Abstract
BACKGROUND
The risk of cardiovascular disease is increased among persons with human immunodeficiency virus (HIV) infection, so data regarding primary prevention strategies in this population are needed.
METHODS
In this phase 3 trial, we randomly assigned 7769 participants with HIV infection with a low-to-moderate risk of cardiovascular disease who were receiving antiretroviral therapy to receive daily pitavastatin calcium (at a dose of 4 mg) or placebo. The primary outcome was the occurrence of a major adverse cardiovascular event, which was defined as a composite of cardiovascular death, myocardial infarction, hospitalization for unstable angina, stroke, transient ischemic attack, peripheral arterial ischemia, revascularization, or death from an undetermined cause.
RESULTS
The median age of the participants was 50 years (interquartile range, 45 to 55); the median CD4 count was 621 cells per cubic millimeter (interquartile range, 448 to 827), and the HIV RNA value was below quantification in 5250 of 5997 participants (87.5%) with available data. The trial was stopped early for efficacy after a median follow-up of 5.1 years (interquartile range, 4.3 to 5.9). The incidence of a major adverse cardiovascular event was 4.81 per 1000 person-years in the pitavastatin group and 7.32 per 1000 person-years in the placebo group (hazard ratio, 0.65; 95% confidence interval [CI], 0.48 to 0.90; P = 0.002). Muscle-related symptoms occurred in 91 participants (2.3%) in the pitavastatin group and in 53 (1.4%) in the placebo group; diabetes mellitus occurred in 206 participants (5.3%) and in 155 (4.0%), respectively.
CONCLUSIONS
Participants with HIV infection who received pitavastatin had a lower risk of a major adverse cardiovascular event than those who received placebo over a median follow-up of 5.1 years. (Funded by the National Institutes of Health and others; REPRIEVE ClinicalTrials.gov number, NCT02344290.)
The risk of atherosclerotic cardiovascular disease, including myocardial infarction and stroke, is up to twice as high among persons with human immunodeficiency virus (HIV) infection as in the general population,1 a risk that underlines the need to prevent excess cardiovascular events. The mechanisms of the increase in risk of cardiovascular disease in this population are not completely understood but may relate to traditional risk factors, as well as to residual inflammation and immune activation.2–4 Large cohort studies have suggested that an increase in the risk of cardiovascular disease persists after controlling for traditional risk factors.5,6 Although prevention guidelines recognize HIV infection as a “risk modifier,” no population-specific cardioprotective recommendations have been offered7 pending the results of large, randomized studies of primary prevention methods in this population.
We designed the phase 3 Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE) to address this knowledge gap and determine whether statin use prevents atherosclerotic cardiovascular disease events in persons with HIV infection who are at low-to-moderate risk for cardiovascular events. This intervention was chosen with the knowledge that statin therapy simultaneously lowers low-density lipoprotein (LDL) cholesterol,8 a main driver of atherosclerotic cardiovascular disease, and has beneficial effects on relevant inflammatory and immune pathways.9–12
METHODS
TRIAL DESIGN AND OVERSIGHT
The rationale and design of REPRIEVE have been published previously13 and are described in the trial protocol, available with the full text of this article at NEJM.org. Pitavastatin calcium was chosen because it does not interact with the drugs that are used in antiretroviral therapy.14
The trial was designed by the principal investigators with guidance and approval from the National Institutes of Health (NIH); additional details are provided in the Supplementary Appendix, also available at NEJM.org. The trial was funded primarily by the NIH with additional support from Kowa Pharmaceuticals America, Gilead Sciences, and ViiV Healthcare. The funders played no role in the conduct of the analyses or the drafting of the manuscript. The protocol was approved by the institutional review board of Massachusetts General Brigham and by the ethics committee at each participating site. An independent data and safety monitoring board was appointed by the NIH to review the trial every 6 months.
The first author had full access to the trial results, supervised the preparation of the manuscript, and made the decision to submit the manuscript for publication with approval from the members of the executive and publication committees consisting of trial leadership and NIH staff members overseeing the trial. The publications committee also included site investigators and community advisors. The trial statisticians had full access to the trial data, wrote the statistical analysis plan, and performed the analyses. The authors vouch for the completeness and accuracy of the data and for the fidelity of the trial to the protocol.
TRIAL POPULATION
The inclusion criteria included a diagnosis of HIV infection, an age of 40 to 75 years, and receipt of stable antiretroviral therapy. All the participants had a low-to-moderate risk of atherosclerotic cardiovascular disease, as determined by the score on the American Heart Association and American College of Cardiology 2013 Pooled Cohort Equation risk calculator15 — a risk of up to 15% for LDL cholesterol (≥70 mg per deciliter [1.81 mmol per liter]) in conjunction with LDL cholesterol levels below specific thresholds. Full eligibility criteria are provided in the Supplementary Appendix. Those with a history of statin use within the previous 90 days and known atherosclerotic cardiovascular disease were excluded from participation in the trial. All the participants provided written informed consent.
RANDOMIZATION AND TREATMENT
Participants were assigned in a 1:1 ratio to receive oral pitavastatin calcium (at a dose of 4 mg per day) or identical placebo (both provided by Kowa Pharmaceuticals America). Computerized randomization was stratified according to sex at birth, CD4 count (≤500 cells or >500 cells per cubic millimeter), and participation in a mechanistic substudy,13 which assessed the effects of pitavastatin calcium on coronary plaque and inflammatory biomarkers; a permuted block size of 8 was used. All the participants received information on lifestyle modifications, as described in the Supplementary Appendix. Pitavastatin or placebo was discontinued and clinically indicated statin therapy was initiated at the discretion of the site investigator or the participant’s care provider, with the intention of following the participant according to the intention-to-treat trial design.
OUTCOMES
The primary outcome was the occurrence of a major adverse cardiovascular event, which was a composite of cardiovascular death; myocardial infarction; hospitalization for unstable angina; stroke; transient ischemic attack (TIA); peripheral arterial ischemia; revascularization of a coronary, carotid, or peripheral artery; or death from an undetermined cause, as measured in a time-to-event analysis. Key secondary outcomes were a composite of a major adverse cardiovascular event or death from any cause; individual components of the primary outcome; death from any cause; LDL and non–high-density lipoprotein (non-HDL) cholesterol; targeted safety events, including incident diabetes mellitus; liver injury, which was defined as an alanine aminotransferase (ALT) level of 5 to less than 10 times the upper limit of the normal range (grade 3) or 10 or more times the upper limit (grade 4); and myalgia, muscle weakness, or myopathy of grade 3 (inability to perform social activities) or grade 4 (disabling) or that was treatment-limiting, according to the Division of AIDS table for the grading of the severity of adverse events.
