Abstract
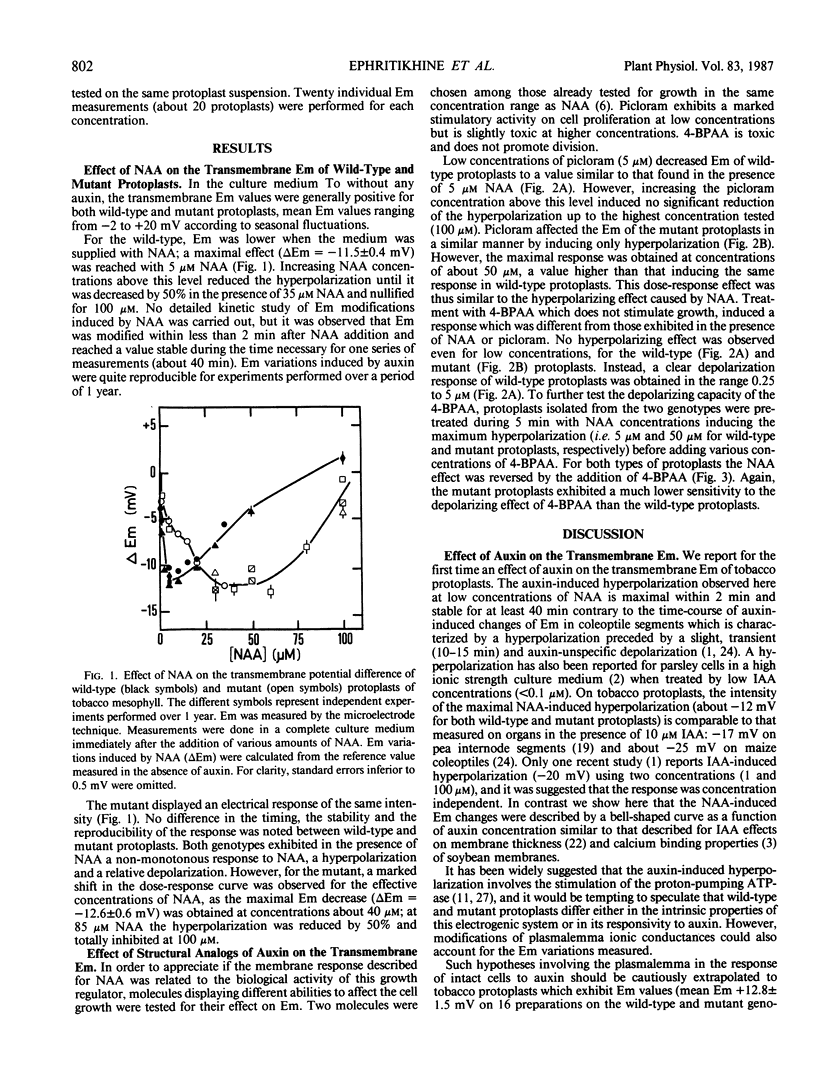
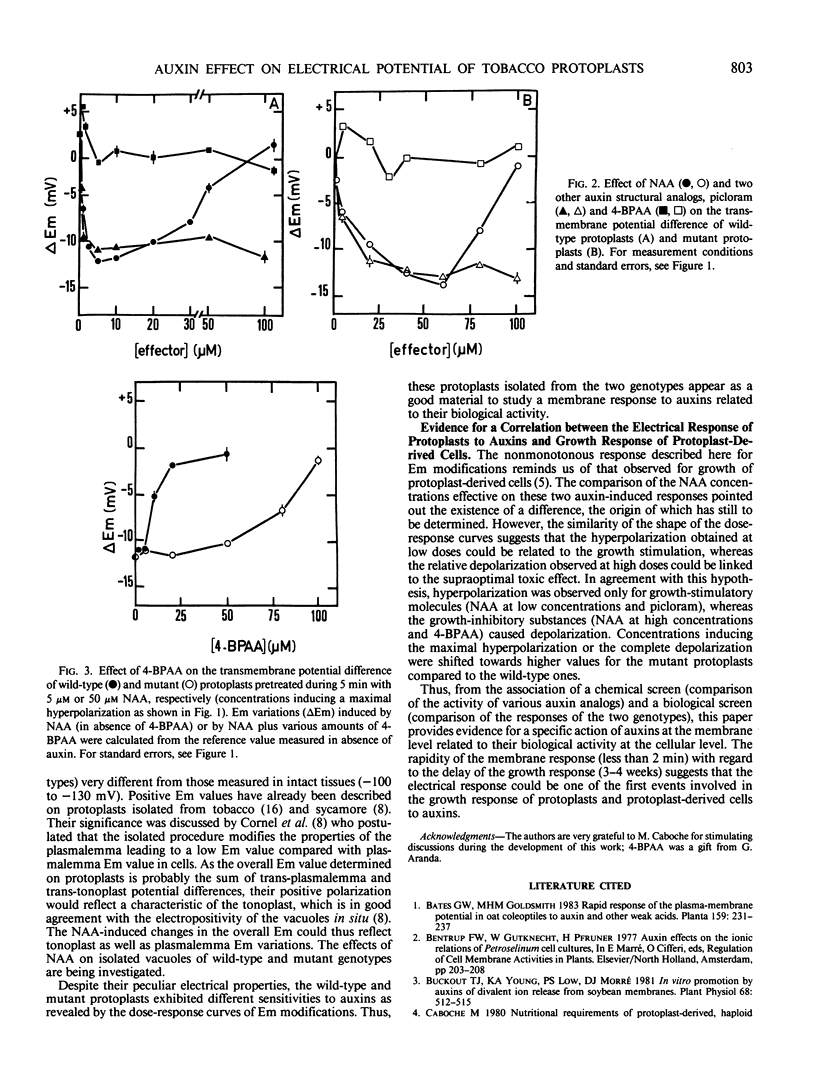
The effects of 1-naphthaleneacetic acid (NAA) and other auxin analogs on the transmembrane potential difference (Em) were compared on tobacco protoplasts isolated from two genotypes differing in their sensitivity to auxins. For both types, NAA modifies Em by inducing at low doses a hyperpolarization, the amplitude of which increased with auxin concentration. Above an optimal concentration this hyperpolarization was reduced and even nullified. However, for the mutant type, this electrical response was shifted toward higher NAA concentrations, as its growth response. In the presence of structural analogs of auxin which have been showed to modify the dose-response curve for growth, the Em was altered: the growth-stimulatory molecule (picloram) initiated hyperpolarization, whereas the growth-inhibitory substance (4-bromophenylacetic acid) caused depolarization. These results provide evidence for a specific action of auxin at the membrane level related to its biological activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buckhout T. J., Young K. A., Low P. S., Morré D. J. In vitro promotion by auxins of divalent ion release from soybean membranes. Plant Physiol. 1981 Aug;68(2):512–515. doi: 10.1104/pp.68.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabathuler R., Cleland R. E. Auxin regulation of a proton translocating ATPase in pea root plasma membrane vesicles. Plant Physiol. 