Abstract
Objectives:
Drug reaction with eosinophilia and systemic symptoms (DRESS) is a severe, delayed hypersensitivity reaction (DHR). We observed DRESS to inhibitors of interleukin 1 (IL-1) or interleukin 6 (IL-6) in a small group of Still’s patients with atypical lung disease. We sought to characterize features of Still’s patients with DRESS compared to drug-tolerant Still’s controls. We analyzed human leukocyte antigen (HLA) alleles for association to inhibitor-related DHR, including in a small Kawasaki disease (KD) cohort.
Methods:
In a case/control study, we collected a multicenter series of Still’s patients with features of inhibitor-related DRESS (n=66) and drug-tolerant Still’s controls (n=65). We retrospectively analyzed clinical data from all Still’s subjects and HLA-typed 94/131. European Still’s-DRESS cases were ancestry-matched to INCHARGE pediatric Still’s cases (n=550) and compared for HLA allele frequencies. HLA association also was analyzed using Still’s-DRESS cases (n=64) compared to drug-tolerant Still’s controls (n=30). KD subjects (n=19) were similarly studied.
Results:
Still’s-DRESS features included eosinophilia (89%), AST-ALT elevation (75%), and non-evanescent rash (95%; 88% involving face). Macrophage activation syndrome during treatment was frequent in Still’s-DRESS (64%) versus drug-tolerant Still’s (3%; p=1.9×10−14). We found striking enrichment for HLA-DRB1*15 haplotypes in Still’s-DRESS cases versus INCHARGE Still’s controls (p=7.5×10−13) and versus self-identified, ancestry-matched Still’s controls (p=6.3×10−10). In the KD cohort, DRB1*15:01 was present only in those with suspected anakinra reactions.
Conclusions:
DRESS-type reactions occur among patients treated with IL-1/IL-6 inhibitors and strongly associate with common HLA-DRB1*15 haplotypes. Consideration of pre-prescription HLA typing and vigilance for serious reactions to these drugs are warranted.
Keywords: Pharmacogenetics, Biologic Therapy, Juvenile Arthritis, Still’s disease
Introduction
Adverse drug reactions are one of the leading causes of morbidity and mortality worldwide.1 Among these reactions, severe, potentially fatal delayed hypersensitivity reactions (DHR) are underrecognized due to their complexity and variable presentation.2–4 Particularly during treatment of inflammatory illnesses, DHR may be misinterpreted as disease flares. The most serious types of DHR classify as severe cutaneous adverse reactions (SCAR), including drug reaction with eosinophilia and systemic symptoms (DRESS). Typical features of DRESS-type DHR are latency (days to months) after drug initiation, fever, extensive rash, hematologic manifestations (eosinophilia and atypical lymphocytosis), involvement of various deep organs, and often an extended time to recovery, even after the offending drug is stopped. Recognition of this serious drug reaction during complex illness is both imperative and challenging.
Increasingly, pharmacogenetic data link drug-specific reaction risk with particular human leukocyte antigen (HLA) class I and/or class II alleles. HLA associations with severe drug reactions have proven to be substantially stronger with much higher odds ratios and more complete penetrance than most of the well-known HLA allelic disease associations in autoimmune disorders.1,5,6 In addition to providing clues to pathogenesis, the finding of an HLA/DHR association allows preventative HLA screening pre-prescription. Some well-characterized HLA associations are specific to alleles found primarily in particular populations; others have been linked to relatively common alleles with a wide global distribution.7 The cost/benefit ratio of HLA screening to prevent a serious drug reaction in at-risk individuals improves as the population frequency of the HLA risk allele increases.1
HLA molecules function to present peptides to T cells through binding to T cell surface receptors for antigen. In some severe reactions, the offending drugs have been shown to interact directly with HLA molecules, which in turn stimulate T cell responses; the drug interaction also can alter the repertoire of peptides bound to HLA.1 Thus, HLA associations with severe DHR implicate T cells as immune effectors. This implication is corroborated by evidence from biopsies of DHR-associated skin rashes, which show infiltration of activated T cells.8
Systemic juvenile idiopathic arthritis (sJIA) is a chronic inflammatory disease of childhood with unknown etiology; parenchymal lung disease is not a typical feature.9,10 We observed DRESS among a small group of sJIA patients who developed an unusual, non-infectious parenchymal diffuse lung disease (DLD) during treatment with inhibitors of IL-1 (anakinra, canakinumab, rilonacept) or of IL-6 (tocilizumab).10 We hypothesized that DRESS reactions, with and without DLD, were underrecognized in sJIA and its adult counterpart, adult onset Still’s disease (AOSD), which are currently considered a single disease, Still’s disease, based on clinical and immunologic studies.11–17 We aimed to characterize clinical features of these drug reactions in Still’s patients and to assess HLA alleles as candidate inherited risk factors for DRESS to these drugs. We also hypothesized that an HLA-associated risk of delayed drug reaction might extend to other disease contexts.
Methods
Subjects:
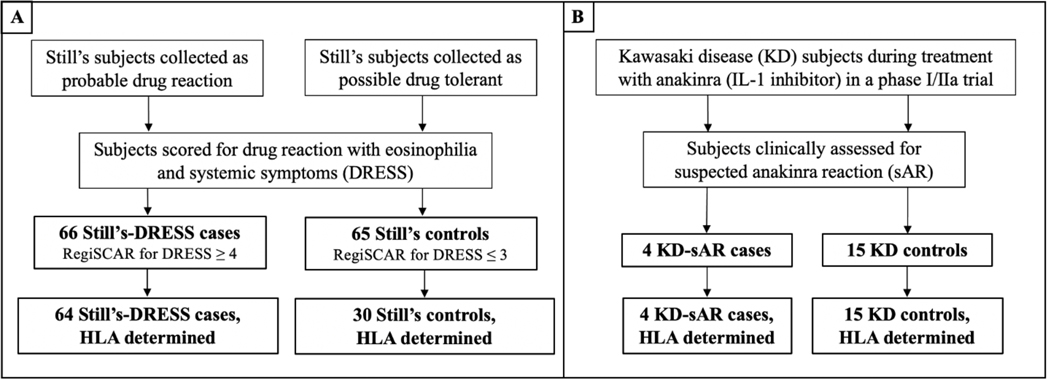
The Still’s disease continuum includes systemic juvenile idiopathic arthritis (sJIA) patients and AOSD patients9,11–17. Still’s disease patients with probable drug reaction to anakinra, canakinumab, rilonacept and/or tocilizumab (cases) or with possible drug-tolerance after exposure to the same drugs (controls) were collected from 37 centers (US, Canada, Australia) through web-based and meeting-based solicitation. Additional Still’s controls from the International Childhood Arthritis Genetics (INCHARGE) Consortium sJIA collection18, the largest available sJIA cohort, and the ancestry-matched, INCHARGE healthy control population were used as sources of genetic data. A small (n=19) cohort of Kawasaki disease (KD) patients in a brief phase I/IIa trial of anakinra (NCT-0217985319 figure 1) also provided cases and controls. In sum, we had 6 major groups of subjects (see table, p7, supplementary information). For sJIA, sJIA-like and AOSD classification criteria used, see supplementary information.
Figure 1: Study design.
Clinical information was collected on Still’s disease subjects with and without clinical suspicion of drug reaction to inhibitors of IL-1 or IL-6 (A). Classification of Still’s patients was verified by RegiSCAR scoring for DRESS. Similar numbers of Still’s-DRESS (n=65 + 1 suspected delayed anakinra reaction Still’s; see methods) and Still’s controls (n=65) subjects were enrolled for case/control comparison and do not reflect the incidence of inhibitor-triggered DRESS in Still’s disease. HLA genotyping was performed on the subset of patients with available sample or sequence data. All 19 Kawasaki disease subjects were enrolled in a Phase I/IIa clinical trial of anakinra in KD patients with coronary artery abnormalities19 (B) and were clinically scored as suspected anakinra reaction or drug-tolerant; all were HLA typed. Details of scoring and HLA genotyping are provided in methods and supplementary information.
