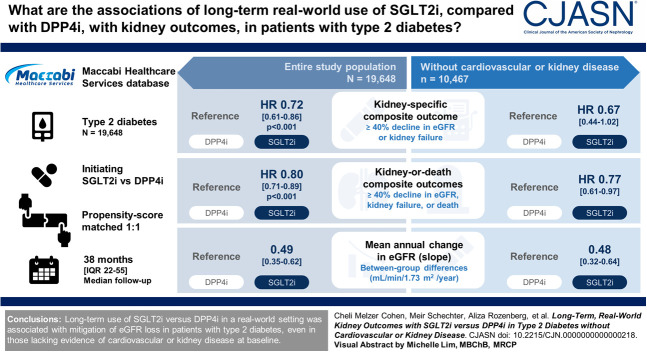
Visual Abstract
Keywords: CKD, diabetes mellitus, SGLT2
Abstract
Background
Contemporary guidelines recommend the use of sodium-glucose cotransporter 2 inhibitors (SGLT2is) independently of glycemic control in patients with type 2 diabetes and those with kidney disease, with heart failure, or at high risk of cardiovascular disease. Using a large Israeli database, we assessed whether long-term use of SGLT2is versus dipeptidyl peptidase 4 inhibitors (DPP4is) is associated with kidney benefits in patients with type 2 diabetes overall and in those without evidence of cardiovascular or kidney disease.
Methods
Patients with type 2 diabetes who initiated SGLT2is or DPP4is between 2015 and 2021 were propensity score-matched (1:1) according to 90 parameters. The kidney-specific composite outcome included confirmed ≥40% decline in eGFR or kidney failure. The kidney-or-death outcome included also all-cause mortality. Risks of outcomes were assessed using Cox proportional hazard regression models. The between-group difference in eGFR slope was also assessed. Analyses were repeated in patients' subgroup lacking evidence of cardiovascular or kidney disease.
Results
Overall, 19,648 propensity score-matched patients were included; 10,467 (53%) did not have evidence of cardiovascular or kidney disease. Median follow-up was 38 months (interquartile range, 22–55). The composite kidney-specific outcome occurred at an event rate of 6.9 versus 9.5 events per 1000 patient-years with SGLT2i versus DPP4i. The respective event rates of the kidney-or-death outcome were 17.7 versus 22.1. Compared with DPP4is, initiation of SGLT2is was associated with a lower risk for the kidney-specific (hazard ratio [HR], 0.72; 95% confidence interval [CI], 0.61 to 0.86; P < 0.001) and kidney-or-death (HR, 0.80; 95% CI, 0.71 to 0.89; P < 0.001) outcomes. The respective HRs (95% CI) in those lacking evidence of cardiovascular or kidney disease were 0.67 (0.44 to 1.02) and 0.77 (0.61 to 0.97). Initiation of SGLT2is versus DPP4is was associated with mitigation of the eGFR slope overall and in those lacking evidence of cardiovascular or kidney disease (mean between-group differences 0.49 [95% CI, 0.35 to 0.62] and 0.48 [95% CI, 0.32 to 0.64] ml/min per 1.73 m2 per year, respectively).
Conclusions
Long-term use of SGLT2is versus DPP4is in a real-world setting was associated with mitigation of eGFR loss in patients with type 2 diabetes, even in those lacking evidence of cardiovascular or kidney disease at baseline.
Introduction
CKD is a common complication of type 2 diabetes, affecting approximately 20%–50% of the patients.1–3 It is commonly defined by a persistent (>3 months) decrease in eGFR, the presence of albuminuria, or other indications of kidney damage.4
Clinical trials in patients with type 2 diabetes at high cardiovascular or kidney risk showed that sodium-glucose cotransporter 2 inhibitors (SGLT2is) improve kidney outcomes.5 On the basis of these studies, current guidelines, including a recent consensus report by the American Diabetes Association and the Kidney Disease Improving Global Outcomes, recommend the use of SGLT2is independent of glycemic control in patients with type 2 diabetes and those with kidney disease, with heart failure, or at high risk of cardiovascular disease.6,7 Real-world studies further assessed the kidney effects of SGLT2is in broader populations of patients with type 2 diabetes that usually have a lower cardiovascular and kidney risk profile than the trial participants. For example, the Kidney Outcomes Associated with Use of SGLT2 Inhibitors in Real-World Clinical Practice (CVD-REAL 3) study demonstrated that initiation of SGLT2is is associated with a lower risk of kidney outcomes and mitigation of eGFR loss in a large, multinational real-world cohort.8 However, this study had a relatively short follow-up (mean 15 months), and the comparators were diverse and included any other glucose-lowering agent. We aimed to test the long-term association between use of SGLT2is, specifically empagliflozin, compared with dipeptidyl peptidase 4 inhibitors (DPP4is), and kidney outcomes, with specific emphasis on populations without evidence of cardiovascular and kidney disease.
Methods
Study Design, Participants, and Follow-Up Definitions
This study was conducted using the Maccabi Healthcare System (MHS) database, which has over 2 million participants with <1% yearly abandon rate. We included adults with type 2 diabetes2 who initiated any of the available SGLT2is in Israel (empagliflozin or dapagliflozin) or any available DPP4is (sitagliptin, linagliptin, vildagliptin, and saxagliptin) between August 2015 and December 2020. In addition, we compared initiators of empagliflozin with any of the available DPP4is. The day of drug dispensation was defined as the index date, and the preceding year was defined as the baseline period. We included patients with at least one eGFR measurement in the baseline period. We excluded patients with type 1 diabetes, eGFR <30 ml/min per 1.73 m2, history of kidney transplantation or dialysis treatment, or those using the comparator drug within the baseline period.
The study was approved by the institutional review board at MHS. Patients' informed consent was not needed because of the deidentified nature of the data.
