Abstract
The chloramphenicol resistance gene catD from Clostridium difficile was shown to be encoded on the transposons Tn4453a and Tn4453b, which were structurally and functionally related to Tn4451 from Clostridium perfringens. Tn4453a and Tn4453b excised precisely from recombinant plasmids, generating a circular form, as is the case for Tn4451. Evidence that this process is mediated by Tn4453-encoded tnpX genes was obtained from experiments which showed that in trans these genes complemented a Tn4451tnpXΔ1 mutation for excision. Nucleotide sequencing showed that the joint of the circular form generated by the excision of Tn4453a and Tn4453b was similar to that from Tn4451. These results suggest that the Tn4453-encoded TnpX proteins bind to similar DNA target sequences and function in a manner comparable to that of TnpX from Tn4451. Furthermore, it has been shown that Tn4453a and Tn4453b can be transferred to suitable recipient cells by RP4 and therefore are mobilizable transposons. It is concluded that, like Tn4451, they must encode a functional tnpZ gene and a target oriT or RSA site. The finding that related transposable elements are present in C. difficile and C. perfringens has implications for the evolution and dissemination of antibiotic resistance genes and the mobile elements on which they are found within the clostridia.
Clostridium difficile is the major etiological agent of pseudomembranous colitis and also causes a more common, but less severe, form of this disease, known as antibiotic-associated diarrhea (13, 17). C. difficile causes disease when the normal intestinal flora is altered as a result of antimicrobial therapy. Although these organisms probably become a part of the normal intestinal flora, during antibiotic treatment they proliferate, which disrupts other endogenous flora (30). Since it is usually necessary to administer further antibiotics to treat the resultant infection, the presence of antibiotic-resistant C. difficile isolates may complicate the treatment of the diseases caused by this organism (13).
Chloramphenicol resistance in C. difficile and Clostridium perfringens may be mediated by the catD (31, 32) and catP (5, 27) genes, respectively, both of which encode chloramphenicol acetyltransferases. The C. perfringens catP gene is located on the transposons Tn4451 and Tn4452 (2). Tn4451 is found on the conjugative tetracycline resistance plasmid pIP401 and excises precisely upon conjugative transfer in C. perfringens and when it is present on multicopy plasmids in both C. perfringens and Escherichia coli (2, 4, 5). The products of both excision events are identical, indicating that the same precise deletion event is occurring in both organisms (3). Transposition of Tn4451 has been demonstrated in E. coli but occurs only at a very low frequency (2). Transposition has not been demonstrated in C. perfringens because of the lack of a detection method with sufficient sensitivity.
Tn4451 has been completely sequenced (6,338 bp) and has been shown to contain six genes (5). One of these genes, tnpX, encodes a trans-acting site-specific recombinase which is responsible for the excision of Tn4451 in both C. perfringens and E. coli (5). The TnpX protein catalyzes the excision of Tn4451 as a circular molecule (5); this molecule may function as the transposition intermediate, as do the equivalent circular molecules from the well-characterized conjugative transposons Tn916 and Tn1545 (24). The TnpX recombinase is a large member of the resolvase-invertase family of site-specific recombinases (5) and site-directed mutagenesis studies have shown that the resolvase-invertase domains are functional in the excision of Tn4451 (8). Tn4451 is flanked by directly repeated GA dinucleotides, and GA residues are also found at the joint of the circular form, where the left and right termini of Tn4451 are fused (3, 5, 8). Analysis of a number of Tn4451 transposition target sites revealed that they resemble the joint of the circular form and that insertion occurs at a GA dinucleotide (8). On the basis of these data a model for the excision and insertion of Tn4451 which involves the resolvase-invertase domain of TnpX that introduces 2-bp staggered cuts at the GA dinucleotides has been proposed (8).
Another gene carried by Tn4451, tnpZ, encodes the 50-kDa TnpZ protein, which has amino acid sequence similarity to those of a group of plasmid mobilization and recombination proteins that comprise the Mob-Pre family (5). These proteins interact with an upstream palindromic sequence known as the RSA site to mediate plasmid mobilization and the formation of plasmid multimers and cointegrates. In the presence of the conjugative IncP plasmid RP4, TnpZ has been shown to promote RSA-dependent plasmid mobilization in cis and the in trans mobilization of a coresident plasmid carrying an RSA site (9). In addition, TnpZ was found to modulate the conjugative transfer of plasmids from E. coli to C. perfringens (9, 15).