Still’s Disease: sJIA, systemic onset juvenile idiopathic arthritis (Still’s onset <16yrs) and AOSD, adult-onset Still’s Disease (Still’s onset ≥16yrs)9,11; RegiSCAR, registry of experts assembled to clinically classify drug induced severe cutaneous reactions20; DRESS, drug reaction with eosinophilia and systemic symptoms
Verification of cases (drug-reactive) and controls (drug-tolerant)
Clinical information required for case/control verification of the Still’s disease subjects was collected by privacy-protected electronic database or by direct communication with the physician case reporter, under approved IRB protocols (see supplementary information). Still’s subjects were verified as cases (n=66, 65 DRESS plus 1 Still’s with suspected delayed anakinra reaction) or controls (drug-tolerant; n=65; hereafter called Still’s controls), using a validated scoring system, the registry for severe cutaneous adverse reactions (RegiSCAR) for DRESS. The RegiSCAR system was validated in the setting of inflammatory diseases and uses clinical parameters allowing differentiation from active Still’s disease.9,20 See supplementary information for RegiSCAR variables. Classification of suspected anakinra reaction (sAR) in Still’s (n=1 subject) required >2 occurrences of unexplained eosinophilia (AEC ≥500) during treatment. Classification as drug- tolerant (Still’s controls) required inhibitor treatment duration of >1 year, RegiSCAR score of <4, and discontinuation of steroids or ≥6wks dosed at <0.2 mg/kg/day of prednisone equivalent; these criteria excluded those with long latency to DRESS or on sufficient steroids to blunt the reaction.
Data for full RegiSCAR scoring were unavailable for KD subjects. Classification of KD subjects as KD-sAR required eosinophilia ≥50% over pre-treatment, study baseline value. Presumed drug-tolerance in KD was defined as absence of eosinophilia during anakinra exposure (9–46 days). Still’s and KD subjects were verified as case or control by a board-certified allergist (VS) prior to HLA determination.
Clinical and demographic data collection
In addition to information for case/control verification, other clinical and demographic (sex, self-identified race) information on the 131 Still’s disease subjects was collected. Laboratory data collected during treatment included eosinophil count, aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Eosinophilia was defined as absolute number or percent of white blood cell count above the laboratory’s upper limit of normal without other cause, e.g., allergic rhinoconjunctivitis in the absence of steroid treatment. AST/ALT elevation was defined as >2x the upper limit of normal more than once without infection or other non-DRESS cause, including macrophage activation syndrome (MAS). MAS was determined by the case reporter using Ravelli classification criteria.21
HLA genotyping determination
As described in supplementary information, genomic DNA was extracted from blood or tissue, and HLA genotyping was performed by one of several methods. For those with limited DNA sample or clinically typed cases, HLA genotyping was limited to Class II [23% (15/64) of Still’s-DRESS cases].
Statistical analyses, including HLA association
As this is a case/control study, we used odds ratios (OR), their 95% confidence intervals and corresponding p-values to summarize the association of various clinical and genetic factors of interest with DRESS.
Six major groups of subjects were studied:
Still’s DRESS cases: Still’s disease patients with DRESS (n=66)
Still’s controls: Still’s disease patients without DRESS [drug-tolerant] (n=65)
INCHARGE childhood-onset Still’s (sJIA) [European ancestry]: Still’s disease patients with drug exposure unknown (n=550)
INCHARGE healthy controls [European ancestry]: Healthy subjects (n=3279)
KD-sAR cases: KD patients with suspected anakinra reaction [sAR] (n=4)
KD controls: KD patients without sAR (n=15)
Groups 3 and 4 (INCHARGE Still’s controls and healthy controls) were constructed to rigorously ancestry-match a subset of Still’s-DRESS cases with European ancestry (White) for unbiased HLA analysis. To this end, two rounds of principal component analysis (PCA) were performed. These used 24 Still’s-DRESS cases with whole exome sequence (WES) data, INCHARGE European Still’s cases (n=773) and European healthy controls (n=6612). Genomic control inflation factors (λGC) were determined to assess robustness of matching.
Analysis of drug exposure, demographic and clinical features:
We compared frequency of exposure to individual inhibitors or any IL-1 inhibitor between Still’s-DRESS cases and Still’s controls (group 1 vs group 2) using Fisher’s exact test. We also compared the demographic and clinical characteristics between Still’s-DRESS cases and Still’s controls (group 1 vs group 2), using Fisher’s exact test. We also compared age of disease onset (<2.5, 2.5–10, 10–16, >16) between Still’s-DRESS cases and Still’s controls (group 1 vs group 2) using proportional odds regression. These four analyses used the entire Still’s case/control collection, excluding 1 or 2 subjects in some analyses of clinical features, due to missing data. In a sensitivity analysis, we compared clinical characteristics (eosinophilia, elevated LFTs and MAS) in the subgroups of Still’s-DRESS cases and Still’s controls with Still’s onset age <16 years (subgroups of group 1 vs group 2) using Fisher’s exact test.
HLA association analysis:
The analysis of HLA allele association with DRESS to the IL-1/IL-6 inhibitors was restricted to subjects who were genotyped for HLA (subsets within groups 1 and 2, as shown in figure 1, and the INCHARGE collection). We compared HLA allele frequencies between Still’s-DRESS cases with European ancestry and INCHARGE Still’s controls (European ancestry patients in group 1 vs group 3) or between Still’s-DRESS cases with European ancestry and INCHARGE healthy controls (European ancestry patients in group 1 vs group 4). Specifically, classical class I and class II HLA alleles were analyzed by logistic regression with sex as a covariate, as described.18 This multi-allelic HLA association analysis was repeated, comparing self-identified White Still’s-DRESS cases and INCHARGE Still’s controls (self-identified White patients in group 1 vs group 3) and comparing self-identified White Still’s-DRESS cases and INCHARGE healthy controls (self-identified White patients in group 1 vs group 4). Bonferroni corrected P value significance threshold, adjusted for multiple comparisons (254 imputed HLA alleles tested), was P<2.0 ×10−4. The identified risk allele (DRB1*15:01) was also tested for association with risk of DRESS to individual inhibitors in self-identified White Still’s-DRESS cases versus INCHARGE Still’s controls (self-identified White patients in group 1 vs group 3) by logistic regression with sex as a covariate. We also compared the frequency of DRB1*15:01 in self-identified White, Still’s-DRESS cases and self-identified White Still’s controls by Fisher’s exact test (self-identified White patients in group 1 vs group 2). In a sensitivity analysis, the latter comparison was repeated using only subjects with sJIA onset age <16 years.
We also compared HLA-DRB1*11:01 frequencies between Still’s-DRESS cases with European ancestry and INCHARGE Still’s controls (European ancestry patients in group 1 vs group 3), between Still’s-DRESS cases with European ancestry and INCHARGE healthy controls (European ancestry patients in group 1 vs group 4), between Still’s-DRESS cases self-reported as White and INCHARGE Still’s controls (self-identified White patients in group 1 vs group 3) and between Still’s-DRESS cases self-reported as White and INCHARGE healthy controls (self-identified White patients in group 1 vs group 4). All analyses used logistic regression, adjusting for sex and the Bonferroni corrected P value significance threshold.
We did not have enough non-White subjects for within ancestry comparisons. Therefore, we reported the allele frequencies in these comparisons without formal statistical analyses (e.g., KD-sAR cases vs KD controls and pooled Still’s-DRESS + KD-sAR cases vs pooled Still’s + KD-sAR controls). Also note that the observed proportion of Still’s-DRESS cases in Still’s disease subjects or the proportion of DLD cases within Still’s-DRESS cases cannot be interpreted as estimates of the prevalence rates due to the case/control study design.
Additional details on methods are in supplementary information.
Results
DRESS, often unrecognized, occurs in a subset of Still’s disease patients treated with IL-1 or IL-6 inhibitors
We collected cases of Still’s disease subjects with probable drug hypersensitivity to IL-1 inhibitors (anakinra, canakinumab, rilonacept) or an IL-6 inhibitor (tocilizumab) and Still’s disease controls with probable drug tolerance. We confirmed classification of 66 subjects as drug-reactive and 65 subjects as drug-tolerant, using specified criteria, including RegiSCAR/DRESS scoring (figure 1; see supplementary materials for details of scoring).
