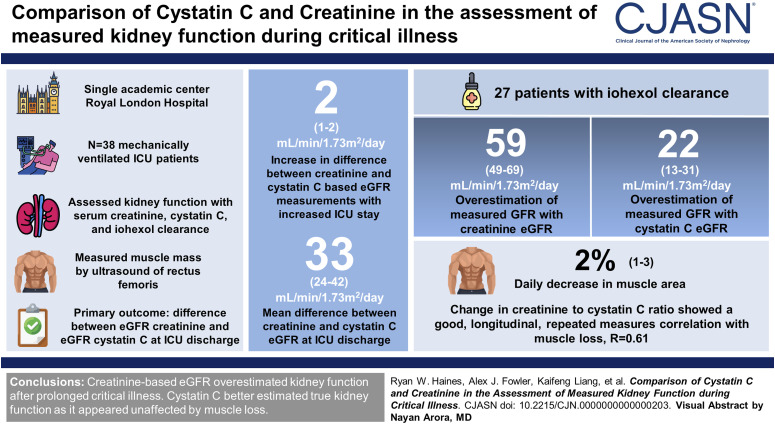
Visual Abstract
Keywords: acute kidney failure, creatinine, GFR, lean body mass, AKI and critical care
Abstract
Background
Incomplete recovery of kidney function is an important adverse outcome in survivors of critical illness. However, unlike eGFR creatinine, eGFR cystatin C is not confounded by muscle loss and may improve identification of persistent kidney dysfunction.
Methods
To assess kidney function during prolonged critical illness, we enrolled 38 mechanically ventilated patients with an expected length of stay of >72 hours near admission to intensive care unit (ICU) in a single academic medical center. We assessed sequential kidney function using creatinine, cystatin C, and iohexol clearance measurements. The primary outcome was difference between eGFR creatinine and eGFR cystatin C at ICU discharge using Bayesian regression modeling. We simultaneously measured muscle mass by ultrasound of the rectus femoris to assess the confounding effect on serum creatinine generation.
Results
Longer length of ICU stay was associated with greater difference between eGFR creatinine and eGFR cystatin C at a predicted rate of 2 ml/min per 1.73 m2 per day (95% confidence interval [CI], 1 to 2). By ICU discharge, the posterior mean difference between creatinine and cystatin C eGFR was 33 ml/min per 1.73 m2 (95% credible interval [CrI], 24 to 42). In 27 patients with iohexol clearance measured close to ICU discharge, eGFR creatinine was on average two-fold greater than the iohexol gold standard, and posterior mean difference was 59 ml/min per 1.73 m2 (95% CrI, 49 to 69). The posterior mean for eGFR cystatin C suggested a 22 ml/min per 1.73 m2 (95% CrI, 13 to 31) overestimation of measured GFR. Each day in ICU resulted in a predicted 2% (95% CI, 1% to 3%) decrease in muscle area. Change in creatinine-to-cystatin C ratio showed good longitudinal, repeated measures correlation with muscle loss, R=0.61 (95% CI, 0.50 to 0.72).
Conclusions
eGFR creatinine systematically overestimated kidney function after prolonged critical illness. Cystatin C better estimated true kidney function because it seemed unaffected by the muscle loss from prolonged critical illness.
Clinical Trial registry name and registration number:
Skeletal Muscle Wasting and Renal Dysfunction After Critical Illness Trauma - Outcomes Study (KRATOS), NCT03736005.
Introduction
An increasing number of critically ill patients require prolonged admission to intensive care unit (ICU).1,2 For those surviving to ICU discharge, many experience prolonged hospital stays, complex rehabilitation, and a higher risk of long-term mortality.3,4 Two organs consistently affected by prolonged critical illness are the kidneys and skeletal muscle; importantly, these are clinically interconnected as the sites of generation and clearance of serum creatinine, respectively.5
There is good epidemiological evidence and pathophysiological rationale linking overt or subclinical episodes of AKI with subsequent persistent decline in kidney function and the development of CKD with an associated higher risk of long-term morbidity and mortality.6,7 Similarly, muscle wasting is a prominent feature of prolonged critical illness8 and is believed to contribute to poor short-term and longer-term outcomes.9,10 These factors suggest that reliance on serum creatinine–based eGFRcreat to assess recovery of kidney function may be confounded by coexistent pathophysiological reduction in muscle mass and creatinine generation, potentially masking persistent kidney dysfunction after critical illness.
Inaccuracy in the measurement of kidney function during and after prolonged critical illness has several clinical implications. First, reconciliation of chronic medications and new administration of pharmacological treatments11 depend on accurate estimations of GFR. Second, poor management of AKI and its recovery through under-recognition of secondary kidney injury may contribute to worse outcomes during and after critical illness.12 Third, nonrecovery of kidney dysfunction may be missed, resulting in missed opportunities for evidence-based interventions to limit CKD progression and reduce cardiovascular risk.6
More generally, it remains unclear as to what extent the trajectory of health after discharge from ICU is determined by the development of underlying comorbidity13 or the ongoing detrimental contribution of prolonged critical illness. In ICU survivors, cystatin C, a marker of GFR independent of muscle mass, has been shown to strongly associate with post-ICU survival and the later development of overt kidney dysfunction.14,15 Furthermore, cystatin C in combination with serum creatinine may enable estimation of muscle mass and thus quantification of muscle wasting.16
Accordingly, we used a sample of general critically ill patients to assess in parallel the effect of critical illness on both kidney function and muscle mass. Our aims were two-fold: First, to assess the difference between cystatin C–based assessment of kidney function and the current best practice using creatinine-based measurements and second, to assess the degree of muscle loss in patients with prolonged critical illness and the effect of this on creatinine and cystatin C measurements.
Methods
Study Population and Setting
This study was conducted at The Royal London Hospital, a tertiary academic ICU serving as a major trauma center in London, United Kingdom, between January 2019 and August 2020 (https://ClinicalTrials.gov/ct2/show/NCT03736005). The Joint Research and Management Office at Queen Mary University of London approved and sponsored the study protocol, and ethical approval was granted by a national research Ethics Committee (Wales REC 6, ref: 18/WA/0304). Informed consent was obtained from patients or their decision makers before enrollment.