The chloramphenicol resistance gene catD from C. difficile has also been cloned (31). Hybridization studies indicated that this chromosomal gene is very closely related to catP (23). The CATD and CATP monomers have 97% amino acid sequence identity (11). In contrast to catP, the catD gene appears to be present in at least two copies on the C. difficile chromosome (31). There is no evidence that catD can be transferred by conjugation, either within or between species (12, 33).
The aim of this study was to determine if the similarity between the catP and catD determinants extended beyond the resistance genes and therefore to determine if catD was located on an element similar to Tn4451. In this paper we report the cloning and detailed genetic analysis of two catD transposons that are closely related to Tn4451.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth media.
The E. coli strains used in this study were derivatives of DH5α (Bethesda Research Laboratories), S17-1 (26), or LT101 (20). The C. perfringens isolate used in this study was CP590, which carries Tn4451 as part of the conjugative plasmid pIP401 (2, 7). The chloramphenicol-resistant C. difficile strains included isolates from Belgium (SGC0545), England (W1), Holland (3026), and Italy (C250) (31) and two isolates from Japan (KZ1606 and KZ1613 [19]). The properties of the plasmids used in this study are presented in Table 1.
TABLE 1.
Properties of recombinant plasmids
| Plasmid(s) | Relevant characteristic(s) | Reference or source |
|---|---|---|
| pUC18 and pUC19 | Apr | 29 |
| pJIR45 | Apr Cmr; Tn4451 in pUC18 | 4 |
| pJIR62 | Apr Cmr; catP from Tn4451 in pUC18 | 4 |
| pJIR639 | Apr; tnpX from Tn4451 in pBluescript-II | 5 |
| pJIR773 | Cmr Kmr; Tn4451tnpXΔ1 in pSU39 | 5 |
| pJIR1377 | Apr Cmr; Tn4453a in pUC18 | This study |
| pJIR1378 | Apr Cmr; Tn4453b in pUC19 | This study |
| pJIR1488 | Apr; tnpX from Tn4453a in pUC18 | This study |
| pJIR1489 | Apr; tnpX from Tn4453b in pUC18 | This study |
E. coli strains were grown on 2YT agar medium (16) supplemented with ampicillin (100 μg/ml), chloramphenicol (20 μg/ml), kanamycin (20 μg/ml), or rifampin (150 μg/ml). C. perfringens strains were cultured in Trypticase-peptone-glucose broth (21), brain heart infusion broth (Oxoid), fluid thioglycolate medium (Difco), or nutrient agar (22) supplemented with chloramphenicol (10 μg/ml). The C. difficile strains were grown in BHIS medium (28) supplemented with chloramphenicol (10 μg/ml). Clostridial agar cultures were grown in an atmosphere of 10% H2–10% CO2–80% N2. All bacterial strains were grown at 37°C.
DNA isolation and general molecular techniques.
Plasmid DNA from E. coli was isolated by an alkaline lysis procedure (18). PCR amplifications were performed with Taq DNA polymerase (Boehringer Mannheim). PCR products for nucleotide sequencing and cloning were purified by isolation from a low-melting-temperature agarose gel (Seaplaque; FMC BioProducts) with the Magic PCR Preps DNA Purification System (Promega). Total genomic DNA from the clostridial isolates was prepared by a method developed for C. perfringens (1). Transformation of E. coli cells was done as described before (25). The primers used for PCR or nucleotide sequencing were synthesized on an Applied Biosystems 392 DNA/RNA Synthesizer and are shown in Table 2 and Fig. 1.
TABLE 2.
