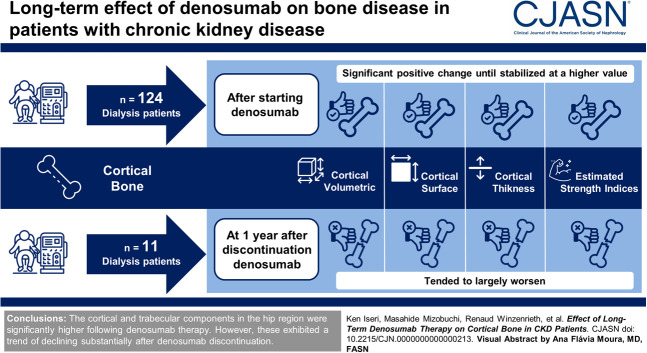
Visual Abstract
Keywords: chronic hemodialysis; mineral metabolism; bones, stones, and mineral metabolism
Abstract
Background
The effect of long-term denosumab therapy and of denosumab discontinuation on the cortical bone of the hip regions in dialysis patients has not been studied.
Methods
This retrospective study investigated the cortical and trabecular compartments and estimated strength indices of the hip region, obtained using 3D-SHAPER software, after a maximum of 5 years of denosumab therapy in 124 dialysis patients. A Wilcoxon signed-rank test was used to identify the differences in each parameter before and after denosumab initiation. Similarly, we investigated the changes in these parameters after denosumab discontinuation in 11 dialysis patients.
Results
Integral and trabecular volumetric bone mineral densities (BMD) were significantly lower at the start of denosumab therapy than those in 1 year before denosumab initiation. After starting denosumab, areal BMD (median change +7.7% [interquartile range (IQR), +4.6 to +10.6]), cortical volumetric BMD (median change +3.4% [IQR, +1.0 to +4.7]), cortical surface BMD (median change +7.1% [IQR, +3.4 to +9.4]), and cortical thickness (median change +3.2% [IQR, +1.8 to +4.9]) showed a significantly higher trend for 3.5 years, which then stabilized at a higher value compared with baseline. A similar trend in the trabecular volumetric BMD (median change +9.8% [IQR, +3.8 to +15.7]) was observed over 2.5 years, with a higher value maintained thereafter. The whole area of the hip region improved after denosumab therapy. Similar trajectories were also found in the estimated strength indices. Conversely, at 1 year after denosumab discontinuation, these 3D parameters and estimated strength indices tended to largely worsen. The lateral aspect of the greater trochanter was the most pronounced location showing volumetric BMD loss.
Conclusions
The BMD of both cortical and trabecular components in the hip region was significantly higher after starting denosumab therapy. However, these measurements exhibited a trend of declining substantially after the discontinuation of denosumab.
Introduction
Bone disorders in patients with CKD have different pathophysiology and features compared with patients with primary osteoporosis.1–6 Although trabecular bone is more rapidly lost than cortical bone in patients with primary osteoporosis initially, rapid cortical bone loss compared with trabecular bone is a unique characteristic in patients with CKD.7,8 Epidemiologic studies have shown that dialysis patients have a higher hip fracture risk than nondialyzed CKD patients and the general population.9–12 Hip fracture is the most prevalent type of fracture in dialysis patients, and subsequent high mortality is well described.11 Therefore, preventing hip fractures is crucial for achieving better clinical outcomes. Despite the importance of fracture prevention, there is currently no consensus on the optimal treatment strategy.
Denosumab is recommended as one of the initial treatments for patients with primary osteoporosis.13 Among dialysis patients, denosumab increased the bone mineral density (BMD) in the spine and hip region.14–16 However, the long-term (>2 years) effect of denosumab therapy on hip BMD, cortical bone, and trabecular bone is unknown. A better understanding of the long-term changes in cortical and trabecular compartments in the hip region before and after denosumab therapy is warranted to prevent fractures and improve clinical outcomes.
Recently, novel methods have been proposed to analyze the bone in 3D using hip dual-energy X-ray absorptiometry (DXA) scans. 3D-DXA methods have been validated against quantitative computed tomography for measuring cortical and trabecular volumetric BMD and geometrical parameters.17,18 3D-DXA has been used to analyze the effect of osteoporosis drug treatments, including denosumab.19–22
This study, which focuses on dialysis patients, aimed to (1) investigate the long-term effect of denosumab therapy on the cortical and trabecular compartments and estimated strength indices in the hip regions; (2) assess the effect of denosumab discontinuation on the cortical and trabecular compartments and estimated strength indices in the hip regions; and (3) analyze the 3D spatial distribution of the changes in cortical bone before and after denosumab treatments and after denosumab discontinuation.
Methods
I. Long-Term Effect of Denosumab on the Cortical and Trabecular Compartments and Estimated Strength Indices in the Hip Regions
The study protocol was approved by the Showa University Ethics Committee (H25-40) and was conducted in accordance with the principles of the Declaration of Helsinki. Informed consent was obtained via an opt-out approach.
