Abstract
Famciclovir (FCV) and valaciclovir (VACV) have previously been shown to be potent inhibitors of herpes simplex virus type 1 (HSV-1) in a murine cutaneous model. In the present study, mice were inoculated in the skin of the left ear pinna with herpes simplex virus (HSV) type 1. Antiviral therapy was started on different days postinoculation (p.i.), terminating at the end of day 10 p.i. The compounds were administered twice daily by oral gavage at 50 mg/kg of body weight/dose. Mice were sampled on day 5 p.i., during the acute phase of the infection, and the titers of infectious virus in the target tissues (ear, brain stem, and trigeminal ganglia) were determined. At 2 to 3 months p.i., the ipsilateral and contralateral trigeminal and cervical dorsal root ganglia were explanted, and four different methods were used to detect latent HSV. The methods were (i) conventional explant culture for 5 days followed by homogenization, (ii) long-term culture (up to 73 days) of whole ganglia, followed by homogenization, (iii) dissociation by enzymatic disaggregation and an infectious center assay, and (iv) in situ hybridization to detect latency-associated transcripts (LATs). The conventional explant culture method was the least sensitive method, while in situ staining for LAT was the most sensitive, and all mice, including those treated from early times with FCV, were shown to be latently infected. Significantly less latent virus was detected by all four methods, however, in ganglia obtained from mice that had been treated with FCV in comparison with the amount detected in ganglia from mice that had been treated with VACV. However, in no case was latency completely eliminated.
The ability to establish latent infections in the neurons of the peripheral nervous system is the hallmark of the alpha herpesviruses, of which herpes simplex virus (HSV) is by far the best studied (13, 28). Following a productive infection in permissive epithelial cells, HSV type 1 (HSV-1) often establishes a quiescent infection within neurons of the peripheral sensory ganglia which innervate the peripheral inoculation site. Thus, infection of the skin or mucosal surfaces is rapidly followed by axonal translocation of virus leading to the establishment of ganglionic infection. The rate of establishment of latent foci is related to the dose of the inoculum (9), although it has been shown previously that following the administration of moderate inoculum doses the ganglia become infected within 24 to 48 h of inoculation (10, 17, 18, 21). It has been known for many years that latent infections can reproducibly be detected by explantation of the affected ganglia (22) and incubation of whole ganglia for a few days followed by homogenization and assay for the presence of infectious virus (9). We have reported previously that this simple technique revealed marked differences in the ability of oral famciclovir (FCV) and valaciclovir (VACV) to affect the establishment of latency in mice with HSV-1 (24) or HSV-2 (25). However, judged by the same reactivation technique, neither drug was able to affect the incidence of virus reactivation when a similar treatment was applied to mice in which latent infection had already been established (11).
The present study extends the observations presented above by using a variety of in vitro reactivation techniques. Methods involving long-term cocultivation were intended to increase the sensitivity of the simple reactivation assay, and the use of an enzymic disaggregation technique was used in order to obtain a quantitative comparison of the number of latently infected foci. Finally, sections of ganglia were stained using in situ hybridization for HSV latency-associated transcripts (LATs) in order to detect the presence of HSV in individual neurons. These more sensitive tests demonstrated the presence of latent infection in groups of mice whose ganglia were scored negative by the simple reactivation test. However, the results also confirmed earlier findings that, using similar doses of the two prodrugs, FCV is superior to VACV in mice in preventing or reducing the number of ganglionic cells in which demonstrable latent infections are established. The differences between the compounds were particularly marked in relation to the contralateral, as opposed to the ipsilateral, ganglia. These findings are discussed in relation to possible differences in the mode of action of the compounds in vivo.
MATERIALS AND METHODS
Virus strain and tissue culture.
The virus strain used was HSV-1 SC16. This strain of virus has been extensively characterized in mice (15) and has been used previously for studying antiviral compounds (5, 9, 11, 23).
Mice and virus inoculation.
The skin of the left ear pinna of each of 650 female 4-week-old BALB/c mice (Bantin and Kingman, Kingston, United Kingdom) was inoculated with 105 PFU of HSV-1 SC16 by previously published methods (11).
Antiviral compounds and treatment regimen.
FCV and VACV were supplied by SmithKline Beecham (Brentford, United Kingdom). The activities of acyclovir (ACV) and penciclovir (PCV), which are the active metabolites of VACV and FCV, respectively, were measured by means of a plaque reduction assay with BALB/c 3T3 cells. The 50% effective doses (± standard deviation) were 0.01 ± 0.01 and 0.02 ± 0.01 μg/ml ACV and PCV, respectively (11). FCV and VACV were dissolved in double-distilled deionized water and were administered by oral gavage (50 mg/kg of body weight/dose) twice daily, with treatment commencing 1, 2, 3, 4, or 5 days postinoculation (p.i.). All treatments were terminated on day 10 p.i.
Measurement of clinical signs.
Mice were assessed once per day when signs of clinical disease were noted. Mice with a flaccid ear pinna which failed to respond to a gentle stimulus were recorded as showing “ear paralysis.” Other neurological signs were recorded, including circling, unsteady gait, and monolateral and bilateral hind limb paralysis. Skin thickness was measured daily in individual mice by means of an engineer’s micrometer screw gauge. Mean values with standard deviations were calculated for groups of eight mice. Mortality was assessed separately by using groups of 20 mice. These methods have previously been described in detail (9).
Titration of virus in tissue samples.
Tissue samples (ear pinna, brain stem, or left trigeminal ganglia) were obtained from three mice in each treatment group on day 5 p.i. only. This included one group of mice (the day 5 to 10 p.i. treatment group) that had been exposed to the drugs for a period of approximately 6 h before tissues were sampled. Each tissue sample was homogenized and titrated independently for infectious virus by a plaque assay on monolayers of BHK-21 cells as described previously (11). All tissues were coded prior to testing, and the code was not broken until all samples had been titrated.
Detection of latent virus in the ganglia by conventional explant culture.