We identified eligible patients by regular screening (Monday–Friday). We enrolled patients 18 years and older admitted to ICU, anticipated to be mechanically ventilated for ≥48 hours, and considered likely to survive to ICU discharge by the treating physician. We excluded patients with major traumatic brain injury (abbreviated injury scale head injury score ≥5), spinal cord injury with paralysis, lower limb amputation, end-stage kidney disease or disseminated cancer, and lack of independence with activities of daily living or nonambulatory status before admission. We defined a subset of patients with major trauma, defined as a new injury severity score of ≥15.
Study Procedures
Serum creatinine and serum cystatin C were used to calculate eGFRcreat and eGFRcys in ml/min per 1.73 m2 using the new Chronic Kidney Disease Epidemiology Collaboration formula.17 Creatinine was measured in mg/dl and analyzed with isotope dilution mass spectrometry–calibrated methods in The Royal London Hospital laboratory. Cystatin C was determined using a turbidimetric method (Gentian Cystatin-c UDR-Kit for Beckman-Coulter Synchron and UniCel Systems, Ref A52761, Moss, Norway). We excluded cystatin C and creatinine measurements during or shortly after KRT. Measurements of eGFR occurred at regular time points (days 1, 3, 5, 7, 10, ICU discharge, and at 1 week after ICU discharge). In a subset of patients, measured GFR at ICU discharge was assessed using a single time point (4 hour) iohexol clearance method. Iohexol plasma clearance has been endorsed as a gold standard method for the evaluation of GFR in patients with CKD by the National Institute of Health and Care Excellence CKD guidelines (G73 1.1.6). Serum iohexol concentration was determined by ultra-high performance liquid chromatography separation and ultraviolet detection in Uppsala, Sweden. The formula for single time point iohexol clearance by Jacobsson was used.18
The rectus femoris cross-sectional area was measured using B-mode ultrasound on study assessment days, as previously described.8 Further sample processing and muscle measurement details are provided in the Supplemental Methods.
Clinical Risk Factors
Demographic data, comorbidities, and admission diagnosis were collected at baseline by investigators. Ethnicity was reported using the National Health Service (NHS) standard categorization because there are potential ethnicity imbalances in the incidence of AKI and CKD in our population.19 We screened electronic health records for historical creatinine measurements and for any prerecorded CKD diagnoses. Severity of Organ Failure Assessment score and AKI stages, using the Kidney Disease Improving Global Outcomes 2012 criteria, were calculated daily for the first 7 days of this study. We assessed patients for acute kidney disease at ICU discharge (defined as the presence of AKI stage, increase in serum creatinine by >50%, or an acute eGFRcreat <60 ml/min per 1.73 m2).20 KRT was delivered as a continuous KRT for all patients. All patients are fed enterally as soon as possible, unless contraindicated. This follows the European Society for Clinical Nutrition and Metabolism guidelines for calories delivered, feed constitution, and use of prokinetic drugs.
Statistical Analysis
Data processing, analyses, and plotting were made in R version 3.6.1 with packages used detailed in the supplement. Data were expressed as mean (SD), median (interquartile range [IQR]), and absolute and relative frequencies, as indicated. The cohort was divided into two groups on the basis of being a trauma or nontrauma admission to ICU. Clinical characteristics, changes in eGFR, and muscle mass trajectory are reported for the whole cohort and compared between groups. We originally planned to enroll 62 mechanically ventilated patients (see online supplement for sample size considerations); however, planned recruitment curtailed because of the coronavirus disease 2019 pandemic.
The primary outcome was the difference between eGFRcreat and eGFRcys at ICU discharge compared using Bayesian regression modeling. The results include the posterior distribution of the difference between eGFRcreat and eGFRcys, given data, and the priors from which we calculated the posterior mean difference and a 95% credible interval (CrI) (95% probability that the value of the unknown parameter falls in this CrI). We adopted a Bayesian approach because the calculated estimates would be less affected by the relatively small sample size and allow clearer interpretation of the results.21
We used the brms22 package in R to define a regression model with a normality parameter to include the t distribution to reduce the effect of outliers23 and chose sceptical priors, representing the prior belief that the results of eGFR equations for creatinine and cystatin C would not normally be expected to markedly differ.
There were no missing data for assessment of the primary outcome. To further investigate difference in eGFRcreat and eGFRcys over multiple time points and explore determinants of difference, we used linear mixed-effects models. This analysis is intended to compare relative magnitude over time between both filtration markers, recognizing that estimated GFR equations will not directly reflect true underlying GFR in the absence of steady state of kidney function, which may be present during the acute phase of critical illness and its recovery.24 Linear mixed-effect models incorporate repeated longitudinal measurements from all patients and allow for patient-specific random effects and can robustly handle missing data.25 We used restricted cubic splines with three knots to capture any nonlinearity from a relatively small sample size.26
As a secondary outcome, we assessed correlation between the creatinine-to-cystatin C ratio and muscle loss, as measured by rectus cross-sectional area. We analyzed within-patient correlation between the creatinine-to-cystatin C ratio using multiple regression as outlined by Bland and Altman27 using the rmcorr package in R.
Results
A total of 38 patients, comprising 22 trauma (58%) and 16 nontrauma (42%) admissions, were enrolled (Supplemental Figure 1). Three patients died and did not complete the primary outcome but were included in the longitudinal modeling. Paired serum creatinine and cystatin C measurements were recorded on 181 patient-days. Rectus femoris measurements were recorded on a total of 169 patient-days. Demographic and clinical characteristics of this study are presented in Table 1. All patients were mechanically ventilated at enrollment with a median ICU admission Severity of Organ Failure Assessment score of 4.5 (IQR, 3.0–6.8) and a median length of ICU stay of 16.5 days (10.3–27.3). A total of 35 of 38 patients were discharged to the ward.
Table 1.
