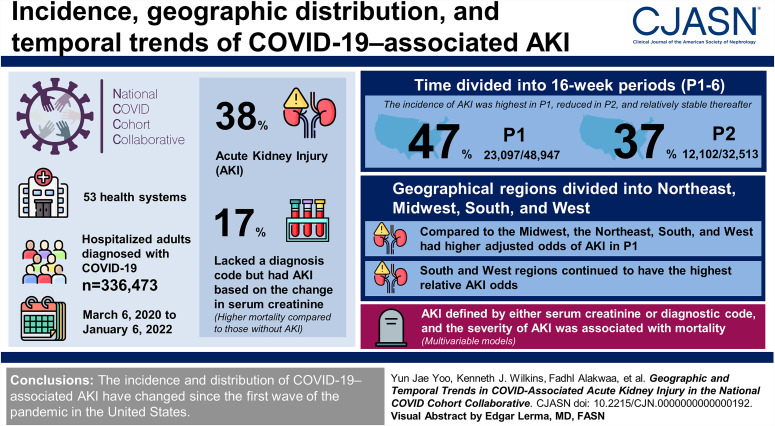
Visual Abstract
Keywords: COVID-19, acute kidney injury, incidence, risk factors
Abstract
Background
AKI is associated with mortality in patients hospitalized with coronavirus disease 2019 (COVID-19); however, its incidence, geographic distribution, and temporal trends since the start of the pandemic are understudied.
Methods
Electronic health record data were obtained from 53 health systems in the United States in the National COVID Cohort Collaborative. We selected hospitalized adults diagnosed with COVID-19 between March 6, 2020, and January 6, 2022. AKI was determined with serum creatinine and diagnosis codes. Time was divided into 16-week periods (P1–6) and geographical regions into Northeast, Midwest, South, and West. Multivariable models were used to analyze the risk factors for AKI or mortality.
Results
Of a total cohort of 336,473, 129,176 (38%) patients had AKI. Fifty-six thousand three hundred and twenty-two (17%) lacked a diagnosis code but had AKI based on the change in serum creatinine. Similar to patients coded for AKI, these patients had higher mortality compared with those without AKI. The incidence of AKI was highest in P1 (47%; 23,097/48,947), lower in P2 (37%; 12,102/32,513), and relatively stable thereafter. Compared with the Midwest, the Northeast, South, and West had higher adjusted odds of AKI in P1. Subsequently, the South and West regions continued to have the highest relative AKI odds. In multivariable models, AKI defined by either serum creatinine or diagnostic code and the severity of AKI was associated with mortality.
Conclusions
The incidence and distribution of COVID-19–associated AKI changed since the first wave of the pandemic in the United States.
Podcast
This article contains a podcast at https://dts.podtrac.com/redirect.mp3/www.asn-online.org/media/podcast/CJASN/2023_08_08_CJN0000000000000192.mp3
Introduction
Coronavirus disease 2019 (COVID-19) caused by the novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has had a devastating effect on individuals and society worldwide.1 Among patients hospitalized with COVID-19, AKI and severity of AKI are associated with in-hospital complications and mortality.2–7
Since the start of the pandemic in December 2019, COVID-19 has varied in incidence, hospitalization, and death rates worldwide8,9 as well as within the United States.10,11 In an early study of 5216 US veterans (94% male) hospitalized with COVID-19, the incidence of AKI declined from 40% in March 2020 to 27% in July 2020 and furthermore varied across regions (10%–56%).12 Still, nearly all previous studies of COVID-19–associated AKI have been limited in sample size, geographic diversity, and observation periods. To address these limitations, we used the National COVID Cohort Collaborative (N3C13), currently the largest public-access enclave of patients with COVID-19 across the United States (nearly 5 million patients), to characterize COVID-19–associated AKI and its geographic heterogeneity period-by-period over the course of the pandemic.
Previous studies have shown limitations in the use of electronic health record (EHR) billing codes to capture the true incidence of AKI.14,15 Furthermore, using creatinine to estimate AKI using the 2012 Kidney Disease Improving Global Outcomes (KDIGO) serum creatinine–based criteria, Grams et al. reported low sensitivity of billing codes to identify milder cases of AKI, which have previously been associated with adverse outcomes.16,17 Hence, a combination of diagnostic billing codes and changes in serum creatinine should lead to a more sensitive estimation of the incidence of AKI and its associated mortality. The objective of this study was to leverage EHR data to explore the temporal and regional variation in AKI incidence and mortality among patients hospitalized with COVID-19.
Methods
N3C Data Ingestion and Harmonization and Study Oversight
N3C collects deidentified EHR data (dating back to January 1, 2018) from 72 US health care institutions into a centralized repository, allowing detailed study of a geographically diverse population of patients with COVID-19.13 The N3C Consortium's data model and cohort information along with data quality and harmonization measures were previously described.11,18
N3C has been approved by the National Institutes of Health's Institutional Review Board (IRB). Each N3C contributor site maintains a data transfer agreement approved by its IRB. The analyses reported in this study were separately approved by the IRB of each participating institution. The IRB reviews included a waiver of informed consent.
Definition of COVID-19 and Other Variables
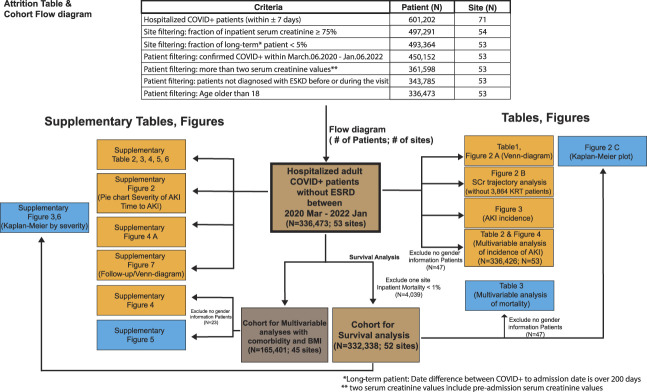
In this study, patients had positive COVID-19 PCR/antigenic tests or a COVID-19 diagnostic code within 7 days of hospital admission (Figure 1). Supplemental Table 1 contains a list of all concepts used to define variables used in our study, also further are described in the Supplemental Methods.
Figure 1.
Flow diagram showing the total number of patients used in the study and the patients used in each table and figure. The study included all adult patients in the National COVID Cohort Collaborative dataset with COVID-19 positive within 7 days of inpatient hospitalization. Other patients were excluded from the study because of site-specific data quality issues, preexisting kidney failure (end-stage kidney disease), or lack of serum creatinine data. Because serum creatinine is routinely measured in most acute care hospitals, we excluded 16 sites where <75% of patients had serum creatinine measured because of the concern that these might not be acute care hospitals. The average hospitalized COVID+patient mortality was ≈12% at all the other sites, except one site, where the mortality was comparatively very low (0.88%). Therefore, we excluded this one site when we performed survival analysis because of the concern that it might not be an acute care hospital. BMI, body mass index; COVID-19, coronavirus disease 2019; SCr, serum creatinine.
Cohort Definition, Inclusion, and Exclusion Criteria
This study is a retrospective analysis of patients who (1) were hospitalized between March 6, 2020, through January 6, 2022; (2) had a contemporary COVID-19 diagnosis; (3) did not have a diagnosis of kidney failure on or before their COVID-19–associated hospitalization (N=17,814); and (4) were at least 18 years old (Figure 1 and Supplemental Figure 1). For all end points, March 6, 2022, was the last day of follow-up. We built the analytic cohort from the April 2, 2022, release of N3C data but additionally applied site-level data quality criteria as described in the Supplemental Methods.
After applying these inclusion and exclusion criteria for data partners, we further excluded patients with no creatinine data during their COVID hospitalization, and patients with fewer than 2 serum creatinine values, that is, those unable to be assessed for AKI (N=88,555), unless they had a diagnostic code of AKI on their COVID-19–associated hospitalization (N=1854). Considering an initial population of 601,202 confirmed COVID-19 hospitalized patients from 71 sites, using the aforementioned criteria, our final cohort consisted of 336,473 adults with COVID-19–associated hospitalizations from 53 sites (Figure 1).
Kidney Measures
Definition of AKI
Patients were required to have ≥2 serum creatinine measurements available to diagnose AKI, except for 1854 patients that carried a diagnosis code for AKI (Figure 2). Patients with any of the 25 diagnosis codes related to AKI (Supplemental Table 1) were designated as “Code-based AKI.”