1985 Dec;79(4):1080–1085. doi: 10.1104/pp.79.4.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgerson S. L., Cramer W. A., Morré D. J. Evidence for an increase in microviscosity of plasma membranes from soybean hypocotyls induced by the plant hormone, indole-3-acetic Acid. Plant Physiol. 1976 Oct;58(4):548–551. doi: 10.1104/pp.58.4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs H. C., Ikegami M., Jobe A. H., Berry D. D., Jones S. Reutilization of surfactant phosphatidylcholine in adult rabbits. Biochim Biophys Acta. 1985 Oct 23;837(1):77–84. doi: 10.1016/0005-2760(85)90087-6. [DOI] [PubMed] [Google Scholar]
- Jacobs M., Ray P. M. Rapid Auxin-induced Decrease in Free Space pH and Its Relationship to Auxin-induced Growth in Maize and Pea. Plant Physiol. 1976 Aug;58(2):203–209. doi: 10.1104/pp.58.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löbler M., Klämbt D. Auxin-binding protein from coleoptile membranes of corn (Zea mays L.). II. Localization of a putative auxin receptor. J Biol Chem. 1985 Aug 15;260(17):9854–9859. [PubMed] [Google Scholar]
- Morré D. J., Bracker C. E. Ultrastructural alteration of plant plasma membranes induced by auxin and calcium ions. Plant Physiol. 1976 Oct;58(4):544–547. doi: 10.1104/pp.58.4.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayle D. L., Cleland R. E. Evidence that Auxin-induced Growth of Soybean Hypocotyls Involves Proton Excretion. Plant Physiol. 1980 Sep;66(3):433–437. doi: 10.1104/pp.66.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]