Definitions of Baseline Variables
Baseline variables were considered as the last measurement within the baseline period. Laboratory and clinical measurements were collected in community settings, and samples were analyzed in the MHS’s certified central laboratory. Creatinine was determined using the Jaffe method, and eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.9 Data of the residential socioeconomic status was obtained from the Israeli Central Bureau of Statistics as previously described.10 The International Classification of Diseases-9 (diagnoses), Anatomical Therapeutic Chemical medications codes, and MHS registries that we used in this study are presented in Supplemental Table 1A.
Follow-Up Definitions
We used intention-to-treat and as-treated follow-up definitions. In the intention-to-treat definition, follow-up continued until the end of data availability, death, or September 2021. The as-treated definition's follow-up criteria were those used for the intention-to-treat definition, added by censoring at study drug discontinuation or the initiation of the comparator drug. In the as-treated definition, follow-up was extended by a grace period of 90 days after the last drug dispensation.
Outcomes and Subgroup Definitions
The study had two main composite outcomes: a kidney-specific outcome composed of confirmed (two consecutive tests) ≥40% reduction from baseline eGFR or new kidney failure and a kidney-or-death outcome that included the components of the kidney-specific outcome or all-cause death. Additional outcomes were confirmed- or single-measurement eGFR reduction of ≥30%, ≥40%, ≥50%, or ≥57% (corresponding to a doubling of serum creatinine), new kidney failure, or all-cause death. We also assessed the risk of a categorical increase in the urine albumin-to-creatinine ratio (UACR) among patients with baseline UACR <300 for the following categories: <30, 30–<300, and ≥300 mg/g. In addition, we assessed the eGFR slopes during follow-up. Safety outcomes were not assessed in this study.
The outcomes were assessed in the whole study population and by the following subgroups: age (younger than 60 years or 60 years and older), sex, years in a diabetes registry (≤10 or >10), presence of cardiovascular disease (defined in the Supplemental Methods and Supplemental Table 1B), HbA1c (<8 or ≥8%), body mass index (<30 or ≥30 kg/m2), eGFR (≥90, 60–<90, or <60 ml/min per 1.73 m2), urine albumin (urine albumin less than detectable levels, UACR (<30, 30–<300, or ≥300 mg/g), and use of angiotensin-converting enzymes or angiotensin II receptor blockers. We specifically focused on a subgroup of patients with low cardiorenal risk defined by lack of all the following: evidence of cardiovascular disease, eGFR <60 ml/min per 1.73 m2, or UACR ≥30 mg/g.
Statistical Analyses
Participants were propensity score-matched in a 1:1 ratio by layers of baseline eGFR (>90, 60–90, and <60 ml/min per 1.73 m2). Matching was performed using greedy matching, as previously described.11 The model included 90 baseline parameters (see the full list of demographics variables, medical history, concomitant medications, and laboratory values in the Supplemental Methods). For the matching process, missing values for continuous variables were imputed by using the mean value per study group. For categorical variables, missing values were classified into a missing category to allow all patients to be matched.
Continuous variables with approximately normal distribution were described as mean and SD, and those with skewed distribution as median and interquartile range (IQR). Categorical variables were described by proportions. Standardized difference was used to assess the baseline differences between the SGLT2i and DPP4i groups, with values >10% considered significant.
Incidence of the categorical outcomes was described using cumulative incidence functions. Cox proportional hazard regression models were applied to estimate hazard ratios (HRs), confidence intervals (CIs), and P values between the treatment arms. Competing risk of death was adjusted in the models using subdistribution hazard function and by using a cause-specific hazard model for cumulative incidence function.12 To test for heterogeneity by subgroups, the Cox models were adjusted to an interaction term of the treatment arm with the baseline subgroups.
Mixed models for repeated measures were used to describe the change in eGFR over time. We defined time windows as follows: every 3 months in the first year, every 6 months between years 1 and 3, and each year thereafter. At each time window, we considered only the eGFR measurement closest to the end of the period for each patient. We calculated the P value of the change in eGFR between groups at each time point, using a mixed-effect model with repeated measures. To compare the change in eGFR over time between the treatment groups using all available eGFR measurements per patient, we calculated an eGFR slope per patient by fitting a linear regression model. We then calculated the mean eGFR slope over time for each group and used a t-test to compare the treatment groups. In this analysis, we did not consider measurements that occurred within 28 days from the index date, to avoid over-representations of the reversible acute eGFR dip on the overall slope estimation.13
Analyses were performed using SAS version 9.4. The analyses did not include formal hypothesis testing, and the P values are presented for descriptive purposes, considering a P value of < 0.05 as statistically significant. No correction for multiple testing was performed.
Role of the Funding Sources
This is an investigator-initiated analysis, and the study was funded by Boehringer Ingelheim. The investigators independently designed the study, analyzed the data, interpreted the findings, and wrote the report. The funder had the right to comment on the manuscript drafts.
Results
Baseline Characteristics
Between August 2015 and December 2020, 16,065 and 23,208 patients initiated SGLT2is and DPP4is, respectively (Supplemental Table 2). After propensity score-matching, there were 19,648 patients included in the analysis, of whom 7688 (39%) were women and 15,050 (77%) did not have evidence of cardiovascular disease. The mean age was 61 years (SD 12); the mean eGFR was 90 ml/min per 1.73 m2 (19); and the median UACR was 12 mg/g (IQR, 0–43). Overall, 10,467 patients (53%) had no evidence of cardiovascular or kidney disease (Table 1). Most (79%) of the participants in the SGLT2i arm initiated empagliflozin, and the rest initiated dapagliflozin. Baseline characteristics after propensity score-matching were balanced between the cohorts (with a standardized difference <10%) (Table 1).
Table 1.