Figure 2.
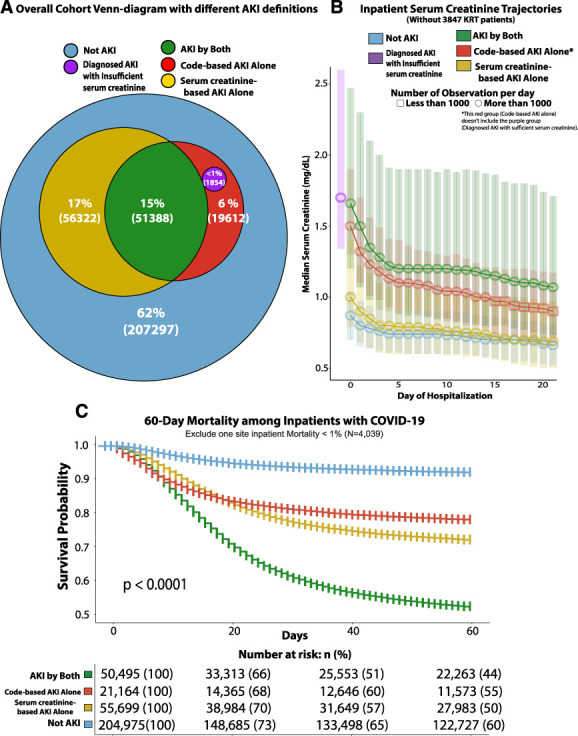
Development of the cohort, definitions of COVID-19–associated AKI, and comparative mortality rates. (A) Overall prevalence of AKI by different AKI definitions in a total of 336,473 adult cohorts. (B) Serum creatinine trajectories by day of admission between patient groups classified in different AKI definitions. (C) 60-day survival after diagnosis of COVID-19 in the patient shown in panel A (this analysis excluded one site with a COVID inpatient mortality rate of <1%).
Patients were classified as having serum creatinine–based AKI if they met one of the following three KDIGO-based definitions:
When the difference in serum creatinine was ≥0.3 mg/dl within any 48-hour period during hospitalization.
When the serum creatinine increased ≥1.5 times from the lowest serum creatinine within any 7-day period during hospitalization.
When the patient's maximum serum creatinine during hospitalization was higher than their baseline serum creatinine value (defined in Supplemental Methods) by at least 1.5 times.
The number of patients for each serum creatinine–based AKI definition is shown in Supplemental Figure 2A. Unless otherwise noted, AKI in this study was defined as meeting the criteria for either serum creatinine–based AKI or code-based AKI.
Any patient requiring KRT was classified as stage 3 AKI with KRT. The severity of AKI is further explained in the Supplemental Methods.
Geographical Regions and Time Periods
Patients included in the study had their index hospitalization with COVID-19 between March 6, 2020, and January 6, 2022. The cohort, representing 96 weeks of enrollment, was divided into six equal 16-week periods: P1: March 6, 2020, to June 25, 2020; P2: after P1–October 16, 2020; P3: after P2–February 5, 2021; P4: after P3–May 28, 2021; P5: after P4–September 16, 2021; P6: after P5–January 6, 2022. The cohort was also divided based on the location of the health care sites into four geographic regions of the United States (Figure 3B): West (10 sites, 29,221 patients), Northeast (12 sites, 61,728 patients), Midwest (18 sites, 183,275 patients), and South (13 sites, 62,249 patients).
Figure 3.
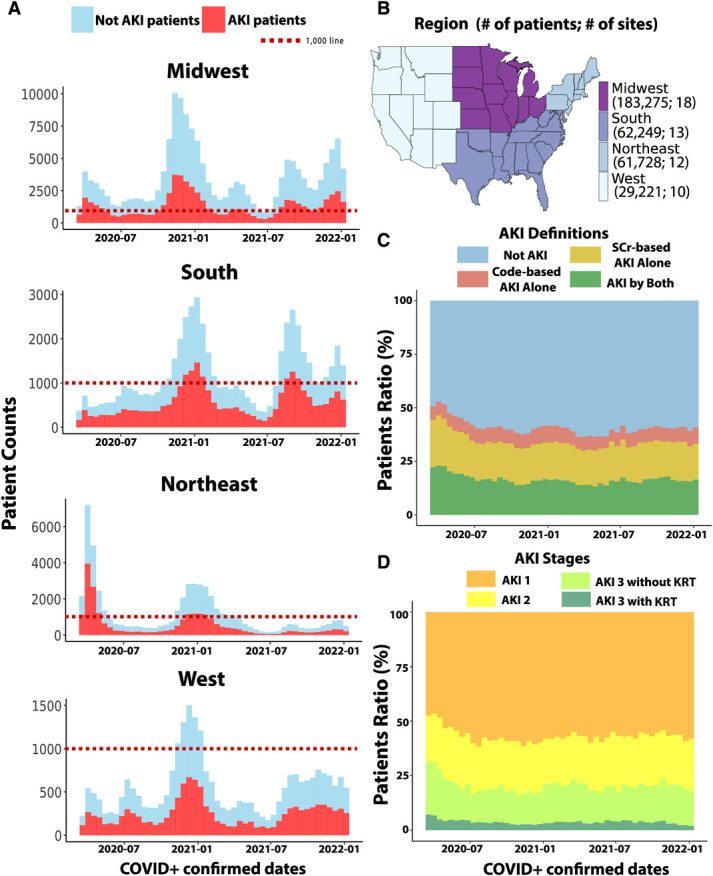
Temporal and geographical distribution of COVID-19–associated AKI. (A) Timeline of patients with and without AKI across four regions of the United States of our cohort of 336,473. (B) Choropleths show the overall number of the patients and sites over region. (C) Timeline of definitions of AKI among those with AKI over time shows a higher level of more AKI by both at earlier times. (D) Timeline of severity of AKI among those with AKI over time shows a higher level of more severe disease at earlier times.
Mortality
Death information was obtained from each contributing N3C site's local research data warehouse. This primarily included inpatient deaths recorded in each individual site's EHR systems along with other sources of death data that each site may use to document both in-hospital and outpatient death, for example, US Social Security Death Master file.
Statistical Analysis
The cohort construction for primary and subgroup analyses is described in Figure 1. Comparison of categorical variables was performed using chi-squared tests. For continuous variables, t tests or ANOVA were used (Table 1, Supplemental Tables 2–6). All survival analyses were performed using the COVID-19 index date as a start date and death date as an end point (Figure 2C, Table 3, Supplemental Figure 3). A total of 336,473 patients (primary cohort) were used to define the geographic distribution and temporal trends of AKI (Figure 3). Multivariable logistic regression estimated the risk of AKI, quantifying relative risk in odds ratio (OR) estimates and Cox proportional hazard model–based estimates of mortality risk in the hazard ratio (HR), with AKI status-specific HRs for mortality as the primary end point (Figure 4, Tables 2 and 3, Supplemental Figures 5 and 6). All analysis and visualization were performed in the N3C enclave using SQL, Python, and R, including ggplot2,19 survival,20 and survminer21 packages. Further statistical details, such as assessing site heterogeneity, are provided in the Supplemental Methods.
Table 1.