All surviving mice were divided into groups of five mice each, with each group being assigned a different reactivation technique. Ganglia innervated by the ear pinna (left [ipsilateral] and right [contralateral] trigeminal ganglia and left and right cervical dorsal root ganglia [CII, CIII and CIV pooled]) were obtained from five mice per group (except the positive control group, from which only three mice were used) on day 44 p.i. Ganglia were placed in 0.5 ml of Eagle’s minimal essential medium (EMEM) in 2-ml glass vials with loose lids, and the vials were incubated at 37°C for 5 days. The ganglia were then processed as described in detail previously (24). Samples were coded before testing and were read blind.
Detection of latent virus in the ganglia by long-term culture.
For detection of latent virus in the ganglia by long-term culture, ganglia from five mice from each treatment group were sampled on day 43 p.i. The contralateral and ipsilateral trigeminal ganglia were removed and placed in serum-free EMEM. Freshly prepared BHK-21 cell monolayers were transferred to medium containing 5% newborn calf serum immediately prior to use. The dissected ganglia were washed in EMEM and were then placed onto the cell sheet. The ganglia remained intact throughout the period of incubation. Every 5 to 7 days the ganglia were carefully transferred to fresh cell sheets if there was no sign of any cytopathic effect (CPE) in the cell monolayer. This continued until all the ganglia obtained from positive control (untreated) mice had shown signs of a CPE in the cell monolayers. The longest period of culture of ganglia by this method was 73 days.
Isolation of infectious centers by disaggregation of ganglia.
The methods for isolation of infectious centers were adapted from those described previously (16, 19). Briefly, a quantity of rat tail collagen was prepared at 4 mg/ml in 0.02 N acetic acid. This was used to coat six-well tissue culture plates. The coated plates were exposed to ammonium hydroxide for 3 min before adding 3 ml of sterile, distilled water to each well and leaving the plates in a fume hood overnight. The plates were washed three times with a weak trypsin solution prior to use.
For this test groups of four mice per treatment (three mice from the infected, untreated controls) were sampled on day 50 p.i., and the left trigeminal ganglia were removed. Each ganglion was placed in a 50-ml conical tube containing 5 ml of EMEM. The tubes were vortexed to wash the ganglia and were then spun at 2,500 × g for 10 min in a refrigerated centrifuge. The supernatant was carefully removed, and 3 ml of dissociation mixture (EMEM, sodium bicarbonate, 0.02 M HEPES, 0.125% trypsin, 0.02% collagenase) was added to each ganglion. All samples were incubated on a shaking platform at 37°C for 1 h before being spun at 2,000 × g for 10 min at 4°C. The pellets were resuspended in 3 ml of EMEM containing 10% fetal calf serum (FCS) and 0.75% sodium bicarbonate. All the ganglia dissociated into single-cell suspensions at this point. The resulting cell suspensions were then placed neat and at a 1:10 dilution onto the collagen-coated plates. For each sample from each mouse, three wells were inoculated with the neat suspension and three were inoculated with the 1:10 dilution. All the plates were placed at 37°C for overnight incubation. The following morning, all the medium was carefully removed from each plate and then 106 BHK cells were added to each well in 3 ml of EMEM containing 10% FCS. The cells were allowed to settle for 5 h, and then the medium was removed and was replaced with 3 ml of EMEM containing 5% FCS and 2% carboxymethyl cellulose per well. The plates were then incubated at 37°C for a further period of 5 days. All wells were checked daily for any signs of developing plaques. The numbers of infectious centers (i.e., plaques) were enumerated from the wells containing the neat suspension and the 1:10 dilution. The CPE was confirmed to be virus specific by further passage in BHK-21 cells. The results for the various treatment groups were then compared by means of the paired and unpaired two-tailed Student’s t test.
Detection of LATs by in situ hybridization.
Probes for the detection of LATs were made by T7 polymerase transcription of HindIII-linearized pSLAT 2 with a digoxigenin (DIG) detection system as previously described in detail (1). The plasmid pSLAT 2 was a gift from Stacey Efstathiou. After transcription, the reaction mixtures were ethanol precipitated and the product was resuspended in 100 μl of 10 mM Tris (pH 8)–1 mM dithiothreitol with RNase inhibitor.
Single ganglia were fixed in periodate-lysine-paraformaldehyde at room temperature for 16 h, transferred to 50% ethanol, and then paraffin embedded. Sections (thickness, 5 μm) were collected onto glutaraldehyde-activated, 3-aminopropyl-triethoxysilane-coated slides and were dewaxed in xylene before use.
Sections were digested with 100 μg of proteinase K per ml at 37°C for 5 min for cervical dorsal root ganglia and 6 min for trigeminal ganglia. Overnight hybridization was carried out at 25°C below the theoretical melting temperature (72°C). One to three microliters of DIG-labelled riboprobe was used in each 100 μl of hybridization solution. One stringent wash in 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–30% formamide–10 mM Tris-HCl (pH 7.5) was carried out at 10°C below the melting temperature (75°C) for 30 min. Bound probe was detected with alkaline phosphatase-conjugated anti-DIG Fab fragments according to the manufacturer’s instructions (Boehringer Mannheim). Positive cells contained brown stain confined to the nucleus. The staining pattern varied among individual neurons, and there was some block-to-block variation. A characteristic nucleoplasmic signal was seen, although individual cells showed various signal distributions and intensities. Cell nuclei which contained a level of brown staining clearly above the background level were scored as positive. The number of LAT-positive cells in each section was recorded. All sections from all tissues were counted, and the mean number of positive neurons per group was enumerated. For the photography, 10 representative sections from each ganglion were examined. Five mice from each treatment group were tested. Every in situ hybridization test with the LAT probe included RNase- and DNase-treated sections to attempt to rule out any spurious staining artifacts.
RESULTS
Clinical signs.
In the absence of antiviral therapy, all mice developed erythematous ears and ear paralysis, and 90% of these mice had ear lesions that were visible macroscopically. Although treatment with neither FCV nor VACV affected the production of erythema, FCV was markedly superior to VACV in reducing the incidence of visible ear lesions (Table 1). Neither compound reduced the incidence of ear paralysis except when VACV therapy commenced on day 1 or 2 p.i. and FCV therapy commenced on day 1, 2, 3, or 4 p.i.