Synthetic oligonucleotide primers
| Primer | Nucleotide sequence | Coordinatesa |
|---|---|---|
| 94 | 5′-GAAAATGTCAAGGACTT-3′ | 72–88 |
| 211 | 5′-CGTTCCTTGTCCTGCT-3′ | 5542–5527 |
| 212 | 5′-TCGGGGACTATTACTA-3′ | 2173–2188 |
| 220 | 5′-CATCAATCACAATCTC-3′ | 5025–5010 |
| 221 | 5′-TGGTGCGGTAGAGTGG-3′ | 4131–4116 |
| 274 | 5′-AACCTGTGGTTATGTAT-3′ | 3407–3391 |
| 300 | 5′-GGGCTATACTTTAATAG-3′ | 1–17 |
| 1727 | 5′-GGGGTCGAGTTTGTCAAG-3′ | 6338–6321 |
| 3277b | 5′-AGTATTCCGCAAAGGTTTTTCTTCTGCGG-3′ | 2220–2200 |
| 4675 | 5′-AATCAAGCAGGACAAGGAACGA-3′ | 5522–5543 |
| 4676 | 5′-TGAAGTCCTTGACATTTTCTTA-3′ | 86–75 |
All primers were derived from Tn4451, the nucleotide sequence of which was published previously (5).
Note that the first 8 nucleotide bases at the 3′ end of this oligonucleotide primer are not derived from Tn4451.
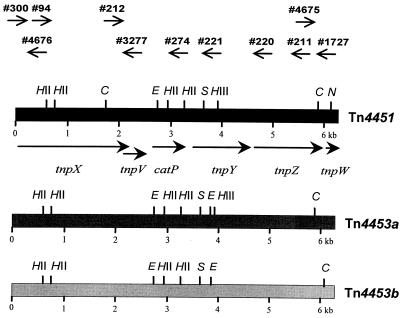
FIG. 1.
Comparative linear maps of Tn4451, Tn4453a, and Tn4453b. The oligonucleotide primers used for PCR analysis are indicated by the short arrows. Restriction sites for CfoI (C), EcoRV (E), HindII (HII), HindIII (HIII), NsiI (N), and Sau3A (S) are indicated. The relative size, location, and direction of transcription of each of the Tn4451-encoded genes are indicated by the longer filled arrows.
Transposon stability assays.
To determine the stabilities of Tn4451, Tn4453a, and Tn4453b on recombinant plasmids, assays were performed as described previously (5, 8), with modifications as follows. Each strain was cultured on solid medium supplemented with ampicillin and chloramphenicol, and a single colony was transferred to 10 ml of broth with the same antibiotics, which select for the vector plasmid and the transposon, respectively. After overnight incubation at 37°C, plasmid DNA was extracted and was used to transform competent E. coli DH5α cells to ampicillin resistance. Single colonies (n = 120) were then patched onto media containing chloramphenicol or ampicillin and were incubated at 37°C overnight. The stability of the transposon carried by each plasmid was defined as the percentage of ampicillin-resistant colonies that were resistant to chloramphenicol. The values presented are the averages of three independent experiments.
To determine the ability of recombinant plasmids carrying tnpX genes from Tn4453a and Tn4453b to complement the Tn4451tnpXΔ1 mutation carried on pJIR773, trans-complementation assays were performed as described previously (8).
E. coli spot mating experiments.
Matings were carried out with late-exponential-phase E. coli cultures as follows (10). A 1-in-2 dilution of the rifampin-resistant recipient LT101 (20) was used to flood the selective agar medium, and the surface was allowed to dry. Samples (20 μl) of serially diluted donor cultures were then spotted onto the surface and, when absorbed, were incubated overnight at 37°C.
Cloning of PCR-generated DNA fragments.
Purified PCR products were treated with T4 polynucleotide kinase and T4 DNA polymerase (Boehringer Mannheim). Following phenol-chloroform extraction and ethanol precipitation (25) the products were ligated to the appropriate restriction endonuclease-digested and alkaline phosphatase-treated vector DNA.
Nucleotide sequencing.
Nucleotide sequence analysis was performed with the PRISM Ready Reaction DyeDeoxy Terminator Cycle Sequencing Kit (Applied Biosystems) and an ABI 373 A automated fluorescent sequencing apparatus (Applied Biosystems). The sequences were compiled with Sequencher software (Gene Codes Corporation).
RESULTS
Identification of a Tn4451-like transposon in C. difficile.
To see if catD was located on an element similar to Tn4451, PCR analysis was performed with C. difficile SGC0545, W1, 3026, and C250, which are known to carry catD (31), as well as chloramphenicol-resistant strains KZ1606 and KZ1613. The Tn4451-carrying strain C. perfringens CP590 was included as a positive control. The Tn4451-derived oligonucleotide primers used for this analysis were chosen so that when combined they would span the entire transposon (Fig. 1). The primers were used in the combinations 300-274, 94-274, and 212-274 to amplify the left side of the transposon and 212-221, 212-220, 212-211, and 212-1727 to amplify the right side. PCR products of the appropriate size were obtained for all primer combinations from each of the six C. difficile strains (data not shown). These data therefore confirmed that the C. difficile isolates carried catD and provided evidence that in each isolate this gene was located on a transposon similar to Tn4451. We have designated this putative element Tn4453.