Clinical characteristics of the study cohort and patient subgroups according to nontrauma and trauma admissions
| Characteristic | Overall Study Population (N=38) | Nontrauma (n=16) | Trauma (n=22) |
|---|---|---|---|
| Age, yr | 51 (38–63) | 52 (48–64) | 48 (29–61) |
| Male sex, n (%) | 25 (66) | 7 (44) | 18 (82) |
| Body mass index, admission, kg/m2 | 26.2 (23.5–28.2) | 27.0 (23.5–29.2) | 25.9 (23.8–27.1) |
| Charlson Comorbidity Index | 1.0 (0.3–1.0) | 1.0 (1.0–2.0) | 1.0 (0.0–1.0) |
| Ethnicity, n (%) | |||
| Black | 3 (8) | 2 (13) | 1 (5) |
| South Asian | 8 (21) | 3 (19) | 5 (23) |
| White | 27 (71) | 11 (68) | 16 (73) |
| Primary diagnosis, n (%) | |||
| Cardiovascular | 1 (3) | 1 (6) | |
| Gastrointestinal | 3 (8) | 3 (19) | |
| Liver | 1 (3) | 1 (6) | |
| Neurological | 5 (13) | 5 (31) | |
| Respiratory | 3 (8) | 3 (19) | |
| Sepsis | 3 (8) | 3 (19) | |
| CKD stage 3, n (%)a | 10 (26) | 7 (44) | 3 (14) |
| Admission eGFR, ml/min per 1.73 m2 | 79 (51–102) | 61 (47–93) | 89.0 (63–111) |
| APACHE 2, admission | 12.5 (9.0–18.8) | 17.0 (12.8–22.0) | 9.5 (8.0–15.5) |
| SOFA, admission | 4.5 (3.0–6.8) | 4.5 (3.0–7.3) | 4.5 (4.0–6.0) |
| AKI stage, maximum, n (%) b | |||
| 0 | 20 (53) | 7 (44) | 13 (59) |
| 1 | 7 (18) | 2 (13) | 5 (23) |
| 2 | 1 (3) | 0 (0.0) | 1 (5) |
| 3 | 10 (26) | 7 (44) | 3 (14) |
| KRT, n (%) | 9 (24) | 6 (38) | 3 (14) |
| Invasive mechanical ventilation days | 9.0 (6.0–14.0) | 8.0 (2.8–14.3) | 9.0 (7.3–13.8) |
| Tracheostomy, n (%) | 11 (29) | 4 (25) | 7 (32) |
| Advanced cardiovascular daysc | 2.0 (0.0–3.0) | 0.0 (0.0–2.0) | 2.0 (0.3–4.8) |
| Basic cardiovascular daysc | 11.5 (6.0–13.8) | 11.0 (6.0–14.5) | 12.0 (7.8–13.0) |
| Pre-ICU length of stay, d | 0.0 (0.0–0.0) | 0.0 (0.0–1.3) | 0.0 (0.0–0.0) |
| ICU length of stay, d | 16.5 (10.3–27.3) | 14.9 (7.2–28.6) | 17.7 (15.4–23.6) |
| Hospital length of stay | 44.0 (20.5–68.3) | 24.5 (16.5–93.8) | 50.5 (31.0–61.5) |
| Hospital discharge location, n (%) d | |||
| Died | 5 (13) | 2 (13) | 3 (14) |
| Home | 20 (53) | 10 (63) | 10 (46) |
| Nursing home | 2 (5) | 0 (0) | 2 (9) |
| Rehabilitation facility | 11 (29) | 4 (25) | 7 (32) |
| 3-mo postdischarge location, n (%) | |||
| Home | 21 (64) | 10 (71) | 11 (58) |
| Nursing home | 3 (9) | 1 (7) | 2 (11) |
| Rehabilitation facility | 9 (27) | 3 (21) | 6 (31) |
The APACHE 2 score ranges from 0 to 71. The SOFA score ranges from 0 to 24. APACHE, Acute Physiology and Chronic Health Evaluation; SOFA, Sequential Organ Failure Assessment; ICU, intensive care unit.
CKD defined by baseline creatinine as first documented in hospital.
AKI stage as per Kidney Disease Improving Global Outcomes criteria.
On the basis of the Intensive Care National Audit and Research Center definitions: Advanced cardiovascular: multiple IV/rhythm controlling drugs (at least one vasoactive), continuous observation of cardiac output, intra-aortic balloon pump, and temporary cardiac pacemaker; basic cardiovascular: central venous catheter, arterial line, and single IV vasoactive/rhythm controlling drug.
Hospital discharge location 3 months after hospital discharge.
Time Course of Changes in Estimation of Kidney Function
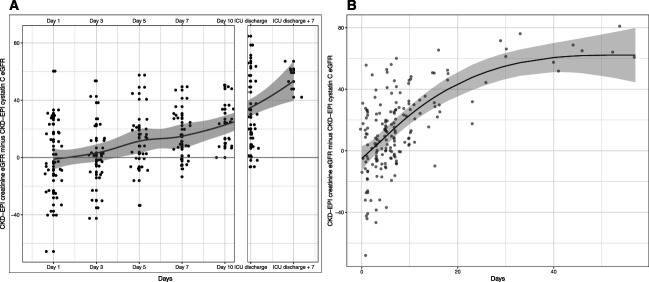
At admission, median eGFRcreat and eGFRcys were comparable, respectively, 79 (IQR, 51–102) and 78 (36–117) ml/min per 1.73 m2; however, over the course of this study, there was an increasing separation between eGFRcreat and eGFRcys (Figure 1A and Supplemental Table 1). Modeling using restricted cubic splines demonstrated a nonlinear relationship with an increase in eGFR difference over the first 30 days from ICU admission, plateauing thereafter (Figure 1B). Each day in ICU in the first 30 days resulted in a predicted 2 ml/min per 1.73 m2 (95% confidence interval [CI], 1 to 2) increase in difference in eGFR between creatinine and cystatin C–based measurements.
Figure 1.
Longitudinal assessment of estimated glomerular filtration. Difference in estimated glomerular filtration measurements (serum creatinine−cystatin C) by study time point (A). Includes a loess smoother with 95% confidence intervals. A marginal-effects plot of predicted modeled difference in eGFR by days (B) fitted with restricted cubic spline. Bands represent 95% confidence interval. Actual data points are overlayed on the modeled trend line. The median length of ICU stay was 16.5 days (interquartile range, 10.3–27.3). CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; ICU, intensive care unit.