TABLE 1.
Clinical signs in mice treated for various times with FCV or VACV
| Drug and time (days) p.i. (n = 20) | % of mice affected by the following clinical signs:
|
|||||
|---|---|---|---|---|---|---|
| Red ear | Ear lesions | Ear paralysis | Neurologic signs | Death | Ear swelling (AUC)a | |
| None | 100 | 90 | 100 | 35 | 40 | 28 |
| VACV | ||||||
| 5–10 | 100 | 70 | 80 | 10 | 10 | 30 |
| 4–10 | 100 | 70 | 80 | 5 | 5 | 31 |
| 3–10 | 100 | 40 | 90 | 5 | 5 | 31 |
| 2–10 | 100 | 50 | 80 | 0 | 0 | 25 |
| 1–10 | 100 | 0 | 30 | 0 | 0 | 21 |
| FCV | ||||||
| 5–10 | 100 | 40 | 80 | 0 | 0 | 23 |
| 4–10 | 100 | 30 | 40 | 0 | 0 | 20 |
| 3–10 | 100 | 0 | 25 | 0 | 0 | 17 |
| 2–10 | 90 | 0 | 10 | 0 | 0 | 12 |
| 1–10 | 90 | 0 | 0 | 0 | 0 | 4 |
For each group, the curve described by time and mean group ear thickness was plotted on graph paper. The area under the curve was then cut out and weighed.
Mortality.
The mortality rate among the infected, untreated controls was 40%, with mice dying between days 7 and 10 p.i. The mortality rate in the VACV-treated groups decreased in relation to the time of onset of therapy (Table 1), and all mice survived when treatment with VACV was commenced on day 2 p.i. or earlier. The mean time to death was similar for VACV-treated mice and for those that died without antiviral therapy. No mice in any of the groups treated with FCV died, even when the onset of therapy was delayed to day 5 p.i.
Weight gain.
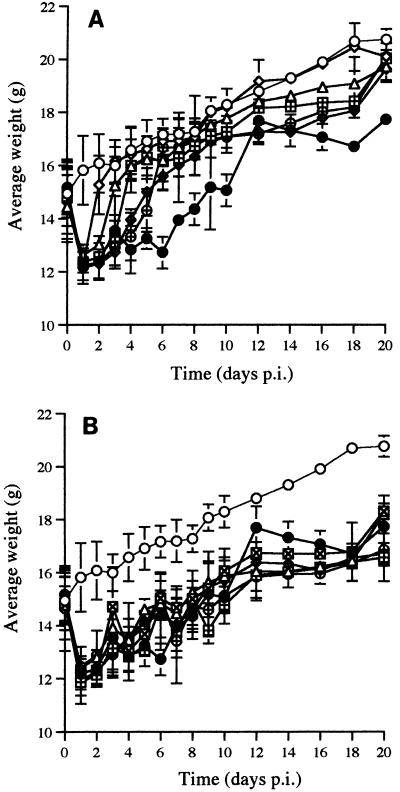
VACV therapy had little effect on restoring weight gain. In contrast, FCV therapy produced a rapid restoration of weight gain to the uninfected control values within 1 day of commencement of therapy (Fig. 1A and B).
FIG. 1.
Effect of antiviral therapy on the weight gain of HSV-1-infected mice. The skin of the left ear pinna of all mice was inoculated with 105 PFU of HSV-1. FCV or VACV was administered by oral gavage at 50 mg/kg/dose twice daily. Antiviral therapy commenced on day 1, 2, 3, 4, or 5 p.i., with all treatments terminating on day 10 p.i. Vertical bars represent standard deviations; error bars have been omitted from those points where the error is very small. (A) FCV-treated mice. (B) VACV-treated mice. ○, uninoculated control; •, infected, untreated control; ⊠, days 1 to 10 p.i.; ▵, days 2 to 10 p.i.; ⊞, days 3 to 10 p.i.; ◊+, days 4 to 10 p.i.; ⊕, days 5 to 10 p.i.
Inflammation of the ear.
For both compounds there was a graded relationship between the time of commencement of therapy and the extent of inflammation, as judged by increased ear thickness (Table 1). VACV therapy was less effective at reducing the ear thickness increase except when therapy commenced on day 1 or 2 p.i. FCV was more effective and, in addition, produced a favorable response even when the commencement of therapy was delayed until day 3 p.i.
Virus growth in the ear on day 5 p.i.
In infected, untreated mice, titers of infectious virus (in the range of 3.5 × 104 to 4.1 × 104 PFU/ear) were detected in the samples on day 5 p.i. A delay of treatment with VACV until 4 days p.i. or later had no effect on the virus titer at day 5. However, when VACV treatment was commenced on day 1, 2, or 3 p.i., the mean virus titers measured on day 5 were reduced by 2.1, 1.2, and 1.1 log10 PFU/ear, respectively. When FCV treatment commenced on day 1 p.i., the level of infectious virus on day 5 p.i. was below the level of detection at <0.5 log10 PFU/ear (representing a reduction in titer of >4.0 log10 PFU/ear). For FCV treatments starting on days 2, 3, and 4, the virus titers measured on day 5 were reduced by 2.8, 1.9, and 1.8 log10 PFU/ear, respectively.
Virus growth in the brain stem on day 5 p.i.
The titer of infectious virus in the infected, untreated control samples at day 5 p.i. was approximately 4 log10 PFU/brain stem. Treatment with either FCV or VACV from day 1 p.i. reduced the titer of infectious virus on day 5 p.i. to below the level of detection. When treatment with FCV was delayed to day 2, 3, or 4, the reductions in virus titer on day 5 p.i. were 2.7, 1.4, and 1.6 log10 PFU, respectively. For VACV the log10 reductions in virus titers were slightly less at 2.4, 1.3, and 1.4 log10 PFU for days 2, 3, and 4, respectively.
Virus growth in the trigeminal ganglia on day 5 p.i.