Cloning and restriction mapping of Tn4453a and Tn4453b from C. difficile W1.
Since the catD gene from strain W1 had previously been cloned and sequenced (31, 32), further studies were restricted to this strain. Southern hybridization analysis of EcoRI-digested chromosomal DNA from strain W1 was carried out with a catD-specific probe. The results confirmed that there are two copies of this gene (31) and therefore two potential copies of Tn4453 on the W1 chromosome (data not shown). These Tn4453 variants were cloned into pUC18 and pUC19 as separate 15.5-kb and 11.0-kb EcoRI fragments, generating the recombinant plasmids pJIR1377 and pJIR1378, respectively. The putative transposons carried on these plasmids were designated Tn4453a and Tn4453b, respectively.
The restriction maps of both transposons were deduced and compared to the known map of Tn4451 (Fig. 1). Both similarities and differences in the restriction profiles of the three elements were evident. These data also indicate that Tn4453a and Tn4453b are not identical, even though they are both found in the same strain. The differences between Tn4451 and the two Tn4453 variants suggest that although these elements probably have a common origin, they have subsequently evolved independently. Further studies were aimed at comparing the functional properties of these transposons.
Tn4453a and Tn4453b are excised as circular molecules in E. coli and C. difficile.
Tn4451 undergoes precise TnpX-mediated excision from multicopy plasmids in both C. perfringens and E. coli (2, 4, 5). To assess whether the C. difficile elements are also excised precisely in E. coli, transposon stability assays were performed with pJIR1377 and pJIR1378. The results (Table 3) showed that the recombinant plasmids carrying the Tn4453 elements were unstable in E. coli, although they were more stable than plasmids carrying Tn4451. The C. difficile elements also differed in their stability, with Tn4453a being more stable than Tn4453b. The variation in transposon stability may reflect differences in flanking sequences rather than differences between the elements.
TABLE 3.
Stabilities of Tn4453a and Tn4453b in E. coli
| Plasmid | Transposon | % Stabilitya |
|---|---|---|
| pJIR62 | Noneb | 100.0 ± 0 |
| pJIR45 | Tn4451 | 1.4 ± 0.9 |
| pJIR1377 | Tn4453a | 80.8 ± 2.2 |
| pJIR1378 | Tn4453b | 19.7 ± 3.3 |
Each value is an average of three independent experiments and refers to the percentage of ampicillin-resistant colonies able to grow in the presence of chloramphenicol.
Carries the catP gene only.
Excision of Tn4451 results in the formation of a circular molecule (5). PCR analysis was used to determine whether similar molecules are produced by the excision of Tn4453a and Tn4453b. These studies used the outward-firing Tn4451-derived oligonucleotide primers 4675 and 4676 (Fig. 1). Binding of these primers to circular forms of Tn4451-like transposons would lead to the formation of 909-bp PCR products. Products of the appropriate size were observed when DNA from E. coli strains carrying pJIR45, pJIR1377, or pJIR1378 or DNA from the parent strain C. difficile W1 was used as the template (data not shown). These results provide clear evidence that the excision of Tn4453a and Tn4453b results in the formation of a circular molecule, as is the case with Tn4451. This process occurs both in E. coli and in the original C. difficile host, implying that excision occurs by a similar mechanism in both organisms.
Nucleotide sequence of the joint of the circular form of Tn4453a and Tn4453b.
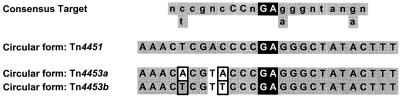
The 909-bp 4675-4676 PCR products generated from pJIR1377 and pJIR1378 were purified, partially sequenced, and compared to the sequence of the circular form of Tn4451. The results showed that both PCR products represented the joint of the circular form of the transposon, where the left and right termini of the elements are fused. At the fusion point these joints contained a GA dinucleotide, as does the circular form of Tn4451 (Fig. 2). These residues are also found in consensus Tn4451 target sequences (Fig. 2) and in the regions flanking Tn4451 insertion sites, which indicates that they are important for Tn4451 excision and insertion (5, 8). Site-directed mutagenesis studies have confirmed that the GA residues are important components of the TnpX target site (8).
