumulate in patients with diabetes, with kidney disease, and in high inflammatory states. Thus, the conclusion offered regarding the lack of toxicity of albumin remains premature. However, this does not lessen the described potential toxic effect of other serum constituents described in their article1 (Figure 1).
Figure 1.
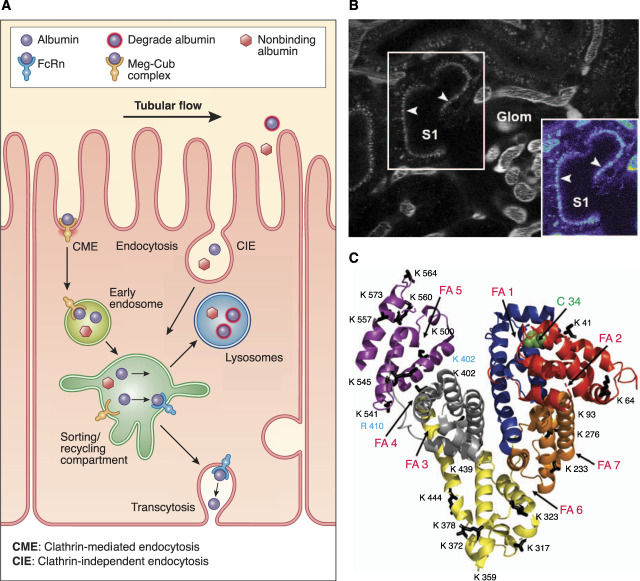
Albumin uptake and processing by the proximal tubule. (A) Albumin filtered across the glomerulus into Bowman's space is reabsorbed by both receptor-mediated endocytosis of clathrin-coated vesicles, mediated by binding to the apical megalin-cubilin receptor, and fluid-phase endocytosis or clathrin-independent endocytosis. After uptake, albumin can be transcytosed, mediated by dissociation from the megalin-cubilin complex and subsequent binding to the neonatal Fc receptor (FcRn) in the acidic endosomal environment, or undergo catabolism by lysosomal trafficking and degradation (nonbinding FcRn albumin). Albumin fragments in the urine result from lysosomal exocytosis of partially degraded albumin or peptide hydrolysis by apical membrane proteases. (B) An intravital two-photon image of an S1 proximal tubule section after infusion of Texas Red-X-rat serum albumin. The inset at the bottom right in the panel shows the S1 segment in a pseudocolor palette to better discern dimmer intensities not readily evident in the black-and-white version. The image, obtained 100 seconds after the start of intravenous albumin infusion, clearly shows small, distinct, early endocytic vesicles lining the subapical region, with a few appearing to have traversed well into the cytosol of the tubular epithelium. (C) Albumin structure (PDB ID 1E78) labeled for many of its various binding moieties and modifications, including carbamylation, glycation, fatty acid, and oxidation. The primary site of both glycation and carbamylation is K525 (blue), whereas R410 is a non-lysine glycated site, and C34 is the oxidation site. Other sites are noted to emphasize the potential effect of modifications/associations on albumin's many interactions. This figure was modified from ref. 2, with permission. CIE, clathrin-independent endocytosis; CME, clathrin-mediated endocytosis.
The recent Chronic Renal Insufficiency Cohort study has refocused on the importance of understanding the role of modified albumins, that is, glycated, carbamylated, and others, in inducing cell injury.3 Over 30 years ago, it was recognized that glycated albumin and nonglycated albumin were metabolized differently, with glycated albumin having a much shorter serum half-life. In addition, a higher percentage of glycated albumin was excreted. These studies led to the hypothesis that processing or editing was distinct for different forms of albumin and further that the site of this editing was likely the proximal tubule.4 The mechanism for these differences was believed to be alterations to albumin's structure or binding sites that altered the physiologic editing events within the kidney. More recent work, by multiple laboratories, has documented direct effects of modified albumins on inflammatory molecules, including reactive oxygen species generation,5,6 messager RNA,7 IL-6,8 receptor for advanced glycation endproducts, NF-κB, PDGF, TGF, EGF, endothelin-1, and on interstitial fibrosis,9 vascular clearance,2 and endocytic pathways in multiple cell types. While many questions remain, albumin's structure is altered by multiple different modifications and ligand binding that can affect its binding to receptors responsible for its metabolism.