The reductions in virus titer observed in trigeminal ganglia were similar to those described above for the brain stem samples. The infected, untreated controls contained an average of 3.2 ± 0.2 log10 PFU per ganglion.
Having ascertained that the acute infection had been established, that this was comparable in terms of clinical signs and the distribution of infectious virus with those found in previous experiments, and that the therapy with each drug had been effective in a dose-dependent manner, the surviving mice were maintained for a period of approximately 8 weeks, by which time all infectious virus would have been cleared from all tissues. Groups of mice were then killed and the relevant ganglia were analyzed for the presence of latent virus by means of a variety of different methods.
Conventional explant culture.
For conventional explant culture, explanted ganglia were incubated whole at 37°C for 5 days and were then homogenized and tested for the presence of infectious virus. In the three control mice from the group which had received no chemotherapy, all ganglia (trigeminal and cervical) were positive for virus reactivation, both for the ipsilateral and for the contralateral ganglia (Table 2). For mice that had received VACV, all ipsilateral ganglia were positive, irrespective of when therapy commenced. For the mice that had commenced therapy with FCV from day 1 or day 2, both trigeminal and cervical ganglia were negative by this test. For the contralateral ganglia, chemotherapy with FCV from any time point yielded negative dorsal root ganglia; however, the contralateral trigeminal ganglia from mice in which FCV therapy was delayed until day 5 were positive for all five mice tested. For mice receiving VACV therapy the pattern of latency in the contralateral ganglia was less clear-cut. Contralateral ganglia were positive for all groups in which treatment was delayed until day 3 p.i. or later, with the exception of the contralateral trigeminal ganglia from mice treated with VACV from day 5, of which only three of five mice yielded positive results.
TABLE 2.
Proportion of mice yielding positive reactivation following conventional explant culture of ganglia after inoculation of the ear pinna and treatment with FCV or VACV
| Drug and time (days) p.i. (n = 3–5) | Proportion of mice positive for isolation of infectious virus from ganglia at 44 days p.i.a
|
|||
|---|---|---|---|---|
| Ipsilateral ganglia (left)
|
Contralateral ganglia (right)
|
|||
| TGb | Dorsal rootc | TG | Dorsal rootc | |
| None | 3/3 | 3/3 | 3/3 | 3/3 |
| VACV | ||||
| 5–10 | 5/5 | 5/5 | 3/5 | 5/5 |
| 4–10 | 5/5 | 5/5 | 5/5 | 5/5 |
| 3–10 | 5/5 | 5/5 | 5/5 | 5/5 |
| 2–10 | 5/5 | 5/5 | 0/5 | 0/5 |
| 1–10 | 5/5 | 5/5 | 0/5 | 0/5 |
| FCV | ||||
| 5–10 | 5/5 | 5/5 | 5/5 | 0/5 |
| 4–10 | 5/5 | 5/5 | 0/5 | 0/5 |
| 3–10 | 5/5 | 5/5 | 0/5 | 0/5 |
| 2–10 | 0/5 | 0/5 | 0/5 | 0/5 |
| 1–10 | 0/5 | 0/5 | 0/5 | 0/5 |
The proportion is indicated as number of mice positive for isolation of infectious virus/total number of mice in the group.
TG, trigeminal ganglia.
CII, CIII, and CIV pooled.
Long-term culture.
For long-term culture, explanted ganglia were maintained in culture for many weeks and were finally homogenized and tested for the presence of infectious virus. In addition to the groups that yielded positive ganglia by the conventional test, all ipsilateral ganglia scored positive when tested by long-term culture. In some cases, however, evidence of infectious virus was not detected in the contralateral ganglia (Table 3).
TABLE 3.
Mice yielding reactivated HSV-1 from long-term cultures of trigeminal ganglia after inoculation of the ear pinna and treatment with FCV or VACV
| Ganglion type, drug, and time (days) p.i. (n = 5) | Proportion of mice with reactivated HSV-1 from long-term cultures of trigeminal ganglia after the following no. of passagesa:
|
||
|---|---|---|---|
| 3 | 14 | Homogenates | |
| Ipsilateral ganglia | |||
| None | 3/5 | 5/5 | NA |
| VACV | |||
| 5–10 | 2/5 | 3/5 | 5/5 |
| 4–10 | 2/5 | 2/5 | 5/5 |
| 3–10 | 3/5 | 3/5 | 5/5 |
| 2–10 | 2/5 | 5/5 | NA |
| 1–10 | 2/5 | 4/5 | 5/5 |
| FCV | |||
| 5–10 | 1/5 | 5/5 | NA |
| 4–10 | 2/5 | 2/5 | 5/5 |
| 3–10 | 1/5 | 4/5 | 5/5 |
| 2–10 | 3/5 | 3/5 | 5/5 |
| 1–10 | 2/5 | 2/5 | 5/5 |
| Contralateral ganglia | |||
| None | 3/5 | 3/5 | 5/5 |
| VACV | |||
| 5–10 | 1/5 | 4/5 | 5/5 |
| 4–10 | 2/5 | 2/5 | 5/5 |
| 3–10 | 2/5 | 2/5 | 5/5 |
| 2–10 | 2/5 | 3/5 | 5/5 |
| 1–10 | 3/5 | 4/5 | 5/5 |
| FCV | |||
| 5–10 | 1/5 | 3/5 | 5/5 |
| 4–10 | 2/5 | 2/5 | 3/5 |
| 3–10 | 0/5 | 1/5 | 1/5 |
| 2–10 | 3/5 | 3/5 | 3/5 |
| 1–10 | 2/5 | 2/5 | 2/5 |
The supernatants of intact trigeminal ganglia cultures were assayed for HSV after 3 and 14 passages. After the last culture, the ganglia were homogenized and another culture was performed. NA, not applicable. Proportion indicates number of mice with reactivated HSV-1/total number of mice.
Disaggregation of ganglia.