Descriptive characteristics of hospitalized coronavirus disease 2019–positive patients with and without AKI: demographics, comorbid status, body mass index, disease severity, and mortality
| Variables | Total (N=336,473) | AKI [Code-Based or Serum Creatinine–Based] (N=129,176) | Not AKI (N=207,297) |
|---|---|---|---|
| Demographics | |||
| Age, mean (SD) | 61 (19) | 65 (17) | 59 (20) |
| Sex (N, %) | |||
| Female | 164,234 (49) | 56,577 (44) | 107,657 (52) |
| Male | 172,192 (51) | 72,581 (56) | 99,611 (48) |
| Race (N, %) | |||
| Asian | 7618 (2) | 3094 (2) | 4524 (2) |
| Black | 63,761 (19) | 28,636 (22) | 35,125 (17) |
| White | 212,779 (63) | 77,764 (60) | 135,015 (65) |
| Others | 7654 (2) | 2799 (2) | 4855 (2) |
| No information | 44,661 (13) | 16,883 (13) | 27,778 (13) |
| Ethnicity (N, %) | |||
| Not Hispanic or Latino | 263,766 (78) | 101,930 (79) | 161,836 (78) |
| Hispanic or Latino | 44,934 (13) | 15,953 (12) | 28,981 (14) |
| No information | 27,773 (8) | 11,293 (9) | 16,480 (8) |
| Comorbid conditions (N, %) | |||
| History available | 261,296 (78) | 94,992 (74) | 166,304 (80) |
| Among those with histories available | |||
| Cardiovascular disease | 101,926 (39) | 44,260 (47) | 57,666 (35) |
| Diabetes mellitus | 77,358 (30) | 35,462 (37) | 41,896 (25) |
| Heart failure | 40,993 (16) | 20,436 (22) | 20,557 (12) |
| Hypertension | 132,690 (51) | 56,801 (60) | 75,889 (46) |
| BMI, kg/m2, mean (SD) | 30.9 (8.6) | 30.6 (8.7) | 31.0 (8.6) |
| Severity of illness (N, %) | |||
| Sepsis | 59,490 (18) | 39,725 (31) | 19,765 (10) |
| Invasive mechanical ventilation | 32,932 (10) | 28,752 (22) | 4180 (2) |
| Length of hospital stay, d, mean (IQRs) | 9.3 (8) | 14.4 (12) | 6.1 (4) |
| Medications (N, %) | |||
| Vasopressors | 43,124 (13) | 30,697 (24) | 12,427 (6) |
| Periods (N, %) | |||
| P1 | 48,947 (15) | 23,097 (18) | 25,850 (12) |
| P2 | 32,513 (10) | 12,102 (9) | 20,411 (10) |
| P3 | 107,744 (32) | 40,583 (31) | 67,161 (32) |
| P4 | 41,236 (12) | 14,430 (11) | 26,806 (13) |
| P5 | 41,845 (12) | 15,103 (12) | 26,742 (13) |
| P6 | 64,188 (19) | 23,861 (18) | 40,327 (19) |
| Death (N, %) | 48,230 (14) | 34,329 (27) | 13,901 (7) |
N, number of patients; BMI, body mass index; IQR, interquartile range; P1–6, time periods 1–6.
Table 3.
Association between AKI and mortality, adjusted for AKI definitions, age, sex, race, ethnicity, severity markers, and timing of initial coronavirus disease infection: raw count and percentage of deaths, unadjusted and adjusted hazard ratios
| Characteristic | Deceased Patients (N, %) | HR (95% CI) | |
|---|---|---|---|
| Unadjusted | Adjusted | ||
| AKI definitions | |||
| Not AKI | 13,872 (7) | Reference | |
| Serum creatinine–based AKI alone | 11,697 (21) | 3.28 (3.19 to 3.36)a | 2.49 (2.43 to 2.56)a |
| Code-based AKI alone | 3821 (18) | 2.83 (2.73 to 2.93)a | 2.54 (2.45 to 2.63)a |
| AKI by both | 18,703 (37) | 6.13 (6.00 to 6.27)a | 3.26 (3.18 to 3.35)a |
| Age—decade levelb | 48,093 (14) | 1.03 (1.03 to 1.03)a | 1.04 (1.04 to 1.04)a |
| Sex | |||
| Female | 20,631 (13) | Reference | |
| Male | 27,462 (16) | 1.35 (1.32 to 1.37)a | 1.08 (1.06 to 1.10)a |
| Race | |||
| White | 32,774 (16) | Reference | |
| Asian | 1146 (15) | 1.03 (0.97 to 1.10) | 0.77 (0.73 to 0.82)a |
| Black | 7450 (12) | 0.74 (0.72 to 0.76)a | 0.67 (0.65 to 0.69)a |
| Other races | 754 (10) | 0.66 (0.61 to 0.70)a | 0.73 (0.68 to 0.78)a |
| No race info | 5969 (13) | 0.88 (0.85 to 0.90)a | 0.95 (0.92 to 0.98)a |
| Ethnicity | |||
| Not Hispanic | 38,907 (15) | Reference | |
| Hispanic | 4968 (11) | 0.75 (0.72 to 0.77)a | 0.67 (0.64 to 0.69)a |
| Others/no ethnicity info | 4218 (15) | 1.10 (1.06 to 1.13)a | 0.94 (0.91 to 0.97)a |
| Severity markers | |||
| Invasive mechanical ventilator | 16,808 (52) | 5.82 (5.71 to 5.93)a | 2.30 (2.23 to 2.36)a |
| Vasopressor on the visit | 16,913 (39) | 3.91 (3.84 to 3.99)a | 1.47 (1.43 to 1.51)a |
| Sepsis on the visit | 18,819 (32) | 3.19 (3.13 to 3.25)a | 1.39 (1.36 to 1.42)a |
| Periods | |||
| P1 | 9663 (20) | Reference | |
| P2 | 4446 (14) | 0.65 (0.62 to 0.67)a | 0.81 (0.78 to 0.84)a |
| P3 | 17,164 (16) | 0.82 (0.79 to 0.84)a | 1.02 (1.00 to 1.05) |
| P4 | 4468 (11) | 0.59 (0.57 to 0.61)a | 0.73 (0.71 to 0.76)a |
| P5 | 4946 (12) | 0.76 (0.73 to 0.79)a | 0.88 (0.85 to 0.91)a |
| P6 | 7406 (12) | 1.11 (1.08 to 1.15)a | 1.24 (1.20 to 1.28)a |
N, number of patients; %, percent of patients; HR, hazard ratio; CI, confidence interval; P1–6, time periods 1–6.
P value < 0.001.
Age was expressed in 10-year increments to better show the odds ratio; analysis excluded one site with a COVID inpatient mortality rate of <1%.
Figure 4.
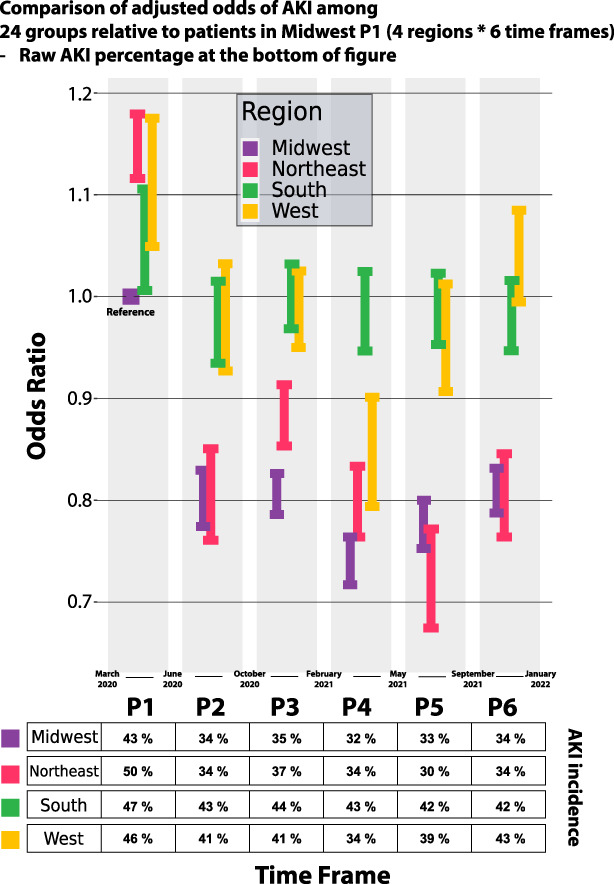
Comparison of adjusted odds of AKI among 24 groups based on Midwest P1 patients with spatial information in multivariable analysis of COVID-19–associated AKI risk in 332,426 patients using a logistic regression model (four regions×six time periods [P1–6]).
Table 2.
