Abstract
Background
Previous studies have reported conflicting results of prolonged antibiotic prophylaxis on infectious complications after pancreatoduodenectomy. This study evaluated the effect of prolonged antibiotics on surgical-site infections (SSIs) after pancreatoduodenectomy.
Methods
A systematic review and meta-analysis was undertaken of SSIs in patients with perioperative (within 24 h) versus prolonged antibiotic (over 24 h) prophylaxis after pancreatoduodenectomy. SSIs were classified as organ/space infections or superficial SSI within 30 days after surgery. ORs were calculated using a Mantel–Haenszel fixed-effect model.
Results
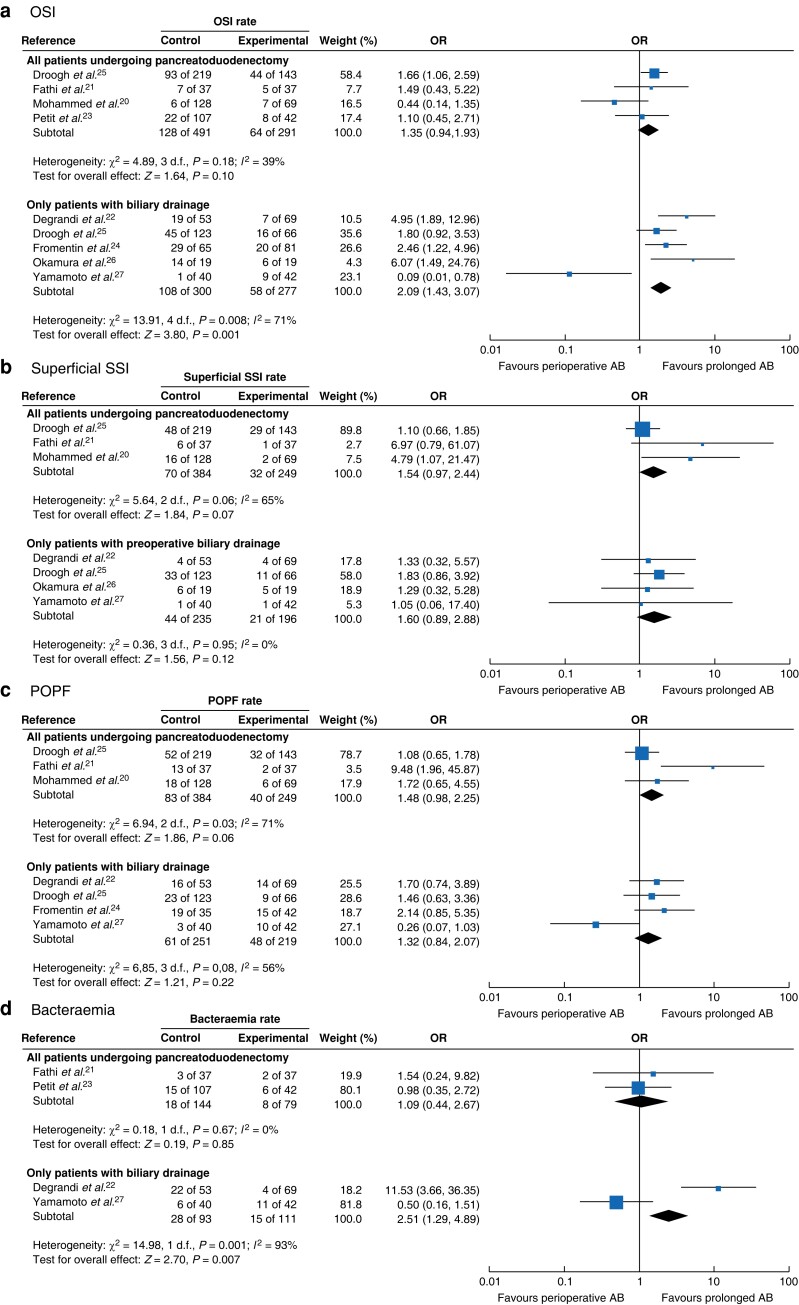
Ten studies were included in the qualitative analysis, of which 8 reporting on 1170 patients were included in the quantitative analysis. The duration of prolonged antibiotic prophylaxis varied between 2 and 10 days after surgery. Four studies reporting on 782 patients showed comparable organ/space infection rates in patients receiving perioperative and prolonged antibiotics (OR 1.35, 95 per cent c.i. 0.94 to 1.93). However, among patients with preoperative biliary drainage (5 studies reporting on 577 patients), organ/space infection rates were lower with prolonged compared with perioperative antibiotics (OR 2.09, 1.43 to 3.07). Three studies (633 patients) demonstrated comparable superficial SSI rates between patients receiving perioperative versus prolonged prophylaxis (OR 1.54, 0.97 to 2.44), as well as in patients with preoperative biliary drainage in 4 studies reporting on 431 patients (OR 1.60, 0.89 to 2.88).
Conclusion
Prolonged antibiotic prophylaxis is associated with fewer organ/space infection in patients who undergo preoperative biliary drainage. However, the optimal duration of antibiotic prophylaxis after pancreatoduodenectomy remains to be determined and warrants confirmation in an RCT.
Antibiotic prophylaxis varies substantially between institutes. The effect of prolonged antibiotic prophylaxis seems promising, particularly in patients undergoing pancreatoduodenectomy with contaminated bile. This systematic review and meta-analysis demonstrated a beneficial effect of prolonged antibiotic prophylaxis after pancreatoduodenectomy in patients who had preoperative biliary drainage.