The mean number of infectious centers obtained for the untreated controls was 82 per ipsilateral trigeminal ganglion (Table 4). Mice treated with both antiviral compounds gave similar results when treatment was delayed to day 5 p.i. However, all other treatment groups showed significant reductions in the numbers of infectious centers. These results confirmed a greater quantitative reduction in latently infected cells in ganglia from mice treated with FCV compared with the reduction for mice treated with VACV. There were highly significant differences (P < 0.001) between FCV-treated and VACV-treated animals when treatment started on day 2 p.i. or day 5 p.i. for experiment 1 and day 1 p.i. or day 2 p.i. for experiment 2 (Table 4). No samples from either of the two early treatment groups (FCV or VACV from days 1 to 10 p.i.) were available for testing.
TABLE 4.
Number of infectious centers obtained from individual latently infected ipsilateral trigeminal ganglia following disaggregation and plating of single cells
| Drug and time (days) p.i. (n = 3–5) | No. of infectious centers from TGa
|
|
|---|---|---|
| Expt 1 | Expt 2 | |
| None | 82 ± 5.3 | 76 ± 3.6 |
| VACV | ||
| 5–10 | 81 ± 5.0b | ND |
| 4–10 | 30 ± 2.5c | ND |
| 3–10 | 42 ± 2.9c | ND |
| 2–10 | 45 ± 2.1b,c | 43.2 ± 1.7b,c |
| 1–10 | ND | 30.1 ± 1.3b,c |
| FCV | ||
| 5–10 | 61 ± 4.2d | ND |
| 4–10 | 33 ± 2.8c | ND |
| 3–10 | 30 ± 5.5c | ND |
| 2–10 | 3.8 ± 1.1c | 4.2 ± 0.4c |
| 1–10 | ND | 1.6 ± 0.6c |
Values are means ± standard errors for three, four, or five mice per group tested independently. The results were obtained by using a two-tailed Student’s t test (unpaired for experiment 1 and paired for experiment 2). All samples were compared with the relevant control. ND, not done.
P value of <0.001 when VACV-treated mice were compared with mice treated with equivalent dose of FCV.
P < 0.001.
P < 0.05.
The experiment was repeated on a smaller scale with a view to confirming the quantitative results obtained by disaggregation and staining for LAT by in situ hybridization (see below). This also enabled the missing data points for disaggregated ganglia (therapy from days 1 to 10) to be addressed.
In the second experiment, 80 mice were inoculated and treatment by oral gavage as described above was initiated from days 1 and 2 p.i. only. The clinical signs and mortality were closely similar to those recorded in the first experiment (data not shown). Ten mice from each experimental group were killed during the period from 7.5 to 8 weeks p.i., and trigeminal and cervical ganglia from five mice were disaggregated and five were tested by in situ hybridization (see below). The mean number of infectious centers obtained from control mice and mice treated from days 2 to 10 was closely similar to those observed in experiment 1. Neither VACV nor FCV completely eradicated the latent infection by this test.
Detection of latently infected neurons by means of in situ hybridization.
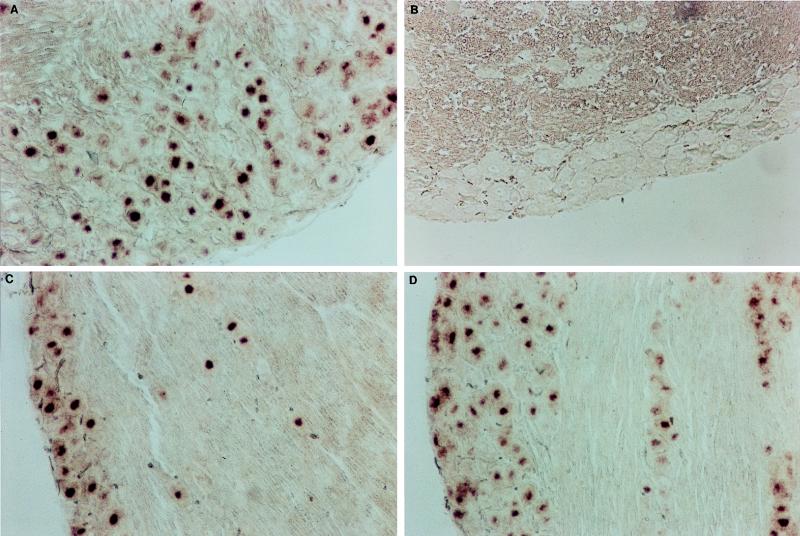
Mice were killed 6 to 7 weeks after inoculation, their ganglia were explanted and fixed, and sections were analyzed by means of a DIG-labelled riboprobe. In the ganglionic sections obtained from survivors of the infection without treatment, numerous neurons stained positive for major LAT (Fig. 2A). The stain was confined to the nucleus and had a punctate distribution characteristic of this probe (1). The DNase-treated, latently infected sections gave results similar to those given by the infected, untreated control sections. All RNase-treated, latently infected sections were negative (data not shown). Sections of ganglia from uninfected mice processed identically were completely negative for major LAT staining (Fig. 2B), and a sense riboprobe also gave no positive neurons, although this probe did show positively staining neurons when it was applied to acutely infected ganglionic sections obtained at 5 days p.i. (data not shown). For each experimental group analyzed by in situ hybridization, a DIG-labelled probe specific for ICPO (an immediate-early gene) was applied to the sections to provide an internal control for nonspecific hybridization or staining artifacts. The number of positively staining neurons/section (± standard error) was 124 ± 2.09 for the left trigeminal ganglion and 58 ± 2.87 for the left CIII (Table 5).
FIG. 2.
Detection of latently infected neurons in the trigeminal ganglia by in situ hybridization with a DIG-labelled major LAT riboprobe. Mice were killed 6 to 8 weeks p.i., and 5-μm sections of ganglia were tested as described in the text. (A) Infected, untreated control trigeminal ganglia showing punctate, nuclear staining. (B) Uninfected control trigeminal ganglia. (C) FCV-treated (days 1 to 10) trigeminal ganglia. (D) VACV-treated (days 1 to 10) trigeminal ganglia. Magnifications, ×100.
TABLE 5.