Baseline characteristics of patients initiating any sodium-glucose cotransporter 2 inhibitor or any dipeptidyl peptidase-4 inhibitor in the Maccabi Healthcare System, after propensity score-matching
| Variable | SGLT2is (n=9824) | DPP4is (n=9824) | |
|---|---|---|---|
| Demographics | |||
| Age, yr, mean (SD) | 61 (11) | 61 (12) | |
| Women, n (%) | 3820 (39) | 3868 (39) | |
| SES,a n (%) | 1–3 | 1227 (13) | 1190 (12) |
| 4–5 | 2911 (30) | 2902 (30) | |
| 6–7 | 3442 (35) | 3466 (35) | |
| 8–10 | 2229 (23) | 2246 (23) | |
| Missing | 15 (0.2) | 20 (0.2) | |
| Year of study entry, n (%) | 2015–2016 | 2129 (22) | 2262 (23) |
| 2017–2018 | 3723 (38) | 3245 (33) | |
| 2019–2020 | 3972 (40) | 4344 (44) | |
| Medical history | |||
| Years in diabetes registry, mean (SD) | 8 (6) | 8 (6) | |
| Established cardiovascular disease history,b n (%) | 2333 (24) | 2265 (23) | |
| Without evidence of cardiovascular or kidney disease, n (%) | 5161 (53) | 5306 (54) | |
| Hypertension registry,b n (%) | 6022 (61) | 6028 (61) | |
| History of smoking, n (%) | Current smoker | 1258 (13) | 1225 (13) |
| Past smoker | 285 (3) | 277 (3) | |
| Never smoker | 4298 (44) | 4292 (44) | |
| Unknown | 3983 (41) | 4030 (41) | |
| BMI, kg/m2, mean (SD), (No. patients with a measurement) | 32 (6), (8651) | 32 (6), (8445) | |
| HbA1c (%), mean (SD), (No. patients with a measurement) | 8 (2), (9774) | 8 (2), (9759) | |
| Fasting plasma glucose, mg/dl, mean (SD), (No. patients with a measurement) | 174 (61), (9268) | 175 (62), (9203) | |
| Low-density lipoprotein, mg/dl, mean (SD), (No. patients with a measurement) | 95 (37), (8848) | 93.5 (35), (8729) | |
| High-density lipoprotein, mg/dl, mean (SD), (No. patients with a measurement) | 43 (11), (9743) | 44 (11), (9706) | |
| Systolic BP, mm Hg, mean (SD), (No. patients with a measurement) | 133 (15), (9331) | 133 (16), (9299) | |
| Diastolic BP, mm Hg, mean (SD), (No. patients with a measurement) | 78 (10), (9331) | 79 (10), (9299) | |
| Diabetes medications | |||
| Metformin, n (%) | 9211 (94) | 9290 (95) | |
| Insulin, n (%) | All | 1933 (20) | 1796 (18) |
| Basal insulin | 1785 (18) | 1694 (17) | |
| Fast-acting insulin | 634 (7) | 610 (6) | |
| Sulfonylureas, n (%) | 1389 (14) | 1413 (14) | |
| Glucagon-like peptide-1 receptor agonists, n (%) | 352 (4) | 342 (4) | |
| Thiazolidinediones, n (%) | 346 (4) | 340 (4) | |
| Cardiovascular medications | |||
| RAAS inhibitors, n (%) | 6294 (64) | 6237 (64) | |
| Statin, n (%) | 7613 (78) | 7599 (77) | |
| β blocker, n (%) | 3473 (35) | 3420 (35) | |
| Antihypertensives, n (%) | 6965 (71) | 6932 (71) | |
| Aldosterone antagonist, n (%) | 450 (5) | 395 (4) | |
| Kidney markers | |||
| eGFR, ml/min per 1.73 m2, n (%) | >90 | 5573 (57) | 5573 (57) |
| 60–90 | 3430 (35) | 3430 (35) | |
| 45–60 | 661 (7) | 661 (7) | |
| 30–45 | 160 (2) | 160 (2) | |
| Mean (SD) | 89 (18) | 90 (19) | |
| UACR, mg/g, n (%) | Urine albumin below detectable levels | 3589 (37) | 3620 (37) |
| <15 | 1467 (15) | 1501 (15) | |
| 15–<30 | 1276 (13) | 1296 (13) | |
| 30–<300 | 2311 (24) | 2251 (23) | |
| ≥300 | 611 (6) | 563 (6) | |
| Missing | 570 (6) | 593 (6) | |
| Median (IQR) | 12 (0–45) | 12 (0–42) | |
SGLT2i, sodium-glucose transporter 2 inhibitor; DPP4i, dipeptidyl peptidase 4 inhibitors; SES, socioeconomic status; BMI, body mass index; HbA1c, glycated hemoglobin A1c; RAAS, renin-angiotensin-aldosterone system; UACR, urine albumin-to-creatinine ratio; IQR, interquartile range.
Socioeconomic status was ranked on a 1 (lowest) to 10 (highest) scale. This parameter was categorized into four groups: low (1–3), low-medium (4–5), medium (6–7), and high (8–10).
Based on Maccabi Healthcare Services's validated registries.