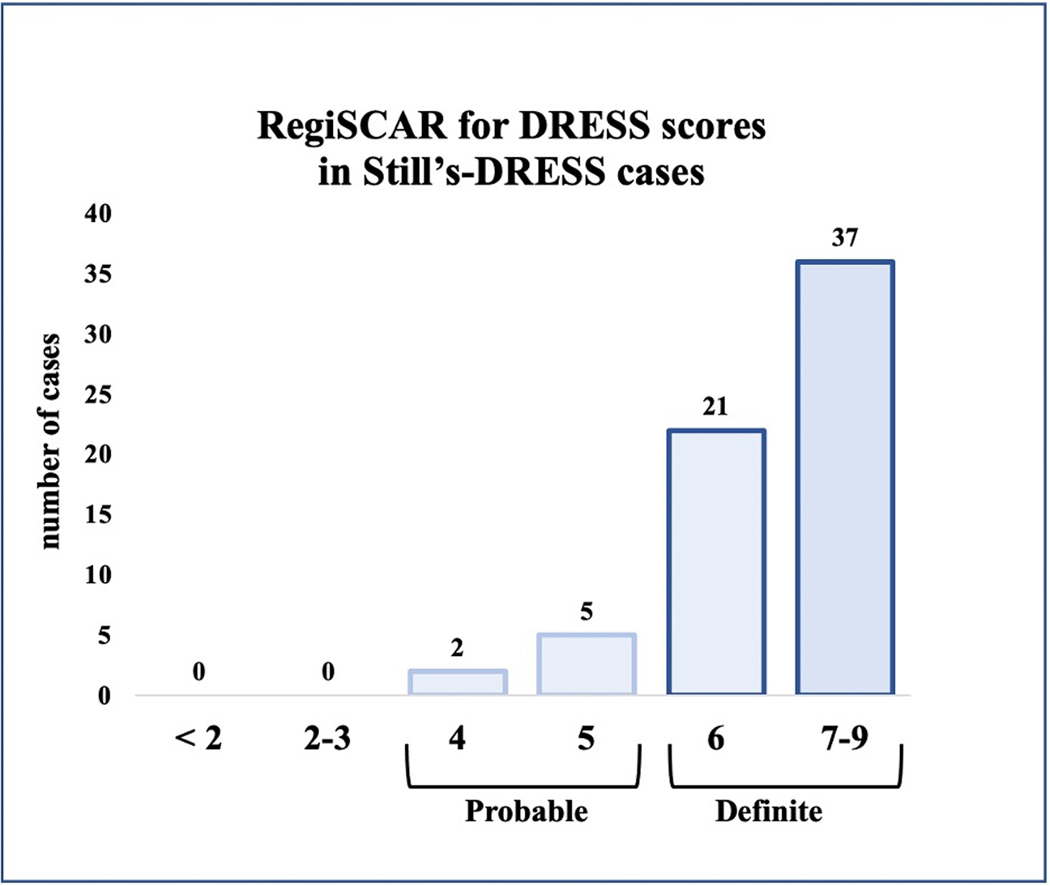
Almost all (65/66) drug-reactive cases were classified as DRESS; the single exception was classified as suspected anakinra reaction (sAR). The majority (89%) of DRESS patients classified as definite DRESS (figure 2); 7 subjects classified a probable DRESS and were included as cases per standard application of RegiSCAR/DRESS.20 We observed a DRESS reaction to anakinra, canakinumab and tocilizumab used alone, indicating that each is capable of triggering DRESS (table S1a–b; rilonacept was not used as the sole drug in any subject). 26/66 drug-reactive subjects reacted to multiple inhibitors. The frequency of drug reaction per exposed subject was not significantly enriched for IL-1 inhibitors compared to tocilizumab (anti-IL-6) or for a particular IL-1 inhibitor (table S1c, part A). For each implicated drug, the frequency of reactions/case was comparable to the frequency of exposures/control (table S1c, part A). These findings supported comparisons of the pooled Still’s-DRESS cases to the pooled Still’s controls in subsequent analyses.
Figure 2: RegiSCAR for DRESS scores in Still’s-DRESS cases.
Numbers of Still’s-DRESS cases with RegiSCAR for DRESS scores of definite or probable are shown (n=65). The Still’s case with suspected delayed anakinra reaction is not included. RegiSCAR classifies a case as definite (6–9), probable (4–5), possible (2–3) or no case (0 to negative 4).20 For DRESS cases reacting to more than one IL-1/IL-6 inhibitor, the highest RegiSCAR value is shown. By definition, no drug-tolerant subject scored ≥4. RegiSCAR scoring elements are shown in supplementary information.
The Still’s-DRESS group and the Still’s control group were similar in having broad ancestral distribution (table S1a–b), as expected in Still’s disease10; they differed modestly in % male subjects. [32% vs 51%; table S2]. Clinical features did not vary systematically based on the particular drug exposure (table S3a) and were similar among Still’s-DRESS patients across the age spectrum, with the exception of increased frequency of DLD in patients with very young onset Still’s disease (table S2).
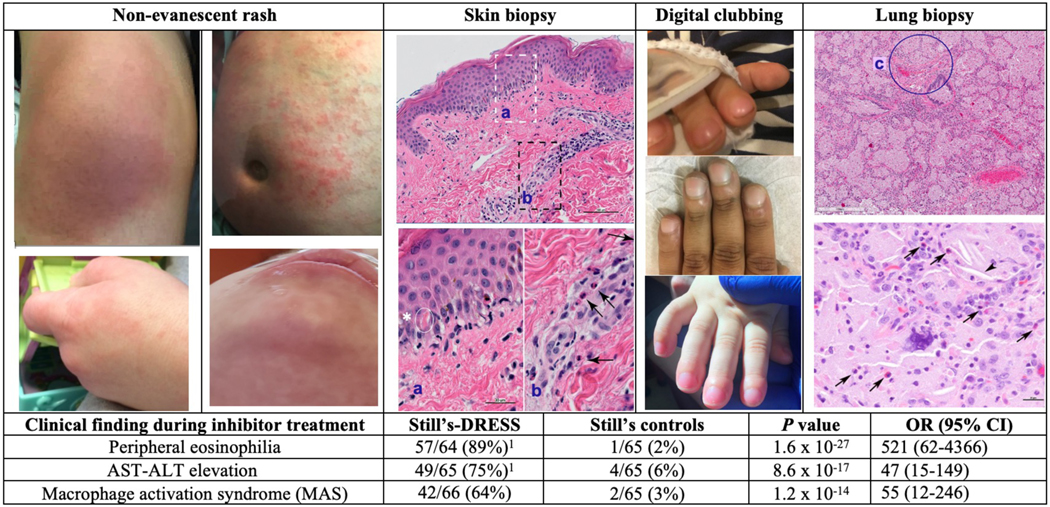
In Still’s-DRESS cases, DRESS features appeared during treatment at FDA-approved doses for autoinflammatory diseases. Clinical DRESS differed from features of Still’s flare9 and notably included eosinophilia and non-Still’s rash (figure 3). Peripheral blood eosinophilia without other cause (e.g., pre-existing atopy) was observed in 57/65 (88%) cases. In >60% of cases, eosinophilia was pronounced despite concurrent steroids. Non-evanescent drug eruptions were observed in 63/66 (95%). In 42/48 (88%) providing detail, rash included facial rash and/or edema, which are typical of DRESS.3 Skin biopsy reports (12 cases) showed features of drug reaction/DRESS7, including interface dermatitis, dyskeratosis and eosinophilia. In 49/65 (75%) Still’s-DRESS cases, AST-ALT elevation was noted in the absence of MAS or other explanation. MAS during inhibitor treatment, which can be a manifestation of DRESS,2,3,23,24 was significantly more common in DRESS cases than in Still’s controls (p=1.9 ×10−14). When MAS occurred during drug treatment, transient eosinophilia typically preceded this by months, consistent with evolution of DRESS-associated features.3,4
Figure 3: Unusual clinical features in inhibitor-treated Still’s patients.
Images of non-evanescent rash, typically pruritic, are shown. Upper left: On anakinra, erythema and prominent edema affecting knee; upper right: on tocilizumab, excoriated and areas of hyperpigmentation on abdomen; lower left: On canakinumab, erythematous, edematous rash on hand [similar rash on face and ear is not shown]; lower right: On anakinra, erythema, edema and non-herpetic vesiculation on face. Skin biopsy of drug-associated rash shows vacuolar interface dermatitis and eosinophils. Higher power images (sections a, b) show lymphocytes, vacuolation at the dermal-epidermal junction, focal dyskeratotic keratinocytes (asterisk) and perivascular eosinophils (arrows). Acute digital clubbing, often erythematous, was frequently the first indication of lung involvement in patients with DRESS and diffuse lung disease. Images of acute clubbing on tocilizumab (top), anakinra (middle), on canakinumab (bottom). Lung biopsy showing variant pulmonary alveolar proteinosis/endogenous lipoid pneumonia and arterial wall thickening (c). Higher power image (below) shows cholesterol clefts (arrowhead) and scattered eosinophils (arrows). 8/16 reviewed cases showed eosinophils in many fields (see supplementary methods). Increased lung eosinophils are consistent with DRESS and also seen in various inflammatory diseases. Table: In DRESS cases, median (interquartile range) of peak absolute eosinophil count was 1500/ul (980,3080) and peak eosinophil % of WBC was 18% (12,33). AST-ALT elevation was defined as aspartate aminotransferase (AST) or alanine aminotransferase (ALT) measuring >2x the upper limit of normal more than once, without alternative (e.g., non-drug) explanation. The frequency of DRESS reactions did not differ significantly when combined anti-IL-1 inhibitors were compared to the IL-6 inhibitor (tocilizumab) or when each inhibitor was analyzed separately (table S1c). Analyses of specific clinical findings yielded similar results when AOSD patients were omitted (table S5). See supplementary information for detailed methods and additional clinical data (tables S1c, S2, S3a–b).