Mean number of LAT-positive cells per ganglionic section from mice inoculated in the ear pinna with HSV-1 SC16 and treated with FCV or VACV
| Drug and time (days) p.i. (n = 5) | No. of LAT-positive cells/sectiona (mean ± SE)
|
|
|---|---|---|
| Left trigeminal ganglia | CIII | |
| None | 124 ± 2.1 | 58 ± 2.9 |
| VACV | ||
| 5–10 | 140 ± 4.8b | 59 ± 1.6 |
| 4–10 | 113 ± 2.0b | 56 ± 0.9 |
| 3–10 | 113 ± 1.5b | 49 ± 1.1b |
| 2–10 | 105 ± 1.5c | 63 ± 1.4 |
| 1–10 | 84 ± 0.9c | 54 ± 0.4 |
| FCV | ||
| 5–10 | 49 ± 1.2c | 41 ± 1.0c |
| 4–10 | 40 ± 0.9c | 34 ± 0.5c |
| 3–10 | 38 ± 0.9c | 22 ± 1.1c |
| 2–10 | 36 ± 1.5c | 20 ± 1.1c |
| 1–10 | 32 ± 0.2c | 16 ± 0.2c |
All sections were examined, and the mean number of positively staining neurons was determined. The means for five mice were then used to obtain the values presented here. The values for all treatment groups were compared with control values by a paired two-tailed Student’s t test. For VACV treatment groups, P was <0.001 compared with the values for mice treated with equivalent dose of FCV.
P < 0.05.
P < 0.001.
The ganglia from mice treated with VACV starting from 4 days p.i. or earlier showed reduced numbers of LAT-positive neurons (Table 5). For FCV there was a reduction in all treatment groups. A second experiment was carried out as described above to cover the treatment time from day 1 to day 10 p.i. These results were consistent with those of experiment 1 and confirmed the results obtained by disaggregation that even when treatment was initiated on day 1 p.i., a small number of positively staining neurons remained in all sections (Table 5 and Table 6; Fig. 2C and D). Sections from ganglia treated with FCV from day 1 p.i. contained approximately 30% positive neurons compared with the number present in ganglion sections from infected, untreated control mice.
TABLE 6.
Reduction in latent infection following antiviral treatment as judged by disaggregation and in situ staining for LATsa
| Drug and time (days) p.i. (n = 3–5) | % of untreated controls with infectious center disaggregation
|
% of untreated controls with LAT-positive cells
|
||||
|---|---|---|---|---|---|---|
| Expt 1, left TGb | Expt 2, left TG | Expt 1, left TG | Expt 2, left TG | Expt 1, CIII | Expt 2, CIII | |
| None | 100 | 100 | 100 | 100 | 100 | 100 |
| VACV | ||||||
| 2–10 | 55 | 56 | 85 | 99 | 109 | 118 |
| 1–10 | NDc | 39 | 68 | 70 | 92 | 92 |
| FCV | ||||||
| 2–10 | 4.6 | 5 | 29 | 30 | 35 | 39 |
| 1–10 | ND | 2 | 26 | 24 | 28 | 24 |
For infectious centers, n is equal to 3 for infected, untreated mice or four for treated mice. For in situ hybridization; n is equal to 5 for all groups.
TG, trigeminal ganglia.
ND, not done.
The results confirmed that of the methods used the in situ method was the most sensitive marker for latency. Furthermore, the relative reduction in latently infected cells in mice treated with FCV compared with the level in mice treated with VACV was also confirmed. In the second experiment, a smaller number of latently infected foci was detected in the trigeminal ganglia by means of LAT (approximately 26 cells/section) in mice that had been treated from day 1 p.i. with FCV. In mice that had received VACV from day 1 there was also a reduction in the number of cells staining positive (approximately 75 compared to 108 cells/section); however, when the start of treatment was delayed to day 2 p.i., FCV-treated animals gave an average of 32 cells/section, while VACV-treated animals gave 106 cells/section.
DISCUSSION
We have reported previously that mice treated from early times after infection with HSV-1 (24) or HSV-2 (25) with equal doses of oral FCV or VACV showed differences in the apparent incidence of latency when the explanted ganglia were analyzed for the presence of latent infections by a simple explant test. By the test, FCV appeared to be markedly superior to VACV in preventing the establishment of latency; moreover, when the commencement of treatment was delayed to up to 4 days after virus inoculation, a reduced incidence of latent infections was detected. In mice infected with moderate doses of HSV-1, latency has been shown to be established before 24 to 48 h after inoculation (10, 17, 18, 21). However, in line with many earlier studies, for example, in which alternative compounds, e.g., ACV (3, 9, 18, 20), brivudine, (10) or ganciclovir (14), were used, neither FCV nor VACV appeared to affect the incidence of latency once it has been established. Thus, treatment for 2 weeks during a 2- to 3-month period after virus inoculation had no effect on the incidence of latency (24).
In view of the apparent difference between FCV and VACV regarding the prevention of latent infections in the murine model, a large experiment was carried out in order to compare the incidence of latency by a variety of different methods to detect the presence of virus. Long-term cultures were attempted to improve the sensitivity of detection, and ganglia were disaggregated in order to obtain more quantitative data on the number of latently infected foci established in the ganglia. The method chosen for administration of the drugs was oral gavage, which allowed a precise daily administration of the antiviral compounds. This was considered necessary for an accurate comparison between the two different compounds to address concern that the difference between FCV and VACV was due to different levels of drug intake. However, we have observed over a number of previous experiments that administration per os is a significantly less effective method of treatment than free administration in the drinking water, although the quantities of drug consumed over the 24-h period were approximately the same. This may explain why the apparent protection from latency in ganglia obtained in the present experiment was less than that reported previously in mice to which drugs had been administered in drinking water (24).