Outcomes regarding major adverse cardiovascular events were independently adjudicated by practitioners who were unaware of trial-group assignments. After January 2020, such adjudication included the contribution of coronavirus disease 2019 (Covid-19) to the event. Heart failure was an adjudicated exploratory outcome.
STATISTICAL ANALYSIS
We determined that the enrollment of 7700 participants would provide the trial with 85% power to detect a 30% reduction in the risk of a major adverse cardiovascular event (hazard ratio, 0.70) in the pitavastatin group on the basis of a maximum of 288 events. We performed interim efficacy and futility analyses after the occurrence of an estimated 50% and 75% of primary-outcome events using an O’Brien–Fleming boundary and Lan–DeMets spending function to control for type I error, according to the trial design. Trial investigators were unaware of the interim results. The data and safety monitoring board recommended stopping the trial for efficacy at the second planned review on March 30, 2023, and concluded that no unexpected safety concerns had been reported. At that time, 225 events had occurred (78.1% information fraction), which provided a P value of 0.02084 at the efficacy stopping boundary. The data presented here are those reviewed by the data and safety monitoring board at the time of the data cutoff.
The primary estimand (i.e., the precisely defined estimated measure of treatment effect) was the cause-specific relative hazard of prescribed pitavastatin as compared with placebo, with statin initiation if clinically indicated. A supportive analysis included the occurrence of major adverse cardiovascular events that were captured during vital-status follow-up. Except for noncardiovascular deaths, intercurrent events were ignored (intention-to-treat policy), and noncardiovascular deaths were censored as competing events.16 Time-to-event analyses used Cox proportional-hazards modeling as stratified according to sex at birth and CD4 count at screening. Nonproportional hazards were evaluated with treatment-by-time interaction. Missing data were assumed to be randomly censored. Sensitivity analyses for the primary outcome excluded deaths of undetermined cause and imputed events for participants who had no contact with trial investigators for more than 10 months. Supportive analyses were adjusted for cardiovascular and HIV-related factors and for the global burden of disease in the region.17 We also performed an as-treated analysis (in which data regarding the discontinuation of a randomized treatment were censored regardless of reason) and a per-protocol analysis (in which treatment discontinuation because of a clinical need for statin therapy was not considered to be a treatment-discontinuation event). Details regarding estimands, handling of missing values, and statistical analysis methods are provided in the Supplementary Appendix.
The primary result is presented with a 95% confidence interval that was adjusted for the group sequential design. For all other analyses, results are reported as point estimates and 95% confidence intervals without adjustment for multiplicity, so the intervals should not be used to infer definitive treatment effects for secondary outcomes. We compared the occurrence of adverse events according to stratified incidence rate ratios using Poisson regression. All analyses were performed with the use of SAS software (version 9.4M7 for Linux).
RESULTS
PARTICIPANTS
From March 26, 2015, to July 31, 2019, a total of 10,865 participants were screened at 145 sites in 12 countries (Fig. S1 in the Supplementary Appendix). A total of 7769 participants were enrolled (3888 in the pitavastatin group and 3881 in the placebo group). The median age was 50 years (interquartile range, 45 to 55); 5065 participants (65.2%) were non-White, and 2419 (31.1%) were women.
The median screening LDL cholesterol level was 108 mg per deciliter (interquartile range, 87 to 128 [2.79 mmol per liter; interquartile range, 2.25 to 3.31 mmol per liter]), and the median CD4 count was 621 cells per cubic millimeter (interquartile range, 448 to 827). The median 10-Year Atherosclerotic Cardiovascular Disease risk score was 4.5% (interquartile range, 2.1 to 7.0) on a scale of low risk (<5%), borderline risk, (5 to <7.5%), intermediate risk (7.5 to <20%), and high risk (≥20%). The HIV viral load was below the lower limit of quantification in 5250 of 5997 participants (87.5%), according to clinical care assays with limits of 20 to 400 copies per milliliter. Among 747 participants with quantifiable viremia, the median viral load was 62 copies per milliliter (interquartile range, 34 to 199). Additional details regarding the participants’ characteristics are provided in Table 1 and Table S1.
Table 1.
Characteristics of the Participants at Baseline.*
| Characteristic | Pitavastatin (N = 3888) | Placebo (N = 3881) | Total (N = 7769) |
|---|---|---|---|
| Region of global burden of disease — no. (%) | |||
| High income | 2044 (52.6) | 2051 (52.8) | 4095 (52.7) |
| Latin America or Caribbean | 709 (18.2) | 714 (18.4) | 1423 (18.3) |
| Southeast or East Asia | 304 (7.8) | 286 (7.4) | 590 (7.6) |
| South Asia | 246 (6.3) | 258 (6.6) | 504 (6.5) |
| Sub‑Saharan Africa | 585 (15.0) | 572 (14.7) | 1157 (14.9) |
| Age | |||
| Median (IQR) — yr | 50 (45–55) | 50 (45–55) | 50 (45–55) |
| Range — yr | 40 to 72 | 40 to 74 | 40 to 74 |
| Distribution — no. (%) | |||
| 40–49 yr | 1842 (47.4) | 1888 (48.6) | 3730 (48.0) |
| 50–59 yr | 1712 (44.0) | 1649 (42.5) | 3361 (43.3) |
| ≥60 yr | 334 (8.6) | 344 (8.9) | 678 (8.7) |
| Sex — no. (%)† | |||
| Male | 2677 (68.9) | 2673 (68.9) | 5350 (68.9) |
| Female | 1211 (31.1) | 1208 (31.1) | 2419 (31.1) |
| Gender identity — no. (%) | |||
| Cisgender | 3687 (94.8) | 3680 (94.8) | 7367 (94.8) |
| Transgender | 63 (1.6) | 64 (1.6) | 127 (1.6) |
| Not reported | 138 (3.5) | 137 (3.5) | 275 (3.5) |
| Race — no. (%)‡ | |||
| Black | 1569 (40.4) | 1639 (42.2) | 3208 (41.3) |
| White | 1364 (35.1) | 1340 (34.5) | 2704 (34.8) |
| Asian | 571 (14.7) | 567 (14.6) | 1138 (14.6) |
| Other | 384 (9.9) | 335 (8.6) | 719 (9.3) |
| Ethnic group in North America — no./total no. (%)§ | |||
| Hispanic or Latino | 366/1957 (18.7) | 332/1961 (16.9) | 698/3918 (17.8) |
| Not Hispanic or Latino | 1575/1957 (80.5) | 1611/1961 (82.2) | 3186/3918 (81.3) |
| Unknown | 16/1957 (0.8) | 18/1961 (0.9) | 34/3918 (0.9) |
| Atherosclerotic Cardiovascular Disease risk score — %¶ | |||
| Median (IQR) | 4.5 (2.1–7.0) | 4.5 (2.2–7.0) | 4.5 (2.1–7.0) |
| Distribution — no. (%) | |||
| 0 to <2.5 | 1096 (28.2) | 1060 (27.3) | 2156 (27.8) |
| 2.5 to <5 | 1030 (26.5) | 1025 (26.4) | 2055 (26.5) |
| 5 to <7.5 | 934 (24.0) | 960 (24.7) | 1894 (24.4) |
| 7.5 to 10 | 540 (13.9) | 561 (14.5) | 1101 (14.2) |
| >10 | 288 (7.4) | 275 (7.1) | 563 (7.2) |
| Nadir CD4 level | |||
| Distribution — no. (%) | |||
| <200 cells/per mm3 | 1890 (48.6) | 1911 (49.2) | 3801 (48.9) |
| 200–349 cells/per mm3 | 1019 (26.2) | 1022 (26.3) | 2041 (26.3) |
| ≥350 cells/per mm3 | 840 (21.6) | 825 (21.3) | 1665 (21.4) |
| Unknown no. of cells/per mm3 | 139 (3.6) | 123 (3.2) | 262 (3.4) |
| CD4 count — no. (%)† | |||
| ≤500 cells/per mm3 | 1257 (32.3) | 1253 (32.3) | 2510 (32.3) |
| >500 cells/per mm3 | 2631 (67.7) | 2628 (67.7) | 5259 (67.7) |
| HIV‑1 RNA — no./total no. (%)‖ | |||
| <LLOQ copies/ml | 2641/3009 (87.8) | 2609/2988 (87.3) | 5250/5997 (87.5) |
| LLOQ to <400 copies/ml | 305/3009 (10.1) | 312/2988 (10.4) | 617/5997 (10.3) |
| ≥400 copies/ml | 63/3009 (2.1) | 67/2988 (2.2) | 130/5997 (2.2) |
Percentages may not total 100 because of rounding. HIV denotes human immunodeficiency virus, and IQR interquartile range.