DRESS, drug reaction with eosinophilia and systemic symptoms; MAS, macrophage activation syndrome, a form of secondary hemophagocytic lymphohistiocytosis22,23; P value, by Fisher’s exact; OR (95% CI), odds ratio (95% confidence interval)
1Eosinophil information was unavailable in 2 cases (n=64); AST-ALT values were unavailable in 1 case, (n=65).
The drug reactions were often unrecognized, as reflected by continuation of inhibitor therapy after DRESS criteria were met. Only 17/66 (27%) Still’s patients with DRESS stopped IL-1/IL-6 inhibitors for ≥3 months without re-introduction. In this group, rash, eosinophilia, and AST-ALT elevation resolved in all cases, consistent with resolution of DRESS. In addition, with removal of DRESS as a contributor, inflammation became easier to manage. For example, 10/17 (59%) discontinued steroids and only 2/17 cases required steroids >6 months after drug stop [median follow-up 14 months (IQR: 6, 36)]. By contrast, of 33 subjects who continued inhibitors after scoring as DRESS, 9 died and only 17% of survivors were off steroids, despite median follow-up of 27 months (IQR: 16, 53). Restarting suspended IL-1 inhibitors was associated with fatal MAS (4 of 6 cases) within two months.
Common HLA-DRB1*15 alleles are risk factors for DHR to IL-1 and IL-6 inhibitors
To test for an HLA association with inhibitor-triggered DRESS, we studied the subset of the Still’s disease subjects (n=94/131) with available HLA data. (Individual HLA data and associated clinical/demographic data on this subset are on tables S1a–e, S3a–b). First, PCA analyses of the 24 Still’s-DRESS subjects with WES data, yielded a tight cluster of every subject of White (European) ancestry (n=14) from the Still’s-DRESS cohort, together with 550 INCHARGE sJIA subjects and 3279 INCHARGE healthy controls, as shown on figure S1. Genomic control inflation factors (λGC) were 1.01–1.05, demonstrating robust matching. Comparing these groups revealed a striking enrichment for HLA-DRB1*15:01 (p=2.7×10−7; table 1, S4a–b), which is part of a common European haplotype, HLA-DRB5*01:01~DRB1*15:01~DQA1*01:02 ~DQB1*06:02. The strong HLA-DRB1*15:01 association was maintained when all self-identified White Still’s-DRESS subjects (n=36) were compared to the European INCHARGE Still’s (sJIA) cohort (p=7.5×10−13) (table 1, S4a–b). We also performed an analysis of the 36 White subjects, stratified by treatment group. The anakinra, canakinumab, and rilonacept groups were each enriched for HLA-DRB1*15:01, relative to the European INCHARGE Still’s (sJIA) cohort. The tocilizumab group was not adequately powered to identify an association; however, the frequency of HLA-DRB1*15:01 among tocilizumab-reactive subjects (80%) was similar to the frequencies observed in the other groups (83–92%). The confidence intervals overlapped with one another, so the effect sizes are statistically indiscernible table S1c, part B). No independent HLA class I association was found (table S4c).
Table 1:
HLA class II allele association with hypersensitivity to IL-1 and IL-6 inhibitors1
| HLA allele | Ancestry | Cases | Controls | |||
|---|---|---|---|---|---|---|
| Still’s-DRESS2 | Still’s controls | INCHARGE sJIA | P value3 | OR (95% CI) | ||
| DRB1*15:01 | European vs European | 13/14 (93%) | 130/550 (24%) | 2.7×10−7 | 40.8 (5.3, 316) | |
| self-ID White vs European | 30/36 (83%) | 130/550 (24%) | 7.5×10−13 | 15.5 (6.3, 38.1) | ||
| DRB1*15:014 | self-ID White | 30/36 (83%) | 0/19 (0%) | 6.3×10−10 | Inf (16.05-Inf) | |
| self-ID non-White | 16/28 (57%) | 0/11 (0%) | ||||
| DRB1*15:XX4 | 21/28 (75%) | 2/11 (18%) | ||||
| Kawasaki disease | KD-sAR | Drug-tolerant KD | ||||
| DRB1*15:01 | All | 2/4 (50%) | 0/15 (0%) | |||
| DRB1*15:XX | All | 3/4 (75%) | 2/15 (13%)5 | |||
| Still’s + Kawasaki disease | Still’s-DRESS + KD-sAR | Drug-tolerant Still’s + KD | ||||
| DRB1*15:01 | All | 48/68 (71%) | 0/45 (0%) | |||
| DRB1*15:XX | All | 54/68 (79%) | 4/45 (9%) | |||
| DQB1*06:02 | All | 47/65 (72%) | 3/45 (7%) | |||
DRESS, Drug reaction with eosinophilia and systemic symptoms classified per RegiSCAR20; INCHARGE, International Childhood Arthritis Genetics Consortium18; OR (95% CI), Odds ratio and 95% confidence interval; European: Still’s-DRESS cases were ancestry-matched by PCA to the INCHARGE Still’s (sJIA) cohort (figure S1); self-ID, self-identified; White, similar to European descent; Inf, infinite; KD, Kawasaki disease; HLA-DRB1*15:XX, all HLA-DRB1*15 alleles
In analyses omitting AOSD patients, similar results were obtained (table S5).
Includes one case with suspected anakinra reaction (see methods).
P value, top two rows are by logistic regression from multi-allelic comparison to the INCHARGE cohort; only DRB1*15:01 result is shown (extended results on tables S4a–c). Bonferroni corrected P<2.0×10−4. P-value in third row is by Fisher’s exact test, comparing Still’s-DRESS to Still’s controls for DRB1*15:01.
Each HLA-DR allele group observed in self-identified White Still’s DRESS subjects initially was interrogated for association; only HLA-DRB1*15 alleles showed significant association (table S6).
HLA-DRB1*15:02 in two individuals, treated briefly (12d and 28d) with anakinra.
As the INCHARGE collection does not include data on drug tolerance, we compared HLA frequency in the self-identified White Still’s-DRESS group to self-identified White Still’s drug-tolerant controls (table 1). Using HLA-DRB1* 15:01 as a haplotype proxy, the comparison (83% vs 0%) showed a highly significant enrichment in the DRESS group (p=6×10−10) with a notable effect size (OR lower bound=16.05).