Associations of clinical factors and period with AKI among 336,426 patientsa infected with coronavirus disease 2019: adjusted by age, sex, race, ethnicity, and timing of initial coronavirus disease infection
| Characteristic | Patients with AKI (N, %) | OR (95% CI) | P Value | |
|---|---|---|---|---|
| Unadjusted | Adjusted | |||
| Age—decade levelb | 129,158 (34) | 1.03 (1.03 to 1.03) | 1.03 (1.03 to 1.03) | <0.001 |
| Sex | ||||
| Female | 56,577 (34) | Reference | ||
| Male | 72,581 (42) | 1.22 (1.21 to 1.24) | 1.23 (1.21 to 1.24) | <0.001 |
| Race | ||||
| White | 77,756 (37) | Reference | ||
| Asian | 3093 (41) | 1.11 (1.07 to 1.15) | 1.08 (1.04 to 1.12) | <0.001 |
| Black | 28,632 (45) | 1.23 (1.21 to 1.25) | 1.24 (1.22 to 1.26) | <0.001 |
| Other races | 2797 (37) | 1.00 (0.96 to 1.04) | 1.03 (0.99 to 1.07) | 0.09 |
| No race info | 16,880 (39) | 1.03 (1.02 to 1.05) | 1.05 (1.03 to 1.07) | <0.001 |
| Ethnicity | ||||
| Not Hispanic | 101,920 (39) | Reference | ||
| Hispanic | 15,950 (36) | 0.92 (0.90 to 0.93) | 0.92 (0.90 to 0.94) | <0.001 |
| Others/no ethnicity info | 11,288 (41) | 1.05 (1.03 to 10.7) | 1.06 (1.03 to 1.08) | <0.001 |
| Periods | ||||
| P1 | 23,093 (47) | Reference | ||
| P2 | 12,100 (37) | 0.79 (0.77 to 0.81) | 0.80 (0.79 to 0.82) | <0.001 |
| P3 | 40,578 (38) | 0.80 (0.79 to 0.81) | 0.81 (0.80 to 0.82) | <0.001 |
| P4 | 14,429 (35) | 0.74 (0.73 to 0.76) | 0.75 (0.74 to 0.77) | <0.001 |
| P5 | 15,102 (36) | 0.77 (0.75 to 0.78) | 0.79 (0.77 to 0.81) | <0.001 |
| P6 | 23,856 (37) | 0.79 (0.77 to 0.80) | 0.80 (0.79 to 0.83) | <0.001 |
N, number of patients; %, percent of patients; OR, odds ratio; CI, confidence interval; P1–6, periods 1–6. Mean age categorized per 10-year (decade) increments.
In the analysis, 47 patients who did not report their sex were excluded.
Age was expressed in 10-year increments to better show the odds ratio.
Results
Characterization of COVID-19–Associated AKI
The study cohort included 336,473 adults (age: mean 61 years, SD 18.8; 49% female and 63% White) (Figure 1, Table 1). One hundred twenty-nine thousand one hundred and seventy-six (38%) patients had AKI by either criterion (Table 1). Seventy-two thousand eight hundred and fifty-four (22%) patients had code-based AKI, and 107,710 (32%) had serum creatinine–based AKI, while 51,388 (15%) met both the criteria (AKI by both). Furthermore, 21,466 patients (7%) only met the code-based AKI criteria (code-based AKI alone), and 56,322 (17%) only met the serum creatinine–based AKI criteria (serum creatinine–based AKI alone) (Figure 2 and Supplemental Table 2).
Figure 2B shows the in-hospital trajectory of serum creatinine in study patients (except those who underwent KRT) during hospitalization. The initial median serum creatinine levels in patients with only code-based AKI were significantly higher than those without AKI (Figure 2B). The 60-day mortality rates of patients with either serum creatinine–based or code-based AKI were significantly higher than patients without AKI (P < 0.0001), similar to each other, and highest in those who met both the AKI criteria (Figure 2C). A similar trend was noted in mortality after 60 days from hospitalization (Supplemental Figure 3B). The mortality rate was higher at a higher AKI stage (Supplemental Figure 3C and D).
Most AKI cases were observed within the first 5 days of hospitalization, reflecting proximity to the COVID-19 diagnosis (Supplemental Figure 2B). Compared with AKI by serum creatinine–based criteria only, the cohort with AKI by both criteria had a significantly higher proportion of more severe AKI (stages 2 and 3) (Supplemental Figure 2C). Six hundred and forty patients (1%) who received KRT were not coded for AKI (Supplemental Figure 2C).
In a secondary analysis of patients with a code-based AKI diagnosis but with <2 serum creatinine measurements (Figure 2A), the single serum creatinine measure (when available) tended to be higher than the rest of the cohort's admission serum creatinine (Figure 2B). Approximately 30% of these patients died within 20 days of hospitalization (Supplemental Figure 3A).
Temporal and Regional Trends of COVID-19–Associated AKI and Mortality
The overall incidence and severity of AKI were highest in P1 and gradually became lower until July 2020 (Figure 3). Most of the AKI cases in the Northeast were observed during P1, while in other regions, the highest number of cases were observed during the winters of 2020 and 2021 (Figure 3A).
The number of deaths in each period correlated with the rates of COVID-19 hospitalization in each region of the United States (Supplemental Figure 4A). The unadjusted mortality rates among patients with all stages of AKI saw peaks in P1, P3, and P5 periods (Supplemental Figure 4B).
The comparative characteristics of patients in the four regions and six periods are provided in Supplemental Tables 3 and 4, respectively. By region, patients in the Northeast were older and had a higher proportion of non-White patients, the West had the highest proportion of Hispanic patients, and the South had the highest proportion of Black patients and patients with comorbidities (Supplemental Table 3). Mortality rates were among the lowest in the Midwest overall.
The periods with the highest death rates (P1 and P3) also had the oldest mean age (Supplemental Table 4). The highest relative numbers of male patients, non-White patients, and patients of Hispanic heritage were in P1. The relative frequency of all comorbid diseases was the highest in P3.
Risk Factors of COVID-19–Associated AKI
Compared with patients without AKI, those with AKI were more likely to be older, male, non-White, have greater comorbidity burden, and have greater severity of illness (Table 1). In a multivariable model based on the primary cohort of 336,473 patients (Figure 1), older (OR, 1.03; 95% confidence interval [CI], 1.03 to 1.03), male sex (OR, 1.25; 95% CI, 1.23 to 1.26), Black (OR, 1.28; 95% CI, 1.26 to 1.30), Asian races (OR, 1.11; 95% CI, 1.07 to 1.16), and P1 showed association with higher odds of AKI (Table 2). In P1, all other regions had higher adjusted AKI odds compared with the Midwest (Figure 4). After P1, the odds of AKI were higher in the South and West compared with the Northeast and Midwest. Multivariable analysis of AKI in a subgroup of 165,401 patients with body mass index and comorbidity data did not differ meaningfully from the unadjusted analysis (Supplemental Figure 5).
Risk Factors of Mortality in Patients Hospitalized with COVID-19
In a multivariable analysis of mortality, AKI defined by both definitions (HR, 3.26; 95% CI, 3.18 to 3.35), serum creatinine–based AKI alone (HR, 2.49; 95% CI, 2.43 to 2.56) and code-based AKI alone (HR, 2.54; 95% CI, 2.45 to 2.63) were all strongly associated with higher hazards compared with patients without AKI (Table 3). Furthermore, mortality risk was higher with greater severity of AKI (Supplemental Table 7), older age (HR, 1.04; 95% CI, 1.04 to 1.04), male sex (HR, 1.08; 95% CI, 1.06 to 1.10), and all indicators of COVID-19 severity. Patients who did not identify themselves as White compared with White and patients who identified themselves as Hispanic compared with patients who did not identify themselves as Hispanic had a lower mortality risk (Table 3). White patients seemed to be older than patients of other races (Supplemental Table 4), but there was no difference in follow-up (Supplemental Figure 7A). Compared with P1, P3 and P6 periods had higher, while P2, P4, and P5 had lower association with mortality.
We also repeated this analysis using body mass index and comorbidity as additional explanatory variables for the subset of patients available (Supplemental Figure 6). Hypertension, which was not associated with mortality in Supplemental Figure 7A, was found to co-occur with other comorbidities on average in more than 85% (Supplemental Figure 7B).
When studying mortality in patients with AKI (Supplemental Table 6), we observed that older age, male sex, White race, and all markers of severity of illness were associated with death in patients with AKI. Those in the Midwest had lower AKI-related death compared with the other three regions.
Discussion
Using a large, geographically diverse cohort of patient data, we rigorously evaluated AKI incidence, geographic and temporal trends, risk factors, and mortality in patients hospitalized with COVID-19 over 2 years since the start of the pandemic. We observed that the incidence and severity of AKI, as well as mortality, were lower after P1 (first wave of the pandemic in the United States). This might be related to the characteristics of the patients within this period (P1), that is, a greater relative proportion of male patients and greater severity of illness compared with the subsequent periods. This observation might also reflect improved management of COVID-19, changes in virulence of SARS-CoV-2, or changing case-mix of hospitalized patients. While the initiation of mass vaccinations since February 2021 might have played a role, the incidence and severity of AKI were already lower in the P2 period, before approval of COVID-19 vaccines in the United States. A trend of higher adjusted AKI odds was noted in the recent P5 and P6 periods, where we saw the emergence of the delta and omicron variants.