Main Outcomes
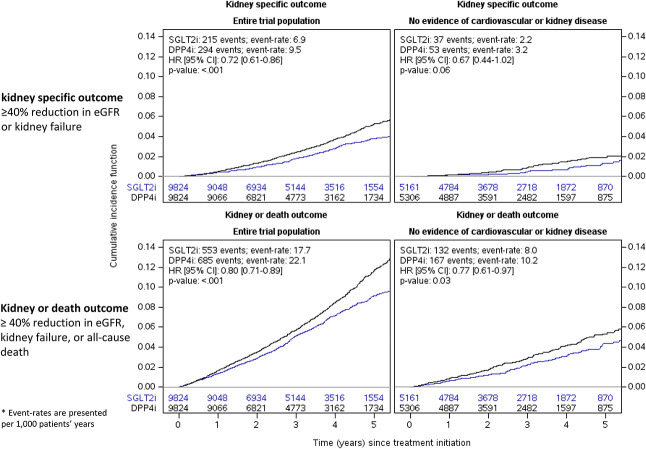
Participants had a median follow-up of 38 months (IQR, 22–55), with an overall duration of 63,145 person-years, and a rate of loss to follow-up of 1% (Supplemental Tables 3 and 4). The composite kidney-specific outcome occurred at an event rate of 6.9 versus 9.5 events per 1000 patient-years with SGLT2i versus DPP4i. The respective event rates of the kidney-or-death outcome were 17.7 versus 22.1. Compared with DPP4is, initiation of SGLT2is was associated with a lower risk for the composite kidney-specific and kidney-or-death outcomes (HR, 0.72; 95% CI, 0.61 to 0.86, and HR, 0.80; 95% CI, 0.72 to 0.89, respectively; P < 0.001) (Figure 1). There was no evidence that these associations varied by most baseline subgroups (P of interaction ranging from 0.11 to 0.87). The association between initiation of SGLT2is versus DPP4is and the kidney-or-death outcome was more pronounced in patients not treated with angiotensin-converting enzymes/angiotensin II receptor blockers compared with those treated (P interaction = 0.04), although reaching separate statistical significance indicated superiority of SGLT2is in each category alone (Supplemental Figure 2). In the subgroup of patients without evidence of cardiovascular or kidney disease, initiation of SGLT2is was associated with a lower risk for the composite kidney-or-death outcome (HR, 0.77; 95% CI, 0.61 to 0.97), but not for the kidney-specific outcome (HR, 0.67; 95% CI, 0.44 to 1.02) (Figure 1 and Supplemental Figure 2). Similar findings were observed in the as-treated analysis (Supplemental Figure 2) or when comparing empagliflozin initiators with DPP4i initiators (Supplemental Tables 5 and 6).
Figure 1.
The association between initiation of sodium-glucose cotransporter 2 inhibitors, compared with dipeptidyl peptidase 4 inhibitors, and the risks of the kidney-specific or kidney-or-death outcomes, in the whole trial population and in patients without evidence of cardiovascular or kidney disease. The kidney-specific composite outcome included confirmed ≥40% eGFR decline or kidney failure. The kidney-or-death outcome included also all-cause mortality. No evidence of cardiovascular or kidney disease was defined by the lack of the following: evidence of cardiovascular disease, eGFR <60 ml/min per 1.73 m2, or urine albumin-to-creatinine ratio ≥30 mg/g. Analysis was performed using the intention-to-treat follow-up. Event rates are presented as events per 1000 patient-years. HR, 95% CI, and P values were assessed using Cox proportional hazard regression models. CI, confidence interval; DPP4i, dipeptidyl peptidase 4 inhibitor; HR, hazard ratio; SGLT2i, sodium-glucose transporter 2 inhibitor.
Other Outcomes
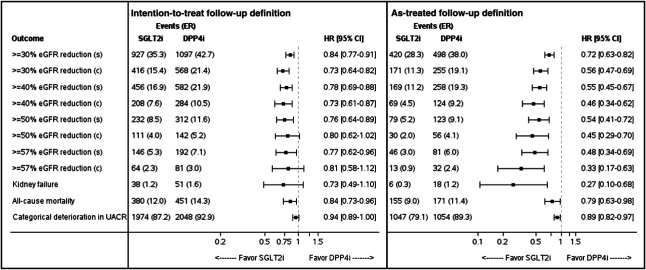
Initiation of SGLT2is versus DPP4is was associated with lower risks of single-measurement ≥30% (HR, 0.84; 95% CI, 0.77 to 0.91), ≥40% (HR, 0.78; 95% CI, 0.69 to 0.88), ≥50% (HR, 0.76; 95% CI, 0.64 to 0.89), and ≥57% (HR, 0.77; 95% CI, 0.62 to 0.96) eGFR loss. Generally, similar findings, although not always reaching statistical significance, were observed for the respective confirmed-measurement eGFR declines (Figure 2). The HRs for a categorical increase in UACR were 0.94 (95% CI, 0.89 to 1.00) and 0.89 (95% CI, 0.82 to 0.97) in the intention-to-treat and as-treated analyses, respectively. Compared with DPP4is, SGLT2i therapy was associated with a lower risk of death from any cause (HR, 0.84; 95% CI, 0.74 to 0.96), but not new kidney failure (38 and 51 patients in SGLT2i and DPP4i groups, respectively; HR, 0.73; 95% CI, 0.49 to 1.10). Generally, similar findings were observed in the as-treated analysis, although reaching statistically significant benefits with SGLT2is versus DPP4is for all tested end points (Figure 2).
Figure 2.
The association between initiation of sodium-glucose cotransporter 2 inhibitors, compared with dipeptidyl peptidase 4 inhibitors, and other kidney outcomes in the intention-to-treat and as-treated follow-up analyses. In the intention-to-treat analysis, follow-up continued until the occurrence of an effectiveness outcome (for the specific end point), end of data availability, death, or September 2021. The as-treated analysis's follow-up criteria were those used by the intention-to-treat analysis, added by censoring at study drug discontinuation or the initiation of the comparator drug. In the as-treated analysis, follow-up was extended by a grace period of 90 days after the last drug dispensation. Outcomes are assessed either as single (s) or confirmed (c) measurements. Categorical increase in UACR was defined using the following categories: <30, 30–<300, and ≥300 mg/g. Event rates are presented per 1000 patient-year. HR, 95% CIs, and P values were assessed using Cox proportional hazard regression models. ER, event rate; UACR, urine albumin-to-creatinine ratio.
eGFR Slopes
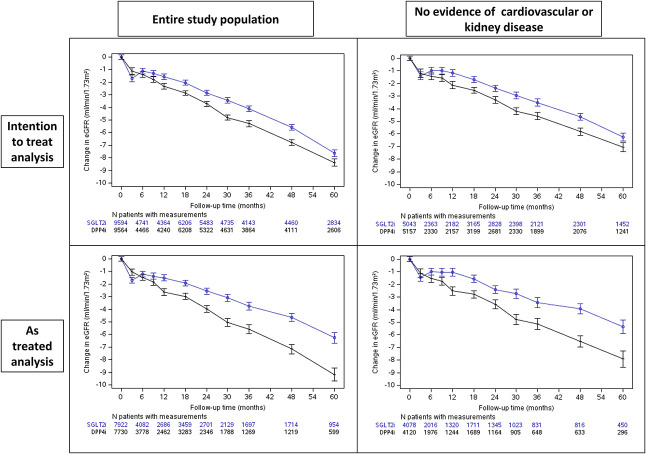
In the SGLT2i group, there was an acute reduction in eGFR followed by stabilization compared with the DPP4i group (Figure 3). Similar findings were observed for the subgroup of patients without evidence of cardiovascular or kidney disease (Figure 3).