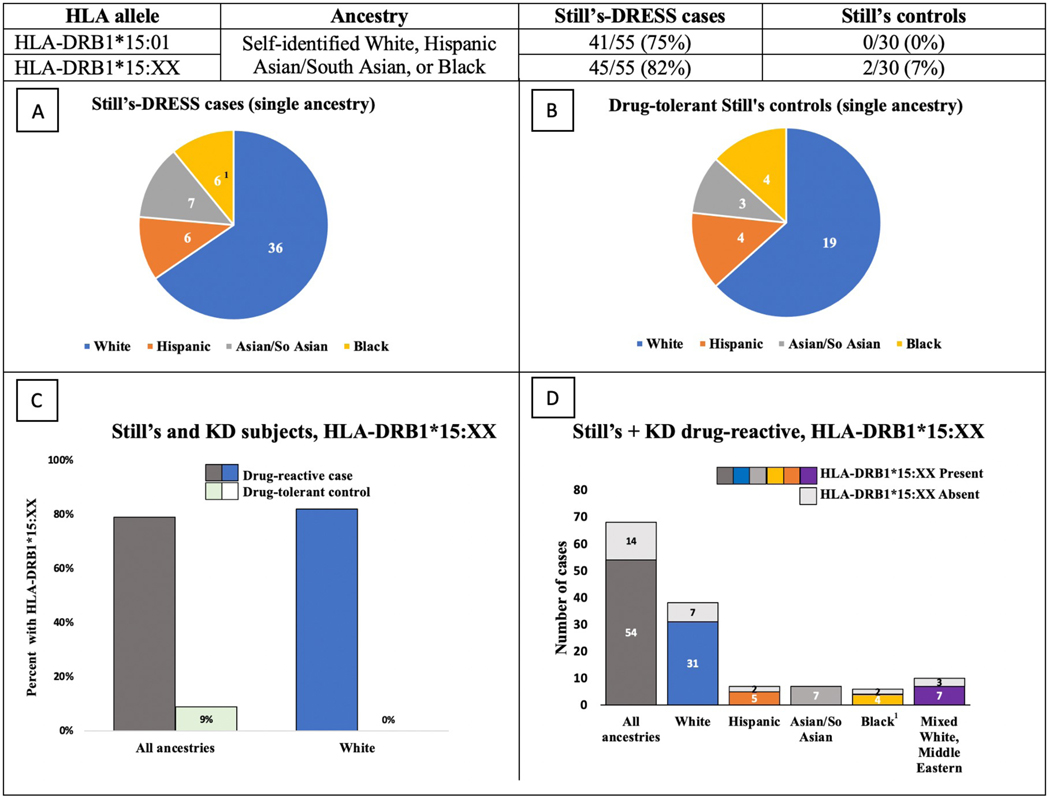
Another 28 subjects with Still’s-DRESS and 11 who were Still’s controls had self-identified ancestry other than White. Although the sample was insufficient to perform within-group analyses, we noted a similarly striking pattern of HLA association. HLA-DRB1*15:01 was observed in 57% non-White subjects with DRESS and 0% of drug-tolerant controls (table 1). Other alleles of the DRB1*15 family are more often present in non-White/European populations, and these appear to be associated with DRESS as well. Together, HLA-DRB1*15 alleles (specifically HLA-DRB1*15:01, *15:03, *15:06) were noted in 75% non-White subjects with Still’s-DRESS compared to 18% Still’s controls (table 2). Comparing the subset of all Still’s DRESS subjects who could be matched for ancestry with Still’s controls also showed HLA-DRB1*15:XX enrichment in the DRESS versus drug-tolerant group (82% vs 7%; figure 4). No independent HLA class I association with Still’s-DRESS was observed (table S1d–e).
Table 2:
HLA-DRB1*11:01 is Still’s-associated in the Still’s-DRESS cohort1
| HLA allele | Ancestry | Still’s-DRESS | Still’s controls | INCHARGE Still’s (sJIA) | INCHARGE healthy controls | P value | OR (95% CI) |
|---|---|---|---|---|---|---|---|
| DRB1*11:01 | European vs | 4/14 (29%) | 103/550 (19%) | 0.43 | |||
| European INCHARGE | 2/19 (10%) | 0.36 | |||||
| 313/3279 (10%) | 0.095 | 3.7 (1.1–11.9)2 | |||||
| DRB1*11:01 | Self-ID White vs European INCHARGE | 7/35 (20%) | 103/550 (19%) | 0.051 | |||
| 2/19 (10%) | 0.46 | ||||||
| 313/3279 (10%) | 0.01 | 2.3 (1.0–5.2)2 | |||||
| DRB1*15:01 | European | 130/550 (24%) | 822/3279 (25%) | 0.59 | |||
P value, by logistic regression with sex as a covariate for INCHARGE comparisons; OR (95% CI), Odds ratio and 95% confidence interval; sJIA, systemic juvenile idiopathic arthritis; DRESS, Drug reaction with eosinophilia and systemic symptoms classified per RegiSCAR20; INCHARGE, International Childhood Arthritis Genetics Consortium13; European, Still’s-DRESS cases were ancestry-matched by PCA to Still’s (sJIA) INCHARGE
Additional information is provided in tables S1a–b and S4a–b.
Results are consistent with published data from INCHARGE consortium study of HLA association with sJIA.18 Drug exposure in INCHARGE sJIA subjects is unknown.
Figure 4: HLA-DRB1*15:XX appears enriched in delayed drug reactions across ancestries.
Table shows carrier frequencies of HLA-DRB1*15:01 and HLA-DRB1*15:XX in Still’s-DRESS cases and Still’s controls. To compare groups with balanced ancestry, 9 Still’s-DRESS cases were excluded from this analysis (leaving n=55), as they could not be matched with Still’s controls (n=30). (A, B) Pie charts of cases (A) and controls (B) indicate the proportions of subjects with each self-identified ancestry; absolute numbers in each group are shown. (C) Percentages are shown of delayed hypersensitivity reaction cases and drug-tolerant controls with HLA-DRB1*15:XX, in Still’s + KD subjects of all ancestries and in self-identified White subjects. (D) The number of cases in Still’s disease + KD subjects (Still’s-DRESS and KD-sAR) with and without HLA-DRB1*15:XX in all ancestries and in each indicated ancestry group are shown. All subjects with HLA-DRB*3/4/5 information (n=34) carry both HLA-DRB1*15 and HLA-DRB5*01:01. Additional information is on tables S1a–b and S7a.
HLA-DRB1*15:XX, any HLA-DRB1*15; self-identified, self-reported ancestry; KD, Kawasaki disease, Mixed White, White + non-White ancestry
1Includes one Still’s drug-reactive case with suspected delayed anakinra reaction.
When the drug-reactive and drug-tolerant cohorts (all ancestries) were analyzed by drug subgroup, the carrier frequencies of HLA-DRB1*15:XX in drug-reactive cases were enriched in each subgroup and similar between groups (table S1c Part C). HLA-DRB1*15:XX was comparably enriched in DRESS subjects with and without DLD (82% vs 72%; table S1a). Clinical features in DRESS subjects with and without the identified HLA risk alleles were similar (table S3a).
We also examined the frequency of the sJIA-associated HLA-DRB1*11:01 allele18 in our cohort. Unsurprisingly, frequencies of this allele in European and self-identified White Still’s-DRESS cases were similar to the European INCHARGE Still’s (sJIA) cases (table 2) and increased compared to INCHARGE healthy controls (tables 2, S4a–b). HLA-DRB1*15:01 was not associated with Still’s in the European INCHARGE cohort (table 2). HLA-DRB1*11:01 frequency did not differ significantly between White Still’s-DRESS cases and Still’s controls. Overall, the results were consistent with the specificity of the HLA-DRB1*11 association for sJIA (young onset Still’s) and of the HLA-DRB1*15 association for DRESS in Still’s disease. The effect size (odds ratio) for the HLA-associated, inhibitor-related DRESS risk is substantially higher than for the HLA-associated Still’s disease risk.
Lastly, we found that our key clinical and genetic findings persisted when the AOSD subjects were removed from the analyses (table S5), supporting our comparison of aggregate Still’s-DRESS cases to Still’s disease controls.
Common HLA-DRB1*15 alleles are also likely risk factors for suspected anakinra reaction in KD.
To determine whether HLA-linked delayed drug hypersensitivity required Still’s-specific immune dysfunction, we studied a small cohort (n=19) of children with KD in a trial of 2–6 weeks of anakinra treatment.19 Four had suspected delayed anakinra reaction (sAR; table S6a). We observed the same striking effect; 3/4 children with sAR carried HLA-DRB1*15 alleles (HLA-DRB1*15:01 and *15:03), whereas a different HLA-DRB1*15 allele, HLA-DRB1*15:02, was observed in 2/15 apparently drug-tolerant children with KD (table 1, S7a). Notably, HLA-DRB1*15:01 was not observed in any drug-tolerant subject (table S7a). No class I association was observed (table S7b).