This single-center retrospective cohort study was conducted at Sekishin-kai Kawasaki Clinic, where annual routine DXA scans were provided to almost all patients for screening fracture risk. Among 197 patients who met the criteria for osteoporosis, denosumab initiation was not commenced in 37 patients due to some factors: patient refusal, decreased Activities of Daily Living, and low medication adherence, and the remaining 160 patients began denosumab therapy.23 The inclusion criteria were dialysis patients (18 years or older) starting denosumab. The exclusion criteria were as follows: (1) patients who had not undergone DXA examination at baseline and (2) patients who have not received any DXA examination at follow-up. Among the 160 dialysis patients starting denosumab therapy (60 mg subcutaneously every 6 months) from January 2016 to December 2020 at Sekishin-kai Kawasaki Clinic and were followed up until October 2021, 36 patients who did not undergo DXA measurements at baseline or follow-up were excluded. Thus, the remaining 124 patients who were assessed by DXA at baseline and follow-up were included (Supplemental Figure 1). We also included DXA data obtained 6 and 12 months before starting denosumab therapy, if available. The median interval (in month) from the starting denosumab therapy to each time point and the number of participants are presented in Supplemental Table 1. DXA scans were performed using a Discovery-A scanner (Hologic Inc., Waltham, MA) at Kawasaki Clinic according to manufacturer's recommendations. Chronic kidney disease–mineral and bone disorder (CKD-MBD) was managed according to the Japanese Society for Dialysis Therapy guidelines.24 Medical treatments used to manage the condition of all patients were similar. We collected demographic and laboratory data at baseline. 3D-DXA analysis was performed using 3D-SHAPER software (v2.11, 3D-Shaper Medical, Barcelona, Spain). In brief, 3D-DXA methods use a 3D statistical shape and density model of the proximal femur that is built from a database of quantitative computed tomography scans. The statistical model is registered onto the DXA scan to obtain a patient-specific 3D model of the proximal femur, including its 3D geometry and bone density distribution. Details on 3D-DXA methods and validation against quantitative computed tomography can be found elsewhere.17,18 3D-SHAPER software provides the following measurements at the hip region.
Cortical compartments at the total hip region are cortical volumetric BMD (mg/cm3), cortical thickness (mm), and cortical surface BMD (mg/cm2), and trabecular compartment at the total hip region is trabecular volumetric BMD (mg/cm3).
Strength indices at the neck region are cross-sectional area (cm2), cross-sectional moment of inertia (cm4), section modulus (cm3), and buckling ratio.
II. The Effect of Denosumab Discontinuation on the Cortical and Trabecular Compartments and Estimated Strength Indices in the Hip Regions
Among the 124 dialysis patients, 12 dialysis patients discontinued denosumab therapy after a median of 2.5-year (interquartile range [IQR], 1.6–2.5) denosumab therapy because of various reasons, such as patient and dentist requests. We excluded one patient who did not undergo DXA measurements at follow-up after discontinuing denosumab. Considering the administration interval of denosumab, we referred to 6 months after the last denosumab injection as the baseline for this (II.) analysis. Therefore, we included the remaining 11 patients who were assessed by central DXA at baseline (×6 months after the last denosumab injection) and at follow-up (Supplemental Figure 2).
III. 3D Spatial Distribution of the Changes in Cortical Bone Before and After Denosumab Treatments and After Denosumab Discontinuation
To analyze the anatomical distribution of changes in bone structure, we used the 3D-DXA data to create one average 3D model at each time point using image registration techniques. The average 3D models obtained at each follow-up time points were compared with the baseline to assess the anatomical distribution of the changes in bone structure. Changes in cortical surface BMD, volumetric BMD, and thickness were displayed at the periosteal surface of the femur using 3D visualizations. Changes in cortical and trabecular volumetric BMD were displayed using cross-sectional images.
Before and After Denosumab Therapy
The purpose of this analysis was to determine which areas of the hip region were affected by the natural course of dialysis therapy and how denosumab treatment altered that effect. Therefore, we defined the baseline as 1 year before starting denosumab therapy and investigated from −1 year before denosumab therapy to 4 years after denosumab therapy. Among the 124 dialysis patients who started with denosumab therapy, 39 were included in this analysis, considering the following time points: baseline (1 year before starting denosumab therapy) and 0 (starting denosumab), 6, 12, 18, 24, 30, 36, 42, and 48 months.
After Denosumab Discontinuation
We aimed to determine which locations in the hip region were affected after discontinuing denosumab therapy. Therefore, we referred to 6 months after the last denosumab injection as the baseline, similar to the (II.) analysis. We analyzed 11 patients who discontinued denosumab therapy (Supplemental Figure 2).
Statistical Analyses
The demographic data are presented as median values (IQR) for continuous variables, unless otherwise noted, and as numbers (percentage) for categorical variables. The change in parameters at each time point was calculated as the difference in parameters between the two time points, baseline measurement, and each measurement of interest. The differences between two different time points were examined by using the Wilcoxon rank-sum test. We applied a complete case analysis because we did not take into account the missing data (mainly coming from loss of follow-up). Furthermore, we conducted a sensitivity analysis to investigate the changes in the cortical and trabecular compartments and estimated strength indices in the hip regions from baseline to a maximum of 5 years of denosumab therapy, using mixed model analysis (treating between-patient variability as a random effect and considering the repeated measurements as a random slope). Similar analyses were performed for investigating the changes in biochemical markers and drug doses over time. Furthermore, we used mixed model to investigate the associations between biochemical markers and medications at baseline and DXA parameters (areal BMD [aBMD], trabecular volumetric BMD, and cortical surface BMD) over time after adjusting age and sex.
For all tests, the level of significance was set at P < 0.05. Statistical analyses were performed using Stata 16.0 (StataCorp LLC, College Station, TX) and SAS version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Characteristics and Clinical Parameters
Table 1 presents the patients' characteristics and clinical parameters. The median age was 71 years (IQR, 64–76), and 54% of the patients were male. The median dialysis duration was 85 months (IQR, 36–166). The most common cause of kidney failure was diabetic kidney disease (37%). Twelve patients had a history of hip fractures. The median T-scores of the whole body and femoral neck were −2.4 (IQR, −3.5 to −1.1) and −2.9 (IQR, −3.2 to −2.6), respectively. The serum calcium, phosphate, and intact-parathyroid hormone (i-PTH) levels were within the target range recommended.24
Table 1.