Figure 3.
The association between initiation of sodium-glucose cotransporter 2 inhibitors, compared with dipeptidyl peptidase 4 inhibitors, and the change in eGFR over time. In the intention-to-treat analysis, follow-up continued until the occurrence of an effectiveness outcome (for the specific end point), end of data availability, death, or September 2021. The as-treated analysis's follow-up criteria were those used by the intention-to-treat analysis, added by censoring at study drug discontinuation or the initiation of the comparator drug. In the as-treated analysis, follow-up was extended by a grace period of 90 days after the last drug dispensation. A subgroup of patients without evidence of cardiovascular or kidney disease was defined by the lack of the following: evidence of cardiovascular disease, eGFR <60 ml/min per 1.73 m2, or urine albumin-to-creatinine ratio ≥30 mg/g. Mixed models for repeated measures were used to describe the change in eGFR over time. We defined time windows as follows: every 3 months in the first year, every 6 months between years 1 and 3, and each year thereafter. At each time window, we considered only the eGFR measurement closest to the end of the period for each patient. We calculated the P value of the change in eGFR between groups at each time point using a mixed-effect model with repeated measures.
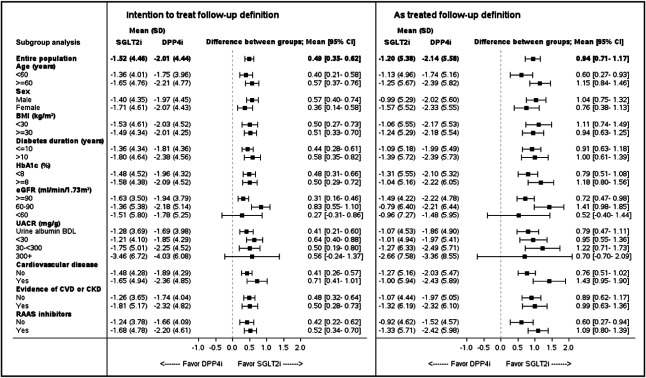
SGLT2i initiation was associated with attenuation of the eGFR slope in both the entire population (between-group difference of 0.49 ml/min per 1.73 m2 per year; 95% CI, 0.35 to 0.62) and those without evidence of cardiovascular or kidney disease (0.48 ml/min per 1.73 m2 per year; 95% CI, 0.32 to 0.64) (Figure 4). Similar findings were observed in the as-treated analysis, where the between-group differences were 0.94 ml/min per 1.73 m2 per year (95% CI, 0.71 to 1.17) and 0.89 ml/min per 1.73 m2 per year (95% CI, 0.62 to 1.17) for the overall population and those without evidence of cardiovascular or kidney disease, respectively. These benefits were observed across most other tested subgroups (Figure 4).
Figure 4.
The association between initiation of sodium-glucose cotransporter 2 inhibitors, compared with dipeptidyl peptidase 4 inhibitors, and annualized eGFR slope overall and by subgroups. In the intention-to-treat analysis, follow-up continued until the occurrence of an effectiveness outcome (for the specific end point), end of data availability, death, or September 2021. The as-treated analysis's follow-up criteria were those used by the intention-to-treat analysis, added by censoring at-study drug discontinuation or the initiation of the comparator drug. In the as-treated analysis, follow-up was extended by a grace period of 90 days after the last drug dispensation. Without evidence of cardiovascular or kidney disease was defined by the lack of the following: evidence of cardiovascular disease, eGFR <60 ml/min per 1.73 m2, or UACR ≥30 mg/g. Mixed models for repeated measures were used to describe the change in eGFR over time. Annualized eGFR slopes were calculated per patient by fitting a linear regression model, followed by calculating the mean eGFR slope for each treatment group, and comparing the groups using a t-test. BMI, body mass index; CVD, cardiovascular disease; HbA1c, glycated hemoglobin A1c; RAAS, renin-angiotensin-aldosterone system.
Discussion
Using over 6 years of real-world data and 63,145 person-years, we compared long-term kidney outcomes between patients with type 2 diabetes who initiated SGLT2is versus DPP4is. Patients treated with SGLT2is had a 28% and 20% relative reduction in the risk of the kidney-specific and kidney-or-death outcomes, respectively, compared with DPP4is, along with attenuation of 0.49 ml/min per 1.73 m2 per year of eGFR slope. Similar effects were observed also in a subgroup of patients without evidence of cardiovascular or kidney disease.