High percentages of all DHR subjects (Still’s + KD) carried DRB1*15 alleles across all ancestries (figure 4). While the HLA-DRB1*15:01~DQA1*01:02~DQB1*06:02 haplotype is in near-complete linkage disequilibrium (LD) in European populations, analysis across ancestries, in which patterns of LD differ, can help to pinpoint the associated locus. Considering the entire Still’s-DRESS + KD-sAR group, HLA-DRB1*15:01 was observed in 71% (46/64 Still’s-DRESS subjects and 2/4 with KD-sAR) and was completely absent in drug-tolerant controls (table 1, S1a–b, S7a). In contrast, HLA-DQB1*06:02 was observed in 7% of controls, in the context of different haplotypes (table 1, S1a, S7a), suggesting HLA-DRB1 as the operative locus. It is important to note that HLA-DRB5*01:01, an allele of a secondary HLA-DRB locus, is found on nearly all haplotypes with HLA-DRB1*15 (tables S1a–b, S7a). We are not able to rule it out as an effector or contributor to DHR risk.24
Discussion
We have uncovered strong evidence in Still’s disease patients for severe delayed hypersensitivity to anakinra, canakinumab, rilonacept (anti-IL-1) and tocilizumab (anti-IL-6). Delayed hypersensitivity reactions occurred with similar frequency after IL-1 or IL-6 inhibition and after any of the IL-1 inhibitors. These reactions met classification criteria for DRESS, a potentially fatal, eosinophilic systemic syndrome. DRESS can lead to organ failure and can stimulate MAS.2,3,20,22,23 Indeed, MAS can be the presenting sign of DRESS.23,25 MAS frequency in Still’s-DRESS cases far exceeded MAS frequency in Still’s controls or in published Still’s disease series.26,27 MAS as part of DRESS to inhibitors suggests a possible etiologic pathway distinct from that of Still’s-associated MAS. In the relatively short-term exposure of KD patients to anakinra, a subset of patients also developed clinical manifestations consistent with drug reaction, arguing that these delayed hypersensitivity reactions can occur in conditions other than Still’s disease.
Importantly, we also discovered a genetic risk factor shared across the delayed reactions to these inhibitors, analyzed individually or as a group. We observed a very strong association of the HLA-DRB1*15:01 allele and the linked HLA-DRB5*01:01 in White Still’s subjects. The numbers of Still’s-DRESS cases, Still’s controls and INCHARGE Still’s (sJIA) controls allowed rigorous analysis of this ancestry group. The effect size we report is substantially greater than those seen in HLA/disease associations28 and instead is comparable to those observed in other HLA associations with severe drug-related delayed hypersensitivity.1,5 Other drug-related HLA associations were initially detected in sample sizes similar to the one reported here and subsequently confirmed.29 We also detected striking penetrance of the risk allele, as evidenced by its complete absence in drug-tolerant controls and the highly significant p-values we report.
Although we were limited by the relative scarcity of non-White subjects in our sample, our findings also suggest that, in addition to HLA-DRB1*15:01, other alleles of the HLA-DRB1*15 family are linked to risk of inhibitor-triggered reaction in these populations. The distribution of subjects with HLA-DRB1*15:XX argues the risk applies across ancestry groups, as found in some other HLA/DHR associations.7 Carriers of DRB1*15:01, *15:03, *15:06 alleles are common [27% (White), 15% (Hispanic), 27% (Black) and 16% (Asians) in US populations].30 Our current cohort does not allow analysis of HLA-DRB1*15:02, a high frequency allele in Asian populations. Approximately 20% of the subjects with a drug reaction do not carry the risk alleles. It will be important to determine if other genetic factors confer risk, both in those with and those without the DRB1*15 risk alleles. Investigation of family history of drug reaction may be useful as regards other risk factors.
In Still’s disease and KD, the drug reactions are delayed type and differ from the immediate, anaphylactic reactions to tocilizumab we observed in association with DLD in sJIA.10 Although some Still’s subjects experienced both types of drug reactions, most did not, and carriers of HLA-DRB1*15 alleles were not enriched among those with anaphylaxis to tocilizumab (table S3a).
The HLA association we observe has some interesting features: it is restricted to HLA class II,1,6,7 and it spans several inhibitors with different chemical structures (figure S2). The latter raises the possibility that an excipient common to these drugs and/or a molecule increased by inhibition of the intersecting IL-1 and IL-6 pathways creates a stimulatory HLA class II molecule, which activates CD4+ T cells. Several molecular mechanisms for the modification of HLA into an immunogenic moiety in drug hypersensitivity have been identified or proposed.7,31 A detailed picture of clinical pathogenesis remains to be elucidated and may involve a complex interplay between viruses, HLA proteins, T cells, cytokine secretion and other genetic polymorphisms.2,7,31
The conditions for which these inhibitors may be used are a large and expanding group.32–34 We found scattered reports of DRESS or hyper-eosinophilia with rash implicating these drugs in RA, polyarthritis, undifferentiated autoinflammatory disorder, giant cell arteritis and COVID-related cytokine storm (table S8). HLA typing was not included in these reports and will be important in future investigations. As an n of 1, our continuing case collection includes DRESS in a DRB1*15:01-positive individual with undifferentiated autoimmune disease (table S8).
Other than a few case reports (table S8), previous studies of IL-1 or IL-6 inhibitors do not mention DRESS. However, it is possible that the reaction was unrecognized. In a recent study of anakinra as first-line therapy for sJIA, 17% of subjects required high dose steroids for clinical deterioration or MAS.29 The pivotal trial of canakinumab for sJIA had a 19% non-response rate.27 A study of tocilizumab in RA had a 15% withdrawal rate for adverse events and/or failure to respond.34 In 24 COVID-19 patients treated with tocilizumab, post-treatment elevation of IL-6 levels identified the 25% who died.35 Further work is needed to determine if hypersensitivity contributes to the rates of drug failures.
There are several limitations to our study. First, our White Still’s control group was small. We addressed this limitation by using the European INCHARGE Still’s (sJIA) cohort as a comparator, although the drug tolerance status of these subjects is unknown. Notably, however, unidentified Still’s-DRESS cases among these subjects would mean the high odds ratio we observe is an underestimate of the true effect size. The number of Still’s-DRESS cases with information for robust ancestry loci-matching with the INCHARGE controls was limited. Nonetheless, the highly significant association with HLA-DRB1*15 alleles was replicated in our total Still’s-DRESS+sAR group (n=64), with and without self-identified ancestry matching, and in KD-sAR. It seems unlikely that the HLA link is indirect. Other limitations include those inherent to a retrospective observational design, such as missing data. For example, we lacked information to determine whether the DRESS subset had a higher frequency of herpes virus reactivation, particularly HHV-6, as reported in DRESS.3,7,36 Our sample had under-representation of non-White subjects, limiting our genetic/HLA analyses. Currently, we are assembling validation cohorts for Still’s-DRESS across ancestry groups.
An unanswered question is how the development of DLD in Still’s disease links to immune-mediated DRESS reactions to the inhibitors.2,3,7 The temporal correlation between increasing use of IL-1 and IL-6 inhibitors and increasing DLD in sJIA raised the question of a relationship.37 In further support of an association, all instances of DLD during inhibitor treatment in our cohort scored as DRESS. Lung involvement occurs in DRESS to other drugs, although specific lung pathology has not been described.38 Cases of drug-induced PAP that resolved on drug withdrawal have been reported.39,40 It will be critical to determine if DLD in Still’s improves by withdrawing the implicated inhibitor, and if there is a window of opportunity for this intervention. As young onset Still’s disease patients appear to be at greater risk for DLD with inhibitor-triggered DRESS (table S2 and ref.10), a possible developmental risk for DRESS-associated DLD requires further study.
Given the same HLA association in hypersensitivity cases with and without DLD, it seems unlikely that DLD is HLA-DRB1*15-associated, independent of the DRESS reaction. The possibility that the clinical features represent a new form of sJIA that is associated with the risk haplotype also seems unlikely, given the HLA-DRB1*15:01 link to anakinra reaction in KD and to DRESS in a case of undifferentiated autoinflammation. We do not know if drug tolerance develops over time, especially with concurrent immune suppression.
The HLA association we report is at least equivalent in effect to the association of HLA-B*57:01 with hypersensitivity to abacavir29 treatment with abacavir is contraindicated in carriers of this risk allele and in the smaller group of risk-allele negative, drug-reactive patients.41Similar to recent reports42 we observed onset of severe delayed drug reaction as early as three days after first exposure but also after months of treatment. Thus, our results are relevant for short-term use of the implicated inhibitors and highlight the need for continued surveillance for DHR over time. Some Still’s patients without the HLA risk alleles also suffered severe inhibitor-triggered DRESS reactions, including fatalities. Attention to signs of hypersensitivity to these drugs is prudent whenever they are used.
The frequency of the risk alleles across populations, the strength of the HLA association, and reaction severity, argue for pre-prescription risk analysis. HLA testing is readily available and typically offered at reasonable cost. However, our data are insufficient as a basis for specific recommendations on when or if it is safe to use these inhibitors in Still’s patients with the reaction-associated HLA haplotypes. Further research is needed to determine underlying mechanisms, additional risk factors for DRESS reactions to inhibitors of IL-1 and IL-6, and relevance in other conditions, particularly inflammatory diseases.
Supplementary Material
Key messages:
What is already known about this subject?