This characteristic was used as a stratification factor in Cox proportional-hazards modeling in time-to-event analyses.
Race was reported by the participants. “Other” race includes participants who identified as native or indigenous to the enrollment region, as having more than one race, or as having an unknown race.
Ethnic group is reported according to the National Institutes of Health definition for participants in the United States (including Puerto Rico) and Canada only and is not applicable to other geographic regions.
The 10-Year Atherosclerotic Cardiovascular Disease risk score is calculated by assessing age, sex, race, systolic blood pressure, total and high-density lipoprotein cholesterol, treatment for hypertension, smoking history, and presence of diabetes. It is measured on a scale of low risk (<5%), borderline risk (5 to <7.5%), intermediate risk (7.5 to <20%), and high risk (≥20%).
The level of HIV-1 RNA was measured if data were available through standard care. The assays that were used for testing varied, including assays with a lower limit of quantification (LLOQ) of 20 to 400 copies per milliliter.
The median duration of follow-up was 5.1 years (interquartile range, 4.3 to 5.9). A total of 6452 participants (83.0%) remained in follow-up; of these participants, 5664 (2910 [74.8%] in the pitavastatin group and 2754 [71.0%] in the placebo group) were continuing to receive their randomized treatment at the time of this report. Treatment discontinuation because of adverse events occurred in 82 participants (2.1%) in the pitavastatin group and in 46 (1.2%) in the placebo group (Fig. S1). Initiation of a statin through clinical care occurred in 223 participants (5.7%) assigned to receive pitavastatin and in 373 (9.6%) assigned to receive placebo. The frequencies of trial discontinuation and statin crossover were below the predetermined thresholds. For each year, more than 80% of the participants evaluated their average adherence to pitavastatin or placebo as very good to excellent (Fig. S2).
PRIMARY OUTCOME
