Abstract
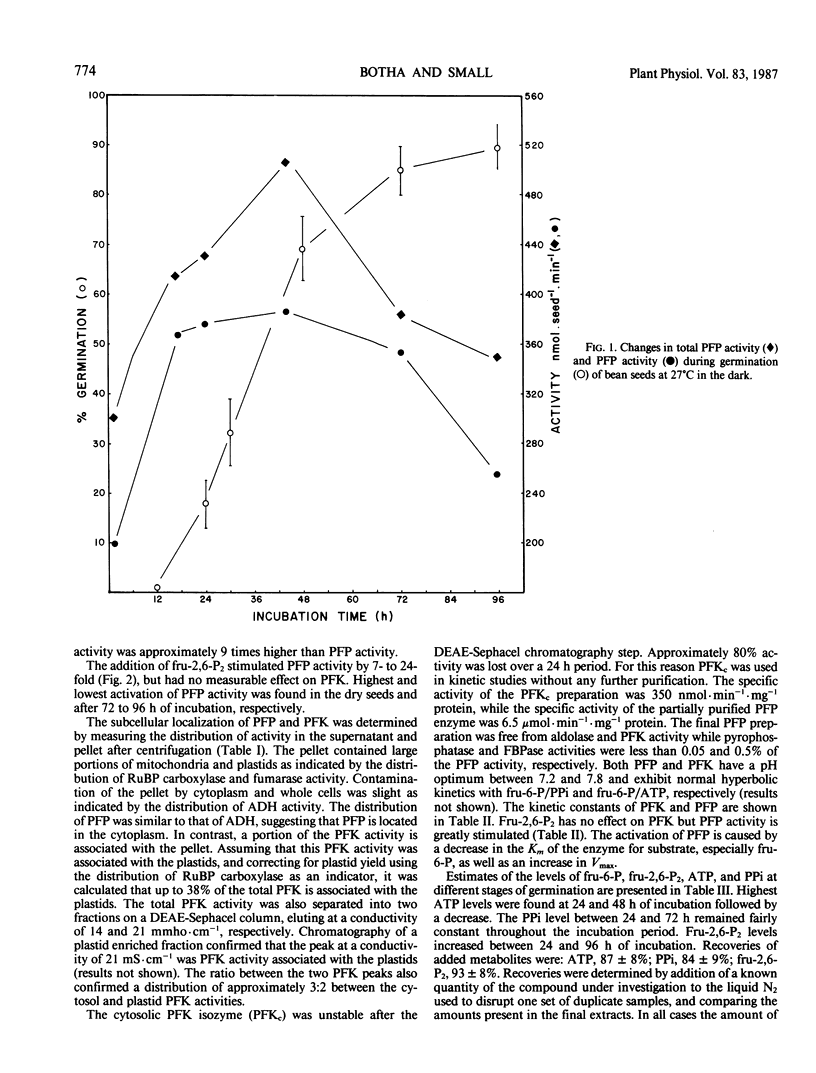
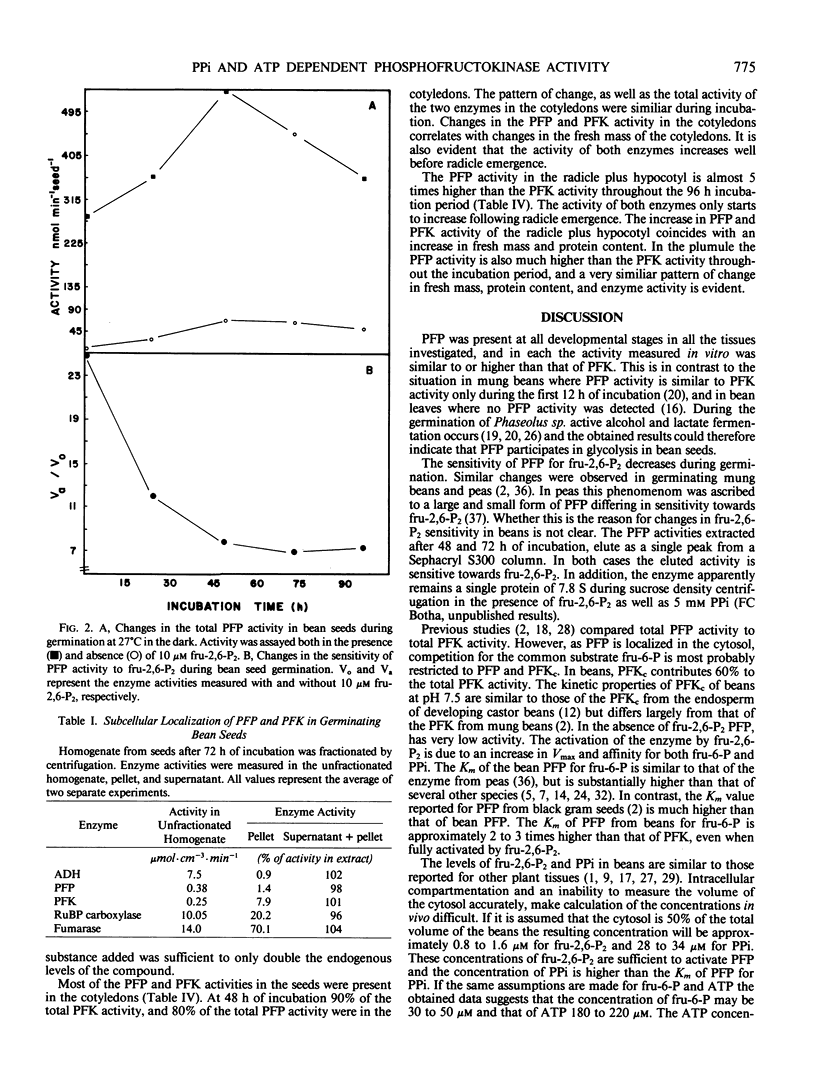
The distribution of pyrophosphate: fructose 6-phosphate phosphotransferase (PFP) and ATP: fructose-6-phosphate 1-phosphotransferase (PFK) was studied in germinating bean (Phaseolus vulgaris cv Top Crop) seeds. In the cotyledons the PFP activity was comparable with that of PFK. However, in the plumule and radicle plus hypocotyl, PFP activity exceeds that of PFK. Approximately 70 to 90%, depending on the stage of germination, of the total PFP and PFK activities were present in the cotyledons. Highest specific activity of both enzymes, however, occurred in the radicle plus hypocotyl (64-90 nanomoles·min·milligram protein). Fractionation studies indicate that 40% of the total PFK activity was associated with the plastids while PFP is apparently confined to the cytoplasm. The cytosolic isozyme of PFK exhibits hyperbolic kinetics with respect to fructose 6-P and ATP with Km values of 320 and 46 micromolar, respectively. PFP also exhibits hyperbolic kinetics both in the presence and absence of the activator fructose-2,6-P2. The activation is caused by lowering the Km for fructose 6-P from 18 to 1.1 millimolar and that for pyrophosphate (PPi) from 40 to 25 micromolar, respectively. Levels of fructose 2,6-P2 and PPi in the seeds are sufficient to activate PFP and thereby enable a glycolytic role for PFP during germination. However, the fructose 6-P content appears to be well below the Km of PFP for this compound and would therefore preferentially bind to the catalytic site of PFK, which has a lower Km for fructose 6-P. The ATP content appears to be at saturating levels for PFK.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Carnal N. W., Black C. C. Phosphofructokinase activities in photosynthetic organisms : the occurrence of pyrophosphate-dependent 6-phosphofructokinase in plants and algae. Plant Physiol. 