FIG. 2.
Alignment of the joint of the circular forms of Tn4451, Tn4453a, and Tn4453b. Tn4451 sequences are as reported previously (5, 8). The consensus target sequence which is cleaved by TnpX (8) is also shown. All of the sequences consist of central GA residues (black boxes). Residues which are the same as those found in Tn4451 are shaded (grey boxes). Residues which differ between Tn4453a and Tn4453b are outlined in black.
Complementation in trans of the Tn4451tnpXΔ1 mutation by the cloned Tn4453a and Tn4453b tnpX genes in E. coli.
On the basis of the previous results it was postulated that Tn4453a and Tn4453b encoded TnpX proteins that functioned in a manner similar to that of TnpX from Tn4451. A trans-complementation assay was used to confirm the presence of functional tnpX genes. This assay is based on the finding that Tn4451 derivatives carrying a tnpX gene that contains the internal tnpXΔ1 deletion are stable on multicopy plasmids in C. perfringens and E. coli. Provision of a wild-type tnpX gene in trans restores the unstable phenotype of the Tn4451tnpXΔ1 element (5).
PCR products encompassing the tnpX gene regions from Tn4453a and Tn4453b were generated with primers 300 and 3277 (Fig. 1). These fragments were cloned into SmaI-digested pUC18 DNA to construct pJIR1488 and pJIR1489, respectively. Partial nucleotide sequencing was used to confirm that the desired fragments had been cloned (data not shown). These plasmids were then introduced into DH5α derivatives carrying Tn4451tnpXΔ1 on a compatible pSU39-derived replicon (5). The stability of the transposon derivative in the resultant transformants was then determined. The cloned C. difficile-derived tnpX genes were shown to be functional in that they could facilitate excision of the Tn4451tnpXΔ1 element in the trans-complementation assay, although to somewhat different extents (Table 4). Excision was confirmed by detection of the circular form after PCR with the oligonucleotides 4675 and 4676 (data not shown).
TABLE 4.
Complementation of Tn4451tnpXΔ1 in E. coli
| Straina | pBS or pUC Replicon:
|
% Stabilityb | |
|---|---|---|---|
| Plasmid | Genotypec | ||
| JIR5509 | pUC18 | 100.0 ± 0 | |
| JIR2771 | pJIR639 | tnpX+ (Tn4451) | 48.0 ± 4.5 |
| JIR5510 | pJIR1488 | tnpX+ (Tn4453a) | 4.4 ± 1.3 |
| JIR5511 | pJIR1489 | tnpX+ (Tn4453b) | 14.0 ± 5.1 |
In addition to the pBluescript-II KS (pBS), pUC18, or pUC19 (pUC) vector indicated, each strain carried the pSU39-based recombinant plasmid pJIR773, which carried Tn4451tnpXΔ1 (5).
Each value is an average of three independent experiments and refers to the percentage of kanamycin-resistant colonies able to grow in the presence of chloramphenicol.
Refers to the tnpX gene carried by the recombinant pBS- or pUC-derived plasmid only.
Tn4453a and Tn4453b mediate mobilization of pJIR1377 and pJIR1378.
To see if Tn4453a and Tn4453b also have a functional TnpZ-RSA mobilization system, plasmids carrying the various transposons were introduced into E. coli S17-1, which carries a chromosomal RP4 derivative. These strains were used as donors in matings with E. coli LT101 (Table 5). The results showed that the Tn4453 plasmids pJIR1377 and pJIR1378 were able to be mobilized to the recipient bacterium, as was the Tn4451 plasmid pJIR45 but not the negative control plasmid pJIR62, which carried only catP (Table 5). These data provide evidence that Tn4453a and Tn4453b carry a TnpZ-RSA mobilization system that is functionally equivalent to that of Tn4451.
TABLE 5.
