Abstract
BACKGROUND:
Physical activity in pregnancy is associated with decreased risks of adverse pregnancy outcomes such as gestational diabetes and preeclampsia. However, the relationship between the amount and type of physical activity during pregnancy and subsequent labor outcomes remains unclear.
OBJECTIVE:
This study aimed to test the hypothesis that higher levels of physical activity across different lifestyle domains in pregnancy are associated with a shorter duration of labor.
STUDY DESIGN:
This study is a secondary analysis of a prospective cohort study in which patients with singleton pregnancies without a major fetal anomaly were administered the Kaiser Physical Activity Survey in each trimester. The Kaiser Physical Activity Survey was designed specifically to quantify various types of physical activities in women and includes 4 summative indices—housework/caregiving, active living habits, sports, and occupation. The study included women at full-term gestations admitted for induction of labor or spontaneous labor. The primary outcome of this analysis was duration of the second stage of labor. Secondary outcomes were duration of the active stage, prolonged first and second stage, mode of delivery, rates of second-stage cesarean delivery, operative vaginal delivery, severe perineal lacerations, and postpartum hemorrhage. These outcomes were compared between patients with and without high physical activity levels, defined as overall Kaiser Physical Activity Survey score ≥75th percentile in the third trimester. Multivariable logistic regression was used to adjust for obesity and epidural use. In addition, a subgroup analysis of nulliparous patients was performed.
RESULTS:
A total of 811 patients with complete Kaiser Physical Activity Survey data in the third trimester were included in this analysis. The median Kaiser Physical Activity Survey score was 9.5 (8.2–10.8). Of the 811 patients, 203 (25%) had higher levels of physical activity in pregnancy. There was no difference in the duration of the second stage of labor between patients with and without higher physical activity levels (1.29±2.94 vs 0.97±2.08 hours; P=15). The duration of active labor was significantly shorter in patients with higher levels of physical activity (5.77±4.97 vs 7.43±6.29 hours; P=.01). Patients with higher physical activity levels were significantly less likely to have a prolonged first stage (9.8% vs 19.4%; P<.01; adjusted relative risk, 0.55; 95% confidence interval, 0.34–0.83). However, rates of prolonged second-stage cesarean delivery, operative vaginal deliveries, and perineal lacerations were similar between the 2 groups.
CONCLUSION:
Patients who are more physically active during pregnancy have a shorter duration of active labor.
Keywords: activity, duration, exercise, KPAS, labor
Introduction
Physical activity during pregnancy has been associated with decreased risks of adverse maternal and neonatal outcomes, including, but not limited to, gestational diabetes, preeclampsia, and macrosomia.1–5 For this reason, regular exercise is strongly encouraged throughout pregnancy. The American College of Obstetrics and Gynecology recommends that women without contraindications should engage in aerobic and strength-conditioning exercises before, during, and after pregnancy.6 However, the World Health Organization describes physical activity as more than intentional exercise. It defines physical activity as “any bodily movement produced by skeletal muscles that requires energy expenditure…including during leisure time, for transport to get to and from places, or as part of a person’s work.” As many forms of physical activity can contribute to a person’s overall health and well-being, it is important to consider all aspects of physical activity rather than only exercise for a global picture of energy expenditure.7
However, the relationship between physical activity before and during pregnancy and labor outcomes is unclear. Previous studies examining the relationship between exercise and labor outcomes show mixed results, with some studies suggesting that increased physical activity may decrease duration of the first and second stages of labor and perineal lacerations, and others with negative results.8–11 These studies measured physical activity using participation in exercise training programs during pregnancy. Few of these studies looked at the impact of diverse physical activities in pregnancy in addition to exercise, such as activity related to occupations or caregiving. In addition, a limited number of these studies used a quantitative assessment tool to quantify physical activity levels during pregnancy.