1983 Jan;71(1):150–155. doi: 10.1104/pp.71.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnal N. W., Black C. C. Pyrophosphate-dependent 6-phosphofructokinase, a new glycolytic enzyme in pineapple leaves. Biochem Biophys Res Commun. 1979 Jan 15;86(1):20–26. doi: 10.1016/0006-291x(79)90376-0. [DOI] [PubMed] [Google Scholar]
- Cséke C., Weeden N. F., Buchanan B. B., Uyeda K. A special fructose bisphosphate functions as a cytoplasmic regulatory metabolite in green leaves. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4322–4326. doi: 10.1073/pnas.79.14.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson S. A., Dennis D. T. Acetyl-coenzyme A carboxylase from the developing endosperm of Ricinus communis. I. Isolation and characterization. Arch Biochem Biophys. 1983 Sep;225(2):576–585. doi: 10.1016/0003-9861(83)90069-3. [DOI] [PubMed] [Google Scholar]
- Finlayson S. A., Dennis D. T. Acetyl-coenzyme A carboxylase from the developing endosperm of Ricinus communis. II. A two-site kinetic mechanism. Arch Biochem Biophys. 1983 Sep;225(2):586–595. doi: 10.1016/0003-9861(83)90070-x. [DOI] [PubMed] [Google Scholar]
- Garland W. J., Dennis D. T. Plastid and cytosolic phosphofructokinases from the developing endosperm of Ricinus communis. II. Comparison of the kinetic and regulatory properties of the isoenzymes. Arch Biochem Biophys. 1980 Oct 1;204(1):310–317. doi: 10.1016/0003-9861(80)90038-7. [DOI] [PubMed] [Google Scholar]
- JOYCE B. K., GRISOLIA S. Purification and properties of a nonspecific acid phosphatase from wheat germ. J Biol Chem. 1960 Aug;235:2278–2281. [PubMed] [Google Scholar]