TnpZ-mediated plasmid mobilization by RP4 in E. coli
| Plasmida | Transposon | Mobilization frequencyb |
|---|---|---|
| pJIR62 | Nonec | <5.0 × 10−8 |
| pJIR45 | Tn4451 | 1.0 × 10−2 |
| pJIR1377 | Tn4453a | 1.8 × 10−5 |
| pJIR1378 | Tn4453b | 1.2 × 10−2 |
All donor strains were derivatives of E. coli S17-1 (26) and contained the indicated plasmid.
Each value is an average of two independent experiments and refers to the number of chloramphenicol-resistant transconjugants per donor cell.
Carries the catP gene only.
DISCUSSION
In this study, two chloramphenicol resistance transposons which carried the catD gene were identified in a single C. difficile strain and were shown to be functionally and structurally related to Tn4451 from C. perfringens. PCR analysis indicated that five other C. difficile isolates carried similar transposons. These strains were from diverse sources, indicating that Tn4451-like transposons not only are found in chloramphenicol-resistant strains of C. perfringens (2, 23) but are also common in C. difficile.
Two closely related but distinct C. difficile-derived elements, Tn4453a and Tn4453b, were cloned and, like Tn4451, were found to be unstable on multicopy plasmids in E. coli. There was variation in the stability levels observed with these three elements (Table 3). These differences could be due to differences in the expression levels of the tnpX gene, sequence differences between the three TnpX proteins, or differences in the TnpX-binding regions flanking the ends of each element.
It was found that excision of Tn4453a and Tn4453b resulted in the production of a circular form of the transposons in both E. coli and C. difficile. This form may represent the transposition intermediate, as has been suggested for the equivalent molecule produced from Tn4451 (5). The joints of these circular molecules were sequenced and were found to be very similar to the corresponding region in the Tn4451 circular form and to the consensus TnpX target site, with a GA dinucleotide located at the fusion point (Fig. 2). A model has been proposed for Tn4451 whereby the resolvase-invertase domain of TnpX introduces 2-bp staggered cuts at GA dinucleotides, leading to the excision or insertion of Tn4451 via a circular intermediate (8). The findings that Tn4453a and Tn4453b have similar GA residues at the joints of their circular forms and similar joint sequences imply that the three TnpX proteins have similar mechanisms of action and similar DNA binding and target sites. The latter suggestions were supported by comparison of the ends of each C. difficile element to those of Tn4451 and also to the consensus target sequence (Fig. 2). A high level of similarity was evident, with only two or three sequence changes, all of which were at one end. The ends of Tn4453a and Tn4453b also closely match the consensus TnpX target sequence. Evidence that the TnpX proteins encoded by these transposons were functionally interchangeable was obtained by cloning the tnpX genes from Tn4453a and Tn4453b and showing that they could substitute for the Tn4451-derived tnpX gene in a trans-complementation excision assay (Table 4). Overall, these data suggest that the TnpX proteins encoded by each transposon bind to similar DNA target sequences and subsequently function in a comparable manner to promote excision or insertion.
The Tn4451-encoded TnpZ protein is the only known Mob-Pre protein encoded on a transposable element from a gram-positive bacterium (9). On the basis of the results of this study, it is concluded that Tn4453a and Tn4453b encode equivalent TnpZ proteins and RSA sites since these transposons also facilitated RP4-mediated mobilization of their host plasmids (Table 5). The observed differences in mobilization frequencies are probably the result of differences in the TnpZ proteins or RSA sites encoded by these transposons. Further studies are required to determine the role that this mobilization system plays in the dissemination of Tn4451- and Tn4453-like transposons to different bacterial genera and species.
The comparative analysis of these chloramphenicol resistance elements provides clear evidence that genetic exchange between C. difficile and C. perfringens may have occurred either directly or through an intermediate bacterial host. Not only is there near identity between the catD and catP genes, but there is also a high degree of similarity between the transposons which carry these genes. The probability that gene transfer may occur directly or indirectly between these species is also supported by the comparative analysis of the erythromycin resistance determinants ermBP and ermBZ from C. perfringens and C. difficile, respectively (6, 14). However, direct and reproducible exchange of genetic information between C. difficile and C. perfringens has not been demonstrated. Further studies are required to elucidate the mechanism of transfer of Tn4451-like elements, especially with regard to the transposition process. Such studies will lead to a greater understanding of how these transposons are disseminated among these important pathogenic bacteria and of the evolutionary relationships between clostridial transposons and those from other bacteria.