We have focused on the mechanism(s) by which albumin modification alters proximal tubule handling, specifically the interactions of modified albumins with cubilin and neonatal Fc receptor (FcRn). The reclamation endocytic pathway for filtered albumin reaching the proximal tubule under physiologic conditions is primarily mediated by the cubilin/megalin receptor and transcytosis by the FcRn.2 The percentage of albumin returned to the vasculature by this mechanism versus being trafficked to the lysosome is dependent on albumin's molecular state. A higher vascular clearance was observed for glycated and carbamylated albumins and other forms having reduced binding to either cubilin or FcRn.2 Interestingly, the glomerular sieving coefficient of these different albumins was unchanged, measured using intravital two-photon microscopy methods.2 This is consistent with the older study supporting the proximal tubule as the site of albumin editing. However, no significant alteration in proximal tubule uptake was seen, although reduced binding to both cubilin and FcRn was quantified. This implies that lysosomal accumulation of modified albumin that does not bind to FcRn could result in heightened production of fibrosis-mediating inflammatory molecules.9
These studies also led to investigations of other mechanisms of albumin uptake and evaluation of modified albumin uptake by other tissues. Interestingly, less carbamylated rat serum albumin was present in the kidney proximal tubules at 16 hours, although distinct accumulations in endothelial/interstitial areas were observed.2 This may reflect the ability of the proximal tubule lysosomes to degrade the carbamylated rat serum albumin more effectively and/or may also be a consequence of modified albumin uptake by endothelial cells. Endothelial FcRn is well established as the primary receptor responsible for salvaging albumin and immunoglobulins from the interstitial space. Other highly specialized endothelial cells involved in filtering the blood are the liver sinusoidal endothelial cells. These highly specialized endocytic cells contain multiple scavenger receptors that have been shown to bind to many modified ligands, including carbamylated LDL and glycated albumin.10 We observed a striking accumulation of carbamylated albumin in these cells and believe it is likely that one or more of these scavenger receptors is responsible for binding and triggering uptake of the carbamylated albumin. Defining which receptor is responsible for this uptake and determining whether this initiates any harmful responses such as has been shown for carbamylated LDL needs further investigation.11
Within the kidney, a pathway has been delineated to transcytose and return albumin to the blood. This reclamation pathway involves the proximal tubule uptake of albumin by clathrin-mediated endocytosis cubulin/megalin receptor complex and/or clathrin-independent endocytosis and release of albumin from this complex in the acidifying conditions within endosomes, followed by binding to the transcytotic albumin receptor FcRn whose affinity for albumin increases under acidic conditions.2 Albumin derivatives that do not bind to FcRn, such as glycated, carbamylated, and oxidized albumin, undergo the default pathway leading to lysosomal accumulation and subsequent catabolism. This reclamation of normal albumin thus acts as a molecular sorting mechanism to preserve functional albumin and eliminate potentially toxic forms of albumin. It also extends the serum half-life of albumin. This proximal tubule molecular sorting pathway is progressively lost as kidney function decreases in CKD and is nonexistent in patients on dialysis with very little or no kidney function, leading to serum accumulation of modified albumins. These conjugated forms of albumin have been demonstrated to be both kidney and systemically toxic to the cardiovascular system and associated with higher mortality in dialysis patients.2,9 Clearly, much remains to be learned about the kidney toxicity of these albumin derivatives and how to minimize the clinical systemic and kidney toxicity they induce.
Acknowledgments
The content of this article reflects the personal experience and views of the author(s) and should not be considered medical advice or recommendation. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or CJASN. Responsibility for the information and views expressed herein lies entirely with the author(s).
Disclosures
B.A. Molitoris reports consultancy for Akebia, AM Pharma, CSL Behring, Ionis, Judo, Novartis, Rayze Bio, Seagan, and Tamarix; ownership interest in FAST BioMedical; research funding from Ionis, NIH-Research Grants, Razebio, and Segen; patents or royalties from FAST BioMedical, GFR and plasma volume measurement, Indiana University C1 and C2 Gentamicin and Soluble Thrombomodulin, and Veterans Administration siRNA for CKD; and advisory or leadership roles for Akebia (SAB), AM Pharma (DSMB comm), CSL Behring (DSMB), FAST BioMedical, and Ionis (DSMB). The remaining author has nothing to disclose.