Baseline clinical and biochemical characteristics of 124 dialysis patients
| Characteristics | Total Patients (n=124) |
|---|---|
| Demography and clinical characteristics | |
| Age, yr | 71 (64–76) |
| Male patients, n (%) | 67 (54) |
| Hemodialysis therapy, n (%) | 122 (98) |
| Dialysis vintage, mo | 85 (36–166) |
| Cause of kidney failure, n (%) | |
| Chronic glomerulonephritis | 34 (27) |
| Diabetic kidney disease | 46 (37) |
| Benign nephrosclerosis | 36 (29) |
| ADPKD | 5 (4) |
| Other | 3 (2) |
| Smoker, n (%) (n=123) | 20 (16) |
| Diabetes mellitus, n (%) (n=117) | 52 (44) |
| Ischemic heart disease, n (%) (n=121) | 21 (17) |
| Cerebral hemorrhage, n (%) (n=123) | 7 (6) |
| Cerebral infarction, n (%) (n=122) | 39 (32) |
| Amputation, n (%) (n=123) | 7 (6) |
| Hip fracture, n (%) (n=123) | 12 (10) |
| Antihypertensive drug, n (%) (n=122) | 78 (64) |
| BMD | |
| Total whole-body BMD, g/cm2 | 0.96 (0.90–1.06) |
| T-score (SD) | −2.4 (−3.5 to −1.1) |
| Z-score (SD) | 0.1 (−0.7 to 1.1) |
| Total hip BMD, g/cm2 | 0.67 (0.59–0.74) |
| Femoral neck BMD, g/cm2 | 0.49 (0.45–0.53) |
| T-score (SD) | −2.9 (−3.2 to −2.6) |
| Z-score (SD) | −1.6 (−2.2 to −1.2) |
| Nutritional status | |
| Body mass index | 21 (18–23) |
| Lean body mass index, kg/m2 (n=122) | 15.5 (13.9–16.8) |
| Total lean mass, kg (n=122) | 38.3 (31.8–44.4) |
| Fat body mass index, kg/m2 (n=122) | 6.1 (4.7–7.6) |
| Total fat mass, kg (n=122) | 15.3 (11.3–18.7) |
| Circulating biomarkers (n=121) | |
| Hemoglobin, g/dl | 11 (10–11) |
| Albumin, g/dl | 3.6 (3.4–3.8) |
| BUN, mg/dl | 64 (55–74) |
| Creatinine, mg/dl | 11 (9.3–12) |
| Calcium, mg/dl | 8.5 (8.1–8.9) |
| Phosphate, mg/dl | 5.3 (4.7–6.0) |
| i-PTH, pg/ml | 194 (136–252) |
| Alkaline phosphatase, U/L | 234 (192–310) |
| Cholesterol, mg/dl | 152 (136–167) |
| CRP, mg/dl | 0.2 (0.1–0.5) |
| Medications | |
| Calcium carbonate, n (%) | 95 (77) |
| Median daily dose, mg/d | 1500 (1500–2000) |
| Vitamin D, n (%) | 101 (82) |
| Alfacalcidol, n (%) | 89 (72) |
| Median daily dose, μg/d | 0.5 (0.5–0.5) |
| Calcitriol, n (%) | 13 (10) |
| Median daily dose, μg/d | 0.5 (0.25–0.5) |
| Cinacalcet, n (%) | 70 (56) |
| Median daily dose, mg/d | 25 (21.9–25) |
Continuous variables are presented as median (25–75 percentiles). Categorical variables are presented as number (n)/percentage (%). ADPKD, autosomal dominant polycystic kidney disease; BMD, bone mineral density; i-PTH, intact-parathyroid hormone; CRP, C-reactive protein.
During the study period, four hip fractures occurred. Supplemental Table 2 presents the demographic data among those who developed hip fracture during the study period. The CKD-MBD parameters remained within the target range in the long run (Supplemental Figure 3). None of the patients developed life-threatening hypocalcemia (defined as albumin-corrected calcium <6.0 mg/dl, according to the Common Terminology Criteria for Adverse Events grade). However, nine patients developed severe hypocalcemia (defined as 6.0 mg/dl <albumin-corrected calcium <7.0 mg/dl), which were immediately attenuated. There were no instances of antiresorptive agents–related osteonecrosis of the jaw. On the basis of mixed model analysis, there were no significant changes in calcium, phosphate, i-PTH, and alkaline phosphatase levels from baseline to a maximum of 5 years of denosumab therapy. However, unexpected positive changes were observed in albumin-corrected calcium levels, whereas negative changes were found in albumin levels. The changes in alkaline phosphatase were negative, but did not reach statistical significance (P = 0.055) (Supplemental Table 3). As regarding medications, doses of alfaclcidol and calcitriol were significantly decreased over time. No significant changes were found in doses of calcium carbonate and cinacalcet (Supplemental Figure 4 and Supplemental Table 4).
The Long-Term Effect of Denosumab Therapy on the Cortical and Trabecular Compartments and Estimated Strength Indices in the Hip Regions
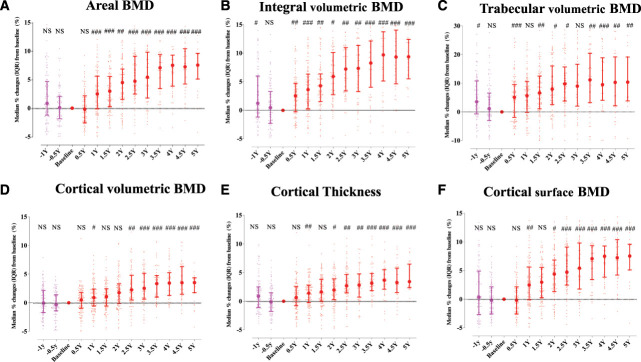
Integral and trabecular volumetric BMD at −1 year before denosumab initiation were significantly higher than those at baseline. The other 3D components and aBMD showed a lower trend but without statistical differences. After the start of denosumab treatment, total hip aBMD, cortical volumetric BMD, cortical surface BMD, and cortical thickness all significantly rose for over 3.5–4 years until settling at higher values as compared with those at baseline (Figure 1, A, B, and D–F). Over a period of 2.5 years, there was a continuous higher trabecular volumetric BMD, which was then maintained (Figure 1C). Any estimated strength indices, with the exception of the buckling ratio, showed a similar pattern (Figure 2). The mixed model analysis as sensitivity analysis showed a significant positive change in all 3D DXA parameters and estimated strength indices (Supplemental Tables 5 and 6). Supplemental Table 7 presented that the serum alkaline phosphatase level at baseline was significantly associated with aBMD over time, but there was no significant association with no biomarkers and medications at baseline and trabecular volumetric BMD and cortical surface BMD over time.