Introduction
Surgical-site infections (SSIs) and postoperative pancreatic fistulas (POPFs) account for approximately 28–48 per cent of the postoperative morbidity after pancreatoduodenectomy1,2. Previous studies2,3 demonstrated an association between preoperative biliary drainage, positive bile cultures, and SSIs, and hypothesized that perioperative spillage of contaminated bile may account for the increased rate of SSIs. Moreover, some studies4–7 suggested a correlation between abdominal contamination and the development of pancreatic fistula. Hence, optimalization of antibiotic prophylactic regimens might not only reduce the rate of SSIs, but also decrease POPF rates.
The additional benefit of prolonged antibiotic prophylaxis after pancreatoduodenectomy has not been determined. Most studies investigating postoperative antibiotic prophylaxis focused on the effect of tailored prophylaxis, predominantly based on bile cultures obtained before operation8. Tailored prophylaxis for each patient has several practical limitations because bile culture results are not available immediately after surgery. Use of standard prolonged antibiotic prophylaxis would be a feasible alternative. The updated enhanced recovery after surgery protocol9 states that ‘postoperative “prophylactic” antibiotics are not recommended but may be considered therapeutic in patients with positive bile cultures’. However, the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Surgical Infection Society, the Society for Healthcare Epidemiology of America, and the European Centre for Disease Prevention and Control guidelines10,11 for perioperative antibiotic prophylaxis recommend against antibiotic prophylaxis prolonged beyond 24 h after abdominal surgery. Consequently, antibiotic regimens vary substantially between institutes, which could imply unnecessary administration of antibiotics, potentially leading to increasing antibiotic resistance12.
This systematic review and meta-analysis evaluated the effect of prolonged antibiotic prophylaxis on infectious complications after pancreatoduodenectomy. Additionally, the effect of prolonged antibiotic prophylaxis was studied separately in patients who underwent preoperative biliary drainage.
Methods
Literature search and study selection
The literature search included the main terms ‘pancreatoduodenectomy’, ‘antibiotics’, and ‘prophylaxis’, and their related concepts and synonyms (Appendix S1). The literature search was performed in PubMed, Embase, Web of Science, the Cochrane library, and Emcare until November 2022. Titles and abstracts were screened independently by two authors for full-text articles written in English investigating prolonged antibiotic prophylaxis after pancreatoduodenectomy. Eligibility criteria for study selection were patients undergoing pancreatoduodenectomy as main subjects, comparison of the duration of postoperative antibiotic prophylaxis (in particular, not only comparison of type of antibiotics), and outcomes related to infectious complications. Case reports, case series, and literature reviews were excluded. This systematic review of the literature was conducted according to the PRISMA statement13. The study protocol was registered in PROSPERO (CRD42022321755).
Data collection
Data extraction was undertaken using a standardized form, including study characteristics, performance of preoperative biliary drainage and acquisition of intraoperative bile cultures, postoperative (infectious) complications, and antibiotic prophylaxis and therapy. Risk of bias was assessed using the Risk Of Bias In Non-randomized Studies—of Interventions (ROBINS-I) tool for cohort studies and the Cochrane tool for randomized trials14,15. Studies that were considered to have a serious risk of bias were excluded.
Outcomes and comparisons
The primary outcome was the rate of abdominal infectious complications, defined as organ/space infections (OSIs). Secondary outcomes were rates of wound infections (hereafter referred to as superficial SSIs), POPF and bacteraemia, duration of hospital stay, and bile culture results. OSIs and superficial SSIs were classified according to the Centers for Disease Control and Prevention definition (Appendix S2)16. POPF was classified according to International Study Group of Pancreatic Surgery definitions17. Only clinically relevant POPF (grade B and C) was considered in the analyses. Bacteraemia was defined by the presence of a positive blood culture. Comparisons were made between patients with perioperative prophylaxis (either perioperative or for 24 h) and those with prolonged antibiotic prophylaxis (longer than 24 h). Additionally, subset analyses were undertaken for patients with preoperative biliary drainage.
Statistical analysis
Quantitative analyses were performed using Review Manager (RevMan version 5.3, Copenhagen: The Nordic Cochrane Centre, Cochrane Collaboration). The I2 statistic was used to assess heterogeneity between studies. An I2 value of more than 50 per cent was considered to represent substantial heterogeneity. A Mantel–Haenszel fixed-effect model was used to calculate pooled effects, presented as ORs and 95 per cent confidence intervals.
Results
Study characteristics
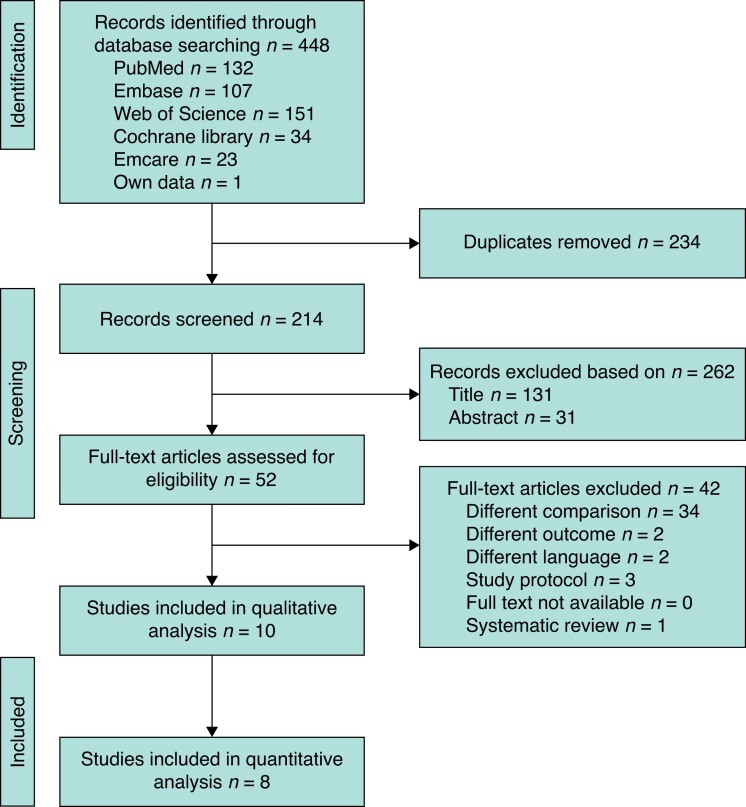
The literature search identified 448 studies. After removal of duplicates and detailed assessment of titles, abstracts, and full text, 10 studies were considered eligible (Fig. 1). Two observational studies18,19 were excluded from the quantitative analysis owing to a serious risk of bias as a result of a substantial baseline differences between patients receiving perioperative versus prolonged prophylaxis (patients without and with preoperative biliary drainage respectively) (Tables 1 and 2). Six observational studies and two RCTs reporting on 1170 patients were included (Table 3). Baseline characteristics, such as age, sex, smoking behaviour, and diabetes were similar between patients who received perioperative versus prolonged prophylaxis.