Funding
B.A. Molitoris is supported by the Division of Diabetes, Endocrinology, and Metabolic Diseases (DK 091623 and 079312).
Author Contributions
Conceptualization: Bruce A. Molitoris, Mark C. Wagner.
Formal analysis: Bruce A. Molitoris.
Funding acquisition: Bruce A. Molitoris.
Project administration: Bruce A. Molitoris.
Writing – original draft: Bruce A. Molitoris, Mark C. Wagner.
Writing – review & editing: Bruce A. Molitoris, Mark C. Wagner.
References
- 1.Lidberg KA, Muthusamy S, Adil M, Mahadeo A, Yang J, Patel RS. Serum protein exposure activates a core regulatory program driving human proximal tubule injury. J Am Soc Nephrol. 2022;33(5):949–965. doi: 10.1681/ASN.2021060751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molitoris BA, Sandoval RM, Yadav SPS, Wagner MC. Albumin uptake and processing by the proximal tubule: physiologic, pathologic and therapeutic implications. Physiol Rev. 2022;102(4):1625–1667. doi: 10.1152/physrev.00014.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalim S, Berg AH, Karumanchi SA, et al. Protein carbamylation and chronic kidney disease progression in the chronic renal insufficiency cohort study. Nephrol Dial Transplant. 2021;37(1):139–147. doi: 10.1093/ndt/gfaa347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kowluru A, Johnson JD, Kowluru R, et al. Suggested mechanism for the selective excretion of glucosylated albumin. The effects of diabetes mellitus and aging on this process and the origins of diabetic microalbuminuria. J Exp Med. 1987;166(5):1259–1279. doi: 10.1084/jem.166.5.1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qi W, Niu J, Qin Q, Qiao Z, Gu Y. Astragaloside IV attenuates glycated albumin-induced epithelial-to- mesenchymal transition by inhibiting oxidative stress in renal proximal tubular cells. Cell Stress Chaperones. 2014;19(1):105–114. doi: 10.1007/s12192-013-0438-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaisson S, Delevallée-Forte C, Touré F, Rieu P, Garnotel R, Gillery P. Carbamylated albumin is a potent inhibitor of polymorphonuclear neutrophil respiratory burst. FEBS Lett. 2007;581(7):1509–1513. doi: 10.1016/j.febslet.2007.03.008 [DOI] [PubMed] [Google Scholar]
- 7.Wang HJ, Lo WY, Lin LJ. Angiotensin-(1-7) decreases glycated albumin-induced endothelial interleukin-6 expression via modulation of miR-146a. Biochem Biophys Res Commun. 2013;430(3):1157–1163. doi: 10.1016/j.bbrc.2012.12.018 [DOI] [PubMed] [Google Scholar]
- 8.Bender O, Weinberg E, Moses O, Nemcovsky CE, Weinreb M. Porphyromonas gingivalis lipopolysaccharide and glycated serum albumin increase the production of several pro-inflammatory molecules in human gingival fibroblasts via NFκB. Arch Oral Biol. 2020;116:104766. doi: 10.1016/j.archoralbio.2020.104766 [DOI] [PubMed] [Google Scholar]
- 9.Gross M-L, Piecha G, Bierhaus A, et al. Glycated and carbamylated albumin are more “nephrotoxic” than unmodified albumin in the amphibian kidney. Am J Physiol Renal Physiol. 2011;301(3):F476–F485. doi: 10.1152/ajprenal.00342.2010 [DOI] [PubMed] [Google Scholar]
- 10.Sørensen KK, McCourt P, Berg T, et al. The scavenger endothelial cell: a new player in homeostasis and immunity. Am J Physiol Regul Integr Comp Physiol. 2012;303(12):R1217–R1230. doi: 10.1152/ajpregu.00686.2011 [DOI] [PubMed] [Google Scholar]
- 11.Alique M, Luna C, Carracedo J, Ramírez R. LDL biochemical modifications: a link between atherosclerosis and aging. Food Nutr Res. 2015;59:29240. doi: 10.3402/fnr.v59.29240 [DOI] [PMC free article] [PubMed] [Google Scholar]