Figure 1.
Time courses of the cortical and trabecular compartments of the hip region. Changes at the total hip region in aBMD (A), integral volumetric BMD (B), trabecular volumetric BMD (C), cortical volumetric BMD (D), cortical thickness (E), and cortical surface BMD (F) before and after denosumab initiation in 124 dialysis patients. Data represent the median (IQR). #P < 0.05; ##P < 0.01; ###P < 0.001 versus baseline (by Wilcoxon signed-rank test). aBMD, areal bone mineral density; BMD, bone mineral density; IQR, interquartile range; Y, year. Figure 1 can be viewed in color online at www.cjasn.org.
Figure 2.
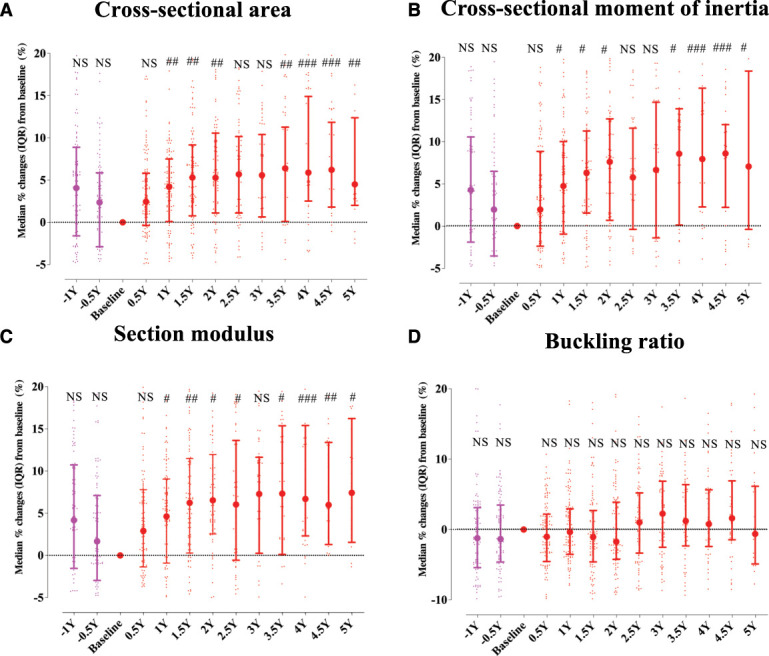
Time courses of the estimated strength indices of the hip region. Changes at the neck region in cross-sectional area (A), cross-sectional moment of inertia (B), section modulus (C), and buckling ratio (D) before and after denosumab initiation in 124 dialysis patients. Data represent the median (IQR). #P < 0.05; ##P < 0.01; ###P < 0.001 versus baseline (by Wilcoxon signed-rank test). Figure 2 can be viewed in color online at www.cjasn.org.
The Effect of Denosumab Discontinuation on the Cortical and Trabecular Compartments and Estimated Strength Indices in the Hip Regions
Altogether, 11 patients with available data after treatment discontinuation were analyzed. The integral volumetric BMD was significantly lower at 1 year after denosumab discontinuation than that at baseline (defined as 6 months after the last denosumab injection as the baseline). Other cortical and trabecular parameters tended to be deteriorated, but the observed changes failed to reach statistical significance, which may be due to the limited sample size (Supplemental Figure 5). Similar trajectories were also seen in the estimated strength indices (Supplemental Figure 6).
3D Spatial Distribution of Changes in the Cortical Bone Before and After Denosumab Treatment and After Denosumab Discontinuation
Before and After Denosumab Therapy
Data from 1 year before to 4 years after denosumab initiation in 39 patients were included in the analysis to clarify the damaged locations under the natural course of dialysis therapy and how denosumab changed it.
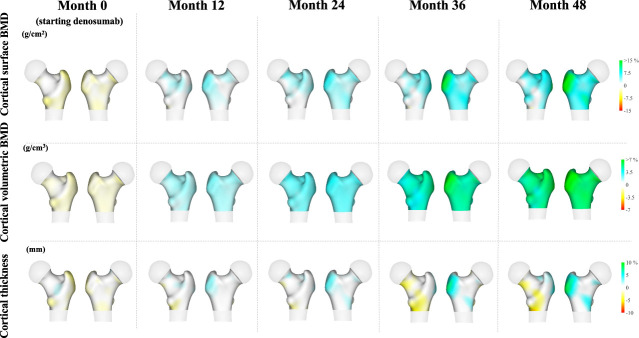
On the basis of calculations using measurements at each vertex of the femoral surface, when starting denosumab therapy (month 0), the lateral aspect of the greater trochanter seemed to be damaged as compared with that baseline (1 year before denosumab therapy), with yellow–orange–red regions reflecting lower in BMD. After denosumab therapy, a significant change in cortical volumetric BMD was seen in the whole area of the hip region, with blue/green regions reflecting BMD gains (Figure 3). Regarding cortical surface BMD and thickness, a more pronounced change was noted at the lateral aspect of the greater trochanter (Figure 3). Supplemental Figure 7 shows cross-sectional images demonstrating that there was a steady higher volumetric BMD in the neck, intertrochanteric, and lower shaft regions for both cortical and trabecular bones. Notably, there was a marked association with higher volumetric BMD in the periosteal bone compared with the endocortical bone.
