Abstract
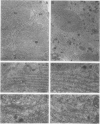
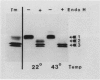
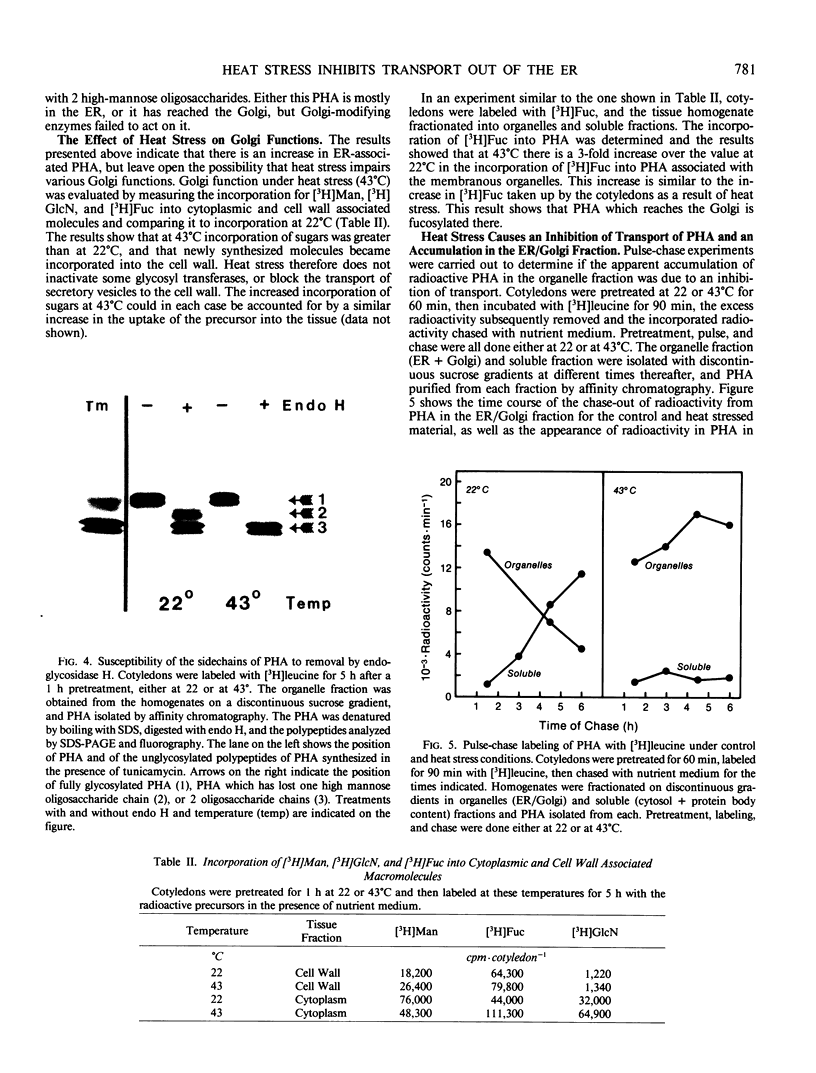
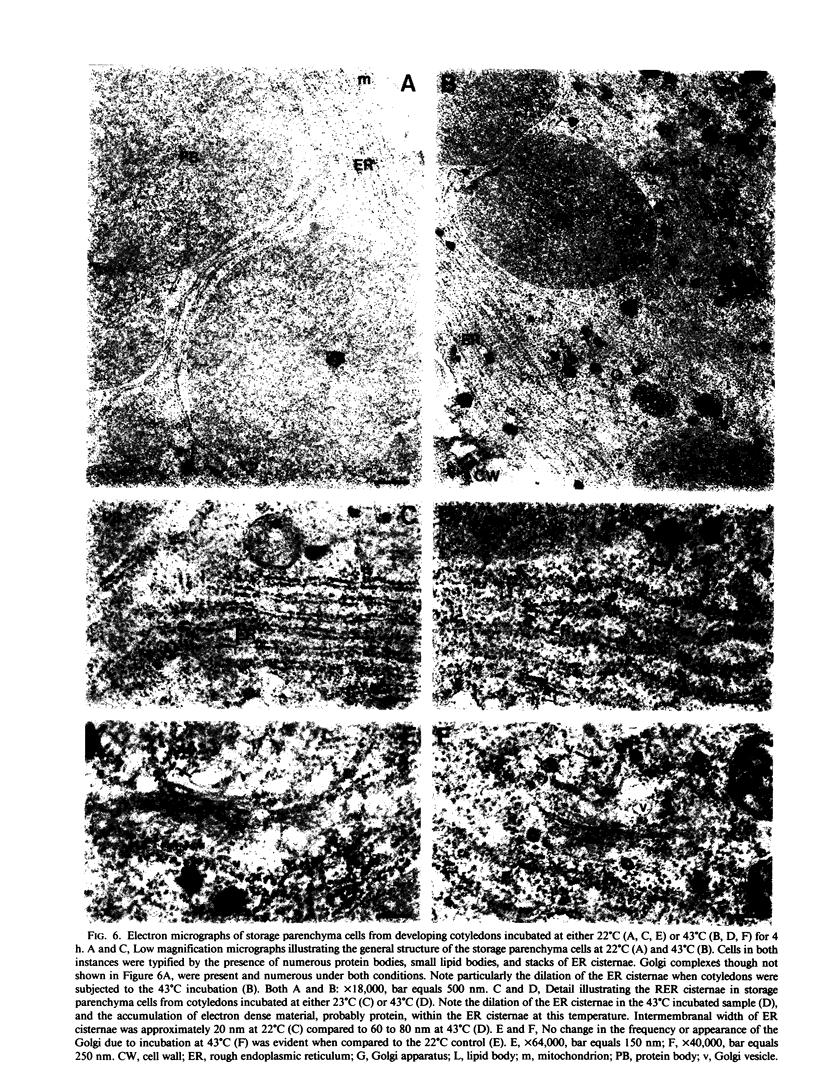
In this study we examined the effect of heat stress (up to 6 hours at 43°C) on the biosynthesis and transport of phytohemagglutinin (PHA) in cotyledons of developing seeds of the common bean, Phaseolus vulgaris. Heat stress resulted in a decrease of total protein synthesis and an enhancement of the synthesis of heat shock proteins and PHA. Pulse chase experiments showed that a considerable proportion of the newly synthesized PHA was present in the endoplasmic reticulum (ER)/Golgi fraction and did not readily chase-out. Analysis with endoglycosidase H showed that the oligosaccharide sidechains of PHA were almost entirely in the high mannose configuration, indicating that most of the newly synthesized PHA was in the ER. However, some of the PHA became fucosylated at 43°C, indicating fucosyltransferase activity. That the biosynthesis and secretion of fucosyl-containing cell wall polymers proceeded normally at 43°C provided evidence that certain Golgi functions (i.e. transport to the cell wall) remained unaffected by heat stress. The ER obtained from these heat stress cotyledons had a greater density (1.16 g· cm−3 at 43°C instead of 1.14 g·cm−3 at 22°C) in sucrose gradients. Ultrastructural observations showed that the width of the lumen of the ER cisternae had increased from 20 nanometers at 22°C to 60 to 80 nanometers at 43°C; the lumen was filled with electrondense material presumed to be protein. The experiments are interpreted as evidence that heat stress imposes a block in the transport of PHA out of the ER. Whether heat stress affects the ER itself or alters the conformation of PHA, thereby preventing its transport, is not clear.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Belanger F. C., Brodl M. R., Ho T. H. Heat shock causes destabilization of specific mRNAs and destruction of endoplasmic reticulum in barley aleurone cells. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1354–1358. doi: 10.1073/pnas.83.5.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chrispeels M. J., Bollini R. Characteristics of Membrane-Bound Lectin in Developing Phaseolus vulgaris Cotyledons. Plant Physiol. 1982 Nov;70(5):1425–1428. doi: 10.1104/pp.70.5.