Eighteen patients had a diagnosis of AKI. Of them, 15 survived to ICU discharge. Two of these patients had acute kidney disease using discharge creatinine values. However, when using eGFRcys, ten patients had a potential diagnosis of acute kidney disease (eGFRcys <60 ml/min per 1.73 m2). These data suggest that eight of the ten patients with potential acute kidney disease on the basis of creatinine criteria (80%) were potentially misclassified as having recovered AKI by ICU discharge.
Only three patients had a reported or recorded diagnosis of CKD before admission. However, ten patients (two trauma) had evidence of acute or chronic kidney impairment at hospital admission with a first eGFRcreat of <60 ml/min per 1.73 m2. By ICU discharge, median eGFRcreat (105 ml/min per 1.73 m2 [IQR, 97–122]) was markedly higher than eGFRcys (70 [37–99]) (Figure 2, Supplemental Figure 2). Iohexol-measured GFR was performed at ICU discharge in 27 of 38 patients, with a median value of 58 ml/min per 1.73 m2 (IQR, 39–70). At ICU discharge, three patients (9%) had an eGFRcreat of <60 ml/min per 1.73 m2 compared with 16 patients (46%) with an eGFRcys of <60 ml/min per 1.73 m2.
Figure 2.
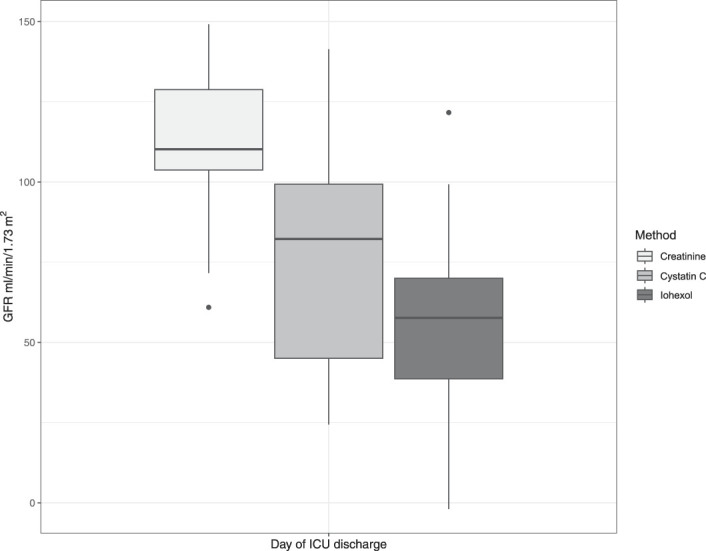
ICU discharge eGFR comparisons, including iohexol measured GFR in (n=27).
In Bayesian models, at ICU admission, the posterior mean for difference between eGFRcreat and eGFRcys was −2 ml/min per 1.73 m2 (95% CrI, −11 to 7) (Supplemental Figure 3A). However, by ICU discharge, the posterior mean for difference between eGFRcreat and eGFRcys was 33 ml/min per 1.73 m2 (95% CrI, 24 to 42) (Supplemental Figure 3B). The posterior mean for eGFRcys suggested a 22 ml/min per 1.73 m2 (95% CrI, 13 to 31) overestimation of measured GFR. Similarly, the posterior mean for eGFRcreat suggested a 59 ml/min per 1.73 m2 (95% CrI, 49 to 69) overestimation of measured GFR. There was a better agreement between eGFRcys and measured GFR than between eGFRcreat and measured GFR, suggesting less systemic bias in eGFRcys values (Supplemental Figure 4).
Of the nine patients who received KRT, two died, and the remaining seven were not dependent on KRT at ICU discharge. Nine paired cystatin C and creatinine measurements were excluded in the cohort on KRT because of ongoing continuous KRT at a study time point. For this KRT cohort, difference between eGFRcreat and eGFRcys at ICU discharge showed a trend similar to the whole cohort (posterior mean for difference, 51 ml/min per 1.73 m2, 95% CrI, 26 to 76).
Time Course of Changes in Muscle Mass and Association with Creatinine and Creatinine-to-Cystatin C Ratio
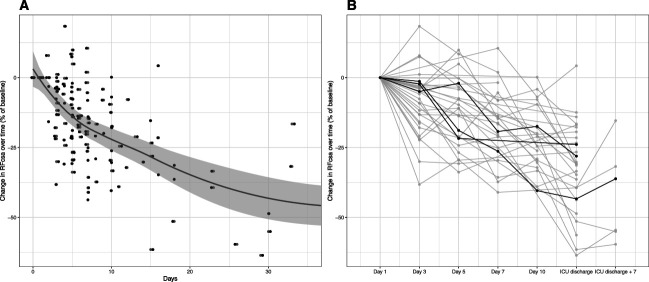
The rectus femoris cross-sectional area decreased over time, Figure 3, A and B. Significant rhabdomyolysis was rare, and only three patients had a creatine kinase measurement of more than 10,000 U/L (median 171 U/L, a total of 473 measurements). Linear mixed-effects modeling suggested that each day in ICU resulted in a predicted 2% (95% CI, 1% to 3%) daily decrease in rectus femoris cross-sectional area (Supplemental Table 2). A decrease of rectus femoris cross-sectional area showed a similar trend for trauma and nontrauma patients (Supplemental Figure 6). The repeated measures correlation between rectus femoris cross-sectional area and change in serum creatinine over time was 0.22 (0.06–0.50) (Supplemental Figure 5A). There was a stronger correlation between change in creatinine-to-cystatin C ratio and change in rectus femoris cross-sectional area, with a repeated measures correlation of 0.61 (95% CI, 0.50 to 0.72) (Supplemental Figure 5B).
Figure 3.
Rectus femoris cross-sectional area decreased over time in ICU patients. Trend line and confidence intervals using loess smoother (A). There was variation in baseline (day 1) rectus femoris cross-sectional area and trajectory of loss over time. Three example patient trajectories are highlighted (B). RFcsa, rectus femoris cross-sectional area.