The Kaiser Physical Activity Survey (KPAS) was designed specifically to quantify physical activity in women. It contains 4 summative activity indices, including housework/caregiving, active living habits, sports, and occupation. Scores range from 4 to 20, with a maximum of 5 points assigned for each category. KPAS has been studied extensively in pregnancy and found to be a valid and reliable tool to measure physical activity levels, specifically in pregnant patients.12,13 It has been used in studies as a means to measure physical activity in multiple facets of life and correlate these scores with common perinatal outcomes, including gestational diabetes, hypertensive disorders of pregnancy, and preterm delivery.5
Because labor is a physically arduous process, this study aimed to determine if higher levels of physical activity in pregnancy, as determined by the KPAS score, are protective against adverse labor outcomes. The association between physical activity in pregnancy and duration of the second stage of labor was studied, as this stage is particularly physically demanding. In addition, duration of the first stage of labor as a secondary outcome was assessed as prolonged labor is associated with adverse outcomes.14
Materials and Methods
This study is a secondary analysis of a prospective cohort study carried out at a single tertiary care center where patients were administered the KPAS each trimester from January 2017 to 2020. This study was approved by the Washington University School of Medicine Human Research Protection Office. Patients could enroll in the study until 20 weeks’ gestation. In the parent study, patients who had a singleton pregnancy, planned to deliver at our center, were 18 years or older, and those who could speak English were included. Exclusion criteria were a major fetal anomaly affecting delivery timing, conception via in vitro fertilization or patients who were incarcerated. In this secondary analysis, patients were also excluded if they had incomplete KPAS data. Gestational age was determined by the last menstrual period or an ultrasound examination.
Baseline maternal demographics and data on the antenatal and intrapartum course were collected from medical records by trained research staff. The primary outcome for this analysis was duration of the second stage of labor. This was selected as the primary outcome given our hypothesis that the second stage of labor requires the most patient-driven physical efforts. Secondary outcomes were duration of the active stage of labor (defined as duration of labor from 6 cm cervical dilation to 10 cm dilation), rates of second-stage cesarean delivery, operative vaginal deliveries, severe perineal lacerations (third or fourth degree), postpartum hemorrhage, prolonged first stage of labor (defined as >75th percentile of the cohort stratified by parity: >20 hours for nulliparous patients and >14 hours for multiparous patients), and prolonged second stage of labor (defined as >3 hours for nulliparous and >2 hours for multiparous patients).15 These outcomes were compared between patients with lower and higher physical activity levels, defined as third-trimester KPAS score 75th percentile and <75th percentile, respectively. The third-trimester KPAS score was used to most accurately reflect the physical activity levels specifically during pregnancy, as the KPAS questions target exercise levels over the previous 12 months.
A subgroup analysis was performed comparing primary and secondary outcomes in nulliparous patients only to determine if parity played a role in the relationship between physical activity and labor outcomes. Additional secondary analyses were performed comparing primary and secondary outcomes using first- and second-trimester KPAS scores.
Baseline characteristics of patients with lower and higher physical activity levels were compared using univariate analyses. Continuous outcomes were compared using the Student’s t test or Mann-Whitney U test. Categorical outcomes were compared using the χ2 or Fisher exact test. Primary and secondary outcomes were compared using the Mann-Whitney U test. Multivariable logistic regression was used to adjust for obesity and epidural use. Confounders were selected on the basis of biological plausibility and results of the univariate analyses. The initial model included maternal age, race, body mass index (BMI), obesity, gestational age at delivery, gestational hypertension, preeclampsia, tobacco use, illicit drug use, oxytocin use, and epidural use. Backward stepwise elimination was used to reduce the number of variables in the model. The final model included obesity and epidural use because other factors were either colinear with obesity or did not have at least a 10% effect on the model. Because the frequency of some outcomes was >10% and the odds ratio would overestimate the relative risks, aRR was estimated using the method proposed by Zhang et al.16
Results
Of the 1200 patients who completed KPAS during pregnancy, 811 patients had complete KPAS data in the third trimester and were included in this analysis. Overall KPAS scores ranged from 4 to 15 with a median score of 9.5. The 75th percentile for KPAS score was 10.8 (Figure 1). Using this cutoff, 25% of the patients (n=203) had higher physical activity levels and 75% of the patients (n=608) had lower physical activity levels (Figure 2). The higher physical activity group had higher scores in all domains within the KPAS. Patients in the higher physical activity group had the highest domain-specific scores in sports, followed by occupation and active living. Patients in the lower physical activity group had the highest domain-specific scores in active living and the lowest scores in sports (Figure 3).