In a previous study, we showed that in patients with type 2 diabetes, initiation of SGLT2is compared with other glucose-lowering agents is associated with a reduction in kidney risk in a real-world setting.11 These trends were observed in subgroups of patients with low kidney risk, although not always reaching statistical significance.11 In this analysis, DPP4is were selected as a comparator because of their neutral effects on eGFR loss.14 Both classes are often used in a similar disease stage, reducing the risk of bias by indication. Like other studies from Scandinavia,15 Hong Kong,16 and the United Kingdom17,18 that used DPP4is as control, we also found that SGLT2is are associated with better kidney outcomes. This analysis adds several novel aspects. First, compared with previous studies,8,15,17,18 we have a relatively long median follow-up (38 months) with a low rate of loss to follow-up, enabling assessment of the long-term effects of the drugs. The between-arm gap of eGFR loss seemed to increase in favor of SGLT2is with longer drug exposure (as-treated analysis). Second, we assessed the risk of an array of commonly used kidney outcomes, including albuminuria progression and eGFR slope over time. Taking advantage of the long-term follow-up, along with comparing eGFR slopes, we were able to find mitigation of eGFR loss, even in patients without evidence of cardiovascular or kidney disease. All in all, these findings indicate that the kidney benefits of SGLT2is are present in various populations treated in the real-world setting.8,11,16–20
eGFR slope is increasingly accepted as a surrogate marker for clinical kidney outcomes. It was shown to have more power to detect kidney benefits in populations with high baseline eGFR.21 A treatment effect of 0.5–1.0 ml/min per 1.73 m2 per year has a strong predictive value for benefits of a composite of ≥57% eGFR loss or kidney failure.22,23 We found attenuation of the eGFR slope by 0.49 and 0.94 ml/min per 1.73 m2 per year in the intention-to-treat and as-treated analyses, respectively. Similar to recent data from a randomized controlled trial,24 SGLT2i therapy was associated with mitigation of eGFR loss in almost all tested subgroups, including those without evidence of cardiovascular or kidney disease.
In cardiovascular outcome trials, SGLT2is improved albuminuria outcomes compared with placebo.25–28 However, there is a paucity of data regarding the effect of SGLT2is on albuminuria in real-world settings. A short study (approximately 6 months) that did not use propensity score-matching found a mild reduction in UACR among 273 patients who received dapagliflozin in the real world.19 Another study found that the association between initiation of SGLT2is versus DPP4is and risk of new microalbuminuria was lower only in an on-treatment analysis but not in the intention-to-treat analysis.16 Similarly, we found a relative reduction (6%–11%) in the risk of categorical albuminuria progression that was statistically significant in the as-treated follow-up and not in the intention-to-treat follow-up. The magnitude of the effect is in line with data from the Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME) and Dapagliflozin Effect on Cardiovascular Events - Thrombolysis in Myocardial Infarction 58 (DECLARE-TIMI 58) trials that found a modest 16% and 14% relative reduction in the risk of single-measurement categorical increase in UACR, respectively.25,27 The lack of statistically significant effects in the intention-to-treat follow-up can be explained by a dilution of the drug effects, irregular sample collection in real-world settings, high interday variability in UACR levels, and the relatively low kidney risk of our study population. In addition, previous data showed that DPP4is also have modest albuminuria-lowering effects.14 Taken together, these analyses fill an important data gap regarding the association between SGLT2i use and albuminuria progression in a real-world setting.
In randomized controlled trials enrolling patients with type 2 diabetes, SGLT2is were shown to improve kidney outcomes compared with placebo. However, these studies included patients with atherosclerotic cardiovascular disease or high cardiovascular risk with limited representation of other populations.29 Thus, there are limited data to support the cardiovascular and kidney protective effects of SGLT2is in lower-risk populations. In a recent post hoc analysis of the DECLARE-TIMI 58 study, dapagliflozin attenuated eGFR slope across all tested populations, including those without evidence of cardiovascular or kidney disease.24 In the current analysis, by combining a large cohort of patients at a relatively low-risk, long-term follow-up, and assessment of eGFR slope, we extend these findings to real-world settings. Taken together, these data suggest that SGLT2is may delay both the onset and progression of kidney disease in patients with type 2 diabetes.
Guidelines recommend using SGLT2is independent of glycemic control in patients with type 2 diabetes and those with kidney disease, with heart failure, or at high risk of cardiovascular disease. There is still a controversy in the field about whether to recommend the use of SGLT2is for kidney protection also in patients with type 2 diabetes and lower cardiovascular risk. This analysis specifically focused on patients without cardiovascular or kidney disease, although some of them were at risk of developing cardiovascular or kidney disease. All in all, our data add to the cumulating findings that support the use of SGLT2is, including empagliflozin, for kidney protection also in patients without evidence of cardiovascular and kidney disease.17,18,24,25
This study has several limitations. It is an observational study. It includes one health care maintenance organization, thus requiring external validation from other cohorts. Some of the baseline characteristics significantly varied between the prematched study cohorts. The propensity score formed comparable cohorts by balancing the baseline characteristics, but at the price of a lower representation of the actual real-world population. For example, 28% and 4% of the initiators of SGLT2is were treated at baseline with glucagon-like peptide-1 receptor agonists before and after matching, respectively. Smoking status was part of the propensity score; however, this variable was available in only around 60% of the participants. Most (79%) of the patients in the SGLT2i arm initiated empagliflozin, and the rest initiated dapagliflozin. Other class members, such as canagliflozin, ertugliflozin, and sotagliflozin, were not tested in this analysis, although in cardiovascular and kidney outcome trials, all tested SGLT2is were shown to have similar kidney protective effects.30 Finally, unlike in a randomized controlled trial setting, where patients usually continue with the index medication, in the real-world setting, patients may stop the index medication or switch to the comparator. We addressed this limitation by using the intention-to-treat and as-treated follow-up definitions—both showing similar findings.
In conclusion, initiation of treatment with SGLT2is versus DPP4is in a real-world setting was associated with continuous long-term mitigation of eGFR loss in patients with type 2 diabetes, even in those without evidence of cardiovascular or kidney disease at baseline.
Supplementary Material
Acknowledgments
This study was supported by Boehringer Ingelheim. Presented is part as poster presentations at the 82nd Scientific Sessions of the American Diabetes Association (ADA), New Orleans, June 3–7, 2022, and at Kidney Week 2022, American Society of Nephrology (ASN) Annual Meeting, Orlando, November 3–6, 2022.
Footnotes
Present address: Regeneron Pharmaceuticals, Inc., Tarrytown, New York.
C.M.C. and M.S. contributed equally to this work.
See related editorial, “Reducing Kidney Disease Burden in Type 2 Diabetes with SGLT2 Inhibitors: Shifting the Goalposts Upstream,” on pages 1119–1121.