Figure 3.
Anatomical distribution of changes in cortical surface BMD (upper), volumetric BMD (middle), and thickness (lower) from 1 year before to 4 years after denosumab initiation denosumab in 39 dialysis patients. Higher values in cortical surface BMD, volumetric BMD, and thickness are presented in blue–green colors, whereas lower values are presented in yellow–red colors.
After Denosumab Discontinuation
Similar to the (II.) analysis, we analyzed the data from a total of 11 patients who discontinued denosumab therapy. A more pronounced change in cortical surface BMD and thickness was observed at the lateral aspect of the greater trochanter, whereas almost all surfaces showed yellow/red regions reflecting lower BMD (Figure 4).
Figure 4.
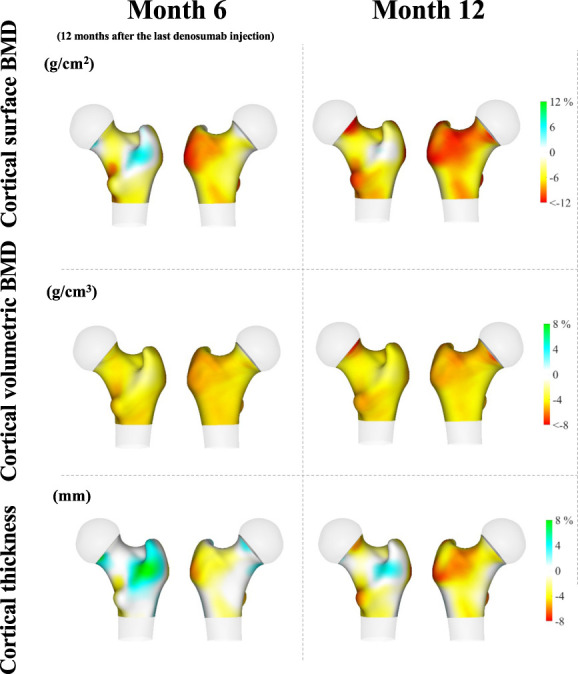
Anatomical distribution of changes in cortical surface BMD (upper), volumetric BMD (middle), and thickness (lower) after denosumab discontinuation in 11 dialysis patients. Higher values in cortical surface BMD, volumetric BMD, and thickness are presented in blue–green colors, whereas lower values are presented in yellow–red colors.
Similarly, although larger volumetric BMD loss seemed to more frequently occur in the periosteal bone than in the endocortical bone at month 6, almost all regions showed yellow/red signs at month 12 (Supplemental Figure 8).
Discussion
In this study, we found a significant association with higher aBMD, cortical volumetric BMD, cortical surface BMD, and cortical thickness over the course of 3.5 years after the initiation of denosumab, before stabilization at a higher value than the baseline. In the course of the 2.5-year observation period, a continuous elevation in trabecular volumetric BMD was documented, eventually stabilizing at a higher level. The estimated strength indices showed similar patterns. However, these 3D parameters and estimated strength indices tended to greatly deteriorate at 1 year after denosumab discontinuation. On the basis of the analysis of the anatomical distribution of the changes in BMD, BMD at the lateral aspect of the greater trochanter was markedly lower due to a 1-year natural course of dialysis therapy. However, BMD at the whole hip region, especially in the lateral aspect of the greater trochanter, was found to be higher after denosumab therapy. After denosumab discontinuation, the lateral aspect of the greater trochanter was the most pronounced location with volumetric BMD loss.
To the best of our knowledge, this study demonstrated the effects of long-term denosumab and denosumab discontinuation on 3D parameters in the hip region of dialysis patients. In the dialysis patients, to date, only up to 2 years of data on the effect of denosumab therapy on BMD in the hip have been reported.16 Although our data suggest that denosumab could be a beneficial treatment for increasing BMD even in the long term in dialysis patients, one of the noteworthy findings was that BMD showed a continuous higher trend for up to 3.5–4 years, after which it reached a plateau, which completely differed from the pattern observed in cases with primary osteoporosis. In patients with primary osteoporosis, a steady increase in BMD over 8 years without reaching a plateau is one of the unique features of denosumab.25 Similarly, although bone microstructure at 5-year denosumab treatment was improved compared with that at 2–3 years of denosumab in patients with primary osteoporosis,26 our results focusing dialysis patients showed that there seemed to be a plateau at 2–3 years of treatment. Thus, we found a different trajectory of bone responses after long-term denosumab treatments between patients with primary osteoporosis and dialysis patients. Although the mechanisms behind this difference are not clear, some factors, including sex differences, uremic substances, acidosis, and hormonal imbalances, such as i-PTH and vitamin D deficiencies, may play a role. These factors may conceivably contribute to not only the difference in the characteristics between CKD-MBD and primary osteoporosis but also a different trajectory of BMD after long-term denosumab treatments.
The cortical bone rather than the trabecular bone is first damaged in cases with a declining kidney function.7,8 Because hip fracture is the most common and critical fracture in dialysis patients,11 investigating the therapeutic effects on cortical and trabecular compartments in the hip region is essential to achieve better clinical outcomes. Our results showing the anatomical distribution of the changes before and after denosumab therapy help better understand how and where the bone was aggravated and whether the impaired bone changed. Under the continuous nature of dialysis therapy without denosumab treatment, cortical surface BMD and volumetric BMD at the lateral aspect of the greater trochanter showed a lower value. After denosumab initiation, BMD at the entire hip region, including cortical surface BMD and volumetric BMD, showed higher values, especially in the lateral aspect of the greater trochanter. Given other findings showing significant changes in the cortical and trabecular compartments and estimated strength indices after denosumab therapy, denosumab may be a useful treatment option for improving impaired bone status even in dialysis patients. Furthermore, continuing denosumab treatment for as long as possible in dialysis patients may be beneficial because discontinuing the medication can lead to significant bone loss. Therefore, as long as denosumab continues to be used, it is likely to help maintain bone density even in long-term use. However, as the primary outcome in previous papers, including this study, assessing the efficacy of the antiosteoporosis drug in dialysis patients was mostly BMD, not fracture incidents, due to the small sample size and/or short follow-up period. Thus, further study is needed to clarify whether denosumab treatment decreases hip fracture risk in dialysis patients as well as the general population.