FIGURE 1. Third-trimester KPAS score.
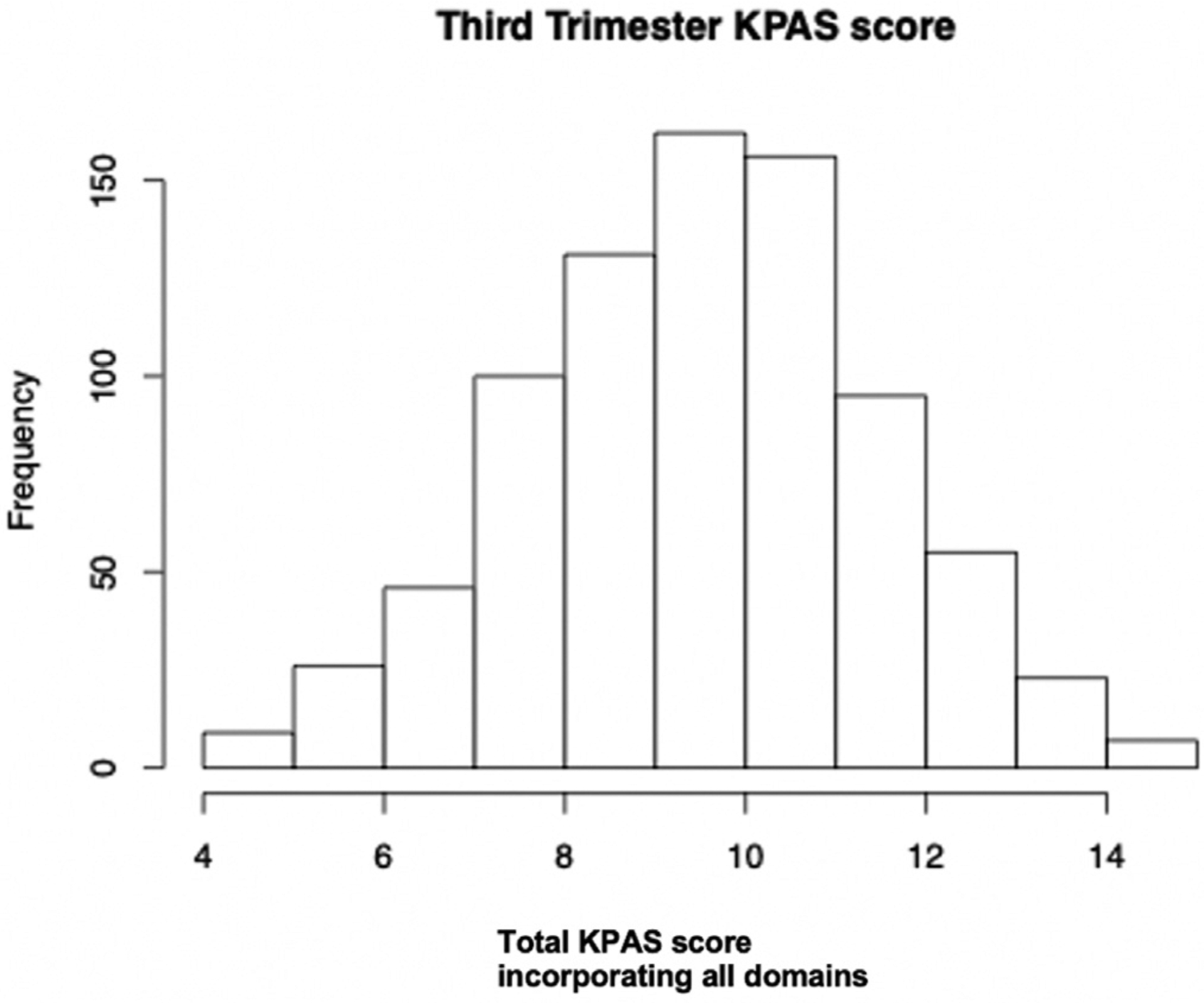
KPAS, Kaiser Physical Activity Survey.