Notwithstanding, the results of the more sensitive tests for latency showed, beyond a doubt, that more ganglia contain latent infections than are shown by the simple tests used in our previous investigation (24, 25). Long-term cultures for up to 73 days demonstrated that all ganglia (both ipsilateral and contralateral) from VACV-treated mice were positive for latent HSV, including mice treated from day 1. It was noted, however, that some virus reactivation cultures remained negative throughout a series of up to 14 passages and became positive only when the ganglion tissue fragment was finally homogenized. It is unlikely that any neurons in the culture remained viable for more than 1 to 2 days; therefore, the implication of these findings is that, assuming that the original latent infection in the ganglion is confined to the neurons, the virus must have passed to other cell types in the culture within the first few days of incubation. Generally, the results of ganglion explant cultivation methods for the assessment of latency are consistent with those of preliminary studies on ganglionic tissue sections by in situ hybridization for the detection of LAT RNA.
Another important finding from this study is that trigeminal ganglia from mice that had received either FCV or VACV from the earliest time at which treatment was commenced (day 1 p.i.) contained LAT-positive cells. In more recent experiments we have found that, using drinking water therapy (which is more efficacious), and starting therapy before virus inoculation, a small number of LAT-positive cells remain detectable (25a).
Despite the increased detection of latent infection by the more sensitive techniques, the distinction between FCV and VACV remained, and in particular, there appeared to be significantly fewer infected foci in the mice that received FCV. Furthermore, when mice were treated with FCV from 4 days p.i. or earlier, some contralateral ganglia were assessed as negative for latency by all four methods. There are several possible explanations for the different effects of the two compounds. First, although the administration of equal doses of FCV or VACV yielded similar concentration-time curves for the active metabolites (PCV and ACV, respectively [11]), it is not known whether the two compounds are distributed to the neural compartments in mice with equal efficiencies. It was notable that FCV completely prevented mortality, even when the start of therapy was delayed until day 5 p.i. This could simply reflect higher neural compartment concentrations of PCV compared with those of ACV. Second, although the two compounds have broadly similar potencies against HSV in tissue culture (4, 6), their mechanisms of action have been shown to differ markedly at the enzyme level (26). Thus, the relative affinities of ACV and PCV for the HSV-coded thymidine kinase and ACV and PCV triphosphates for HSV-coded DNA polymerase differ by up to 2 orders of magnitude (reviewed by Field [8]). Third, we have observed repeatedly that recurrence of infectious virus occurs in the central and peripheral nervous system following the cessation of VACV therapy in mice (12, 24, 25) and that the virus titers during recurrence are frequently of a magnitude similar to the peak titers obtained during the acute phase of the infection without treatment. This (brief) period of active virus replication could allow the colonization of further neurons or enable spread of the virus to contralateral ganglia. No such recurrence has been detected on cessation of FCV treatment.
There exist further important differences between the compounds; these include the fact that ACV is an obligate chain terminator, whereas PCV is not (7), and the observation that the intracellular half-life of PCV triphosphate (approximately 10 h) is significantly greater than that of ACV triphosphate, which has been shown to be approximately 0.7 h (7, 27). This difference in the stability of the metabolic products of the two compounds in infected cells appears to correlate with a more sustained antiviral effect in both tissue culture (2) and animal infections (5, 23). These are also factors that may help to explain the differences between the compounds apparent from the present murine model.
Although the data are not shown in the present report, antiviral therapy starting at day 3 or later had very little effect on the virus titers in the ear pinna (i.e., the local infection site); therefore, we believe that the effects of the inhibitors on the establishment of latency must operate within the nervous system itself and are not simply reflecting a reduction in axonal supply of virus from the periphery during therapy.
It is noteworthy that the studies described in this paper were carried out with equal doses and identical dosing regimens for the two compounds being tested. We have not carried out experiments to optimize the dosing schedules for either compound; however, our previous tests showed toxicity, manifested by marked weight loss, when VACV was given per os at 150 or 200 mg/kg twice daily or administered in the drinking water at 5 mg/ml. Although we accept that effects on the establishment of latency similar to those shown here for FCV may be achieved by using increased doses or more frequent administration of doses of VACV, to date, we have obtained no data to support this possibility.
Further work is required to establish which, if any, of the factors described above are the most important. Only when these have been properly elucidated will it be possible to determine whether or not the present findings concerning the superior effects of FCV in comparison with the effects of VACV may be extrapolated to humans and whether the apparent advantage of one drug over the other is simply correctable by the administration of higher doses of VACV. Alternatively, the results may remain an interesting phenomenon but one that is unique to the murine infection model.
Finally, it remains to be determined whether the quantitative reduction in latent foci reported in the present study is associated with a reduced ability to reactivate the infection in vivo following appropriate stimuli and whether the apparently reduced burden of latently infected neurons would improve the prognosis for humans with primary herpes simplex treated early after exposure to the infection.
ACKNOWLEDGMENT
We thank Stacey Efstathiou for expert help with the in situ hybridization protocol, the gift of plasmid pSLAT 2, and critical evaluation of the manuscript.