Discussion
In this study, we found that in patients surviving to ICU discharge, serum creatinine and eGFRcreat overestimated underlying kidney function by a large margin of clinical significance, including missed diagnosis of a large proportion of patients with persistent kidney dysfunction. Our findings suggest that creatinine-based assessment of kidney function is inadequate to guide clinical management and stratify follow-up of patients after critical illness. By contrast, cystatin C–based estimations provided a more accurate reflection of kidney function and identified more patients with persistent kidney dysfunction. In addition, we found that the ratio of creatinine to cystatin C may be a useful surrogate of muscle mass changes during critical illness.
The limitations of serum creatinine to estimate kidney function during critical illness have consistently been demonstrated.20,28–30 The bias and imprecision associated with eGFRcreat seem most evident in patients with prolonged ICU stays.31 Acute muscle wasting has been proposed as a potential cause of the inaccuracies associated with eGFRcreat.32 The findings in our study of consistent longitudinal reductions in muscle mass support reduced muscle generation of serum creatinine as the dominant mechanism for inaccuracies in eGFRcreat at ICU discharge. In addition, reduced creatinine generation due to altered de novo hepatic synthesis of creatinine precursors (creatine and phosphocreatine)5 or the transient dilutional effects of intravenous fluid33,34 are likely, if at all, to have a greater effect during the acute phase of critical illness rather than at the end of an ICU admission.
In previous studies of a combined total of 190 patients with near-normal kidney function (eGFR 80–96 ml/min per 1.73 m2) and minimal exposure to AKI, eGFRcys consistently outperformed eGFRcreat in relation to measured GFR.31,35–37 Importantly, we confirmed these findings in critically ill patients with variable exposure to AKI with a median measured GFR of <60 ml/min per 1.73 m2 representing a cohort at greater risk of persistent decline in kidney function and subsequent CKD.
There are limitations to eGFRcys in recovering critically ill patients. In our study, eGFRcys overestimated measured GFR. Factors behind inaccuracies in eGFRcys, such as diabetes, body size, and inflammation, have been previously identified.38 Particularly, overestimation of GFR by cystatin C might be associated with loss of adipose tissue mass during critical illness, a significant determinant of cystatin C production.39 In addition, eGFR equations were designed and tested in outpatients settings with kidney function in steady state. However, these limitations of cystatin C are small compared with the consistent and dramatic overestimations of kidney function provided by creatinine during and after critical illness. For example, when measured by eGFRcreat, patients who survive prolonged critical illness seem to have better kidney function by ICU discharge than at premorbid baseline, a biologically implausible finding.28 In addition, inaccuracies in eGFRcys that are associated with the acute phase of illness, such as inflammation, should become less prominent as patients are discharged from ICU and hospital. Similarly, during recovery from critical illness, most weight gained is acquisition of fat rather than muscle.40 Thus, the interpretation of eGFRcreat would be expected to remain confounded because of persistent reduction of muscle mass in patients during the weeks and months after critical illness, persistently precluding clinical assessment of kidney function.
The combination of cystatin C and serum creatinine to assess muscle mass has been investigated in ICU cohorts and has been associated with malnutrition41 and adverse ICU outcomes.16 Previous research has examined a ratio of creatinine to cystatin C or sarcopaenic index but has been limited to single time point assessments of muscle mass in the context of stable kidney function to stratify patients risk near admission to ICU. Evidence from our study suggests that longitudinal measurements of muscle mass strongly correlate with creatinine-cystatin C ratio. Importantly, owing to the limited preexisting comorbidities and defined beginning of critical illness episode in the trauma cohort, our data suggest that changes in muscle mass due to the effect of critical illness led to measurable changes in creatinine-to-cystatin C ratio. Strategies to address changes in muscle mass attributable to critical illness could benefit from a simple, cheap surrogate marker of muscle loss, and with further validation, creatinine-to-cystatin C ratio could fulfill this role.
The consensus report of the Acute Disease Quality Initiative on kidney recovery recommends against creatinine-based formula estimates of GFR and highlights a need for better biomarkers to diagnose and risk-stratify patients recovering from AKI.14 Despite this, current national treatment guidelines recommend basing follow-up of kidney function on hospital discharge eGFRcreat.42 Our data suggest that this approach is misinformed and will miss opportunities to detect and treat many patients with or at risk of CKD. Poor longer-term outcomes of ICU survivors are consistently linked to cardiovascular mortality, as demonstrated in observational studies after sepsis.43,44 Decreases in GFR are associated with a higher risk of cardiovascular events in patients with CKD,45 and eGFRcys better associates with clinical outcomes in both the general population and ICU cohorts.15 The diagnosis and progression of CKD is likely underestimated in survivors of critical illness. Early detection and treatment of modifiable risk factors in ICU survivors is a potential target to reduce the excess morbidity and mortality. Furthermore, substantial reductions in GFR were seen in subgroup of major trauma admissions with little prior comorbidity, suggesting considerable potential to intervene to alter health care trajectory. In addition, during ICU admission, drug dosing on the basis of creatinine-based equations could potentially be harmful and may be improved by incorporation of cystatin C.46
Cystatin C is widely available and is recommended by the National Institute for Health and Clinical Excellence to confirm CKD.47 However, it remains to be used in clinical practice potentially because of cost per test (US$3.4) compared with creatinine at US$0.3545 and clinical uncertainty as to the optimal cohorts to benefit.48 Considering the severe limitations of eGFRcreat in critically ill patients and risk of progressive or new CKD, wider use of cystatin C could be beneficial. However, the implementation of cystatin C in clinical practice requires further research, and these data should highlight to clinicians its potential role in a cohort of patients with a specific phenotype.