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J., Higgins T. J., Craig S., Spencer D. Role of the endoplasmic reticulum in the synthesis of reserve proteins and the kinetics of their transport to protein bodies in developing pea cotyledons. J Cell Biol. 1982 Apr;93(1):5–14. doi: 10.1083/jcb.93.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J., Higgins T. J., Spencer D. Assembly of storage protein oligomers in the endoplasmic reticulum and processing of the polypeptides in the protein bodies of developing pea cotyledons. J Cell Biol. 1982 May;93(2):306–313. doi: 10.1083/jcb.93.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsted R. L., Leavitt R. D., Bachur N. R. Purification of the phytohemagglutinin family of proteins from red kidney beans (Phaseolus vulgaris) by affinity chromatography. Biochim Biophys Acta. 1975 Sep 9;405(1):72–81. doi: 10.1016/0005-2795(75)90316-5. [DOI] [PubMed] [Google Scholar]
- Gething M. J., McCammon K., Sambrook J. Expression of wild-type and mutant forms of influenza hemagglutinin: the role of folding in intracellular transport. Cell. 1986 Sep 12;46(6):939–950. doi: 10.1016/0092-8674(86)90076-0. [DOI] [PubMed] [Google Scholar]
- Gibson R., Schlesinger S., Kornfeld S. The nonglycosylated glycoprotein of vesicular stomatitis virus is temperature-sensitive and undergoes intracellular aggregation at elevated temperatures. J Biol Chem. 1979 May 10;254(9):3600–3607. [PubMed] [Google Scholar]
- Munro S., Pelham H. R. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986 Jul 18;46(2):291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell. 1986 Sep 26;46(7):959–961. doi: 10.1016/0092-8674(86)90693-8. [DOI] [PubMed] [Google Scholar]
- Spencer D., Higgins T. J., Button S. C., Davey R. A. Pulse-labeling Studies on Protein Synthesis in Developing Pea Seeds and Evidence of a Precursor Form of Legumin Small Subunit. Plant Physiol. 1980 Sep;66(3):510–515. doi: 10.1104/pp.66.3.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Wilden W., Gilkes N. R., Chrispeels M. J. The Endoplasmic Reticulum of Mung Bean Cotyledons: ROLE IN THE ACCUMULATION OF HYDROLASES IN PROTEIN BODIES DURING SEEDLING GROWTH. Plant Physiol. 1980 Sep;66(3):390–394. doi: 10.1104/pp.66.3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale A., Ceriotti A., Bollini R., Chrispeels M. J. Biosynthesis and processing of phytohemagglutinin in developing bean cotyledons. Eur J Biochem. 1984 May 15;141(1):97–104. doi: 10.1111/j.1432-1033.1984.tb08162.x. [DOI] [PubMed] [Google Scholar]