The spikes in AKI cases in each geographical region of the United States correlated with waves of COVID-19 in each region in the last 2 years. Compared with the Midwest, the Northeast, South, and West had higher adjusted AKI odds in P1, subsequently the South and West regions continued to have the highest AKI odds. These observations might also reflect the patients' characteristics. The South had the highest proportions of Black patients and those with comorbidities. The West had the highest proportions of male patients and patients who identified themselves as Hispanic. Both regions had patients with greater severity of illness compared with the Northeast and Midwest. It is possible that other regional differences such as the type of medical centers included in the N3C database, rates of vaccinations, and prevalence of specific SARS-CoV-2 variants might have also played a role in the geographical variations.
In multivariable models, we observed that established risk factors such as older age, male sex, and Black race were associated with AKI in patients with COVID-19. AKI was highly associated with mortality, and higher stage of AKI was associated with a greater risk of death. Consistent with previous studies,22 older age, male sex, and severity of illness were associated with higher mortality overall and in patients with AKI. AKI-associated death was the highest in periods P1 and P3, which correlated with the first and second national wave of the COVID pandemic. Similar to findings from a recent study,23 our multivariable models showed that non-White race and Hispanic ethnicity were associated with a lower adjusted mortality risk. However, because this analysis included AKI as an independent variable (with a HR near 2.5), this finding should be interpreted in the context of the 1.28 OR for developing AKI that we saw for Black patients (Table 2). In addition, these observations should be interpreted considering the limitations of this study. Importantly, a significant number of patients in our cohort had no information on race (13%) or ethnicity (8%), as explained in Supplemental Methods. In addition, our cohort consisted of only hospitalized patients, and we do not have data on AKI incidence and mortality before index hospitalization or in those who were never hospitalized. It is possible that Black and Hispanic patients with less access to care had higher out-of-hospital mortality or were hospitalized in centers not part of the N3C and therefore not represented in our data.
Compared with published reports using only diagnostic codes, studies using a serum creatinine–based definition reported a higher incidence of AKI.14,24 In our study, 17% of cases met the serum creatinine–based definition but were not coded for AKI, of whom 70% had only stage 1 AKI. It is possible that patients with mild AKI were clinically diagnosed with AKI, but these diagnoses were not accurately captured as billing codes. Uncoded AKI cases had a similar association with mortality as those coded for AKI and significantly higher mortality than those without AKI, thereby confirming clinical significance. By contrast, we observed that almost 7% of cases were coded for AKI but not identified by the serum creatinine criteria. However, as evident in Figure 2B, the mean serum creatinine at admission for those with code-based AKI only was around 1.5 mg/dl, suggesting objective evidence of AKI. It is possible that some of these patients were coded for AKI by clinicians using information not available in our dataset, for example, urine output or baseline serum creatinine obtained through other resources. Previous studies of COVID-19–associated AKI estimated 32% of hospitalized veterans had AKI in 2020.12 The incidence rates of AKI in our study were higher (38%); however, our estimates would be lower (22%) if restricted to use of diagnostic codes alone. We propose that future EHR-based studies of COVID-19–associated AKI use both criteria of AKI diagnosis to capture the maximum number of clinically significant cases.
Our study has several limitations in addition to those mentioned above. This is an observational study; hence, causality cannot be determined. While excluding patients without serum creatinine values may affect our estimates of AKI, our population is hospitalized patients. Presumably, routine blood work should measure serum creatinine levels at least one time per person (Figure 1). The number of patients excluded for lack of serum creatinine data were low (N=88,555). While we controlled for the major risk factors for AKI and mortality in our multivariable models, residual confounding due to other factors such as laboratory biomarkers and medications is possible. We show that using both serum creatinine–based and code-based criteria for AKI diagnosis reduces false-negative cases of AKI; however, we might have missed a significant number of AKI cases because of the lack of data on urine output. While data from all geographical regions of the United States are represented, there is a relative lack of data from nonacademic medical centers. N3C data are a diverse but not necessarily representative mixture of individual patient experiences, risks, providers, and system practice patterns, as viewed through the lens of EHR-based data ingestion and harmonization processes. Additional survival analyses that modeled intersite heterogeneity did not alter findings. Our study also did not include data from other countries that may show different patients and viral characteristics25; hence, our findings might not be reflective of COVID-19–associated AKI patterns in other world regions. We are also likely missing many deaths occurring after hospital discharge. At the time of this study, we did not have reliable complete data on vaccinations.
In conclusion, we report that the incidence of COVID-19–associated AKI has decreased after the first wave of the pandemic in the United States, and regional and temporal differences in AKI and mortality rates were observed.
Supplementary Material
Acknowledgments
The primary study sponsors are multiple institutes of the National Institutes of Health. The NCATS is the primary steward of the N3C data, created the underlying architecture of the N3C Data Enclave (covid.cd2h.org/enclave), manages the Data Transfer Agreements and Data Use Agreements, houses the Data Access Committee, and supports contracts to vendors to help build various aspects of the N3C Data Enclave. This research was possible because of the patients whose information is included within the data from participating organizations (covid.cd2h.org/dtas) and the organizations and scientists (covid.cd2h.org/duas) who have contributed to the ongoing development of this community resource.15 The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The analyses described in this publication were conducted with data or tools accessed through the NCATS N3C Data Enclave and supported by NCATS U24 TR002306 and NHLBI OTA OT2HL161847 grants. Authorship was determined using ICMJE recommendations.
The contents, views or opinions expressed in this publication or presentation are those of the author(s) and do not necessarily reflect official policy or position of Uniformed Services University of the Health Sciences or the National Institutes of Health, the Departments of Health & Human Services (HHS) or Defense (DoD), or Departments of the Army, Navy, or Air Force. Mention of trade names, commercial products, or organizations does not imply endorsement by the US Government.
Footnotes
Members of the N3C and RECOVER Consortia: Andrew Girvin, Christopher Chute, Davera Gabriel, Emily Pfaff, Harold Lehmann, Jacob Wooldridge, Janos Hajagos, Katie Bradwell, Melissa Haendel, Peter DeWitt, Siao Sun, and Tellen Bennett.
R.A.M. and F.M.K. jointly supervised this work.