Strengths of this study include prospective design and testing of a prespecified hypothesis. We have demonstrated that cystatin C is closer to gold standard measured GFR and that changes in muscle mass can account for the developing discrepancy in estimation of GFR. In the acute setting, we are novel in assessing measured GFR and muscle mass changes simultaneously. However, there are several limitations. First, the estimate of the population difference between eGFRcys and eGFRcreat in patients with prolonged critical care admissions cannot be certain from these data because of risk of confounding in a sample of patients from a single center. The coronavirus disease 2019 pandemic curtailed recruitment, and the initial sample size was not achieved. In addition, seven patients (18%) were discharged from ICU before day 10, and three died before evaluation, further restricting our cohort. However, using a Bayesian approach with a weakly informative prior model provides substantial evidence to support a clinically significant difference that exists between eGFRcys and eGFRcreat at ICU discharge. This is supported by longitudinal modeling that robustly incorporated patient-specific trajectories, varying baseline differences, and repeated measurements of eGFR.25 Second, we were unable to determine the reasons behind the overestimation of measured eGFR by cystatin C. Third, measured GFR was only available for a subgroup of patients (discharged from ICU between 0800 and 1700 on week days), and a single time point method was used, which may have reduced precision compared with multiple time point approaches. However, any systemic bias in single time point iohexol methods would be expected in patients with low GFR (<30 ml/min per 1.73 m2),49 likely in only a small proportion of patients in this study. Fourth, ultrasonographers were not blinded to creatinine measurements during the ICU admission. However, the reported muscle loss is similar in magnitude to other studies.8
Finally, we cannot make conclusions regarding the effect of eGFRcreat inaccuracies on clinical outcomes. By providing a more reliable measurement of eGFR and estimate of muscle mass, cystatin C adds additional insights into one part of the phenotype of patients discharged from ICU.
We have provided further evidence that cystatin C can detect clinically important changes in kidney function and muscle mass in survivors of prolonged critical illness, while conversely, the standard test, creatinine, is not fit for purpose in this context. The use of cystatin C could enhance the clinical management of such patients throughout the recovery phases and warrants investigation in studies aimed at optimizing interventions for the prevention and mitigation of CKD in this vulnerable patient population.
Supplementary Material
Acknowledgments
The funders of this study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in this study and had final responsibility for the decision to submit for publication.
We thank the patients and their families for their individual contributions during difficult times. We thank the Critical Care and Perioperative Medicine Research Group (CCPMG) staff including Maria Fernandez, Tim Jones Mari-Liis Pakats, Tim Martin, Filipa Alexandra Pereira dos Santos, Tasnin Shahid, and Tim Stephens. We also thank the Royal London ICNARC audit office and Royal London critical care consultants.
Footnotes
See related editorial, “Should We Really Still Be Using Creatinine in the Critical Care Setting?” on pages 988–990.
Disclosures
A.J. Fowler reports research funding from Barts Charity and National Institutes of Health Research. R.M. Pearse reports employment with Bart Health NHS Trust; research funding from Edwards Life Sciences, GlaxoSmithKline, and Intersurgical; and honoraria from Edwards Life Sciences, GlaxoSmithKline, and Intersurgical. J.R. Prowle reports employment with Barts Health NHS Trust; consultancy for Baxter, Nikkiso Europe, Jafron Biomedical Co Ltd, Mission Therapeutics Ltd, Cambridge UK, Nephrolytx GmbH, and Paion Ltd; research funding from Barts and the London Charity, bioMérieux SA, Jafron Biomedical Ltd, National Institue of Health Research, and Rosetrees Trust; honoraria from Baxter Inc, BBraun, Fresenius Kabi, and Nikkiso Europe GmbH; a US patent application “Markers of Acute Kidney Injury” in conjunction with Dr M. Westerman, Intrinsic LifeSciences LLC, La Jolla, CA, USA; and other interests or relationships as UK Kidney Research Consortium (UKKRC) Acute Kidney Injury Clinical Specialties Group colead and European Society of Intensive Care Medicine Acute Kidney Injury Speciality Section Chair Elect. Z. Puthucheary reports employment with Puthucheary Medical Consultancy Ltd; consultancy for Bioage, Faraday Pharmaceuticals, Fresenius Kabi, Nestle, and Nutricia; ownership interest in Puthucheary Medical Consultancy Ltd; research funding from Baxter, Fresenius Kabi, and Vitaflo (Nestle); and honoraria from Baxter, Sedana, Fresenius Kabi, Nestle, and Nutricia. All remaining authors have nothing to disclose.
Funding
This work was supported by research grant M918 from the Rosetrees Trust (J.R. Prowle).
Author Contributions
Conceptualization: Ryan W. Haines, Rupert M. Pearse, John R. Prowle.
Data curation: Ryan W. Haines, Kaifeng Liang, John R. Prowle.
Formal analysis: Alex J. Fowler, Ryan W. Haines, Anders O. Larsson, John R. Prowle.
Funding acquisition: Ryan W. Haines, Rupert M. Pearse, John R. Prowle.
Investigation: Ryan W. Haines, Anders O. Larsson, Rupert M. Pearse, John R. Prowle, Zudin Puthucheary.
Methodology: Alex J. Fowler, Ryan W. Haines, Anders O. Larsson, John R. Prowle, Zudin Puthucheary.
Project administration: Ryan W. Haines, Kaifeng Liang, John R. Prowle.
Resources: Ryan W. Haines, Anders O. Larsson, Rupert M. Pearse, John R. Prowle, Zudin Puthucheary.
Software: Ryan W. Haines, John R. Prowle.
Supervision: John R. Prowle, Zudin Puthucheary.
Validation: John R. Prowle.
Visualization: Ryan W. Haines, John R. Prowle.
Writing – original draft: Ryan W. Haines, John R. Prowle, Zudin Puthucheary.
Writing – review & editing: Alex J. Fowler, Ryan W. Haines, Anders O. Larsson, Kaifeng Liang, Rupert M. Pearse, John R. Prowle, Zudin Puthucheary.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/CJN/B776.
Supplemental Table 1. Difference in eGFR creatinine and eGFR cystatin C at each of study time points.
Supplemental Table 2. Linear mixed-effects model of change in rectus femoris cross-sectional area over time.
Supplemental Figure 1. Study flow diagram.
Supplemental Figure 2. ICU discharge eGFR comparisons, including iohexol measured GFR in (n=27).