Fig. 1.
PRISMA diagram showing selection of articles for review
Table 1.
Risk-of-bias assessment according to ROBINS-I tool for cohort studies
| Confounding | Selection of participants | Classification of intervention | Deviation from intended interventions | Missing data | Measurement of outcomes | Selection of reported results | Overall risk of bias | |
|---|---|---|---|---|---|---|---|---|
| Sourrouille et al.19 | Serious | Moderate | Low | Low | Low | Moderate | Low | Serious |
| Mohammed et al.20 | Moderate | Moderate | Low | Moderate | Low | Low | Low | Moderate |
| Fathi et al.21 | Low | Moderate | Low | Low | Low | Moderate | Low | Moderate |
| Degrandi et al.22 | Low | Moderate | Low | Moderate | Low | Moderate | Low | Moderate |
| Sánchez Acedo et al.18 | Serious | Moderate | Low | Low | Low | Low | Moderate | Serious |
| Petit et al.23 | Moderate | Moderate | Low | Low | Moderate | Moderate | Low | Moderate |
| Fromentin et al.24 | Moderate | Moderate | Moderate | Low | Low | Low | Low | Moderate |
| Droogh et al.25 | Low | Moderate | Low | Low | Moderate | Low | Low | Moderate |
ROBINS-I, Risk Of Bias In Non-randomized Studies—of Interventions
Table 2.
Risk-of-bias assessment according to the Cochrane tool for randomised studies
Table 3.
Study characteristics
| Study design | Country | Inclusion interval | Inclusion criteria | Total sample size | Preoperative biliary drainage (%) | ||
|---|---|---|---|---|---|---|---|
| Perioperative prophylaxis | Prolonged prophylaxis | ||||||
| Sourrouille et al.19* | Prospective | France | 2004–2009 | Pancreatoduodenectomy | 175 | 0 | 100 |
| Mohammed et al.20 | Retrospective | USA | 2005–2011 | Pancreatoduodenectomy | 197 | 48 | 67 |
| Fathi et al.21 | Retrospective with PSM | USA | 2006–2001 | Pancreatoduodenectomy | 74 | 59 | 78 |
| Okamura et al.26 | RCT | Japan | 2008–2013 | HPB surgery with biliary reconstruction† | 38 | 100 | 100 |
| Yamamoto et al.27 | RCT | Japan | 2012–2016 | Pancreatoduodenectomy with preoperative biliary drainage‡ | 82 | 100 | 100 |
| Degrandi et al.22 | Retrospective | France | 2008–2017 | Pancreatoduodenectomy with preoperative biliary drainage | 122 | 100 | 100 |
| Sánchez Acedo et al.18* | Retrospective | Spain | 2015–2018 | Pancreatoduodenectomy | 90 | 0 | 100 |
| Petit et al.23 | Retrospective | France | 2007–2018 | Major pancreatic surgery (77% pancreatoduodenectomy) | 149 | 18 | 55 |
| Fromentin et al.24 | Retrospective | France | 2010–2016 | Pancreatoduodenectomy | 146 | 100 | 100 |
| Droogh et al.25 | Retrospective | Netherlands | 2016–2019 | Pancreatoduodenectomy | 362 | 56 | 46 |
Only included in qualitative analysis. †Only patients who underwent pancreatoduodenectomy included for analysis in this review. ‡Patients with cholangitis were excluded. PSM, propensity score matching.
The percentage of patients who underwent preoperative biliary drainage differed between the studies (Table 3). Four studies20,21,23,25 (782 patients) included all patients undergoing pancreatoduodenectomy. Preoperative biliary drainage was performed in 45.3 per cent of patients receiving perioperative prophylaxis and in 62.7 per cent of those receiving prolonged prophylaxis. Five studies22,24–27 (577 patients) reported the results for patients who underwent biliary drainage before pancreatoduodenectomy separately.
Choice of antibiotic prophylaxis
Type of perioperative and prolonged antibiotics differed between the studies (Table 4). Perioperative antibiotic prophylaxis consisted of a second- or third-generation cephalosporin in seven studies. In eight studies, patients received a different antibiotic agent as prolonged prophylaxis compared with the perioperative antibiotic agent. The different antibiotic agent for the prolonged prophylaxis was based on individually obtained bile cultures (3 studies), bile cultures analysed in a former cohort of patients (4), or surgeon’s preference (1). The indication for prolonged antibiotic prophylaxis in the observational studies was based on positive bile cultures (4), perioperative observations (1), year of surgery (1) or was centre-specific (2). The duration of prolonged antibiotic prophylaxis was either 2 days (1), 5 days (4), or 10 days (2), or based on surgeon’s preference (1).
Table 4.