Drug reaction with eosinophilia and systemic symptoms (DRESS), a severe delayed hypersensitivity reaction (DHR), is underrecognized, especially in inflammatory conditions.
Secondary hemophagocytic lymphohistiocytosis, indistinguishable from macrophage activation syndrome (MAS), is reported in DRESS.
Strong HLA allelic associations are common in severe, drug-related DHR.
What does this study add?
A subset of Still’s patients develop DRESS to anakinra, canakinumab, rilonacept or tocilizumab.
MAS during treatment with inhibitors of IL-1 or IL-6 appears to be a manifestation of this DRESS reaction.
Diffuse lung disease occurs in some Still’s patients with this DRESS reaction.
Delayed hypersensitivity reactions to inhibitors of IL-1 and IL-6 exhibit a striking association with a common HLA class II haplotype.
How might this impact on clinical practice or future developments?
Our findings argue for consideration of HLA testing for pre-prescription risk assessment.
As 20% of subjects with a reaction do not carry the risk alleles and relevance in other conditions is unknown, vigilance for a DRESS-type delayed reaction is recommended during treatment with these inhibitors.
Acknowledgments:
This work utilized in silico genetic data generated by the International Childhood Arthritis Genetics Consortium; we gratefully acknowledge the INCHARGE investigators. We also thank Debra D. Hiraki, Farkhondeh Ziaei, Linda Gojenola and Drs. James Zehnder and Bing M. Zhang of Stanford Pathology Department for assisting with sample procurement, processing and HLA genotyping, Jamie L. Duke (Children’s Hospital of Philadelphia) for assistance with HLA sequence analysis, Elizabeth Moreno (Rady Children’s Hospital) for assistance with Kawasaki disease samples and clinical data, Lori Ponder (Children’s Healthcare of Atlanta) for patient consents and facilitating sample collection, and Carol Lake (National Institute of Arthritis and Musculoskeletal and Skin Diseases) for accessing and aggregating clinical and laboratory data. We thank the Stanford Blood Center for support performing HLA tests and the following individuals at Stanford University: Drs. Tzielan Lee, Joyce Hsu, Imelda Balboni, Rajdeep Puni, Uptej Khalsa, Christy Sandborg, Quoc Du, Dana Gerstbacher, Claudia Macaubas and Bernice Kwong for facilitating this project, and Dr. Ann Leung for reviewing CT scans.
Funding sources:
This work was funded by The Lucile Packard Foundation for Children’s Health, Stanford Maternal and Child Health Research Institute, the Division of Intramural Research of the National Institutes of Health (National Institute of Arthritis and Musculoskeletal and Skin Diseases [Z01-AR041198] and National Human Genome Research Institute [Z01-HG200370]), the Gordon and Marilyn Macklin Foundation, RK Mellon Institute for Pediatric Research, and The Marcus Foundation Inc., Atlanta, GA. This study utilized the computational resources of the NIH HPC Biowulf cluster (http://hpc.nih.gov).
Footnotes
Competing interests: VS, GD report personal fees from Novartis, SP reports fees from Novartis outside the current work, SC reports personal fees from Novartis and grants from AB2 Bio, and EM reports grants from Novartis. Drug Hypersensitivity Consortium: SL, GS, SS, MS report personal fees from Novartis, AG reports grants and personal fees from Novartis and grants from NovImmune. INCHARGE Consortium: No conflicts of interest
Patient and public involvement statement: Patients were not involved in the research process of this study. Results will be shared via CARRA patient communication mechanisms
Patient consent for publication: Not applicable
Ethics approval: Ethics approval was obtained through the Stanford University School of Medicine institutional review board. Other approvals were per local institutional review board requirements.
Public/Private: Not applicable
A list of members of each Consortium is provided in the supplementary information.
Data availability:
All relevant data generated for this study are included in the article or uploaded as supplementary information. The INCHARGE consortium dataset, which was previously published (Ombrello MJ et al. PNAS 2015) and used in silico for this study, is available through Dr. Michael Ombrello (ORCID ID 0000-0003-3322-4089).
References
- 1.Bharadwaj M, Illing P, Theodossis A, et al. Drug hypersensitivity and human leukocyte antigens of the major histocompatibility complex. Annu Rev Pharmacol Toxicol 2012;52:401–31. doi: 10.1146/annurev-pharmtox-010611-134701 [published Online First: 2011/10/17] [DOI] [PubMed] [Google Scholar]
- 2.Duong TA, Valeyrie-Allanore L, Wolkenstein P, et al. Severe cutaneous adverse reactions to drugs. Lancet 2017;390(10106):1996–2011. doi: 10.1016/S0140-6736(16)30378-6 [published Online First: 2017/05/02] [DOI] [PubMed] [Google Scholar]
- 3.Yang CW, Cho YT, Hsieh YC, et al. The interferon-γ-induced protein 10/CXCR3 axis is associated with human herpesvirus-6 reactivation and the development of sequelae in drug reaction with eosinophilia and systemic symptoms. Br J Dermatol 2020;183(5):909–919. doi: 10.1111/bjd.18942 [published Online First 2020/03/20] [DOI] [PubMed] [Google Scholar]
- 4.Kim GY, Anderson KR, Davis DMR, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS) in the pediatric population: A systematic review of the literature. J Am Acad Dermatol 2020. doi: 10.1016/j.jaad.2020.03.081 [published Online First: 2020/04/02] [DOI] [PubMed] [Google Scholar]
- 5.Howell WM. HLA and disease: guilt by association. Int J Immunogenet 2014;41(1):1–12. doi: 10.1111/iji.12088 [published Online First: 2013/09/04] [DOI] [PubMed] [Google Scholar]
- 6.Usui T, Naisbitt DJ. Human leukocyte antigen and idiosyncratic adverse drug reactions. Drug Metab Pharmacokinet 2017;32(1):21–30. doi: 10.1016/j.dmpk.2016.11.003 [published Online First: 2016/11/18] [DOI] [PubMed] [Google Scholar]
- 7.Bellón T. Mechanisms of Severe Cutaneous Adverse Reactions: Recent Advances. Drug Saf 2019;42(8):973–92. doi: 10.1007/s40264-019-00825-2 [DOI] [PubMed] [Google Scholar]
- 8.Ortonne N, Valeyrie-Allanore L, Bastuji-Garin S, et al. Histopathology of drug rash with eosinophilia and systemic symptoms syndrome: a morphological and phenotypical study. Br J Dermatol 2015;173(1):50–8. doi: 10.1111/bjd.13683 [published Online First: 2015/04/16] [DOI] [PubMed] [Google Scholar]
- 9.Mellins ED, Macaubas C, Grom AA. Pathogenesis of systemic juvenile idiopathic arthritis: some answers, more questions. Nat Rev Rheumatol. 2011;7(7):416–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saper VE, Chen G, Deutsch GH, et al. Emergent high fatality lung disease in systemic juvenile arthritis. Ann Rheum Dis 2019;78(12):1722–31. doi: 10.1136/annrheumdis-2019-216040 [published Online First: 2019/09/27] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Efthimiou P, Kontzias A, Hur P, Rodha K, Ramakrishna GS, Nakasato P. Adult-onset Still’s disease in focus: Clinical manifestations, diagnosis, treatment, and unmet needs in the era of targeted therapies. Seminars in Arthritis and Rheumatism. 2021;51(4):858–74. [DOI] [PubMed] [Google Scholar]
- 12.Jamilloux Y, Gerfaud-Valentin M, Martinon F, Belot A, Henry T, Sève P. Pathogenesis of adult-onset Still’s disease: new insights from the juvenile counterpart. Immunol Res. 2015;61(1–2):53–62. [DOI] [PubMed] [Google Scholar]
- 13.Pardeo M, Rossi MN, Pires Marafon D, Sacco E, Bracaglia C, Passarelli C, et al. Early Treatment and IL1RN Single-Nucleotide Polymorphisms Affect Response to Anakinra in Systemic Juvenile Idiopathic Arthritis. Arthritis Rheumatol. 2021;73(6):1053–61. [DOI] [PubMed] [Google Scholar]
- 14.Kaneko Y, Takeuchi T. Interleukin-6 inhibition: a therapeutic strategy for the management of adult-onset Still’s disease. Expert Opinion on Biological Therapy. 2021:in press. [DOI] [PubMed] [Google Scholar]