REFERENCES
- 1.Arthur J, Efstathiou S, Simmons A. Intranuclear foci containing low abundance herpes simplex virus latency-associated transcripts visualized by non-isotopic in situ hybridization. J Gen Virol. 1993;74:1363–1370. doi: 10.1099/0022-1317-74-7-1363. [DOI] [PubMed] [Google Scholar]
- 2.Bacon T H, Howard B A. Further characterization of the potent and prolonged inhibition of herpes simplex virus replication in human cell lines by penciclovir. Antivir Chem Chemother. 1996;7:128–137. [Google Scholar]
- 3.Blyth W A, Harbour D A, Hill T J. Effect of acyclovir on recurrence of herpes simplex skin lesions in mice. J Gen Virol. 1980;48:417–419. doi: 10.1099/0022-1317-48-2-417. [DOI] [PubMed] [Google Scholar]
- 4.Boyd M R, Bacon T H, Sutton D, Cole M. Antiherpesvirus activity of 9-(4-hydroxy-3-hydroxymethylbut-1-yl)guanine (BRL 39123) in cell culture. Antimicrob Agents Chemother. 1987;31:1238–1242. doi: 10.1128/aac.31.8.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyd M R, Bacon T H, Sutton D. Antiherpes activity of 9-(4-hydroxy-3-hydroxymethylbut-1-yl)guanine (BRL 39123) in animals. Antimicrob Agents Chemother. 1988;32:358–363. doi: 10.1128/aac.32.3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyd M R, Safrin S, Kern E R. Penciclovir: a review of its spectrum of activity, selectivity, and cross-resistance pattern. Antivir Chem Chemother. 1993;4:3–11. [Google Scholar]
- 7.Earnshaw D L, Bacon T H, Darlison S J, Edmonds K, Perkins R M, Vere Hodge R A. Mode of antiviral action of penciclovir in MRC-5 cells infected with herpes simplex virus type 1 (HSV-1), HSV-2, and varicella-zoster virus. Antimicrob Agents Chemother. 1992;36:2747–2757. doi: 10.1128/aac.36.12.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Field H J. Famciclovir—origins, progress and prospects. Exp Opin Invest Drugs. 1996;5:925–938. [Google Scholar]
- 9.Field H J, Bell S E, Elion G B, Nash A A, Wildy P. Effect of acycloguanosine treatment on acute and latent herpes simplex infections in mice. Antimicrob Agents Chemother. 1979;15:554–561. doi: 10.1128/aac.15.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Field H J, De Clercq E. Effects of oral treatment with acyclovir and bromovinyldeoxyuridine on the establishment and maintenance of latent herpes simplex virus infection in mice. J Gen Virol. 1981;56:259–265. doi: 10.1099/0022-1317-56-2-259. [DOI] [PubMed] [Google Scholar]
- 11.Field H J, Tewari D, Sutton D, Thackray A M. Comparison of efficacies of famciclovir and valaciclovir against herpes simplex virus type 1 in a murine immunosuppression model. Antimicrob Agents Chemother. 1995;39:1114–1119. doi: 10.1128/aac.39.5.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Field H J, Thackray A M. Valaciclovir and famciclovir—differences between two similar guanosine analogue prodrugs emerge from laboratory models. Int Antivir News. 1996;4:23–27. [Google Scholar]
- 13.Fraser N, Valyi-Nagy T. Viral, neuronal, and immune factors which may influence herpes simplex virus (HSV) latency and reactivation. Microb Pathog. 1993;15:83–91. doi: 10.1006/mpat.1993.1059. [DOI] [PubMed] [Google Scholar]
- 14.Goldthorpe S E, Boyd M R, Field H J. Effects of penciclovir and famciclovir in a murine model of encephalitis induced by intranasal inoculation of herpes simplex virus type 1. Antivir Chem Chemother. 1992;3:37–47. [Google Scholar]
- 15.Hill T J, Field H J, Blyth W A. Acute and recurrent infection with herpes simplex virus in the mouse: a model for studying latency and recurrent disease. J Gen Virol. 1975;28:341–353. doi: 10.1099/0022-1317-28-3-341. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy P G E, Lisak R P, Raff M C. Cell type-specific markers for human glial and neuronal cells in culture. Lab Invest. 1980;43:342–351. [PubMed] [Google Scholar]
- 17.Klein R J, Friedman-Kien A E, Yellin P B. Orofacial herpes simplex virus infection in hairless mice; latent virus in trigeminal ganglia after topical antiviral treatment. Infect Immun. 1978;20:130–135. doi: 10.1128/iai.20.1.130-135.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein R J, Friedman-Kien A E, de Stefano E. Latent herpes simplex virus infection in sensory ganglia of hairless mice prevented by acycloguanosine. Antimicrob Agents Chemother. 1979;15:723–729. doi: 10.1128/aac.15.5.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leib D A, Coen D M, Bogard C L, Hicks K A, Yager D R, Knipe D M, Tyler K L, Schaffer P A. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J Virol. 1989;63:759–768. doi: 10.1128/jvi.63.2.759-768.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park N-H, Pavan-Langston D, McLean S L. Acyclovir in oral and ganglionic herpes simplex virus infections. J Infect Dis. 1979;140:802–806. doi: 10.1093/infdis/140.5.802. [DOI] [PubMed] [Google Scholar]
- 21.Pavan-Langston D, Park N H, Lass J H. Herpetic ganglionic latency. Aciclovir and vidarabine therapy. Arch Ophthalmol. 1979;97:1508–1510. doi: 10.1001/archopht.1979.01020020170017. [DOI] [PubMed] [Google Scholar]
- 22.Stevens J G, Cook M L. Latent herpes simplex virus in spinal ganglia of mice. Science. 1971;173:843–845. doi: 10.1126/science.173.3999.843. [DOI] [PubMed] [Google Scholar]
- 23.Sutton D, Boyd M R. Comparative activity of penciclovir and acyclovir in mice infected intraperitoneally with herpes simplex virus type 1 SC16. Antimicrob Agents Chemother. 1993;37:642–645. doi: 10.1128/aac.37.4.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thackray A M, Field H J. Differential effects of famciclovir and valaciclovir on the pathogenesis of herpes simplex virus in a murine infection model including reactivation from latency. J Infect Dis. 1996;173:291–299. doi: 10.1093/infdis/173.2.291. [DOI] [PubMed] [Google Scholar]
- 25.Thackray A M, Field H J. Comparison of effects of famciclovir and valaciclovir on pathogenesis of herpes simplex virus type 2 in a murine infection model. Antimicrob Agents Chemother. 1996;40:846–851. doi: 10.1128/aac.40.4.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25a.Thackray, A. M., and H. J. Field. Unpublished data.
- 26.Vere Hodge R A, Cheng Y-C. The mode of action of penciclovir. Antivir Chem Chemother. 1993;4:13–24. [Google Scholar]
- 27.Vere Hodge R A, Perkins R M. Mode of action of 9-(4-hydroxy-3-hydroxymethylbut-1-yl)guanine (BRL 39123) against herpes simplex virus in MRC-5 cells. Antimicrob Agents Chemother. 1989;33:223–229. doi: 10.1128/aac.33.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wildy P, Field H J, Nash A A. Virus persistence, Symposium 33, Society for General Microbiology). Cambridge, United Kingdom: Cambridge University Press; 1982. Classical herpes latency revisited; pp. 133–167. [Google Scholar]