Study antibiotics
| Sample size | Type of antibiotics | Duration of antibiotics | |||||
|---|---|---|---|---|---|---|---|
| Perioperative | Prolonged | Indication for prolonged prophylaxis | Perioperative | Prolonged | Perioperative | Prolonged | |
| Sourrouille et al.19* | 76 | 99 | Positive bile culture | Cefoxitin | Gentamicin, piperacillin, tazobactam (or ticarcillin + clavulanic acid) | Perioperative | 5 days (2 days for negative IOBC) |
| Mohammed et al.20 | 128 | 69 | Positive bile culture | Carbapenem | Tailored (IOBC) | 24 h | 10 days (3 days for negative IOBC) |
| Fathi et al.21 | 37 | 37 | Positive bile culture | Third-generation cephalosporin + piperacillin, tazobactam + fluconazole | Tailored (IOBC) | 24 h | 10 days (3 days for negative IOBC) |
| Okamura et al.26 | 19 | 19 | Randomization | Cefmetazole | Tailored (preoperative bile culture) | Perioperative | 48 h |
| Yamamoto et al.27 | 40 | 42 | Randomization | Cefozopran | Cefozopran | 24 h | 5 days |
| Degrandi et al.22 | 53 | 69 | Year of surgery | Cefazolin | Piperacillin + tazobactam | Perioperative | 5 days |
| Sánchez Acedo et al.18* | 39 | 51 | Preoperative biliary drainage | Cefoxitin | Piperacillin + tazobactam | Perioperative | 5 days |
| Petit et al.23 | 107 | 42 | Perioperative signs of infection | Cefuroxime | Surgeon’s preference | Perioperative | Not protocolized |
| Fromentin et al.24 | 65 | 81 | Centre-specific | Cefazolin, cefoxitin or cefamandole | Piperacillin + tazobactam, in one centre additional dose of gentamicin | Perioperative | 5 days |
| Droogh et al.25 | 219 | 143 | Centre-specific | Cefazoline + metronidazole | Cefuroxime + metronidazole | Perioperative | 5 days |
Only included in qualitative analysis. IOBC, intraoperative bile culture.
Organ/space infections
Eight studies (1170 patients) were included in the quantitative analysis of OSIs (Figs 2 and S2). Four studies (782 patients) included all patients undergoing pancreatoduodenectomy, and showed comparable OSI rates in patients who had perioperative versus prolonged prophylaxis (pooled OR 1.35, 95 per cent c.i. 0.94 to 1.93; I2 = 39 per cent). Five studies (577 patients) included patients with biliary drainage performed before pancreatoduodenectomy, and observed a lower OSI rate in patients receiving prolonged antibiotic prophylaxis (pooled OR 2.09, 1.43 to 3.07; I2 = 71 per cent). Owing to substantial heterogeneity of the studies, an additional analysis using a Mantel–Haenszel random-effects model was carried out for OSIs in patients with biliary drainage, which demonstrated an OR of 2.19 (0.94 to 5.07) (Fig. S1).
Fig. 2.
Forest plots showing occurrence of organ/space infection, superficial surgical-site infection, postoperative pancreatic fistula, and bacteraemia in patients with perioperative versus prolonged prophylaxis
a Organ/space infection (OSI), b superficial surgical-site infection (SSI), c postoperative pancreatic fistula (POPF), and c bacteraemia. For each complication, results are shown for all patients undergoing pancreatoduodenectomy and only in those who underwent preoperative biliary drainage. A Mantel–Haenszel fixed-effect model was used for meta-analysis. ORs are shown with 95% confidence intervals. AB, antibiotics.
Superficial surgical-site infections
Six studies (875 patients) were included in the quantitative analysis of superficial SSIs (Figs 2 and S2). Three studies (633 patients) included all patients undergoing pancreatoduodenectomy, and did not show a difference in superficial SSI rates between patients with perioperative versus prolonged prophylaxis (pooled OR 1.54, 95 per cent c.i. 0.97 to 2.44; I2 = 65 per cent). Four studies (431 patients) included only patients with preoperative biliary drainage, and did not show a difference in superficial SSI rates in patients with perioperative versus prolonged prophylaxis (pooled OR 1.60, 0.89 to 2.88; I2 = 0 per cent).
Postoperative pancreatic fistula
Six studies (914 patients) were included in the quantitative analysis for POPF (Figs 2 and S2). Three studies (633 patients) included all patients undergoing pancreatoduodenectomy, and observed comparable POPF rates in patients who received perioperative versus prolonged prophylaxis (pooled OR 1.48, 95 per cent c.i. 0.98 to 2.25; I2 = 71 per cent). Four studies (470 patients) included only patients who had preoperative biliary drainage, and showed similar POPF rates in patients with perioperative versus prolonged prophylaxis (pooled OR 1.32, 0.84 to 2.07; I2 = 56 per cent).
Bacteraemia
Four studies (427 patients) were included in the quantitative analysis of bacteraemia (Figs 2 and S2). Two studies (223 patients) included all patients undergoing pancreatoduodenectomy, and did not show a difference in bacteraemia rates between patients who had perioperative versus prolonged prophylaxis (pooled OR 1.09, 95 per cent c.i. 0.44 to 2.67; I2 = 0 per cent). Two studies (204 patients) included only patients who had preoperative biliary drainage and reported more bacteraemia in patients with perioperative prophylaxis only (pooled OR 2.51, 1.29 to 4.89; I2 = 93 per cent).
Duration of hospital stay
Four studies (475 patients) were included in the quantitative analysis of hospital stay (Fig. S3). Two studies (271 patients) included all patients undergoing pancreatoduodenectomy, and did not report a difference in duration of hospital stay for patients who received perioperative versus prolonged prophylaxis (pooled mean difference −0.09 (95 per cent c.i. −0.38 to 0.20) days; I2 = 0 per cent). Two studies (204 patients) analysed duration of hospital stay in patients who had preoperative biliary drainage, and reported only median (i.q.r.) values. Yamamoto et al.27 documented a median duration of hospital stay of 10 (8–33 days) days for patients who received perioperative prophylaxis and 15 (8–44) days for those with prolonged prophylaxis (P = 0.018). Degrandi et al.22 reported 17 (13–27) and 13 (10–14) days respectively (P < 0.001).