ACKNOWLEDGMENTS
We thank Pauline Howarth for excellent technical assistance.
This research was supported by a grant from the Australian National Health and Medical Research Council.
REFERENCES
- 1.Abraham L J, Rood J I. Cloning and analysis of the Clostridium perfringens tetracycline resistance plasmid, pCW3. Plasmid. 1985;13:155–162. doi: 10.1016/0147-619x(85)90038-1. [DOI] [PubMed] [Google Scholar]
- 2.Abraham L J, Rood J I. Identification of Tn4451 and Tn4452, chloramphenicol resistance transposons from Clostridium perfringens. J Bacteriol. 1987;169:1579–1584. doi: 10.1128/jb.169.4.1579-1584.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abraham L J, Rood J I. The Clostridium perfringens chloramphenicol resistance transposon Tn4451 excises precisely in Escherichia coli. Plasmid. 1988;19:164–168. doi: 10.1016/0147-619x(88)90055-8. [DOI] [PubMed] [Google Scholar]
- 4.Abraham L J, Wales A J, Rood J I. Worldwide distribution of the conjugative Clostridium perfringens tetracycline resistance plasmid, pCW3. Plasmid. 1985;14:37–46. doi: 10.1016/0147-619x(85)90030-7. [DOI] [PubMed] [Google Scholar]
- 5.Bannam T L, Crellin P K, Rood J I. Molecular genetics of the chloramphenicol-resistance transposon Tn4451 from Clostridium perfringens: the TnpX site-specific recombinase excises a circular transposon molecule. Mol Microbiol. 1995;16:535–551. doi: 10.1111/j.1365-2958.1995.tb02417.x. [DOI] [PubMed] [Google Scholar]
- 6.Berryman D I, Rood J I. The closely related ermB/AM genes from Clostridium perfringens, Enterococcus faecalis (pAMβ1), and Streptococcus agalactiae (pIP501) are flanked by variants of a directly repeated sequence. Antimicrob Agents Chemother. 1995;39:1830–1834. doi: 10.1128/aac.39.8.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brefort G, Magot M, Ionesco H, Sebald M. Characterization and transferability of Clostridium perfringens plasmids. Plasmid. 1977;1:52–66. doi: 10.1016/0147-619x(77)90008-7. [DOI] [PubMed] [Google Scholar]
- 8.Crellin P K, Rood J I. The resolvase/invertase domain of the site-specific recombinase TnpX is functional and recognizes a target sequence that resembles the junction of the circular form of the Clostridium perfringens transposon Tn4451. J Bacteriol. 1997;179:5148–5156. doi: 10.1128/jb.179.16.5148-5156.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crellin P K, Rood J I. Tn4451 from Clostridium perfringens is a mobilizable transposon that encodes the functional Mob protein, TnpZ. Mol Microbiol. 1998;27:631–642. doi: 10.1046/j.1365-2958.1998.00712.x. [DOI] [PubMed] [Google Scholar]
- 10.Fong S T, Stanisich V A. Location and characterization of two functions on RP1 that inhibit the fertility of the IncW plasmid R388. J Gen Microbiol. 1989;135:499–502. doi: 10.1099/00221287-135-3-499. [DOI] [PubMed] [Google Scholar]
- 11.Huggins A S, Bannam T L, Rood J I. Comparative sequence analysis of the catB gene from Clostridium butyricum. Antimicrob Agents Chemother. 1992;36:2548–2551. doi: 10.1128/aac.36.11.2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ionesco H. Transfert de la résistance à la tétracycline chez Clostridium difficile. Ann Inst Pasteur Microbiol. 1980;131A:171–179. [PubMed] [Google Scholar]
- 13.Johnson S, Gerding D N. Enterotoxemic infections. In: Rood J I, McClane B A, Songer J G, Titball R W, editors. The clostridia: molecular biology and pathogenesis. London, United Kingdom: Academic Press; 1997. pp. 117–140. [Google Scholar]
- 14.Lyras D, Rood J I. Transposable genetic elements and antibiotic resistance determinants from Clostridium perfringens and Clostridium difficile. In: Rood J I, McClane B A, Songer J G, Titball R W, editors. The clostridia: molecular biology and pathogenesis. London, United Kingdom: Academic Press; 1997. pp. 73–92. [Google Scholar]