The incidence of major adverse cardiovascular events was 4.81 per 1000 person-years in the pitavastatin group and 7.32 per 1000 person-years in the placebo group (hazard ratio, 0.65; 95% confidence interval [CI], 0.48 to 0.90; P = 0.002) (Fig. 1A and 1B and Fig. S3). Nonproportional-hazard assumptions were not violated. Similar results were seen in analyses that included outcomes for major adverse cardiovascular events captured during vital-status follow-up (Table S3). The effect of pitavastatin appeared to be generally consistent among subgroups, with some apparent differences according to hypertension status (Fig. 2).
Figure 1. Treatment Effect of Pitavastatin on Major Adverse Cardiovascular Events.
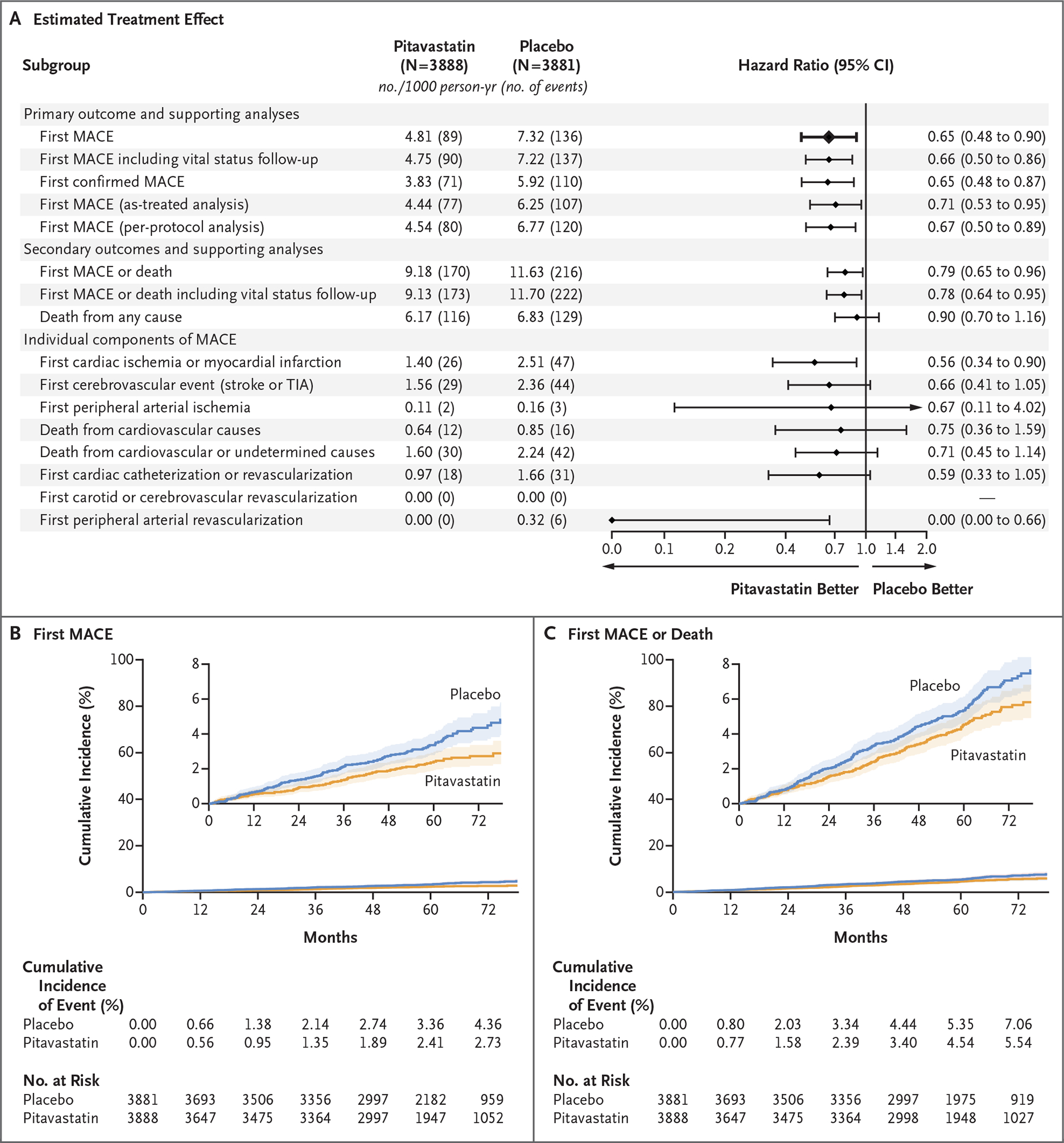
Shown is the incidence rate of a major adverse cardiovascular event (MACE) among trial participants with human immunodeficiency virus (HIV) infection in the pitavastatin group and the placebo group and the estimated treatment effect, according to stratified Cox proportional-hazards analysis (Panel A). Also shown are the cumulative incidence of the primary outcome (first MACE) (Panel B) and a key secondary outcome (first MACE or death from any cause) (Panel C). In Panels B and C, the insets show the data on an expanded y axis. At the top of Panel A, the primary outcome of the trial is shown in bold text. Panel A also shows the treatment effect for secondary and supportive analyses. Cox proportional-hazards models were stratified according to sex at birth and the CD4 cell count at screening. Aside from the primary result, the widths of the confidence intervals have not been adjusted for multiplicity and therefore may not be used in place of hypothesis testing. TIA denotes transient ischemic attack.
Figure 2. Treatment Effect on First MACE in Predefined Subgroups.
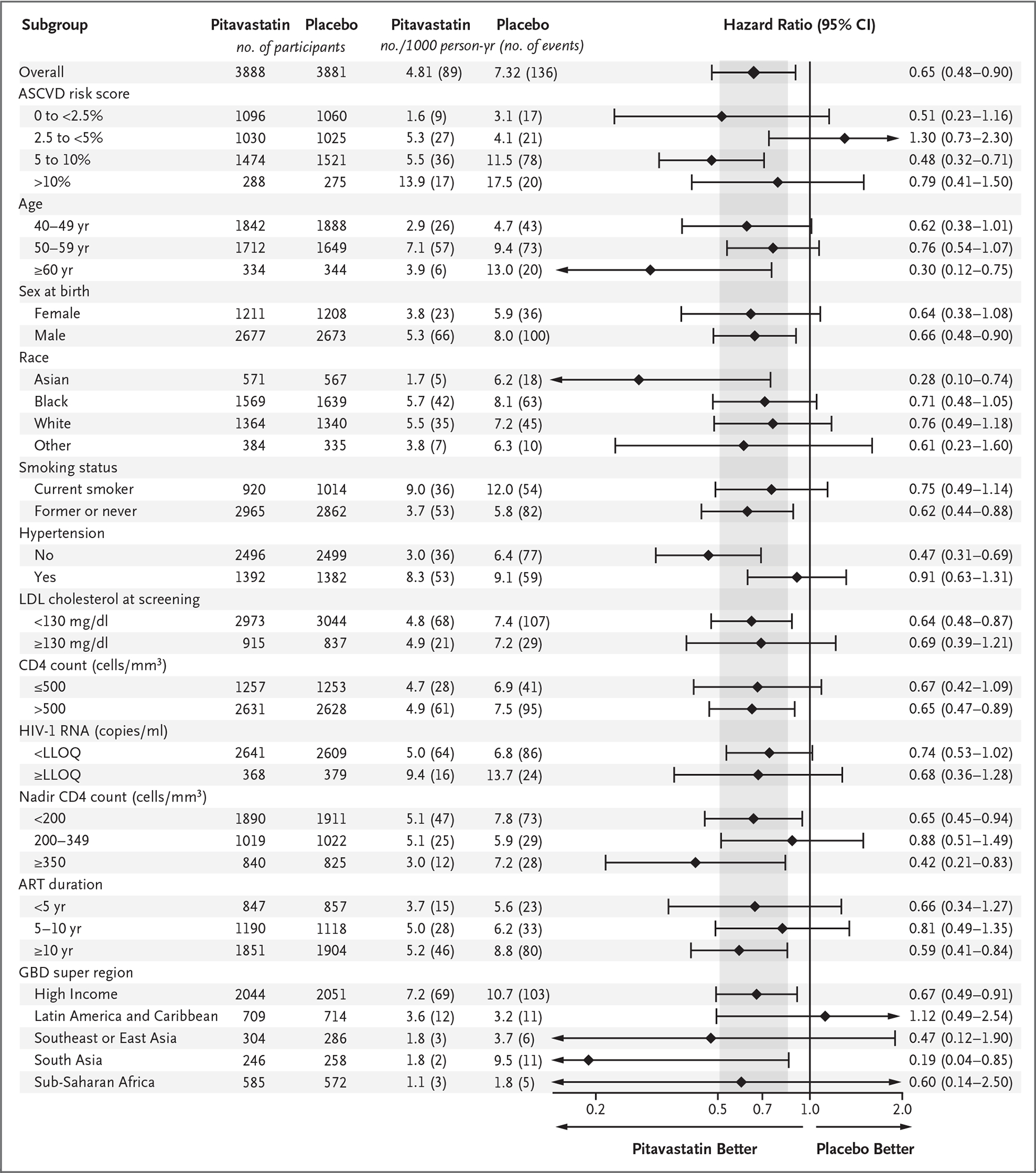
Each subgroup factor was included individually in a cause-specific Cox proportional-hazards model that was stratified according to sex at birth and the CD4 count at screening. For reference, the overall treatment effect in the primary analysis is shown at the top of the graph. The widths of the confidence intervals have not been adjusted for multiplicity and therefore may not be used in place of hypothesis testing. The 95% confidence intervals in subgroups with small numbers of events have been truncated, as indicated by arrows. To convert the values for cholesterol to millimoles per liter, multiply by 0.02586. ART denotes antiretroviral therapy, ASCVD atherosclerotic cardiovascular disease, GBD global burden of disease, LDL low-density lipoprotein, and LLOQ lower limit of quantification.