Supplemental Figure 3. Density plot of the prior and posterior distribution of the mean difference in eGFR for creatinine and cystatin C at intensive care admission (A) and discharge (B) (n=35).
Supplemental Figure 4. Bland–Altman plots for eGFR on the basis of CKD-EPI creatinine (A) and CKD-EPI cystatin C (B) versus measured iohexol GFR.
Supplemental Figure 5. Rectus femoris cross-sectional area measurements correlated with serum creatinine (A) and creatinine-cystatin C ratio (B).
Supplemental Figure 6. Rectus femoris cross-sectional area decreased over time in ICU trauma patients.
References
- 1.Bagshaw SM, Stelfox HT, Iwashyna TJ, Bellomo R, Zuege D, Wang X. Timing of onset of persistent critical illness: a multi-centre retrospective cohort study. Intensive Care Med. 2018;44(12):2134–2144. doi: 10.1007/s00134-018-5440-1 [DOI] [PubMed] [Google Scholar]
- 2.Iwashyna TJ Hodgson CL Pilcher D, et al. Timing of onset and burden of persistent critical illness in Australia and New Zealand: a retrospective, population-based, observational study. Lancet Respir Med. 2016;4(7):566–573. doi: 10.1016/S2213-2600(16)30098-4 [DOI] [PubMed] [Google Scholar]
- 3.Lone NI Gillies MA Haddow C, et al. Five-year mortality and hospital costs associated with surviving intensive care. Am J Respir Crit Care Med. 2016;194(2):198–208. doi: 10.1164/rccm.201511-2234OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herridge MS Cheung AM Tansey CM, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348(8):683–693. doi: 10.1056/NEJMoa022450 [DOI] [PubMed] [Google Scholar]
- 5.Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80(3):1107–1213. doi: 10.1152/physrev.2000.80.3.1107 [DOI] [PubMed] [Google Scholar]
- 6.Kellum JA, Sileanu FE, Bihorac A, Hoste EA, Chawla LS. Recovery after acute kidney injury. Am J Respir Crit Care Med. 2017;195(6):784–791. doi: 10.1164/rccm.201604-0799OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chawla LS, Amdur RL, Shaw AD, Faselis C, Palant CE, Kimmel PL. Association between AKI and long-term renal and cardiovascular outcomes in United States veterans. Clin J Am Soc Nephrol. 2014;9(3):448–456. doi: 10.2215/CJN.02440213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puthucheary ZA Rawal J McPhail M, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310(15):1591–1600. doi: 10.1001/jama.2013.278481 [DOI] [PubMed] [Google Scholar]
- 9.Friedrich O Reid MB Van den Berghe G, et al. The sick and the weak: neuropathies/myopathies in the critically ill. Physiol Rev. 2015;95(3):1025–1109. doi: 10.1152/physrev.00028.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desai SV, Law TJ, Needham DM. Long-term complications of critical care. Crit Care Med. 2011;39(2):371–379. doi: 10.1097/CCM.0b013e3181fd66e5 [DOI] [PubMed] [Google Scholar]
- 11.Ostermann M Chawla LS Forni LG, et al. Drug management in acute kidney disease—report of the Acute Disease Quality Initiative XVI meeting. Br J Clin Pharmacol. 2018;84(2):396–403. doi: 10.1111/bcp.13449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prescott HC, Langa KM, Iwashyna TJ. Readmission diagnoses after hospitalization for severe sepsis and other acute medical conditions. JAMA. 2015;313(10):1055–1057. doi: 10.1001/jama.2015.1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garland A, Olafson K, Ramsey CD, Yogendran M, Fransoo R. Distinct determinants of long-term and short-term survival in critical illness. Intensive Care Med. 2014;40(8):1097–1105. doi: 10.1007/s00134-014-3348-y [DOI] [PubMed] [Google Scholar]
- 14.Forni LG Darmon M Ostermann M, et al. Renal recovery after acute kidney injury. Intensive Care Med. 2017;43(6):855–866. doi: 10.1007/s00134-017-4809-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravn B, Prowle JR, Mårtensson J, Martling CR, Bell M. Superiority of serum cystatin C over creatinine in prediction of long-term prognosis at discharge from ICU. Crit Care Med. 2017;45(9):e932–e940. doi: 10.1097/CCM.0000000000002537 [DOI] [PubMed] [Google Scholar]
- 16.Kashani KB Frazee EN Kukrálová L, et al. Evaluating muscle mass by using markers of kidney function: development of the sarcopenia index. Crit Care Med. 2017;45(1):e23–e29. doi: 10.1097/CCM.0000000000002013 [DOI] [PubMed] [Google Scholar]
- 17.Inker LA Eneanya ND Coresh J, et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737–1749. doi: 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobsson L. A method for the calculation of renal clearance based on a single plasma sample. Clin Physiol. 1983;3(4):297–305. doi: 10.1111/j.1475-097x.1983.tb00712.x [DOI] [PubMed] [Google Scholar]
- 19.Grams ME Matsushita K Sang Y, et al. Explaining the racial difference in AKI incidence. J Am Soc Nephrol. 2014;25(8):1834–1841. doi: 10.1681/ASN.2013080867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chawla LS Bellomo R Bihorac A, et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13(4):241–257. doi: 10.1038/nrneph.2017.2 [DOI] [PubMed] [Google Scholar]
- 21.Ruberg SJ Beckers F Hemmings R, et al. Application of Bayesian approaches in drug development: starting a virtuous cycle. Nat Rev Drug Discov. 2023;22(3):235–250. doi: 10.1038/s41573-023-00638-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bürkner P-C. brms: an R package for Bayesian multilevel models using stan. J Stat Softw. 2017;80(1):1–28. doi: 10.18637/jss.v080.i01 [DOI] [Google Scholar]
- 23.Kruschke JK. Bayesian estimation supersedes the t test. J Exp Psychol Gen. 2013;142(2):573–603. doi: 10.1037/a0029146 [DOI] [PubMed] [Google Scholar]
- 24.Levey AS Stevens LA Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shou H Hsu JY Xie D, et al. Analytic considerations for repeated measures of eGFR in cohort studies of CKD. Clin J Am Soc Nephrol. 2017;12(8):1357–1365. doi: 10.2215/CJN.11311116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. Springer; 2001. [Google Scholar]