Contributor Information
Collaborators: Andrew Girvin, Christopher Chute, Davera Gabriel, Emily Pfaff, Harold Lehmann, Jacob Wooldridge, Janos Hajagos, Katie Bradwell, Melissa Haendel, Peter DeWitt, Siao Sun, and Tellen Bennett
Disclosures
J.B. Byrd reports consultancy for Nonin and PhaseBio; patents US15/611,427 Filing date: 2017-06-01—Legal status Pending; serving as an editorial board member of the Journal of Clinical and Translational Endocrinology (Elsevier) and an Associate Editor of the Journal of Human Hypertension (Springer-Nature) and an Associate Editor of Hypertension Research (Springer-Nature). J.B. Byrd has served on an advisory board for PhaseBio and is funded by the NIH and PCORI. D.H. Ellison reports employment with Portland VA Medical Center; honoraria from Renaissance School of Medicine, Boston University School of Medicine; royalties as an author for UpToDate; serving as Consulting Editor for Hypertension; and serving on the Editorial Boards of American Journal of Physiology-Renal Physiology and JASN. Y. He reports ownership interest in Ontmed, Inc.; serving as an advisory committee member of China Biomedical Ontology Consortium (i.e., OntoChina) 2017–present, host and co-chair for ICBO 2022: International Conference on Biomedical Ontology, and 2020–2022 President elect of Overseas Chinese Society for Microbiology (Sino-Micro); and serving as an Associate Editor for Frontier in Cellular and Infection Microbiology and on the Editorial Board of Scientific Data (a Nature journal). S.S. Hong's spouse is a founder of ProtonBio, Inc.; spouse holds patents and have pending patents on chemical compounds that have potential to cure diseases and has received royalties from Partners Health, MGH, for licensed assets to Keros Therapeutics; spouse is a board member of American Heart Association of Greater Maryland and Baltimore, board of directors of Sarnoff Cardiovascular Research Foundation, and editorial board member of Frontiers in Drug Discovery Journal—he is not paid for these roles. S.L. Kane-Gill reports advisory or leadership roles for Executive Committee of Society of Critical Care Medicine and on the Editorial Board of Annals of Pharmacotherapy and Critical Care Medicine and grant funding from NIDDK. F. Liu reports ownership interest in Alibaba, Amazon, Apple, Costco, Palantir Technologies, and Robinhood. S.K. Mallipattu reports consultancy for L.E.K and Wildwood Therapeutics, Inc. Consulting; research funding from Dialysis Clinic Inc. and Spectral Medical Inc.; patents Krüppel-like factor 15 (KLF15) Small Molecule Agonists in Kidney Disease. US 63/018.247. April 30, 2021; and advisory or leadership role for Clinically Integrated Network, Board Member (Accountable Care Organization, LLC Stony Brook Medicine). R.A. Moffitt reports employment with Cold Spring Harbor Laboratories; consultancy for Data Driven Biosciences and Stottler Henke Associates; ownership interest in Aflac, Global Payments Inc; and patents or royalties from GeneCentric. C.R. Parikh reports consultancy for Genfit Biopharmaceutical Company and Novartis; is a member of the advisory board of and owns equity in RenalytixAI; reports research funding from National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and National Heart, Lung and Blood Institute (NHLBI); and serves advisory or leadership roles for Genfit Biopharmaceutical Company and Renalytix. A. Sakhuja reports advisory or leadership role for Carolinas/Virginia's chapter of SCCM—unpaid and current funding from NIH/NIDDK 1K08DK131286-01A1 (PI: Ankit Sakhuja) and past funding from NIH/NIGMS 5U54GM104942 (PI: Sally Hodder). J.H. Saltz reports honoraria from Mark Foundation and Memorial Sloan Kettering and advisory or leadership roles for Morehouse School of Medicine and Mark Foundation—both are academic, neither are companies. R. Saran reports serving as an American Nephrologists of Indian Origin (ANIO) Steering Committee Member and World Federation of Non Communicable Diseases International Advisory Council member. R. Saran is PI on subcontract on a grant awarded to the National Kidney Foundation of Michigan (NKFM). S. Setoguchi reports consultancy for BMS, Medtronic, Merck Inc., Pfizer Inc., and Pfizer Japan; research funding from BMS, Daiichi Sankyo, Pfizer Inc., and Pfizer Japan; and serving on the Editorial Board for Pharmacoepidemiology and Drug Safety. L.A. Torre-Healy reports an advisory or leadership role for Latino Medical Student Association (unpaid). K.J. Wilkins reports employment with National Institute of Diabetes & Digestive & Kidney Diseases; advisory or leadership role for International Journal of Obesity editorial board (unpaid); and previously made commitments to involve members of kidney patient/advocacy organizations in kidney research conferences or technical expert panels: American Association of Kidney Patients (through Board member Jenny Kitsen), Renal Support Network (through President and Founder Lori Hartwell), and only correspondence with similar organizations or advocates within last 24 months for Voice of the Patient (through Kevin Fowler) and Hassanah Consulting (through Janice Tufte). Y.J. Yoo reports research funding from NIH. All remaining authors have nothing to disclose.
Funding
This research was funded by the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) Grant/Award Number: “OTA OT2HL161847” as part of the Researching COVID to Enhance Recovery (RECOVER) research program. This work was supported by National Center for Advancing Translational Sciences (NCATS) Grant/Award Number: “U24 TR002306” (Y.J.Y., S.K., R.A.M., F.M.K.), as well as the National Institute for Diabetes & Digestive & Kidney Diseases (Office of the Director, K.J.W.), as part of the N3C program.
Author Contributions
Conceptualization: Fadhl Alakwaa, James Brian Byrd, David H. Ellison, Yongqun He, Stephanie S. Hong, Sandra L. Kane-Gill, Farrukh M. Koraishy, Spencer Krichevsky, Feifan Liu, Sandeep K. Mallipattu, Richard A. Moffitt, Chirag R. Parikh, Chetan K. Potu, Ankit Sakhuja, Joel H. Saltz, Rajiv Saran, Soko Setoguchi, Luke A. Torre-Healy, Kenneth J. Wilkins, Yun Jae Yoo, Richard L. Zhu.
Data curation: Stephanie S. Hong, Spencer Krichevsky, Richard A. Moffitt, Chetan K. Potu, Kenneth J. Wilkins, Yun J. Yoo.
Formal analysis: Fadhl Alakwaa, James Brian Byrd, David H. Ellison, Stephanie S. Hong, Feifan Liu, Sandra L. Kane-Gill, Farrukh M. Koraishy, Spencer Krichevsky, Richard A. Moffitt, Chirag R. Parikh, Chetan K. Potu, Luke A. Torre-Healy, Rajiv Saran, Soko Setoguchi, Kenneth J. Wilkins, Yun J. Yoo.
Funding acquisition: Richard A. Moffitt.
Investigation: Fadhl Alakwaa, James Brian Byrd, David H. Ellison, Yongqun He, Stephanie S. Hong, Sandra L. Kane-Gill, Farrukh M. Koraishy, Spencer Krichevsky, Feifan Liu, Sandeep K. Mallipattu, Richard A. Moffitt, Chirag R. Parikh, Chetan K. Potu, Ankit Sakhuja, Joel H. Saltz, Rajiv Saran, Soko Setoguchi, Luke A. Torre-Healy, Kenneth J. Wilkins, Yun Jae Yoo, Richard L. Zhu.
Methodology: Stephanie S. Hong, Farrukh M. Koraishy, Spencer Krichevsky, Chirag R. Parikh, Soko Setoguchi, Richard A. Moffitt, Kenneth J. Wilkins, Yun Jae Yoo.
Project administration: Farrukh M. Koraishy, Richard A. Moffitt.
Resources: Stephanie S. Hong, Farrukh M. Koraishy, Richard A. Moffitt, Chetan K. Potu, Kenneth J. Wilkins, Yun J. Yoo.
Software: Stephanie S. Hong, Spencer Krichevsky, Luke A. Torre-Healy, Kenneth J. Wilkins, Yun J. Yoo.
Supervision: Farrukh M. Koraishy, Richard A. Moffitt.
Validation: Fadhl Alakwaa, James Brian Byrd, David H. Ellison, Yongqun He, Stephanie S. Hong, Sandra L. Kane-Gill, Farrukh M. Koraishy, Spencer Krichevsky, Feifan Liu, Sandeep K. Mallipattu, Richard A. Moffitt, Chirag R. Parikh, Chetan K. Potu, Ankit Sakhuja, Joel H. Saltz, Rajiv Saran, Soko Setoguchi, Luke A. Torre-Healy, Kenneth J. Wilkins, Yun Jae Yoo, Richard L. Zhu.
Visualization: Richard A. Moffitt, Yun Jae Yoo.
Writing – original draft: Farrukh M. Koraishy, Richard A. Moffitt, Kenneth J. Wilkins, Yun J. Yoo.
Writing – review & editing: Fadhl Alakwaa, James B. Byrd, David H. Ellison, Yongqun He, Sandra L. Kane-Gill, Farrukh M. Koraishy, Feifan Liu, Sandeep K. Mallipattu, Richard A. Moffitt, Chirag R. Parikh, Ankit Sakhuja, Joel H. Saltz, Rajiv Saran, Soko Setoguchi, Kenneth J. Wilkins, Yun J. Yoo, Richard L. Zhu.
Data Sharing Statement
All data are included in the manuscript and/or supporting materials.