Myocardial infarction was diagnosed in 63 participants; 50 myocardial infarctions (79.4%) were determined to be type 1, and 13 myocardial infarctions (20.6%) were type 2 (Table S2). The incidence of major adverse cardiovascular events did not appear to be affected by cases of Covid-19; only one ischemic stroke was adjudicated as being definitely related to Covid-19. Sensitivity and supporting analyses that excluded deaths with an undetermined cause also favored the pitavastatin group (hazard ratio, 0.65; 95% CI, 0.48 to 0.87), as did analyses that were adjusted for cardiovascular and HIV risk factors (Fig. S4). Sensitivity analyses that accounted for missing data appeared to have results that were consistent with those for the primary outcome under realistic assumptions for the missing data but not under extreme assumptions (Fig. S5). In the per-protocol analysis, the effect of pitavastatin as compared with placebo was also similar to the primary results (hazard ratio, 0.67; 95% CI, 0.50 to 0.89).
SECONDARY OUTCOMES
The incidence of a major adverse cardiovascular event or death from any cause was 9.18 per 1000 person-years in the pitavastatin group and 11.63 per 1000 person-years in the placebo group (hazard ratio, 0.79; 95% CI, 0.65 to 0.96) (Fig. 1A and 1C and Fig. S3). Deaths from noncardiovascular causes occurred in 82 participants in the pitavastatin group and in 81 in the placebo group (Fig. S3 and Table S4). Nonfatal heart-failure events occurred in 13 participants in the pitavastatin group and in 12 in the placebo group (Table S5).
LDL cholesterol levels appeared to be similar at trial entry in the two groups and decreased from a median of 107 to 74 mg per deciliter (2.77 to 1.91 mmol per liter) in the pitavastatin group and from a median of 106 to 105 mg per deciliter (2.74 to 2.72 mmol per liter) in the placebo group at 12 months (Fig. 3). Similar effects were seen regarding non-HDL cholesterol. The effect of pitavastatin on LDL and non-HDL cholesterol appeared to be durable throughout the follow-up period.
Figure 3. Fasting Cholesterol Levels.
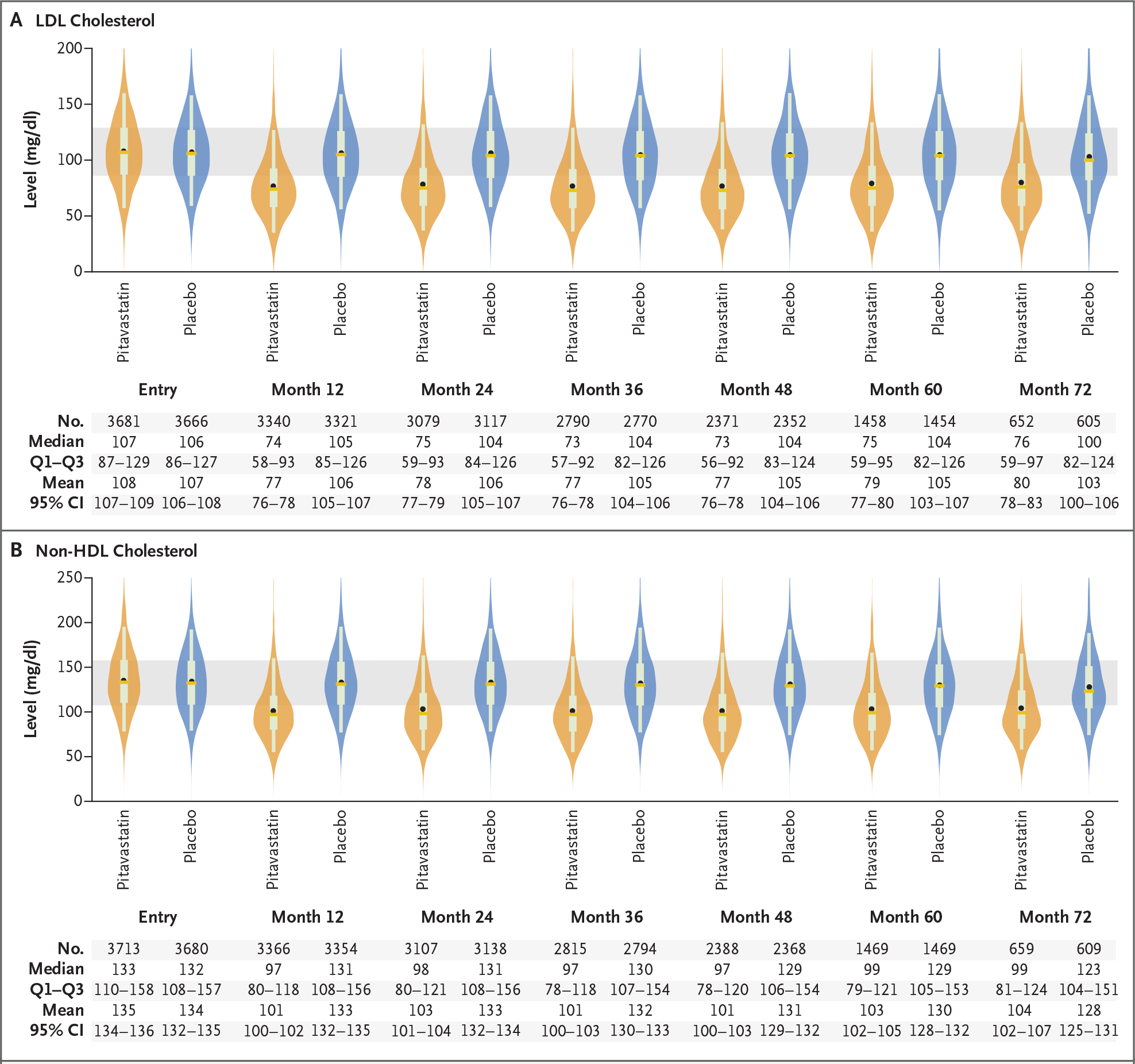
Shown are violin plots of data regarding LDL cholesterol (Panel A) and non–high-density lipoprotein (HDL) cholesterol (Panel B) in the pitavastatin group and the placebo group. In each plot, the mean value is indicated by a circle, the median by a horizontal line, and the interquartile range (Q1–Q3) by the top and bottom of a box; whiskers indicate the 5th and 95th percentiles, and the tapering points reflect the shape of the distribution. For reference, the shaded area indicates the matching interquartile ranges in the pitavastatin and placebo groups at trial entry. To convert the values for cholesterol to millimoles per liter, multiply by 0.02586.