Disclosures
G. Chodick reports employment with Maccabi Healthcare Services. A. Karasik reports consultancy for, research funding from, and honoraria from AstraZeneca, Boheringer Ingelheim, and Novo Nordisk, and speakers bureau for Abbott, AstraZeneca, Boheringer Ingelheim, and Novo Nordisk. C. Melzer Cohen reports employment with Maccai Healthcare Services and research funding from Boehringer Ingelheim. O. Mosenzon reports employment with Regeneron Pharmaceuticals, NY, since May 1, 2023; consultancy for AstraZeneca, Bayer, Boehringer Ingelheim, BOL Pharma, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk, and Sanofi; research funding through Hadassah Hebrew University Hospital from AstraZeneca and Novo Nordisk; honoraria from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk, and Sanofi; role on Advisory Boards for AstraZeneca, Bayer, Boehringer Ingelheim, BOL Pharma, Eli Lilly and Company, Merck Sharp & Dohme, Novo Nordisk, and Sanofi; and speakers bureau for AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly and Company, Merck Sharp & Dohme, Novo Nordisk, and Sanofi. D. Rosenzweig is an employee of Boehringer Ingelheim RCV GmbH & Co KG. Boehringer Ingelheim RCV GmbH & Co KG funded the study, which was managed independently, as disclosed by the named investigator. A. Rozenberg reports hourly payment from AstraZeneca through Hadassah Medical Center and from Novo Nordisk. M. Schechter reports honoraria from AstraZeneca and support to participate in a conference from AstraZeneca and Novo Nordisk through Hadassah Medical Center. I. Yanuv reports hourly payment from AstraZeneca through Hadassah Medical Center and from Novo Nordisk. All remaining authors have nothing to disclose.
Funding
This work was supported by Boehringer Ingelheim.
Author Contributions
Conceptualization: Avraham Karasik, Cheli Melzer Cohen, Ofri Mosenzon, Meir Schechter.
Data curation: Gabriel Chodick, Avraham Karasik, Cheli Melzer Cohen.
Formal analysis: Cheli Melzer Cohen.
Funding acquisition: Avraham Karasik, Doron Rosenzweig.
Investigation: Ofri Mosenzon, Aliza Rozenberg, Meir Schechter, Ilan Yanuv.
Methodology: Ofri Mosenzon, Doron Rosenzweig, Aliza Rozenberg, Meir Schechter, Ilan Yanuv.
Project administration: Doron Rosenzweig.
Supervision: Gabriel Chodick, Avraham Karasik, Ofri Mosenzon.
Visualization: Alisa Fishkin, Cheli Melzer Cohen, Meir Schechter, Dvora R. Sehtman-Shachar.
Writing – original draft: Ofri Mosenzon, Meir Schechter.
Writing – review & editing: Gabriel Chodick, Alisa Fishkin, Avraham Karasik, Cheli Melzer Cohen, Doron Rosenzweig, Aliza Rozenberg, Dvora R. Sehtman-Shachar, Ilan Yanuv.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/CJN/B788.
Supplemental Table 1A. Variable definitions on the basis of ICD-9 codes, ATC codes, and Maccabi Healthcare Services's registries.
Supplemental Table 1B. International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes and current procedural terminology used for defining the cardiovascular disease registry.
Supplemental Table 2. Baseline characteristics of any SGLT2i or DPP4i initiators before propensity score-matching.
Supplemental Table 3. Censoring causes during the trials per each cohort, follow-up definition, and by treatment arm.
Supplemental Table 4. Follow-up durations in the intention-to-treat and as-treated analyses.
Supplemental Table 5. Baseline characteristics before and after propensity score-matching with empagliflozin versus any DPP4i.
Supplemental Table 6. The association between initiation of empagliflozin, compared with any DPP4i, and the risks of the kidney-specific or kidney-or-death outcomes, in the whole trial population.
Supplemental Figure 1. A CONSORT diagram describing the formation of the study population.
Supplemental Figure 2. The association between initiation of SGLT2is, compared with DPP4is, and the risk of (A) the kidney-specific or (B) kidney-or-death outcomes, by subgroups.
References
- 1.Afkarian M Zelnick LR Hall YN, et al. Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014. JAMA. 2016;316(6):602–610. doi: 10.1001/jama.2016.10924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schechter M Melzer Cohen C Yanuv I, et al. Epidemiology of the diabetes-cardio-renal spectrum: a cross-sectional report of 1.4 million adults. Cardiovasc Diabetol. 2022;21(1):104. doi: 10.1186/s12933-022-01521-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoogeveen EK. The epidemiology of diabetic kidney disease. Kidney Dial. 2022;2(3):433–442. doi: 10.3390/kidneydial2030038 [DOI] [Google Scholar]
- 4.Kidney Disease Improving Global Outcomes (KDIGO). KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):136–150. doi: 10.1038/kisup.2012.64 [DOI] [PubMed] [Google Scholar]
- 5.McGuire DK Shih WJ Cosentino F, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol. 2021;6(2):148–158. doi: 10.1001/jamacardio.2020.4511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies MJ Aroda VR Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45(11):2753–2786. doi: 10.2337/dci22-0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Boer IH Khunti K Sadusky T, et al. Diabetes management in chronic kidney disease: a consensus report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Diabetes Care. 2022;102(5):974–989. doi: 10.1016/j.kint.2022.08.012 [DOI] [PubMed] [Google Scholar]
- 8.Heerspink HJL Karasik A Thuresson M, et al. Kidney outcomes associated with use of SGLT2 inhibitors in real-world clinical practice (CVD-REAL 3): a multinational observational cohort study. Lancet Diabetes Endocrinol. 2020;8(1):27–35. doi: 10.1016/s2213-8587(19)30384-5 [DOI] [PubMed] [Google Scholar]
- 9.Schwandt A Denkinger M Fasching P, et al. Comparison of MDRD, CKD-EPI, and Cockcroft-Gault equation in relation to measured glomerular filtration rate among a large cohort with diabetes. J Diabetes Complications. 2017;31(9):1376–1383. doi: 10.1016/j.jdiacomp.2017.06.016 [DOI] [PubMed] [Google Scholar]