Denosumab discontinuation causes a rapid increase in bone turnover and BMD loss and, as a consequence, an increase in fracture incidents.27–30 These facts could be expanded to dialysis patients. Thus, after the discontinuation of denosumab, the 3D parameters and estimated strength indices in the hip region deteriorated rapidly and abruptly at 1 year after denosumab discontinuation, although not significant, probably due to the small number of samples. The lateral aspect of the greater trochanter was one of the most damaged locations in the hip region. Although we could not assess the fracture incidents after denosumab discontinuation, our results suggested that denosumab discontinuation may cause more hip fracture incidents even in dialysis patients.
Some limitations of this study should be noted. First, as with any observational study, it is not possible to establish causality or eliminate the potential for survivorship bias. Other medications and advances in routine medical care may also contribute to the higher BMD after denosumab therapy, although the BMD was lower once discontinuation of denosumab. Second, the retrospective single-center study design may limit the generalizability of the findings. Third, the sample size was not large enough, although our sample size and follow-up duration were the best among the previously published articles, to the best of our knowledge, for investigating 3D components and BMD after treatment with antiosteoporosis drugs in dialysis patients. Fourth, the prevention of new fractures was not evaluated. Fifth, we had no information about bone turnover markers and the bone biopsies for analyzing the histologic changes. However, bone biopsy taken from the iliac bone may not fully represent the condition of the hip region, which was the most important location in dialysis patients. The bone turnover rate differs between different bones because of the differences in the ratio of cortical to trabecular content.31,32 Contrarily, our methodology analyzing the hip region would provide important implications for the management of bone diseases in dialysis patients. Lastly, after initiating denosumab, severe hypocalcemia (with albumin-corrected calcium levels between 6.0 mg/dl and 7.0 mg/dl) was effectively managed within 1–2 weeks in an outpatient setting, eliminating the need for hospitalization. However, careful monitoring and potential calcium supplementation are crucial when beginning denosumab treatment.
In conclusion, under the continuous nature of dialysis therapy before denosumab initiation, integral and trabecular volumetric BMD significantly showed a lower value. The lateral aspect of the greater trochanter seemed to be mainly aggravated. After starting denosumab therapy, surface BMD, volumetric BMD, and thickness of cortical bone, volumetric BMD of the trabecular bone consistently showed higher values over a span of 3–4 years, eventually reaching a stable state. The visual analysis showed that BMD at the whole location in the hip region was higher compared with the baseline, especially in the lateral aspect of the greater trochanter. Similar trends were seen in any estimated strength indices except for the buckling ratio. After denosumab discontinuation, cortical and trabecular compartments and estimated strength indices were aggravated. The lateral aspect of the greater trochanter was largely damaged based on the visual analysis. These findings suggest that denosumab may help dialysis patients with impaired bone conditions by improving their cortical and trabecular bones in the hip region. However, heightened surveillance is required to achieve better clinical outcomes once denosumab is discontinued.
Supplementary Material
Acknowledgments
The Kidney Foundation, Japan (Grant No. JKFB 20-20) and Japanese Association of Dialysis Physicians (Grant No. 2021-5) funded K. Iseri.
Footnotes
See related editorial, “Treating Osteoporosis with Denosumab in Patients on Hemodialysis: The Good, the Bad, and the Ugly,” on pages 1116–1118.
Disclosures
H. Honda reports research funding from Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., and Kyowa Hakko Kirin Co., Ltd.; honoraria from Astellas Pharma Inc., Chugai Pharmaceutical Co., Ltd., Kissei Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co., Ltd., Mitsubishi Tanabe Pharma, and Torii Pharmaceutical Co., Ltd.; patents or royalties from Mitsubishi Tanabe Pharma; and advisory or leadership roles for Astellas Pharma Inc., Mitsubishi Tanabe Pharma, and Torii Pharmaceutical Co., Ltd. L. Humbert reports employment with 3D-Shaper Medical; ownership interest in 3DShaper Medical and Galgo Medical; research funding from Amgen, Radius Health, and Zimmer Biomet; and patents or royalties from 3D-Shaper Medical and Galgo Medical. T. Kato reports research funding from Baxter Ltd. M. Mizobuchi reports research funding from Kyowa Kirin and honoraria from Astellas, Bayer, Daiichi Sankyo, Fuso Yakuhin Kogyo, Kaneka, Kissei, Kyowa Kirin, Ono Yakuhin Kogyo, Sanwa Kagaku, and Torii Yakuhin. R. Winzenrieth is an employee of Molzym GmbH & Co. KG and reports other interests or relationships as a former employee of 3D-SHAPER Medical. All remaining authors have nothing to disclose.
Funding
None.
Author Contributions
Conceptualization: Ken Iseri, Masahide Mizobuchi.
Data curation: Hirokazu Honda, Ken Iseri, Tadashi Kato, Yutaka Nakajima, Tomohiro Saitou, Kanji Shishido, Mikio Wakasa.
Formal analysis: Ludovic Humbert, Ken Iseri, Renaud Winzenrieth.
Funding acquisition: Masahide Mizobuchi.
Investigation: Ken Iseri.
Methodology: Ludovic Humbert, Ken Iseri.
Project administration: Ken Iseri, Yutaka Nakajima.
Resources: Hirokazu Honda, Masahide Mizobuchi.
Software: Ken Iseri, Renaud Winzenrieth.