SAFETY EVENTS
The incidence of nonfatal serious adverse events appeared to be similar in the two groups (Table 2 and Tables S6 and S7). Among targeted adverse events, a higher rate of diabetes mellitus was seen in the pitavastatin group (incidence rate ratio, 1.35; 95% CI, 1.09 to 1.66). One participant in the pitavastatin group and two in the placebo group withdrew because of the development of diabetes mellitus. There was no apparent treatment effect on glucose levels (Fig. S6).
Table 2.
Adverse Events.
| Event | Pitavastatin (N = 3888) | Placebo (N = 3881) | Incidence Rate Ratio (95% CI)* |
||
|---|---|---|---|---|---|
| No. with Event | Incidence Rate (95% CI) | No. with Event | Incidence Rate (95% CI) | ||
|
no./100
person-yr |
no./100
person-yr |
||||
| Nonfatal serious adverse event | 695 | 4.16 (3.86–4.48) | 694 | 4.13 (3.84–4.45) | 1.01 (0.91–1.12) |
| Diabetes mellitus† | 206 | 1.13 (0.99–1.30) | 155 | 0.84 (0.72–0.99) | 1.35 (1.09–1.66) |
| Myalgia, muscle weakness, or myopathy of grade ≥3 or treatment‑limiting‡ | 91 | 0.49 (0.40–0.61) | 53 | 0.28 (0.22–0.37) | 1.74 (1.24–2.45) |
| Rhabdomyolysis of grade ≥3 or treatment‑limiting | 3 | 0.02 (0.01–0.05) | 4 | 0.02 (0.01–0.06) | 0.75 (0.17–3.37)§ |
| Alanine aminotransferase elevation of grade ≥3 | 11 | 0.06 (0.03–0.11) | 8 | 0.04 (0.02–0.08) | 1.38 (0.56–3.43)§ |
| Any adverse event¶ | 1304 | 8.88 (8.41–9.38) | 1256 | 8.37 (7.92–8.84) | 1.06 (0.98–1.15) |
Incidence rate ratios have been adjusted for sex at birth and CD4 cell count at screening, except as indicated.
The evaluation of diabetes incidence was limited to participants without preexisting diabetes at baseline (3865 in the pitavastatin group and 3867 in the placebo group). Diabetes events were defined as diabetes diagnoses with an initiation of antidiabetic therapy. Incident diagnosis of diabetes without initiation of antidiabetic therapy occurred in 11 participants in the pitavastatin group and in 14 in the placebo group. The percentages of participants with diabetes mellitus were 5.3% in the pitavastatin group and 4.0% in the placebo group.
Myopathy of grade 3 or higher occurred in 3 participants in the pitavastatin group and in 1 in the placebo group. The percentages of participants with muscle-related symptoms were 2.3% in the pitavastatin group and 1.4% in the placebo group.
An unadjusted incidence rate ratio is shown because no events were reported in a stratum according to sex at birth and CD4 count at screening.
Any adverse event was defined according to the protocol collection criteria (grade 3 or higher, leading to a change in trial treatment regardless of grade, serious adverse event, or targeted adverse event).
A higher frequency of myalgia or myopathy with a severity of grade 3 or higher or that resulted in a change in treatment occurred in the pitavastatin group (incidence rate ratio, 1.74; 95% CI, 1.24 to 2.45). The majority of muscle-related symptoms in the two groups were myalgias and low-grade muscle weakness with a low incidence of myopathy (Table S8 and Fig. S7). Muscle-related symptoms led to withdrawal in 44 participants (1.1%) in the pitavastatin group and in 21 (0.5%) in the placebo group. Myopathy, rhabdomyolysis, and liver dysfunction (grade 3 or higher ALT level) occurred rarely and had similar frequencies in the two groups.
DISCUSSION
The risk of atherosclerotic cardiovascular disease is increased among persons with HIV infection, including among younger persons and those at lower traditional risk for cardiovascular disease.18 In the REPRIEVE trial, we enrolled a representative global sample of such participants at low-to-moderate cardiovascular risk for whom the benefits of statin therapy for primary prevention are unknown19–21 in order to determine whether the receipt of statins would reduce the incidence of major adverse cardiovascular events. The trial was stopped early after we found that participants in the pitavastatin group had a lower incidence of major adverse cardiovascular events than those in the placebo group, with a hazard reduction of 35% over a median of 5.1 years of follow-up.
This observed reduction was larger than that predicted by the Cholesterol Treatment Trialists’ Collaboration on the basis of the achieved reduction in LDL cholesterol levels.22 This finding suggests effects on cardiovascular risk beyond those associated with the lowering of LDL cholesterol alone. The degree of reduction is consistent with that observed in a study of rosuvastatin23 involving older persons (median age, 66 years) who did not have HIV infection but who had increased C-reactive protein levels without elevated LDL cholesterol levels. In REPRIEVE, the median screening LDL cholesterol level was 108 mg per deciliter, and the incidence of major adverse cardiovascular events was similar among participants in the pitavastatin group regardless of the screening LDL cholesterol level. In addition to the lowering of LDL cholesterol levels, statin therapy reduces measures of immune activation and inflammation in persons with HIV infection,9–12 which suggests potential mechanisms by which statins benefit this population beyond the effects of LDL-cholesterol lowering.
Using available historical data in the trial design, we hypothesized an incidence of 12 major adverse cardiovascular events per 1000 person-years in the placebo group and a hazard ratio of 0.70, with a planned number needed to treat of 58 to prevent one major adverse cardiovascular event. The observed event rate in the placebo group was lower than projected at 7.32 per 1000 person-years, but the treatment effect was larger than projected (hazard ratio, 0.65), which translated to a 5-year number needed to treat of 106 (95% CI, 64 to 303). Although the observed event rate in the placebo group was lower than anticipated, it was consistent with contemporary estimates of cardiovascular disease among persons with HIV infection. Shah et al. found a pooled cardiovascular disease event rate of 6.18 per 1000 person-years among young persons with HIV infection, which was twice the rate in the general population in a global meta-analysis.1 In our trial, we calculated the number needed to treat in participants who were younger and had a lower absolute predicted cardiovascular risk than did those who were enrolled in other primary-prevention studies involving statins, such as JUPITER.23 Moreover, the number needed to treat with pitavastatin to prevent one major adverse cardiovascular event compares favorably with a range of 80 to 160 for hypertension treatment and a value of more than 300 for aspirin, as shown in other studies.24 Overall, event rates increased with increasing risk categories for atherosclerotic cardiovascular disease in the trial. As such, the number needed to treat decreased with an increasing risk category for atherosclerotic cardiovascular disease, which suggests a potentially greater benefit among the participants in our trial who were at higher cardiovascular risk at baseline (Fig. S8).