- 27.Bland JM, Altman DG. Statistics notes: calculating correlation coefficients with repeated observations: part 1—correlation within subjects. BMJ. 1995;310(6977):446. doi: 10.1136/bmj.310.6977.446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prowle JR, Kolic I, Purdell-Lewis J, Taylor R, Pearse RM, Kirwan CJ. Serum creatinine changes associated with critical illness and detection of persistent renal dysfunction after AKI. Clin J Am Soc Nephrol. 2014;9(6):1015–1023. doi: 10.2215/CJN.11141113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu KD Thompson BT Ancukiewicz M, et al. Acute kidney injury in patients with acute lung injury: impact of fluid accumulation on classification of acute kidney injury and associated outcomes. Crit Care Med. 2011;39(12):2665–2671. doi: 10.1097/ccm.0b013e318228234b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doi K Yuen PS Eisner C, et al. Reduced production of creatinine limits its use as marker of kidney injury in sepsis. J Am Soc Nephrol. 2009;20(6):1217–1221. doi: 10.1681/ASN.2008060617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ravn B Rimes-Stigare C Bell M, et al. Creatinine versus cystatin C based glomerular filtration rate in critically ill patients. J Crit Care. 2019;52:136–140. doi: 10.1016/j.jcrc.2019.04.007 [DOI] [PubMed] [Google Scholar]
- 32.Vijayan A Abdel-Rahman EM Liu KD, et al. Recovery after critical illness and acute kidney injury. Clin J Am Soc Nephrol. 2021;16(10):1601–1609. doi: 10.2215/CJN.19601220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pickering JW, Ralib AM, Endre ZH. Combining creatinine and volume kinetics identifies missed cases of acute kidney injury following cardiac arrest. Crit Care. 2013;17(1):R7. doi: 10.1186/cc11931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prowle JR, Leitch A, Kirwan CJ, Forni LG. Positive fluid balance and AKI diagnosis: assessing the extent and duration of ‘creatinine dilution’. Intensive Care Med. 2015;41(1):160–161. doi: 10.1007/s00134-014-3538-7 [DOI] [PubMed] [Google Scholar]
- 35.Delanaye P Cavalier E Morel J, et al. Detection of decreased glomerular filtration rate in intensive care units: serum cystatin C versus serum creatinine. BMC Nephrol. 2014;15(1):9. doi: 10.1186/1471-2369-15-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carlier M Dumoulin A Janssen A, et al. Comparison of different equations to assess glomerular filtration in critically ill patients. Intensive Care Med. 2015;41(3):427–435. doi: 10.1007/s00134-014-3641-9 [DOI] [PubMed] [Google Scholar]
- 37.Eiamcharoenying J, Kulvichit W, Lumlertgul N, Chaiwatanarat T, Peerapornratana S, Srisawat N. The role of serum cystatin C in estimation of renal function in survivors of critical illness. J Crit Care. 2020;59:201–206. doi: 10.1016/j.jcrc.2020.07.005 [DOI] [PubMed] [Google Scholar]
- 38.Stevens LA Schmid CH Greene T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75(6):652–660. doi: 10.1038/ki.2008.638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chew-Harris JS, Florkowski CM, George PM, Elmslie JL, Endre ZH. The relative effects of fat versus muscle mass on cystatin C and estimates of renal function in healthy young men. Ann Clin Biochem. 2013;50(1):39–46. doi: 10.1258/acb.2012.011241 [DOI] [PubMed] [Google Scholar]
- 40.Chan KS Mourtzakis M Aronson Friedman L, et al. Evaluating muscle mass in survivors of acute respiratory distress syndrome: a 1-year multicenter longitudinal study. Crit Care Med. 2018;46(8):1238–1246. doi: 10.1097/CCM.0000000000003183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barreto EF Kanderi T DiCecco SR, et al. Sarcopenia index is a simple objective screening tool for malnutrition in the critically ill. JPEN J Parenter Enteral Nutr. 2019;43(6):780–788. doi: 10.1002/jpen.1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.NICE. Acute Kidney Injury Clinical Practice Summary. 2020. Accessed January 9, 2020. https://cks.nice.org.uk/acute-kidney-injury#!topicSummary [Google Scholar]
- 43.Prescott HC, Angus DC. Enhancing recovery from sepsis: a review. JAMA. 2018;319(1):62–75. doi: 10.1001/jama.2017.17687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prescott HC Iwashyna TJ Blackwood B, et al. Understanding and enhancing sepsis survivorship. Priorities for research and practice. Am J Respir Crit Care Med. 2019;200(8):972–981. doi: 10.1164/rccm.201812-2383cp [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lees JS Welsh CE Celis-Morales CA, et al. Glomerular filtration rate by differing measures, albuminuria and prediction of cardiovascular disease, mortality and end-stage kidney disease. Nat Med. 2019;25(11):1753–1760. doi: 10.1038/s41591-019-0627-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frazee E Rule AD Lieske JC, et al. Cystatin C-guided vancomycin dosing in critically ill patients: a quality improvement project. Am J Kidney Dis. 2017;69(5):658–666. doi: 10.1053/j.ajkd.2016.11.016 [DOI] [PubMed] [Google Scholar]
- 47.Carville S, Wonderling D, Stevens P, Group GD. Early identification and management of chronic kidney disease in adults: summary of updated NICE guidance. BMJ. 2014;349:g4507. doi: 10.1136/bmj.g4507 [DOI] [PubMed] [Google Scholar]
- 48.Markos JR Schaepe KS Teaford HR, et al. Clinician perspectives on inpatient cystatin C utilization: a qualitative case study at Mayo Clinic. PLoS One. 2020;15(12):e0243618. doi: 10.1371/journal.pone.0243618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Delanaye P Flamant M Dubourg L, et al. Single- versus multiple-sample method to measure glomerular filtration rate. Nephrol Dial Transplant. 2018;33(10):1778–1785. doi: 10.1093/ndt/gfx345 [DOI] [PubMed] [Google Scholar]