- Kruger N. J., Beevers H. Synthesis and degradation of fructose 2,6-bisphosphate in endosperm of castor bean seedlings. Plant Physiol. 1985 Feb;77(2):358–364. doi: 10.1104/pp.77.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- Sabularse D. C., Anderson R. L. D-Fructose 2,6-bisphosphate: a naturally occurring activator for inorganic pyrophosphate:D-fructose-6-phosphate 1-phosphotransferase in plants. Biochem Biophys Res Commun. 1981 Dec 15;103(3):848–855. doi: 10.1016/0006-291x(81)90888-3. [DOI] [PubMed] [Google Scholar]
- Smyth D. A., Black C. C. Measurement of the pyrophosphate content of plant tissues. Plant Physiol. 1984 Jul;75(3):862–864. doi: 10.1104/pp.75.3.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaftingen E., Lederer B., Bartrons R., Hers H. G. A kinetic study of pyrophosphate: fructose-6-phosphate phosphotransferase from potato tubers. Application to a microassay of fructose 2,6-bisphosphate. Eur J Biochem. 1982 Dec;129(1):191–195. doi: 10.1111/j.1432-1033.1982.tb07039.x. [DOI] [PubMed] [Google Scholar]
- Wu M. X., Smyth D. A., Black C. C. Fructose 2,6-bisphosphate and the regulation of pyrophosphate-dependent phosphofructokinase activity in germinating pea seeds. Plant Physiol. 1983 Sep;73(1):188–191. doi: 10.1104/pp.73.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. X., Smyth D. A., Black C. C. Regulation of pea seed pyrophosphate-dependent phosphofructokinase: Evidence for interconversion of two molecular forms as a glycolytic regulatory mechanism. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5051–5055. doi: 10.1073/pnas.81.16.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ap Rees T., Green J. H., Wilson P. M. Pyrophosphate:fructose 6-phosphate 1-phosphotransferase and glycolysis in non-photosynthetic tissues of higher plants. Biochem J. 1985 Apr 1;227(1):299–304. doi: 10.1042/bj2270299. [DOI] [PMC free article] [PubMed] [Google Scholar]