The participants in our trial were all receiving antiretroviral therapy at baseline, with good virologic control. In the Strategies for Management of Antiretroviral Therapy (SMART) trial, investigators found that viral suppression that was induced by antiretroviral therapy not only reduced the risk of death but also reduced the risk of cardiovascular disease.25 Antiretroviral therapy improves, but does not fully normalize, measures of immune activation26 and arterial inflammation,27 which may affect the risk of ongoing cardiovascular disease in treated persons with HIV infection. Statin therapy combined with antiretroviral therapy may provide further benefit in reducing cardiovascular disease risk.
Our trial provides important information about pitavastatin among key groups of interest. Some variability in effect was seen among subgroups that were categorized according to the risk of atherosclerotic cardiovascular disease because of small numbers in some subgroups. The effect size in men and women appeared to be similar. The high risk of HIV-associated cardiovascular disease among women is an important concern in the field,28,29 and these data provide reassurance about relative statin effectiveness in this understudied population.30 Effect sizes also appeared to be generally similar across regions with different global burdens of disease, which suggests that the need for more aggressive primary prevention extends to both low-income and high-income countries. There were no apparent differences among groups according to CD4 levels either at baseline or at nadir levels, and the pitavastatin benefit was apparent in the small subgroup with low-level viremia.
Cardiovascular outcomes consisted mainly of cardiac ischemia and stroke or TIA; peripheral arterial ischemia was rare. Previous studies have shown increases in stroke among participants with HIV infection,31,32 and we found that cerebrovascular disease is an important component of cardiovascular disease in this population.31,32 Previous studies have suggested that type 2 myocardial infarction is seen equally as often as type 1 myocardial infarction among participants with HIV infection in primary care.33 In contrast, in our global trial population, type 1 myocardial infarction was most common, which is consistent with previous findings of the high prevalence of subclinical vulnerable plaque in this population.34–36
Pitavastatin appeared to have an acceptable side-effect profile in most participants in our trial, which resulted in low levels of discontinuation because of adverse events. Incident diabetes mellitus was more frequent in the pitavastatin group than in the placebo group, a finding that was consistent with the results of other trials.23 In our trial, the frequencies of diabetes mellitus in both the pitavastatin and placebo groups were similar to those in the general U.S. population for persons between the ages of 45 and 64 years: 1.01 per 100 person-years (95% CI, 0.81 to 1.25).37 Muscle-related symptoms were more frequent in the pitavastatin group but resulted in discontinuation in only 1.1% of the participants. Myopathy, rhabdomyolysis, and substantial ALT elevations were extremely rare in the two groups.
Our results may be generalizable to the large global population of persons with HIV infection between the ages of 40 and 75 years who are receiving antiretroviral therapy and who are at low-to-moderate risk for atherosclerotic cardiovascular disease (Table S9). Our trial population was 65.2% non-White and 31.1% female and was thus representative of these important groups. Although persons with HIV infection who have known cardiovascular disease or who are at higher risk should be receiving statin therapy on the basis of revised existing guidelines, further evidence has been needed to support recommendations for the prescribing of statins in those at low or moderate risk. Our identification of benefit in the groups at lower or moderate risk now establishes the need to expand this recommendation. It also remains important to optimize lifestyle factors beyond lipids, including smoking, in this population.
Our trial has several limitations. Although our results are specific to pitavastatin, other statins may have similar protective effects. For persons who are living in areas where pitavastatin is not available, the use of other statins that do not interact with antiretroviral therapy may be a reasonable choice.21,38 Other strategies that lower LDL cholesterol may also be useful and will need to be tested in large trials and compared with results achieved with statin therapy alone, including with respect to cost, efficacy, and safety.
In participants with HIV infection at low-to-moderate risk for cardiovascular disease who were receiving antiretroviral therapy, those who received pitavastatin had a lower risk of a major adverse cardiovascular event than those who received placebo over a median follow-up of 5.1 years.
Supplementary Material
Acknowledgments
Supported by grants from the National Institutes of Health, Kowa Pharmaceuticals America, Gilead Sciences, and ViiV Healthcare.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
A list of the REPRIEVE trial investigators is provided in the Supplementary Appendix, available at NEJM.org.
Contributor Information
Steven K. Grinspoon, Metabolism Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA
Kathleen V. Fitch, Metabolism Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA
Markella V. Zanni, Metabolism Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA
Carl J. Fichtenbaum, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati, OH, USA
Triin Umbleja, Center for Biostatistics in AIDS Research, Harvard T.H. Chan School of Public Health, Boston, MA, USA
Judith A. Aberg, Division of Infectious Diseases, Icahn School of Medicine at Mount Sinai, New York, NY, USA
Edgar T. Overton, Division of Infectious Diseases, University of Alabama at Birmingham, Birmingham, AL, USA
Carlos D. Malvestutto, Division of Infectious Diseases, Ohio State University Medical Center, Columbus, OH, USA
Gerald S. Bloomfield, Department of Medicine, Duke Global Health Institute and Duke Clinical Research Institute, Duke University, Durham, NC, USA
Judith S. Currier, Division of Infectious Diseases, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA
Esteban Martinez, Infectious Diseases Service, Hospital Clinic and University of Barcelona, Barcelona, Spain
Jhoanna C. Roa, DLH Corporation, Silver Spring, MD
Marissa R. Diggs, Metabolism Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA
Evelynne S. Fulda, Metabolism Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA
Kayla Paradis, Cardiovascular Imaging Research Center, Department of Radiology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA
Stephen D. Wiviott, Thrombolysis in Myocardial Infarction (TIMI) Study Group, Cardiovascular Division, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
Borek Foldyna, Cardiovascular Imaging Research Center, Department of Radiology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA
Sara E. Looby, Metabolism Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA Yvonne L. Munn Center for Nursing Research, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Patrice Desvigne-Nickens, Division of Cardiovascular Sciences, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, USA
Beverly Alston-Smith, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA
Jorge Leon-Cruz, Center for Biostatistics in AIDS Research, Harvard T.H. Chan School of Public Health, Boston, MA, USA
Sara McCallum, Metabolism Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA
Udo Hoffmann, Cleerly, Denver, CO, USA
Michael T. Lu, Cardiovascular Imaging Research Center, Department of Radiology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA
Heather J. Ribaudo, Center for Biostatistics in AIDS Research, Harvard T.H. Chan School of Public Health, Boston, MA, USA