Microbiology
Six studies examined the results of culture of bile samples obtained during surgery; the cultures were predominantly polymicrobial (range 54–69 per cent)18–22,26. The most frequently cultured microorganisms were Enterococci (range 14–63 per cent), Enterobacter (range 22–30 per cent), and Klebsiella (range 18–39 per cent) species. Three studies21,22,26 compared microbiological profiles of bile and abdominal drain cultures, and reported a concordance of 12–39 per cent. Degrandi et al.22 showed more extensive resistance rates for microorganisms cultured from bile in patients receiving only perioperative antibiotics (first-generation cephalosporin) compared with prolonged antibiotics (piperacillin with tazobactam): 64 versus 14 per cent. Three studies23,26,27 reported on resistance patterns of postoperative cultures from SSIs, and reported similar resistance rates between patients with perioperative versus prolonged prophylaxis, including the proportion of multidrug-resistant microorganisms.
Discussion
This systematic review and meta-analysis did not demonstrate a difference in OSI between patients receiving perioperative versus prolonged antibiotics (OR 1.35, 95 per cent c.i. 0.94 to 1.93). However, there was a lower proportion of OSIs in patients who had preoperative biliary drainage receiving prolonged antibiotic prophylaxis after pancreatoduodenectomy (OR 2.09, 1.43 to 3.07). The rate of superficial SSIs and POPF was comparable between patients receiving perioperative versus prolonged antibiotic prophylaxis, also after stratification for preoperative biliary drainage. Moreover, antibiotic resistance rates were comparable between patients receiving perioperative or prolonged prophylaxis.
SSIs and POPF occur frequently after pancreatoduodenectomy, and previous research2,4,28 related these complications to contaminated bile. In line with these studies, the present overview showed an association between positive bile cultures and the occurrence of OSIs and bacteraemia. However, an association between contaminated bile and pancreatic fistula was not demonstrated. Optimization of postoperative antibiotic prophylaxis potentially decreases the rate of OSIs and bacteraemia, which might lead to a shorter hospital admission and faster time to functional recovery; this was reported by only one study22 in this review. However, duration of hospital admission was not extended by the prolonged administration of antibiotics. This review accentuated the varying administration and duration of antibiotic prophylaxis between institutes. Current guidelines lack clear recommendations regarding type and duration of antibiotic prophylaxis; as with the current evidence, the optimal antibiotic prophylaxis for patients with a high risk of contaminated bile remains undetermined9.
Perioperative antibiotic prophylaxis is used widely to prevent SSIs, whereas prolonged antibiotic prophylaxis could be used as pre-emptive treatment to prevent OSIs in surgical procedures with a risk of perioperative contamination. A recent meta-analysis8 evaluated the effect of targeted antibiotic prophylaxis based on bile cultures obtained from a former cohort of patients, and reported a 21 per cent decrease in SSIs. However, not only type but also duration of the antibiotic regimens differed substantially between the included studies. Standard use of prolonged antibiotic prophylaxis in patients with contaminated bile could replace individually tailored antibiotic prophylaxis to reduce abdominal infectious complications, as supported by the results of this review. Preoperative biliary instrumentation and the presence of an ampullary malignancy are highly associated with contaminated bile, as approximately 95 per cent of these patients have positive bile cultures2–4,29. As use of preoperative biliary drainage is likely to increase owing to neoadjuvant therapies, antibiotic prophylaxis should be optimized for these patients. Hence, prolonged antibiotic prophylaxis should be considered for patients with a high risk of contaminated bile to reduce OSIs.
Various other interventions have been investigated to prevent SSIs. Recently, a multicentre RCT30 including 13 301 patients demonstrated a 3 per cent lower SSI rate after routine change of gloves before wound closure in abdominal surgery with contamination of the abdominal space. Wound management devices have also been suggested to reduce SSIs in abdominal and biliary surgery31. However, an RCT32 of 212 patients undergoing pancreatoduodenectomy did not demonstrate an effect of an intraoperative wound protector device on the superficial SSI rate. Additionally, a meta-analysis33 including 4 studies reporting on 309 patients did not demonstrate a benefit of negative pressure wound therapy on superficial SSIs. Fewer interventions to prevent OSIs have been evaluated. A small RCT34 including 40 patients evaluated bile duct clamping during pancreatoduodenectomy, and did not demonstrate a difference in OSI between patients with or without bile duct clamping (4 versus 11 OSIs; P = 0.23). Antibiotic versus saline irrigation during pancreatoduodenectomy was evaluated in a RCT35 including 190 patients, which reported comparable superficial SSI and OSI rates. Overall, preventive interventions, aside from adequate antibiotic prophylaxis, have not been confirmed to substantially reduce OSIs after pancreatoduodenectomy.
In most of the studies included in this review, type of prolonged antibiotic prophylaxis was based on bile cultures, which were either patient-specific (based on cultures obtained during surgery which are generally available after 3–5 postoperative days), or standardized based on bile culture results of a former cohort of patients. The microbiological profile of bile cultures was commonly polymicrobial, and predominantly showed Enterococcus, Enterobacter, and Klebsiella species. Enterococcus and Enterobacter species are intrinsically resistant to antibiotic agents frequently used as antibiotic prophylaxis (for example first- to third-generation cephalosporins and nitroimidazole derivates), whereas Klebsiella species could develop acquired resistance to cephalosporins36–38. Although Enterococcus species were often present in (mainly polymicrobial) bile and abdominal drain fluid cultures, Enterococci are considered low-virulence microorganisms36,39. Previous studies40,41 supported a sufficient effect of cephalosporins combined with metronidazole as prophylaxis with regard to SSIs. In the present review, there was a comparable prevalence of the abovementioned bacteria in patients receiving perioperative versus prolonged prophylaxis. Despite the presence of these bacteria, the overall rate of abdominal infectious complications was lower in patients receiving prolonged prophylaxis. It is plausible that adequate antibiotic prophylaxis does not necessarily require coverage of Enterococcus species.