Data Partners with Released Data: Stony Brook University—U24TR002306 • University of Oklahoma Health Sciences Center—U54GM104938: Oklahoma Clinical and Translational Science Institute (OCTSI) • West Virginia University—U54GM104942: West Virginia Clinical and Translational Science Institute (WVCTSI) • University of Mississippi Medical Center—U54GM115428: Mississippi Center for Clinical and Translational Research (CCTR) • University of Nebraska Medical Center—U54GM115458: Great Plains IDeA-Clinical & Translational Research • Maine Medical Center—U54GM115516: Northern New England Clinical & Translational Research (NNE-CTR) Network • Wake Forest University Health Sciences—UL1TR001420: Wake Forest Clinical and Translational Science Institute • Northwestern University at Chicago—UL1TR001422: Northwestern University Clinical and Translational Science Institute (NUCATS) • University of Cincinnati—UL1TR001425: Center for Clinical and Translational Science and Training • The University of Texas Medical Branch at Galveston—UL1TR001439: The Institute for Translational Sciences • Medical University of South Carolina—UL1TR001450: South Carolina Clinical & Translational Research Institute (SCTR) • University of Massachusetts Medical School Worcester—UL1TR001453: The UMass Center for Clinical and Translational Science (UMCCTS) • University of Southern California—UL1TR001855: The Southern California Clinical and Translational Science Institute (SC CTSI) • Columbia University Irving Medical Center—UL1TR001873: Irving Institute for Clinical and Translational Research • George Washington Children's Research Institute—UL1TR001876: Clinical and Translational Science Institute at Children's National (CTSA-CN) • University of Kentucky—UL1TR001998: UK Center for Clinical and Translational Science • University of Rochester—UL1TR002001: UR Clinical & Translational Science Institute • University of Illinois at Chicago—UL1TR002003: UIC Center for Clinical and Translational Science • Penn State Health Milton S. Hershey Medical Center—UL1TR002014: Penn State Clinical and Translational Science Institute •The University of Michigan at Ann Arbor—UL1TR002240: Michigan Institute for Clinical and Health Research • Vanderbilt University Medical Center—UL1TR002243: Vanderbilt Institute for Clinical and Translational Research • University of Washington • UL1TR002319: Institute of Translational Health Sciences • Washington University in St. Louis—UL1TR002345: Institute of Clinical and Translational Sciences • Oregon Health & Science University—UL1TR002369: Oregon Clinical and Translational Research Institute • University of Wisconsin-Madison—UL1TR002373: UW Institute for Clinical and Translational Research • Rush University Medical Center—UL1TR002389: The Institute for Translational Medicine (ITM) • The University of Chicago—UL1TR002389: The Institute for Translational Medicine (ITM) • University of North Carolina at Chapel Hill—UL1TR002489: North Carolina Translational and Clinical Science Institute • University of Minnesota—UL1TR002494: Clinical and Translational Science Institute • Children's Hospital Colorado—UL1TR002535: Colorado Clinical and Translational Sciences Institute • The University of Iowa—UL1TR002537: Institute for Clinical and Translational Science • The University of Utah—UL1TR002538: Uhealth Center for Clinical and Translational Science • Tufts Medical Center—UL1TR002544: Tufts Clinical and Translational Science Institute • Duke University—UL1TR002553: Duke Clinical and Translational Science Institute • Virginia Commonwealth University—UL1TR002649: C. Kenneth and Dianne Wright Center for Clinical and Translational Research • The Ohio State University—UL1TR002733: Center for Clinical and Translational Science • The University of Miami Leonard M. Miller School of Medicine—UL1TR002736: University of Miami Clinical and Translational Science Institute • University of Virginia—UL1TR003015: iTHRIV Integrated Translational health Research Institute of Virginia • Carilion Clinic—UL1TR003015: iTHRIV Integrated Translational health Research Institute of Virginia • University of Alabama at Birmingham—UL1TR003096: Center for Clinical and Translational Science • Johns Hopkins University—UL1TR003098: Johns Hopkins Institute for Clinical and Translational Research • University of Arkansas for Medical Sciences—UL1TR003107: UAMS Translational Research Institute • Nemours—U54GM104941: Delaware CTR ACCEL Program • University Medical Center New Orleans—U54GM104940: Louisiana Clinical and Translational Science (LA CaTS) Center • University of Colorado Denver, Anschutz Medical Campus—UL1TR002535: Colorado Clinical and Translational Sciences Institute • Mayo Clinic Rochester—UL1TR002377: Mayo Clinic Center for Clinical and Translational Science (CCaTS) • Tulane University—UL1TR003096: Center for Clinical and Translational Science • Loyola University Medical Center—UL1TR002389: The Institute for Translational Medicine (ITM) • Advocate Health Care Network—UL1TR002389: The Institute for Translational Medicine (ITM) • OCHIN—INV-018455: Bill and Melinda Gates Foundation grant to Sage Bionetworks.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/CJN/B769.
Supplemental Table 1. The defined list of concepts including visit type, race, ethnicity, comorbidities (AKI, diabetes mellitus, hypertension, cardiovascular disease, heart failure, kidney failure, sepsis), procedures (invasive mechanical ventilation), drugs (vasopressors, dexamethasone, remdesivir, tocilizumab), and additional ICD codes for AKI diagnosis.
Supplemental Table 2. Descriptive characteristics of hospitalized COVID-positive patients with and without AKI by two different AKI definitions.
Supplemental Table 3. Descriptive characteristics of each region.
Supplemental Table 4. Descriptive characteristics of each period.
Supplemental Table 5. Descriptive characteristics of each racial group.
Supplemental Table 6. Descriptive characteristics of deceased and survived patients with AKI.
Supplemental Table 7. Association between AKI and mortality, adjusted for the severity of AKI, any code-based AKI, age, sex, race, ethnicity, severity markers, and timing of initial COVID infection: raw count and percentage of deaths, unadjusted and adjusted hazard ratios.
Supplemental Figure 1. A bar chart visualizing site-level filtering visualizes the characteristics of each data partner and their status before and after filtering.
Supplemental Figure 2. (A) Venn diagrams showing patients meeting different SCr-based AKI definitions. (B) Comparison between the time of onset of AKI from the date of hospitalization and the length of hospitalization of patients (code-based AKI for all patients with AKI onset longer than the length of hospitalization). Color means the number of patients, darker the more patients. (C) Comparison of severity between patients meeting both AKI criteria versus those meeting only serum creatinine criteria.
Supplemental Figure 3. (A) First 60-day survival for patients diagnosed with AKI but with insufficient serum creatinine data to calculate a change. (B) Post–60-day survival for different AKI definitions. (C) First 60-day survival by KDIGO-based AKI stages and code-based AKI. (D) Post–60-day survival by KDIGO-based AKI stages and code-based AKI.
Supplemental Figure 4. (A) Comparison of unadjusted mortality rates (and 95% confidence intervals), between regional and time frames. (B) Observed mortality rates (and 95% confidence intervals), within different severities of AKI, for six periods.
Supplemental Figure 5. Multivariable analysis of COVID-19–related AKI risk in patients with BMI.
Supplemental Figure 6. (A) Multivariable survival analysis of 165,401 patients using the Cox proportional hazards (CoxPH) model BMI and comorbidity. (B) CoxPH multivariable analysis including only hypertension with BMI.
Supplemental Figure 7. (A) Follow-up comparison between race groups. (B) Venn diagram of comorbidities (hypertension, diabetes mellitus, heart failure, cardiovascular disease).