Pamela S. Douglas, Duke University Research Institute, Duke University School of Medicine, Durham, NC
REFERENCES
- 1.Shah ASV, Stelzle D, Lee KK, et al. Global burden of atherosclerotic cardiovascular disease in people living with HIV: systematic review and meta-analysis. Circulation 2018;138:1100–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zanni MV, Schouten J, Grinspoon SK, Reiss P. Risk of coronary heart disease in patients with HIV infection. Nat Rev Cardiol 2014;11:728–41. [DOI] [PubMed] [Google Scholar]
- 3.Longenecker CT, Sullivan C, Baker JV. Immune activation and cardiovascular disease in chronic HIV infection. Curr Opin HIV AIDS 2016;11:216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feinstein MJ, Bogorodskaya M, Bloomfield GS, et al. Cardiovascular complications of HIV in endemic countries. Curr Cardiol Rep 2016;18:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007;92:2506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freiberg MS, Chang C-CH, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013;173:614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;74:1376–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aberg JA, Sponseller CA, Ward DJ, Kryzhanovski VA, Campbell SE, Thompson MA. Pitavastatin versus pravastatin in adults with HIV-1 infection and dyslipidaemia (INTREPID): 12 week and 52 week results of a phase 4, multicentre, randomised, double-blind, superiority trial. Lancet HIV 2017;4(7):e284–e294. [DOI] [PubMed] [Google Scholar]
- 9.Eckard AR, Jiang Y, Debanne SM, Funderburg NT, McComsey GA. Effect of 24 weeks of statin therapy on systemic and vascular inflammation in HIV-infected subjects receiving antiretroviral therapy. J Infect Dis 2014;209:1156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Funderburg NT, Jiang Y, Debanne SM, et al. Rosuvastatin reduces vascular inflammation and T-cell and monocyte activation in HIV-infected subjects on antiretroviral therapy. J Acquir Immune Defic Syndr 2015;68:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toribio M, Fitch KV, Sanchez L, et al. Effects of pitavastatin and pravastatin on markers of immune activation and arterial inflammation in HIV. AIDS 2017;31:797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toribio M, Fitch KV, Stone L, et al. Assessing statin effects on cardiovascular pathways in HIV using a novel proteomics approach: analysis of data from INTREPID, a randomized controlled trial. EBioMedicine 2018;35:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grinspoon SK, Fitch KV, Overton ET, et al. Rationale and design of the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE). Am Heart J 2019;212:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malvestutto CD, Ma Q, Morse GD, Underberg JA, Aberg JA. Lack of pharmacokinetic interactions between pitavastatin and efavirenz or darunavir/ritonavir. J Acquir Immune Defic Syndr 2014;67:390–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:25Pt B:2935–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang M, Kendall MA, Ribaudo H, et al. Incorporating estimands into clinical trial statistical analysis plans. Clin Trials 2022;19:285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020;396:1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feinstein MJ, Hsue PY, Benjamin LA, et al. Characteristics, prevention, and management of cardiovascular disease in people living with HIV: a scientific statement from the American Heart Association. Circulation 2019;140(2):e98–e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mangione CM, Barry MJ, Nicholson WK, et al. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force recommendation statement. JAMA 2022;328:746–53. [DOI] [PubMed] [Google Scholar]
- 20.European AIDS Clinical Society Guidelines, version 11.1. October 2022. (https://www.eacsociety.org/media/guidelines-11.1_final_09-10.pdf).
- 21.Jacobson TA, Maki KC, Orringer CE, et al. National lipid association recommendations for patient-centered management of dyslipidemia. J Clin Lipidol 2015;9:Suppl:S1.e1–122.e1. [DOI] [PubMed] [Google Scholar]
- 22.Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ridker PM, Danielson E, Fonseca FAH, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195–207. [DOI] [PubMed] [Google Scholar]
- 24.Ridker PM, MacFadyen JG, Fonseca FA, et al. Number needed to treat with rosuvastatin to prevent first cardiovascular events and death among men and women with low low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein: justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin (JUPITER). Circ Cardiovasc Qual Outcomes 2009;2:616–23. [DOI] [PubMed] [Google Scholar]
- 25.The Strategies for Management of Antiretroviral Therapy (SMART) Study Group. CD4+ count–guided interruption of antiretroviral treatment. N Engl J Med 2006;355:2283–96. [DOI] [PubMed] [Google Scholar]
- 26.Lederman MM, Funderburg NT, Sekaly RP, Klatt NR, Hunt PW. Residual immune dysregulation syndrome in treated HIV infection. Adv Immunol 2013;119:51–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zanni MV, Toribio M, Robbins GK, et al. Effects of antiretroviral therapy on immune function and arterial inflammation in treatment-naive patients with human immunodeficiency virus infection. JAMA Cardiol 2016;1:474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abelman RA, Mugo BM, Zanni MV. Conceptualizing the risks of coronary heart disease and heart failure among people aging with HIV: sex-specific considerations. Curr Treat Options Cardiovasc Med 2019;21:41. [DOI] [PubMed] [Google Scholar]
- 29.Kentoffio K, Temu TM, Shakil SS, Zanni MV, Longenecker CT. Cardiovascular disease risk in women living with HIV. Curr Opin HIV AIDS 2022;17:270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curno MJ, Rossi S, Hodges-Mameletzis I, Johnston R, Price MA, Heidari S. A systematic review of the inclusion (or exclusion) of women in HIV research: from clinical studies of antiretrovirals and vaccines to cure strategies. J Acquir Immune Defic Syndr 2016;71:181–8. [DOI] [PubMed] [Google Scholar]
- 31.Ransley G, Zimba S, Gadama Y, Saylor D, Benjamin L. Trends and clinical characteristics of hiv and cerebrovascular disease in low- and middle-income countries (LMICs) between 1990 and 2021. Curr HIV/AIDS Rep 2022;19:548–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chow FC. HIV infection, vascular disease, and stroke. Semin Neurol 2014;34:35–46. [DOI] [PubMed] [Google Scholar]
- 33.Crane HM, Paramsothy P, Drozd DR, et al. Types of myocardial infarction among human immunodeficiency virus-infected individuals in the United States. JAMA Cardiol 2017;2:260–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zanni MV, Abbara S, Lo J, et al. Increased coronary atherosclerotic plaque vulnerability by coronary computed tomography angiography in HIV-infected men. AIDS 2013;27:1263–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffmann U, Lu MT, Foldyna B, et al. Assessment of coronary artery disease with computed tomography angiography and inflammatory and immune activation biomarkers among adults with HIV eligible for primary cardiovascular prevention. JAMA Netw Open 2021;4(6):e2114923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karady J, Lu M, Bergström G, et al. Coronary artery disease in low- to intermediate-risk asymptomatic people with HIV: comparison to asymptomatic community and stable chest pain populations without known HIV. J Am Coll Cardiol 2023;81:Suppl:1801. abstract. [Google Scholar]
- 37.Centers for Disease Control and Prevention. Incidence of newly diagnosed diabetes (https://www.cdc.gov/diabetes/data/statistics-report/newly-diagnosed-diabetes.html).
- 38.Wiggins BS, Lamprecht DG Jr, Page RL II, Saseen JJ. Recommendations for managing drug-drug interactions with statins and HIV medications. Am J Cardiovasc Drugs 2017;17:375–89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