Supervision: Ludovic Humbert, Renaud Winzenrieth.
Validation: Ludovic Humbert, Masahide Mizobuchi.
Visualization: Ken Iseri, Masahide Mizobuchi.
Writing – original draft: Ken Iseri.
Writing – review & editing: Hirokazu Honda, Ludovic Humbert, Ken Iseri, Masahide Mizobuchi, Renaud Winzenrieth.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/CJN/B782.
Supplemental Table 1. The median interval (months) from starting denosumab therapy to each measurement.
Supplemental Table 2. Baseline clinical and biochemical characteristics of four dialysis patients who developed hip fracture during studied period.
Supplemental Table 3. Linear mixed model of CKD-MBD parameters from baseline to a maximum of 5 years of denosumab therapy.
Supplemental Table 4. Linear mixed model of medication doses from baseline to a maximum of 5 years of denosumab therapy.
Supplemental Table 5. Linear mixed model of 3D DXA parameters from baseline to a maximum of 5 years of denosumab therapy.
Supplemental Table 6. Linear mixed model of bone strength indices at the neck region from baseline to a maximum of 5 years of denosumab therapy.
Supplemental Table 7. Predictors of changes in areal BMD, trabecular volumetric BMD, and cortical surface BMD from multivariable mixed analysis.
Supplemental Figure 1. Patient disposition.
Supplemental Figure 2. Patient disposition for discontinuation analysis.
Supplemental Figure 3. Time courses of the CKD-MBD parameters from baseline to a maximum of 5 years of denosumab therapy.
Supplemental Figure 4. Stacked bar chart of medication dose from baseline to a maximum of 5 years of denosumab therapy.
Supplemental Figure 5. Changes at the total hip region in areal BMD (a), integral volumetric BMD (b), trabecular volumetric BMD (c), cortical volumetric BMD (d), cortical thickness (e), and cortical surface BMD (f) after denosumab discontinuation in 11 dialysis patients.
Supplemental Figure 6. Changes at the neck region in cross-sectional area (a), cross-sectional moment of inertia (b), section modulus (c), and buckling ratio (d) after denosumab discontinuation in 11 dialysis patients.
Supplemental Figure 7. Cross-sectional images showing the time course of the average changes in volumetric BMD from 1 year before to 4 years after denosumab initiation in 39 dialysis patients.
Supplemental Figure 8. Cross-sectional images showing the time course of the average changes in volumetric BMD after denosumab discontinuation in 11 dialysis patients.
References
- 1.Mizobuchi M, Ogata H, Koiwa F, Kinugasa E, Akizawa T. Research on kidney and mineral metabolism in Japan: past, present, and future. Clin Exp Nephrol. 2017;21(S1):4–8. doi: 10.1007/s10157-016-1366-5 [DOI] [PubMed] [Google Scholar]
- 2.Komaba H, Ketteler M, Cunningham J, Fukagawa M. Old and new drugs for the management of bone disorders in CKD. Calcif Tissue Int. 2021;108(4):486–495. doi: 10.1007/s00223-020-00788-y [DOI] [PubMed] [Google Scholar]
- 3.Eknoyan G, Moe SM. Renal osteodystrophy: a historical review of its origins and conceptual evolution. Bone Rep. 2022;17:101641. doi: 10.1016/j.bonr.2022.101641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evenepoel P Cunningham J Ferrari S, et al. European Consensus Statement on the diagnosis and management of osteoporosis in chronic kidney disease stages G4-G5D. Nephrol Dial Transplant. 2021;36(1):42–59. doi: 10.1093/ndt/gfaa192 [DOI] [PubMed] [Google Scholar]
- 5.Malluche HH, Monier-Faugere MC, Blomquist G, Davenport DL. Two-year cortical and trabecular bone loss in CKD-5D: biochemical and clinical predictors. Osteoporos Int. 2018;29(1):125–134. doi: 10.1007/s00198-017-4228-4 [DOI] [PubMed] [Google Scholar]
- 6.Parfitt AM. A structural approach to renal bone disease. J Bone Miner Res. 1998;13(8):1213–1220. doi: 10.1359/jbmr.1998.13.8.1213 [DOI] [PubMed] [Google Scholar]
- 7.Iseri K Dai L Chen Z, et al. Bone mineral density and mortality in end-stage renal disease patients. Clin Kidney J. 2020;13(3):307–321. doi: 10.1093/ckj/sfaa089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nickolas TL Stein EM Dworakowski E, et al. Rapid cortical bone loss in patients with chronic kidney disease. J Bone Miner Res. 2013;28(8):1811–1820. doi: 10.1002/jbmr.1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Runesson B Trevisan M Iseri K, et al. Fractures and their sequelae in non-dialysis-dependent chronic kidney disease: the Stockholm CREAtinine Measurement project. Nephrol Dial Transplant. 2019;35(11):1908–1915. doi: 10.1093/ndt/gfz142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iseri K Carrero JJ Evans M, et al. Fractures after kidney transplantation: incidence, predictors, and association with mortality. Bone. 2020;140:115554. doi: 10.1016/j.bone.2020.115554 [DOI] [PubMed] [Google Scholar]
- 11.Iseri K Carrero JJ Evans M, et al. Major fractures after initiation of dialysis: incidence, predictors and association with mortality. Bone. 2020;133:115242. doi: 10.1016/j.bone.2020.115242 [DOI] [PubMed] [Google Scholar]
- 12.Iseri K Carrero JJ Evans M, et al. Incidence of fractures before and after dialysis initiation. J Bone Miner Res. 2020;35(12):2372–2380. doi: 10.1002/jbmr.4141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watts NB, Camacho PM, Lewiecki EM, Petak SM. American association of clinical endocrinologists/American College of endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis—2020 update. Endocr Pract. 2021;27(4):379–380. doi: 10.1016/j.eprac.2021.02.001 [DOI] [PubMed] [Google Scholar]