References
- 1.Adam D.. The pandemic's true death toll: millions more than official counts. Nature. 2022;601(7893):312–315. doi: 10.1038/d41586-022-00104-8 [DOI] [PubMed] [Google Scholar]
- 2.Stony Brook COVID-19 Research Consortium. Geospatial distribution and predictors of mortality in hospitalized patients with COVID-19: a cohort study. Open Forum Infect Dis. 2020;7(10):ofaa436. doi: 10.1093/ofid/ofaa436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirsch JS, Ng JH, Ross DW, et al. Acute kidney injury in patients hospitalized with covid-19. Kidney Int. 2020;98(1):209–218. doi: 10.1016/j.kint.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020;323(20):2052. doi: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubin S, Orieux A, Prevel R, et al. Characterization of acute kidney injury in critically ill patients with severe coronavirus disease 2019. Clin Kidney J. 2020;13(3):354–361. doi: 10.1093/ckj/sfaa099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chao CT, Tsai HB, Wu CY, et al. The severity of initial acute kidney injury at admission of geriatric patients significantly correlates with subsequent in-hospital complications. Sci Rep. 2015;5(1):13925. doi: 10.1038/srep13925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anesi GL, Jablonski J, Harhay MO, et al. Characteristics, outcomes, and trends of patients with COVID-19-related critical illness at a learning health system in the United States. Ann Intern Med. 2021;174(5):613–621. doi: 10.7326/m20-5327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Youfei Y, Tian G, Thomas SV, Bhramar M, Lars GF, et al. Changes in COVID-19-related outcomes, potential risk factors and disparities over time. Epidemiol Infect. 2021;149(e192):1–11. doi: 10.1017/S0950268821001898 [DOI] [Google Scholar]
- 10.CDC Prevention. Trends in Number of COVID-19 Cases and Deaths in the US Reported to CDC, by State/Territory. COVID Data Tracker Web Site. 2021. Accessed November 1, 2021. https://covid.cdc.gov/covid-data-tracker/#trends_dailydeaths [Google Scholar]
- 11.Bennett TD, Moffitt RA, Hajagos JG, et al. Clinical characterization and prediction of clinical severity of SARS-CoV-2 infection among US adults using data from the US National COVID Cohort Collaborative. JAMA Netw Open. 2021;4(7):e2116901. doi: 10.1001/jamanetworkopen.2021.16901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowe B, Cai M, Xie Y, Gibson AK, Maddukuri G, Al-Aly Z, et al. Acute kidney injury in a national cohort of hospitalized US veterans with COVID-19. Clin J Am Soc Nephrol. 2020;16(1):14–25. doi: 10.2215/CJN.09610620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haendel MA, Chute CG, Bennett TD, et al. The National COVID Cohort Collaborative (N3C): rationale, design, infrastructure, and deployment. J Am Med Inform Assoc. 2021;28(3):427–443. doi: 10.1093/jamia/ocaa196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J, et al. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol. 2014;9(4):682–689. doi: 10.2215/CJN.07650713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waikar SS, Wald R, Chertow GM, et al. Validity of international classification of diseases, ninth revision, clinical modification codes for acute renal failure. J Am Soc Nephrol. 2006;17(6):1688–1694. doi: 10.1681/ASN.2006010073 [DOI] [PubMed] [Google Scholar]
- 16.Turan A, Cohen B, Adegboye J, et al. Mild acute kidney injury after noncardiac surgery is associated with long-term renal dysfunction: a retrospective cohort study. Anesthesiology. 2020;132(5):1053–1061. doi: 10.1097/aln.0000000000003109 [DOI] [PubMed] [Google Scholar]
- 17.Yang Y, Ma J, et al. Mild AKI is associated with mortality of patients who underwent cardiopulmonary bypass surgery. Exp Ther Med. 2020;20(4):2969–2974. doi: 10.3892/etm.2020.9039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin B, DeWitt PE, Russell S, et al. Characteristics, Outcomes, and Severity Risk Factors Associated With SARS-CoV-2 Infection Among Children in the US National COVID Cohort Collaborative. JAMA Netw Open. 2022; 5(2):e2143151. doi: 10.1001/jamanetworkopen.2021.43151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wickham H, et al. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag; New York; 2016. https://ggplot2.tidyverse.org [Google Scholar]
- 20.Therneau T. (2023). A Package for Survival Analysis in R. R package version 3.2-13. https://CRAN.R-project.org/package=survival. [Google Scholar]
- 21.Kassambara A. (2021). survminer: Drawing Survival Curves using 'ggplot2.' R package version 0.4.9. Accessed December 6, 2021. https://rpkgs.datanovia.com/survminer/index.html. [Google Scholar]
- 22.Goodman KE, Magder LS, Baghdadi JD, et al. Impact of sex and metabolic comorbidities on coronavirus disease 2019 (COVID-19) mortality risk across age groups: 66 646 inpatients across 613 U.S. hospitals. Clin Infect Dis. 2021;73(11):e4113–e4123. doi: 10.1093/cid/ciaa1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenthal N, Cao Z, Gundrum J, Sianis J, Safo S, et al. Risk factors associated with in-hospital mortality in a US national sample of patients with COVID-19. JAMA Netw Open. 2020;3(12):e2029058. doi: 10.1001/jamanetworkopen.2020.29058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu CY, McCulloch CE, Fan D, Ordonez JD, Chertow GM, Go AS, et al. Community-based incidence of acute renal failure. Kidney Int. 2007;72(2):208–212. doi: 10.1038/sj.ki.5002297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y Zhang F Byrd JB Yu H Ye X He Y.. Differential COVID-19 symptoms given pandemic locations, time, and comorbidities during the early pandemic. Front Med (Lausanne). 2022;9:770031. doi: 10.3389/fmed.2022.770031 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are included in the manuscript and/or supporting materials.
Data Partners with Released Data: Stony Brook University—U24TR002306 • University of Oklahoma Health Sciences Center—U54GM104938: Oklahoma Clinical and Translational Science Institute (OCTSI) • West Virginia University—U54GM104942: West Virginia Clinical and Translational Science Institute (WVCTSI) • University of Mississippi Medical Center—U54GM115428: Mississippi Center for Clinical and Translational Research (CCTR) • University of Nebraska Medical Center—U54GM115458: Great Plains IDeA-Clinical & Translational Research • Maine Medical Center—U54GM115516: Northern New England Clinical & Translational Research (NNE-CTR) Network • Wake Forest University Health Sciences—UL1TR001420: Wake Forest Clinical and Translational Science Institute • Northwestern University at Chicago—UL1TR001422: Northwestern University Clinical and Translational Science Institute (NUCATS) • University of Cincinnati—UL1TR001425: Center for Clinical and Translational Science and Training • The University of Texas Medical Branch at Galveston—UL1TR001439: The Institute for Translational Sciences • Medical University of South Carolina—UL1TR001450: South Carolina Clinical & Translational Research Institute (SCTR) • University of Massachusetts Medical School Worcester—UL1TR001453: The UMass Center for Clinical and Translational Science (UMCCTS) • University of Southern California—UL1TR001855: The Southern California Clinical and Translational Science Institute (SC CTSI) • Columbia University Irving Medical Center—UL1TR001873: Irving Institute for Clinical and Translational Research • George Washington Children's Research Institute—UL1TR001876: Clinical and Translational Science Institute at Children's National (CTSA-CN) • University of Kentucky—UL1TR001998: UK Center for Clinical and Translational Science • University of Rochester—UL1TR002001: UR Clinical & Translational Science Institute • University of Illinois at Chicago—UL1TR002003: UIC Center for Clinical and Translational Science • Penn State Health Milton S. Hershey Medical Center—UL1TR002014: Penn State Clinical and Translational Science Institute •The University of Michigan at Ann Arbor—UL1TR002240: Michigan Institute for Clinical and Health Research • Vanderbilt University Medical Center—UL1TR002243: Vanderbilt Institute for Clinical and Translational Research • University of Washington • UL1TR002319: Institute of Translational Health Sciences • Washington University in St. Louis—UL1TR002345: Institute of Clinical and Translational Sciences • Oregon Health & Science University—UL1TR002369: Oregon Clinical and Translational Research Institute • University of Wisconsin-Madison—UL1TR002373: UW Institute for Clinical and Translational Research • Rush University Medical Center—UL1TR002389: The Institute for Translational Medicine (ITM) • The University of Chicago—UL1TR002389: The Institute for Translational Medicine (ITM) • University of North Carolina at Chapel Hill—UL1TR002489: North Carolina Translational and Clinical Science Institute • University of Minnesota—UL1TR002494: Clinical and Translational Science Institute • Children's Hospital Colorado—UL1TR002535: Colorado Clinical and Translational Sciences Institute • The University of Iowa—UL1TR002537: Institute for Clinical and Translational Science • The University of Utah—UL1TR002538: Uhealth Center for Clinical and Translational Science • Tufts Medical Center—UL1TR002544: Tufts Clinical and Translational Science Institute • Duke University—UL1TR002553: Duke Clinical and Translational Science Institute • Virginia Commonwealth University—UL1TR002649: C. Kenneth and Dianne Wright Center for Clinical and Translational Research • The Ohio State University—UL1TR002733: Center for Clinical and Translational Science • The University of Miami Leonard M. Miller School of Medicine—UL1TR002736: University of Miami Clinical and Translational Science Institute • University of Virginia—UL1TR003015: iTHRIV Integrated Translational health Research Institute of Virginia • Carilion Clinic—UL1TR003015: iTHRIV Integrated Translational health Research Institute of Virginia • University of Alabama at Birmingham—UL1TR003096: Center for Clinical and Translational Science • Johns Hopkins University—UL1TR003098: Johns Hopkins Institute for Clinical and Translational Research • University of Arkansas for Medical Sciences—UL1TR003107: UAMS Translational Research Institute • Nemours—U54GM104941: Delaware CTR ACCEL Program • University Medical Center New Orleans—U54GM104940: Louisiana Clinical and Translational Science (LA CaTS) Center • University of Colorado Denver, Anschutz Medical Campus—UL1TR002535: Colorado Clinical and Translational Sciences Institute • Mayo Clinic Rochester—UL1TR002377: Mayo Clinic Center for Clinical and Translational Science (CCaTS) • Tulane University—UL1TR003096: Center for Clinical and Translational Science • Loyola University Medical Center—UL1TR002389: The Institute for Translational Medicine (ITM) • Advocate Health Care Network—UL1TR002389: The Institute for Translational Medicine (ITM) • OCHIN—INV-018455: Bill and Melinda Gates Foundation grant to Sage Bionetworks.