One of the main drawbacks of prolonged antibiotic prophylaxis is the potential acquisition of antibiotic resistance by selective antibiotic pressure at both the individual and population level42. Many hospitals have antibiotic stewardship programmes to tackle the threat of increasing antibiotic resistance by restrictive and appropriate antibiotic use43. The benefit of prolonged antibiotic prophylaxis should be clearly established before widespread implementation. Increasing antibiotic resistance in turn might limit the arsenal of antibiotics suited for prophylaxis, particularly in the event of two different standards regarding type of antibiotic prophylaxis for patients with and without contaminated bile. Recently, two Dutch studies3,29 showed low resistance rates of microorganisms cultured from bile obtained during surgery towards perioperative prophylaxis (cefazolin and metronidazole). In three of the studies included in this review, susceptibility patterns of bacteria cultured from bile and abdominal fluid also demonstrated low acquired resistance rates to the antibiotics used for perioperative or prolonged prophylaxis23,26,27. Nevertheless, resistance patterns vary substantially by region and should therefore be monitored when administering prolonged antibiotic prophylaxis. Long-term outcomes regarding antimicrobial resistance and the effect of prolonged antibiotics on the intestinal microbiome should be evaluated when administering prolonged prophylaxis, particularly with regard to pancreatic cancer outcomes44.
The heterogeneity of the included studies in terms of patient selection, and type and duration of antibiotic prophylaxis is one of the main limitations of this review. In addition, data on the clinical impact of fewer abdominal infections in terms of reinterventions and readmissions was limited. Furthermore, six of the included studies were observational and the only two RCTs reported conflicting results. The negative effect of prolonged antibiotics on OSIs reported by Yamamoto et al.27 might be explained by the high POPF rate in the prolonged prophylaxis group, and could be affected by the small sample size. Nevertheless, antibiotic prophylaxis was administered for 5 days in four of five studies evaluating patients with contaminated bile, and a substantial difference was measured in primary outcome.
Prolonged antibiotic prophylaxis seems to lower the rate of OSIs in patients undergoing pancreatoduodenectomy with contaminated bile, without increasing short-term bacterial resistance rates. The promising effect of prolonged antibiotic prophylaxis for patients with preoperative biliary drainage warrants evaluation in an adequately powered RCT.
Supplementary Material
Contributor Information
Daphne H M Droogh, Department of Surgery, Leiden University Medical Centre, Leiden, the Netherlands.
Jesse V Groen, Department of Surgery, Leiden University Medical Centre, Leiden, the Netherlands.
Mark G J de Boer, Departments of Infectious Diseases and Clinical Epidemiology, Leiden University Medical Centre, Leiden, the Netherlands.
Joffrey van Prehn, Department of Medical Microbiology, Leiden University Medical Centre, Leiden, the Netherlands.
Hein Putter, Department of Biomedical Data Sciences, Leiden University Medical Centre, Leiden, the Netherlands.
Bert A Bonsing, Department of Surgery, Leiden University Medical Centre, Leiden, the Netherlands.
Casper H J van Eijck, Department of Surgery, Erasmus MC Cancer Institute, Rotterdam, the Netherlands.
Alexander L Vahrmeijer, Department of Surgery, Leiden University Medical Centre, Leiden, the Netherlands.
Hjalmar C van Santvoort, Department of Surgery, Regional Academic Cancer Centre Utrecht, Utrecht, the Netherlands.
Bas Groot Koerkamp, Department of Surgery, Erasmus MC Cancer Institute, Rotterdam, the Netherlands.
J Sven D Mieog, Department of Surgery, Leiden University Medical Centre, Leiden, the Netherlands.
Funding
The authors have no funding to declare.
Author contributions
Daphne Droogh (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing—original draft, Writing—review & editing), Jesse Groen (Conceptualization, Methodology, Writing—original draft, Writing—review & editing), Mark de Boer (Conceptualization, Methodology, Supervision, Writing—review & editing), Joffrey van Prehn (Conceptualization, Investigation, Writing—review & editing), Hein Putter (Formal analysis, Methodology, Validation, Visualization, Writing—review & editing), Bert Bonsing (Conceptualization, Supervision, Writing—review & editing), C. van Eijck (Supervision, Writing—review & editing), Alexander Vahrmeijer (Conceptualization, Funding acquisition, Supervision, Writing—review & editing), Hjalmar van Santvoort (Methodology, Supervision, Writing—review & editing), Bas Grootkoerkamp (Conceptualization, Investigation, Methodology, Writing—review & editing), and Sven Mieog (Conceptualization, Funding acquisition, Investigation, Methodology, Supervision, Visualization, Writing—review & editing).
Disclosure
The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS online.
Data availability
All data analysed during this study are included in this article (and its supplementary material) as references to published articles, or are available from the corresponding authors on reasonable request.