- 14.Iseri K Watanabe M Yoshikawa H, et al. Effects of denosumab and alendronate on bone health and vascular function in hemodialysis patients: a randomized, controlled trial. J Bone Miner Res. 2019;34(6):1014–1024. doi: 10.1002/jbmr.3676 [DOI] [PubMed] [Google Scholar]
- 15.Kunizawa K Hiramatsu R Hoshino J, et al. Denosumab for dialysis patients with osteoporosis: a cohort study. Sci Rep. 2020;10(1):2496. doi: 10.1038/s41598-020-59143-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiramatsu R, Ubara Y, Sawa N, Sakai A. Hypocalcemia and bone mineral changes in hemodialysis patients with low bone mass treated with denosumab: a 2-year observational study. Nephrol Dial Transplant. 2021;36(10):1900–1907. doi: 10.1093/ndt/gfaa359 [DOI] [PubMed] [Google Scholar]
- 17.Clotet J, Martelli Y, Di Gregorio S, Del Río Barquero LM, Humbert L. Structural parameters of the proximal femur by 3-dimensional dual-energy X-ray absorptiometry software: comparison with quantitative computed tomography. J Clin Densitom. 2018;21(4):550–562. doi: 10.1016/j.jocd.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 18.Humbert L Martelli Y Fonolla R, et al. 3D-DXA: assessing the femoral shape, the trabecular macrostructure and the cortex in 3D from DXA images. IEEE Trans Med Imaging. 2017;36(1):27–39. doi: 10.1109/TMI.2016.2593346 [DOI] [PubMed] [Google Scholar]
- 19.Winzenrieth R, Ominsky MS, Wang Y, Humbert L, Weiss RJ. Differential effects of abaloparatide and teriparatide on hip cortical volumetric BMD by DXA-based 3D modeling. Osteoporos Int. 2021;32(3):575–583. doi: 10.1007/s00198-020-05806-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winzenrieth R, Kostenuik P, Boxberger J, Wang Y, Humbert L. Proximal femur responses to sequential therapy with abaloparatide followed by alendronate in postmenopausal women with osteoporosis by 3D modeling of hip dual-energy X-ray absorptiometry (DXA). JBMR Plus. 2022;6(4):e10612. doi: 10.1002/jbm4.10612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winzenrieth R, Humbert L, Boxberger JI, Weiss RJ, Wang Y, Kostenuik P. Abaloparatide effects on cortical volumetric BMD and estimated strength indices of hip subregions by 3D-DXA in women with postmenopausal osteoporosis. J Clin Densitom. 2022;25(3):392–400. doi: 10.1016/j.jocd.2021.11.007 [DOI] [PubMed] [Google Scholar]
- 22.Winzenrieth R, Humbert L, Di Gregorio S, Bonel E, García M, Del Rio L. Effects of osteoporosis drug treatments on cortical and trabecular bone in the femur using DXA-based 3D modeling. Osteoporos Int. 2018;29(10):2323–2333. doi: 10.1007/s00198-018-4624-4 [DOI] [PubMed] [Google Scholar]
- 23.Soen S Fukunaga M Sugimoto T, et al. Diagnostic criteria for primary osteoporosis: year 2012 revision. J Bone Miner Metab. 2013;31(3):247–257. doi: 10.1007/s00774-013-0447-8 [DOI] [PubMed] [Google Scholar]
- 24.Fukagawa M Yokoyama K Koiwa F, et al. Clinical practice guideline for the management of chronic kidney disease-mineral and bone disorder. Ther Apher Dial. 2013;17(3):247–288. doi: 10.1111/1744-9987.12058 [DOI] [PubMed] [Google Scholar]
- 25.Papapoulos S Lippuner K Roux C, et al. The effect of 8 or 5 years of denosumab treatment in postmenopausal women with osteoporosis: results from the FREEDOM Extension study. Osteoporos Int. 2015;26(12):2773–2783. doi: 10.1007/s00198-015-3234-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farlay D Rizzo S Dempster DW, et al. Bone mineral and organic properties in postmenopausal women treated with denosumab for up to 10 years. J Bone Miner Res. 2022;37(5):856–864. doi: 10.1002/jbmr.4538 [DOI] [PubMed] [Google Scholar]
- 27.Cosman F, Huang S, McDermott M, Cummings SR. Multiple vertebral fractures after denosumab discontinuation: FREEDOM and FREEDOM extension trials additional post hoc analyses. J Bone Miner Res. 2022;37(11):2112–2120. doi: 10.1002/jbmr.4705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burckhardt P, Faouzi M, Buclin T, Lamy O. Fractures after denosumab discontinuation: a retrospective study of 797 cases. J Bone Miner Res. 2021;36(9):1717–1728. doi: 10.1002/jbmr.4335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anastasilakis AD, Makras P, Yavropoulou MP, Tabacco G, Naciu AM, Palermo A. Denosumab discontinuation and the rebound phenomenon: a narrative review. J Clin Med. 2021;10(1):152–228. doi: 10.3390/jcm10010152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cummings SR Ferrari S Eastell R, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension. J Bone Miner Res. 2018;33(2):190–198. doi: 10.1002/jbmr.3337 [DOI] [PubMed] [Google Scholar]
- 31.Recker R, Lappe J, Davies KM, Heaney R. Bone remodeling increases substantially in the years after menopause and remains increased in older osteoporosis patients. J Bone Miner Res. 2004;19(10):1628–1633. doi: 10.1359/JBMR.040710 [DOI] [PubMed] [Google Scholar]
- 32.Seeman E. Age- and menopause-related bone loss compromise cortical and trabecular microstructure. J Gerontol A Biol Sci Med Sci. 2013;68(10):1218–1225. doi: 10.1093/gerona/glt071 [DOI] [PubMed] [Google Scholar]