- 15.Nirmala N, Brachat A, Feist E, et al. Gene-expression analysis of adult-onset Still’s disease and systemic juvenile idiopathic arthritis is consistent with a continuum of a single disease entity. Pediatr Rheumatol Online J. Nov 2015;13:50. doi: 10.1186/s12969-015-0047-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inoue N, Shimizu M, Tsunoda S, et al. Cytokine profile in adult-onset Still’s disease: Comparison with systemic juvenile idiopathic arthritis. Clinical Immunology 2016;169:8–13. doi: 10.1016/j.clim.2016.05.010 [DOI] [PubMed] [Google Scholar]
- 17.Feist E, Mitrovic S, Fautrel B. Mechanisms, biomarkers and targets for adult-onset Still’s disease. Nat Rev Rheumatol. 10 2018;14(10):603–618. doi: 10.1038/s41584-018-0081-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ombrello MJ, Remmers EF, Tachmazidou I, et al. HLA-DRB1*11 and variants of the MHC class II locus are strong risk factors for systemic juvenile idiopathic arthritis. Proc Natl Acad Sci U S A 2015;112(52):15970–5. doi: 10.1073/pnas.1520779112 [published Online First: 2015/11/23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tremoulet AH, Jain S, Kim S, et al. Rationale and study design for a phase I/IIa trial of anakinra in children with Kawasaki disease and early coronary artery abnormalities (the ANAKID trial). Contemp Clin Trials 2016;48:70–5. doi: 10.1016/j.cct.2016.04.002 [published Online First: 2016/04/11] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol 2013;169(5):1071–80. doi: 10.1111/bjd.12501 [DOI] [PubMed] [Google Scholar]
- 21.Ravelli A, Minoia F, Davi S, Horne A, Bovis F, Pistorio A, et al. 2016 Classification Criteria for Macrophage Activation Syndrome Complicating Systemic Juvenile Idiopathic Arthritis A European League Against Rheumatism/American College of Rheumatology/Paediatric Rheumatology International Trials Organisation Collaborative Initiative. ResearchGate. 2016;75(3):481–9. [DOI] [PubMed] [Google Scholar]
- 22.Yang JJ, Lei DK, Ravi V, et al. Overlap between hemophagocytic lymphohistiocytosis and drug reaction and eosinophilia with systemic symptoms: a review. Int J Dermatol 2020. doi: 10.1111/ijd.15196 [published Online First: 2020/09/21] [DOI] [PubMed] [Google Scholar]
- 23.Jordan MB, Allen CE, Greenberg J, et al. Challenges in the diagnosis of hemophagocytic lymphohistiocytosis: Recommendations from the North American Consortium for Histiocytosis (NACHO). Pediatr Blood Cancer. Nov 2019;66(11):e27929. doi: 10.1002/pbc.27929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J, Jelcic I, Mühlenbruch L, et al. HLA-DR15 Molecules Jointly Shape an Autoreactive T Cell Repertoire in Multiple Sclerosis. Cell 2020;183(5):1264–81.e20. doi: 10.1016/j.cell.2020.09.054 [published Online First: 2020/10/21] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fajgenbaum DC, June CH. Cytokine Storm. N Engl J Med 2020;383(23):2255–73. doi: 10.1056/NEJMra2026131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ter Haar NM, van Dijkhuizen EHP, Swart JF, et al. Treat-to-target using first-line recombinant interleukin-1 receptor antagonist monotherapy in new-onset systemic juvenile idiopathic arthritis: results from a five year follow-up study. Arthritis Rheumatol 2019. doi: 10.1002/art.40865 [published Online First: 2019/03/08] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruperto N, Brunner HI, Quartier P, et al. Two randomized trials of canakinumab in systemic juvenile idiopathic arthritis. N Engl J Med 2012;367(25):2396–406. doi: 10.1056/NEJMoa1205099 [DOI] [PubMed] [Google Scholar]
- 28.Matzaraki V, Kumar V, Wijmenga C, et al. The MHC locus and genetic susceptibility to autoimmune and infectious diseases. Genome Biol 2017;18(1):76. doi: 10.1186/s13059-017-1207-1 [published Online First: 2017/04/27] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hetherington S, Hughes AR, Mosteller M, et al. Genetic variations in HLA-B region and hypersensitivity reactions to abacavir. Lancet 2002;359(9312):1121–2. doi: 10.1016/S0140-6736(02)08158-8 [DOI] [PubMed] [Google Scholar]
- 30.Maiers M, Gragert L, Klitz W. High-resolution HLA alleles and haplotypes in the United States population. Hum Immunol 2007;68(9):779–88. doi: 10.1016/j.humimm.2007.04.005 [published Online First: 2007/05/24] [DOI] [PubMed] [Google Scholar]
- 31.Naisbitt DJ, Olsson-Brown A, Gibson A, et al. Immune dysregulation increases the incidence of delayed-type drug hypersensitivity reactions. Allergy 2020;75(4):781–97. doi: 10.1111/all.14127 [published Online First: 2019/12/23] [DOI] [PubMed] [Google Scholar]
- 32.Dinarello CA. The IL-1 family of cytokines and receptors in rheumatic diseases. Nature Reviews Rheumatology 2019;15(10):612–32. doi: 10.1038/s41584-019-0277-8 [DOI] [PubMed] [Google Scholar]
- 33.Kaur S, Bansal Y, Kumar R, et al. A panoramic review of IL-6: Structure, pathophysiological roles and inhibitors. Bioorg Med Chem 2020;28(5):115327. doi: 10.1016/j.bmc.2020.115327 [published Online First: 2020/01/20] [DOI] [PubMed] [Google Scholar]
- 34.Gabay C, Emery P, van Vollenhoven R, et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet 2013;381(9877):1541–50. doi: 10.1016/S0140-6736(13)60250-0 [published Online First: 2013/03/18] [DOI] [PubMed] [Google Scholar]
- 35.Quartuccio L, Sonaglia A, Pecori D, et al. Higher levels of IL-6 early after tocilizumab distinguish survivors from nonsurvivors in COVID-19 pneumonia: A possible indication for deeper targeting of IL-6. J Med Virol 2020;92(11):2852–56. doi: 10.1002/jmv.26149 [published Online First: 2020/07/22] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barbaud A, Waton J, Herbeth B, et al. Comparison of cytokine gene polymorphism in drug-induced maculopapular eruption, urticaria and drug reaction with eosinophilia and systemic symptoms (DRESS). J Eur Acad Dermatol Venereol 2014;28(4):491–9. doi: 10.1111/jdv.12130 [published Online First: 2013/03/06] [DOI] [PubMed] [Google Scholar]
- 37.Kimura Y, Weiss JE, Haroldson KL, et al. Pulmonary hypertension and other potentially fatal pulmonary complications in systemic juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2013;65(5):745–52. doi: 10.1002/acr.21889 [published Online First: 2012/11/10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taweesedt PT, Nordstrom CW, Stoeckel J, Dumic I. Pulmonary Manifestations of Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome: A Systematic Review. Biomed Res Int. 2019;2019:7863815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Darley DR, Malouf MA, Glanville AR. A rare case of everolimus-induced pulmonary alveolar proteinosis. J Heart Lung Transplant 2016;35(1):147–48. doi: 10.1016/j.healun.2015.10.001 [published Online First: 2015/10/09] [DOI] [PubMed] [Google Scholar]
- 40.Wardwell NR, Miller R, Ware LB. Pulmonary alveolar proteinosis associated with a disease-modifying antirheumatoid arthritis drug. Respirology 2006;11(5):663–5. doi: 10.1111/j.1440-1843.2006.00905.x [DOI] [PubMed] [Google Scholar]
- 41.Dean L. Abacavir Therapy and HLA-B*57:01 Genotype. 2015. Sep 1 [Updated 2018 Apr 18]. In: Pratt VM, Scott SA, Pirmohamed M, et al. , editors. Medical Genetics Summaries [Internet]. Bethesda (MD): National Center for Biotechnology Information (US) https://www.ncbi.nlm.nih.gov/books/NBK315783/ [PubMed] [Google Scholar]
- 42.Sasidharanpillai S, Ajithkumar K, Khader A, et al. Drug reaction with eosinophilia and systemic symptoms within 1 week of exposure to the drug. J Am Acad Dermatol 2020;83(1):e17–e18. doi: 10.1016/j.jaad.2020.03.019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data generated for this study are included in the article or uploaded as supplementary information. The INCHARGE consortium dataset, which was previously published (Ombrello MJ et al. PNAS 2015) and used in silico for this study, is available through Dr. Michael Ombrello (ORCID ID 0000-0003-3322-4089).