References
- 1. Elliott IA, Chan C, Russell TA, Dann AM, Williams JL, Damato Let al. Distinction of risk factors for superficial vs organ-space surgical site infections after pancreatic surgery. JAMA Surg 2017;152:1023–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Limongelli P, Pai M, Bansi D, Thiallinagram A, Tait P, Jackson Jet al. Correlation between preoperative biliary drainage, bile duct contamination, and postoperative outcomes for pancreatic surgery. Surgery 2007;142:313–318 [DOI] [PubMed] [Google Scholar]
- 3. Groen JV, Droogh DHM, de Boer MGJ, van Asten SAV, van Prehn J, Inderson Aet al. Clinical implications of bile cultures obtained during pancreatoduodenectomy: a cohort study and meta-analysis. HPB (Oxford) 2020;23:1123–1133 [DOI] [PubMed] [Google Scholar]
- 4. Cortes A, Sauvanet A, Bert F, Janny S, Sockeel P, Kianmanesh Ret al. Effect of bile contamination on immediate outcomes after pancreaticoduodenectomy for tumor. J Am Coll Surg 2006;202:93–99 [DOI] [PubMed] [Google Scholar]
- 5. Loos M, Strobel O, Legominski M, Dietrich M, Hinz U, Brenner Tet al. Postoperative pancreatic fistula: microbial growth determines outcome. Surgery 2018;164:1185–1190 [DOI] [PubMed] [Google Scholar]
- 6. Ohgi K, Sugiura T, Yamamoto Y, Okamura Y, Ito T, Uesaka K. Bacterobilia may trigger the development and severity of pancreatic fistula after pancreatoduodenectomy. Surgery 2016;160:725–730 [DOI] [PubMed] [Google Scholar]
- 7. Nakamura K, Sho M, Kinoshita S, Akahori T, Nagai M, Nakagawa Ket al. New insight into the association between bile infection and clinically relevant pancreatic fistula in patients undergoing pancreatoduodenectomy. J Hepatobiliary Pancreat Sci 2020;27:992–1001 [DOI] [PubMed] [Google Scholar]
- 8. Pham H, Chen A, Nahm CB, Lam V, Pang T, Richardson AJ. The role of targeted versus standard antibiotic prophylaxis in pancreatoduodenectomy in reducing postoperative infectious complications: a systematic review and meta-analysis. Ann Surg 2022;275:315–323 [DOI] [PubMed] [Google Scholar]
- 9. Melloul E, Lassen K, Roulin D, Grass F, Perinel J, Adham Met al. Guidelines for perioperative care for pancreatoduodenectomy: Enhanced Recovery After Surgery (ERAS) recommendations 2019. World J Surg 2020;44:2056–2084 [DOI] [PubMed] [Google Scholar]
- 10. Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, Bolon MKet al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect (Larchmt) 2013;14:73–156 [DOI] [PubMed] [Google Scholar]
- 11. European Centre for Disease Prevention and Control (ECDC) . Systematic Review and Evidence-based Guidance on Perioperative Antibiotic Prophylaxis. European Centre for Disease Prevention and Control, 2013. https://www.ecdc.europa.eu/en/publications-data/systematic-review-and-evidence-based-guidance-peri-operative-antibiotic
- 12. Macedo FIB, Mowzoon M, Parikh J, Sathyanarayana SA, Jacobs MJ. Disparities in the management and prophylaxis of surgical site infection and pancreatic fistula after pancreatoduodenectomy. J Hepatobiliary Pancreat Sci 2017;24:268–280 [DOI] [PubMed] [Google Scholar]
- 13. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535 [PMC free article] [PubMed] [Google Scholar]
- 14. Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan Met al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman ADet al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Am J Infect Control 1992;20:271–274 [DOI] [PubMed] [Google Scholar]
- 17. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham Met al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery 2017;161:584–591 [DOI] [PubMed] [Google Scholar]
- 18. Sánchez Acedo P, Zazpe Ripa C, Eguaras Cordoba I, Herrera Cabezon J, Tarifa Castilla A, Camarero Triana B. The effect of a preoperative biliary prosthesis on the infectious complications of the pancreaticoduodenectomy. Rev Esp Enferm Dig 2019;111:817–822 [DOI] [PubMed] [Google Scholar]
- 19. Sourrouille I, Gaujoux S, Lacave G, Bert F, Dokmak S, Belghiti Jet al. Five days of postoperative antimicrobial therapy decreases infectious complications following pancreaticoduodenectomy in patients at risk for bile contamination. HPB (Oxford) 2013;15:473–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mohammed S, Evans C, VanBuren G, Hodges SE, Silberfein E, Artinyan Aet al. Treatment of bacteriobilia decreases wound infection rates after pancreaticoduodenectomy. HPB (Oxford) 2014;16:592–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fathi AH, Jackson T, Barati M, Eghbalieh B, Siegel KA, Siegel CT. Extended perioperative antibiotic coverage in conjunction with intraoperative bile cultures decreases infectious complications after pancreaticoduodenectomy. HPB Surg 2016;2016:3031749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Degrandi O, Buscail E, Martellotto S, Gronnier C, Collet D, Adam JPet al. Perioperative antibiotherapy should replace prophylactic antibiotics in patients undergoing pancreaticoduodenectomy preceded by preoperative biliary drainage. J Surg Oncol 2019;120:639–645 [DOI] [PubMed] [Google Scholar]
- 23. Petit M, Geri G, Salomon E, Victor M, Peschaud F, Vieillard-Baron Aet al. Risk factors for surgical site infection after pancreatic surgery: a better postoperative antibiotic strategy is possible. J Hosp Infect 2021;107:28–34 [DOI] [PubMed] [Google Scholar]
- 24. Fromentin M, Mullaert J, Gille B, Tchalla A, Lavollay M, Boyer-Besseyre Met al. Extended antibiotic prophylaxis after pancreatoduodenectomy reduces postoperative abdominal infection in high-risk patients: results from a retrospective cohort study. Surgery 2022;172:205–211 [DOI] [PubMed] [Google Scholar]