- 15.Lyras D, Rood J I. Conjugative transfer of RP4-oriT shuttle vectors from Escherichia coli to Clostridium perfringens. Plasmid. 1998;39:160–164. doi: 10.1006/plas.1997.1325. [DOI] [PubMed] [Google Scholar]
- 16.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. pp. 352–355. [Google Scholar]
- 17.Moncrief J S, Lyerly D M, Wilkins T D. Molecular biology of the Clostridium difficile toxins. In: Rood J I, McClane B A, Songer J G, Titball R W, editors. The clostridia: molecular biology and pathogenesis. London, United Kingdom: Academic Press; 1997. pp. 369–392. [Google Scholar]
- 18.Morelle G. A plasmid extraction procedure on a miniprep scale. Focus. 1989;11:7–8. [Google Scholar]
- 19.Nakamura S, Yamakawa K, Nakhio S, Kamiya S, Nishida S. Correlation between susceptibility to chloramphenicol, tetracycline and clindamycin, and serogroups of Clostridium difficile. Med Microbiol Immunol. 1987;176:79–82. doi: 10.1007/BF00200678. [DOI] [PubMed] [Google Scholar]
- 20.Palombo E A, Yusoff K, Stanisich V A, Krishnapillai V, Willetts N S. Cloning and genetic analysis of tra cistrons of the Tra 2/Tra 3 region of plasmid RP1. Plasmid. 1989;22:59–69. doi: 10.1016/0147-619x(89)90036-x. [DOI] [PubMed] [Google Scholar]
- 21.Rood J I, Maher E A, Somers E B, Campos E, Duncan C L. Isolation and characterization of multiply antibiotic-resistant Clostridium perfringens strains from porcine feces. Antimicrob Agents Chemother. 1978;13:871–880. doi: 10.1128/aac.13.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rood J I. Transferable tetracycline resistance in Clostridium perfringens strains of porcine origin. Can J Microbiol. 1983;29:1241–1246. doi: 10.1139/m83-193. [DOI] [PubMed] [Google Scholar]
- 23.Rood J I, Jefferson S, Bannam T L, Wilkie J M, Mullany P, Wren B W. Hybridisation analysis of three chloramphenicol resistance determinants from Clostridium perfringens and Clostridium difficile. Antimicrob Agents Chemother. 1989;33:1569–1574. doi: 10.1128/aac.33.9.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salyers A A, Shoemaker N B, Stevens A M, Li L-Y. Conjugative transposons: an unusual and diverse set of integrated gene transfer elements. Microbiol Rev. 1995;59:579–590. doi: 10.1128/mr.59.4.579-590.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 26.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Biotechnology. 1983;1:37–45. [Google Scholar]
- 27.Sloan J, Warner T A, Scott P T, Bannam T L, Berryman D I, Rood J I. Construction of a sequenced Clostridium perfringens-Escherichia coli shuttle plasmid. Plasmid. 1992;27:207–219. doi: 10.1016/0147-619x(92)90023-4. [DOI] [PubMed] [Google Scholar]
- 28.Smith C J, Markowitz S M, Macrina F L. Transferable tetracycline resistance in Clostridium difficile. Antimicrob Agents Chemother. 1981;19:997–1003. doi: 10.1128/aac.19.6.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vieira J, Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982;19:259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 30.Wilson, K. H. 1993. The microecology of Clostridium difficile. Clin. Infect. Dis. 16(Suppl.4):S214–S218. [DOI] [PubMed]
- 31.Wren B W, Mullany P, Clayton C, Tabaqchali S. Molecular cloning and genetic analysis of a chloramphenicol acetyltransferase determinant from Clostridium difficile. Antimicrob Agents Chemother. 1988;32:1213–1217. doi: 10.1128/aac.32.8.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wren B W, Mullany P, Clayton C, Tabaqchali S. Nucleotide sequence of a chloramphenicol acetyl transferase gene from Clostridium difficile. Nucleic Acids Res. 1989;17:4877. doi: 10.1093/nar/17.12.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wüst J, Hardegger U. Transferable resistance to clindamycin, erythromycin, and tetracycline in Clostridium difficile. Antimicrob Agents Chemother. 1983;23:784–786. doi: 10.1128/aac.23.5.784. [DOI] [PMC free article] [PubMed] [Google Scholar]