- 10.State of Israel Central Bureau of Statistics. Characterization and Classification of Geographical Units by the Socio-Economic Level of the Population, 2017. https://www.cbs.gov.il/en/publications/Pages/2021/socio-2017-e.aspx [Google Scholar]
- 11.Schechter M Melzer-Cohen C Rozenberg A, et al. Cardiorenal outcomes with sodium/glucose cotransporter-2 inhibitors in patients with type 2 diabetes and low kidney risk: real world evidence. Cardiovasc Diabetol. 2021;20(1):169. doi: 10.1186/s12933-021-01362-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133(6):601–609. doi: 10.1161/circulationaha.115.017719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wanner C Heerspink HJL Zinman B, et al. Empagliflozin and kidney function decline in patients with type 2 diabetes: a slope analysis from the EMPA-REG outcome trial. J Am Soc Nephrol. 2018;29(11):2755–2769. doi: 10.1681/ASN.2018010103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mosenzon O Leibowitz G Bhatt DL, et al. Effect of saxagliptin on renal outcomes in the SAVOR-TIMI 53 trial. Diabetes Care. 2017;40(1):69–76. doi: 10.2337/dc16-0621 [DOI] [PubMed] [Google Scholar]
- 15.Pasternak B Wintzell V Melbye M, et al. Use of sodium-glucose co-transporter 2 inhibitors and risk of serious renal events: Scandinavian cohort study. BMJ. 2020;369:m1186. doi: 10.1136/bmj.m1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Au PCM, Tan KCB, Cheung BMY, Wong ICK, Li H-L, Cheung C-L. Association between SGLT2 inhibitors vs DPP4 inhibitors and renal outcomes among patients with type 2 diabetes. J Clin Endocrinol Metab. 2022;107(7):e2962–e2970. doi: 10.1210/clinem/dgac164 [DOI] [PubMed] [Google Scholar]
- 17.Idris I Zhang R Mamza JB, et al. Lower risk of hospitalization for heart failure, kidney disease and death with sodium-glucose co-transporter-2 inhibitors compared with dipeptidyl peptidase-4 inhibitors in type 2 diabetes regardless of prior cardiovascular or kidney disease: a retrospective cohort study in UK primary care. Diabetes Obes Metab. 2021;23(10):2207–2214. doi: 10.1111/dom.14437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Idris I Zhang R Mamza JB, et al. Significant reduction in chronic kidney disease progression with sodium-glucose cotransporter-2 inhibitors compared to dipeptidyl peptidase-4 inhibitors in adults with type 2 diabetes in a UK clinical setting: an observational outcomes study based on international guidelines for kidney disease. Diabetes Obes Metab. 2022;24(11):2138–2147. doi: 10.1111/dom.14799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fadini GP Solini A Manca ML, et al. Effectiveness of dapagliflozin versus comparators on renal endpoints in the real world: a multicentre retrospective study. Diabetes Obes Metab. 2019;21(2):252–260. doi: 10.1111/dom.13508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fadini GP, Del Prato S, Avogaro A, Solini A. Challenges and opportunities in real-world evidence on the renal effects of sodium-glucose cotransporter-2 inhibitors. Diabetes Obes Metab. 2022;24(2):177–186. doi: 10.1111/dom.14599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greene T Ying J Vonesh EF, et al. Performance of GFR slope as a surrogate end point for kidney disease progression in clinical trials: a statistical simulation. J Am Soc Nephrol. 2019;30(9):1756–1769. doi: 10.1681/ASN.2019010009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inker LA Heerspink HJL Tighiouart H, et al. GFR slope as a surrogate end point for kidney disease progression in clinical trials: a meta-analysis of treatment effects of randomized controlled trials. J Am Soc Nephrol. 2019;30(9):1735–1745. doi: 10.1681/ASN.2019010007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grams ME Sang Y Ballew SH, et al. Evaluating glomerular filtration rate slope as a surrogate end point for ESKD in clinical trials: an individual participant meta-analysis of observational data. J Am Soc Nephrol. 2019;30(9):1746–1755. doi: 10.1681/ASN.2019010008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mosenzon O Raz I Wiviott SD, et al. Dapagliflozin and prevention of kidney disease among patients with type 2 diabetes: post hoc analyses from the DECLARE-TIMI 58 trial. Diabetes Care. 2022;45(10):2350–2359. doi: 10.2337/dc22-0382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mosenzon O Wiviott SD Heerspink HJL, et al. The effect of dapagliflozin on albuminuria in DECLARE-TIMI 58. Diabetes Care. 2021;44(8):1805–1815. doi: 10.2337/dc21-0076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perkovic V de Zeeuw D Mahaffey KW, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018;6(9):691–704. doi: 10.1016/s2213-8587(18)30141-4 [DOI] [PubMed] [Google Scholar]
- 27.Cherney DZI Zinman B Inzucchi SE, et al. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from the EMPA-REG OUTCOME randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5(8):610–621. doi: 10.1016/s2213-8587(17)30182-1 [DOI] [PubMed] [Google Scholar]
- 28.Cherney DZI Charbonnel B Cosentino F, et al. Effects of ertugliflozin on kidney composite outcomes, renal function and albuminuria in patients with type 2 diabetes mellitus: an analysis from the randomised VERTIS CV trial. Diabetologia. 2021;64(6):1256–1267. doi: 10.1007/s00125-021-05407-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birkeland KI Bodegard J Norhammar A, et al. How representative of a general type 2 diabetes population are patients included in cardiovascular outcome trials with SGLT2 inhibitors? A large European observational study. Diabetes Obes Metab. 2019;21(4):968–974. doi: 10.1111/dom.13612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nuffield Department of Population Health Renal Studies Group, SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists' Consortium. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet. 2022;400(10365):1788–1801. doi: 10.1016/S0140-6736(22)02074-8 [DOI] [PMC free article] [PubMed] [Google Scholar]