- 25. Droogh DHM, van Dam JL, Groen JV, de Boer MGJ, van Prehn J, van Eijck CHJet al. Prolonged antibiotics after pancreatoduodenectomy reduce abdominal infections in patients with positive bile cultures: a dual-center cohort study. HPB (Oxford) 2023:S1365-182X(23)00162-4. doi: 10.1016/j.hpb.2023.05.008 [DOI] [PubMed] [Google Scholar]
- 26. Okamura K, Tanaka K, Miura T, Nakanishi Y, Noji T, Nakamura Tet al. Randomized controlled trial of perioperative antimicrobial therapy based on the results of preoperative bile cultures in patients undergoing biliary reconstruction. J Hepatobiliary Pancreat Sci 2017;24:382–393 [DOI] [PubMed] [Google Scholar]
- 27. Yamamoto T, Satoi S, Fujii T, Yamada S, Yanagimoto H, Yamaki Set al. Dual-center randomized clinical trial exploring the optimal duration of antimicrobial prophylaxis in patients undergoing pancreaticoduodenectomy following biliary drainage. Ann Gastroenterol Surg 2018;2:442–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Howard TJ, Yu J, Greene RB, George V, Wairiuko GM, Moore SAet al. Influence of bactibilia after preoperative biliary stenting on postoperative infectious complications. J Gastrointest Surg 2006;10:523–531 [DOI] [PubMed] [Google Scholar]
- 29. Hentzen J, Smit MA, Bruins MJ, Rupert C, Schreinemakers J, Ruijs Get al. Efficacy of pre-operative antimicrobial prophylaxis in patients undergoing pancreatoduodenectomy: a multi-center retrospective analysis. Surg Infect (Larchmt) 2018;19:608–613 [DOI] [PubMed] [Google Scholar]
- 30. Routine sterile glove and instrument change at the time of abdominal wound closure to prevent surgical site infection (ChEETAh): a pragmatic, cluster-randomised trial in seven low-income and middle-income countries. Lancet 2022;400:1767–1776 [DOI] [PubMed] [Google Scholar]
- 31. Edwards JP, Ho AL, Tee MC, Dixon E, Ball CG. Wound protectors reduce surgical site infection: a meta-analysis of randomized controlled trials. Ann Surg 2012;256:53–59 [DOI] [PubMed] [Google Scholar]
- 32. De Pastena M, Marchegiani G, Paiella S, Fontana M, Esposito A, Casetti Let al. Use of an intraoperative wound protector to prevent surgical-site infection after pancreatoduodenectomy: randomized clinical trial. Br J Surg 2020;107:1107–1113 [DOI] [PubMed] [Google Scholar]
- 33. Lenet T, Gilbert RWD, Abou-Khalil J, Balaa FK, Martel G, Brind’Amour Aet al. The impact of prophylactic negative pressure wound therapy on surgical site infections in pancreatic resection: a systematic review and meta-analysis. HPB (Oxford) 2022;24:2035–2044 [DOI] [PubMed] [Google Scholar]
- 34. Singh H, Krishnamurthy G, Kumar H, Gorsi U, Kumar MP, Mandavdhare Het al. Effect of bile duct clamping versus no clamping on surgical site infections in patients undergoing pancreaticoduodenectomy: a randomized controlled study. ANZ J Surg 2020;90:1434–1440 [DOI] [PubMed] [Google Scholar]
- 35. Maatman TK, Weber DJ, Timsina LR, Qureshi B, Ceppa EP, Nakeeb Aet al. Antibiotic irrigation during pancreatoduodenectomy to prevent infection and pancreatic fistula: a randomized controlled clinical trial. Surgery 2019;166:469–475 [DOI] [PubMed] [Google Scholar]
- 36. Kwon W, Jang JY, Kim EC, Park JW, Han IW, Kang MJet al. Changing trend in bile microbiology and antibiotic susceptibilities: over 12 years of experience. Infection 2013;41:93–102 [DOI] [PubMed] [Google Scholar]
- 37. Grundmann H, Glasner C, Albiger B, Aanensen DM, Tomlinson CT, Andrasević ATet al. Occurrence of carbapenemase-producing Klebsiella pneumoniae and Escherichia coli in the European survey of carbapenemase-producing Enterobacteriaceae (EuSCAPE): a prospective, multinational study. Lancet Infect Dis 2017;17:153–163 [DOI] [PubMed] [Google Scholar]
- 38. Mora-Guzmán I, Rubio-Perez I, Domingo-Garcia D, Martin-Perez E. Intra-abdominal infections by carbapenemase-producing Enterobacteriaceae in a surgical unit: counting mortality, stay, and costs. Surg Infect (Larchmt) 2021;22:266–273 [DOI] [PubMed] [Google Scholar]
- 39. Murray BE. The life and times of the Enterococcus. Clin Microbiol Rev 1990;3:46–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Said SA, Hossain MS, DeMare A, Perlmutter BC, McMichael J, Joyce Det al. Long term assessment of antibiotic prophylaxis and biliary microbiome in pancreaticoduodenectomy. HPB (Oxford) 2022;24:1861–1868 [DOI] [PubMed] [Google Scholar]
- 41. Maxwell DW, Jajja MR, Ferez-Pinzon A, Pouch SM, Cardona K, Kooby DAet al. Bile cultures are poor predictors of antibiotic resistance in postoperative infections following pancreaticoduodenectomy. HPB (Oxford) 2020;22:969–978 [DOI] [PubMed] [Google Scholar]
- 42. Lipsitch M, Samore MH. Antimicrobial use and antimicrobial resistance: a population perspective. Emerg Infect Dis 2002;8:347–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baur D, Gladstone BP, Burkert F, Carrara E, Foschi F, Döbele Set al. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis 2017;17:990–1001 [DOI] [PubMed] [Google Scholar]
- 44. Riquelme E, Zhang Y, Zhang L, Montiel M, Zoltan M, Dong Wet al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell 2019;178:795–806.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data analysed during this study are included in this article (and its supplementary material) as references to published articles, or are available from the corresponding authors on reasonable request.