FIGURE 2. Flowchart of study participants.
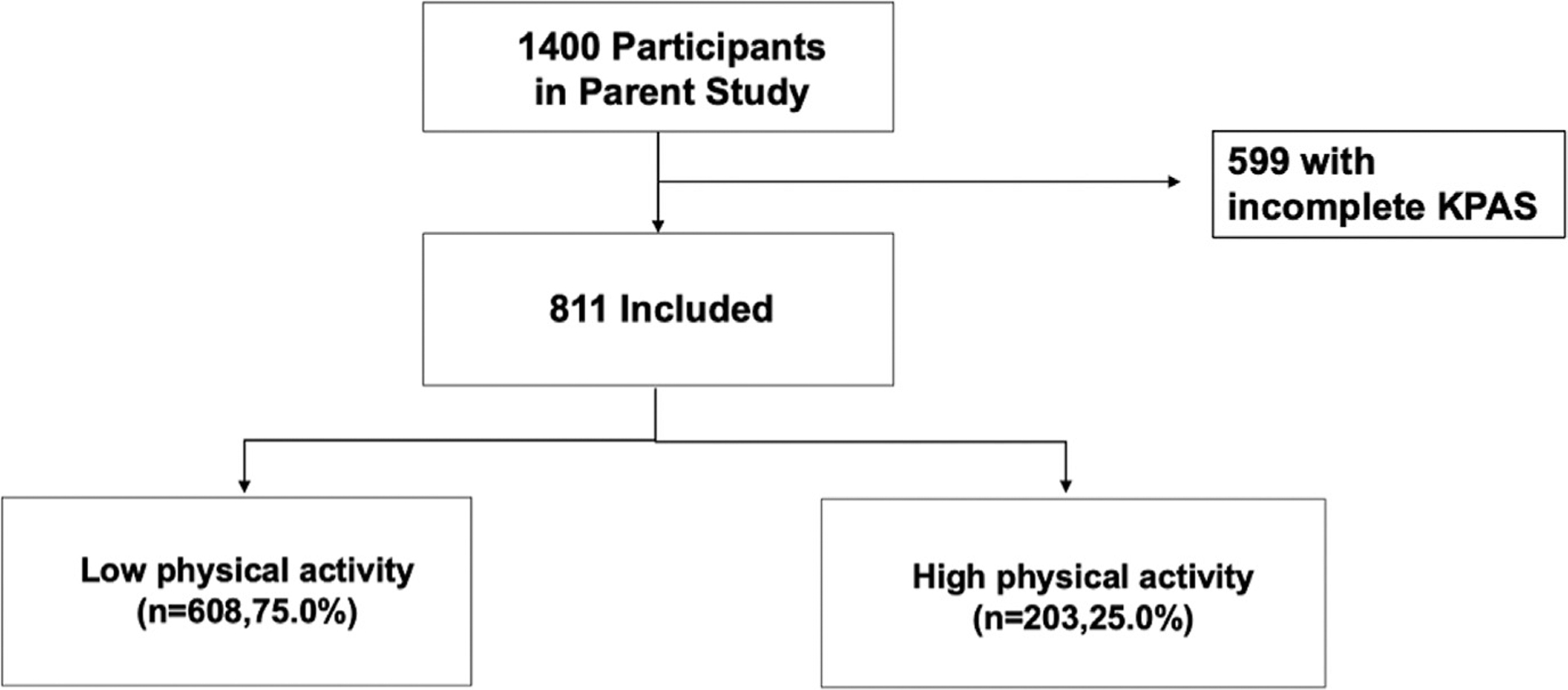
KPAS, Kaiser Physical Activity Survey.
FIGURE 3. Breakdown of KPAS domains by group.
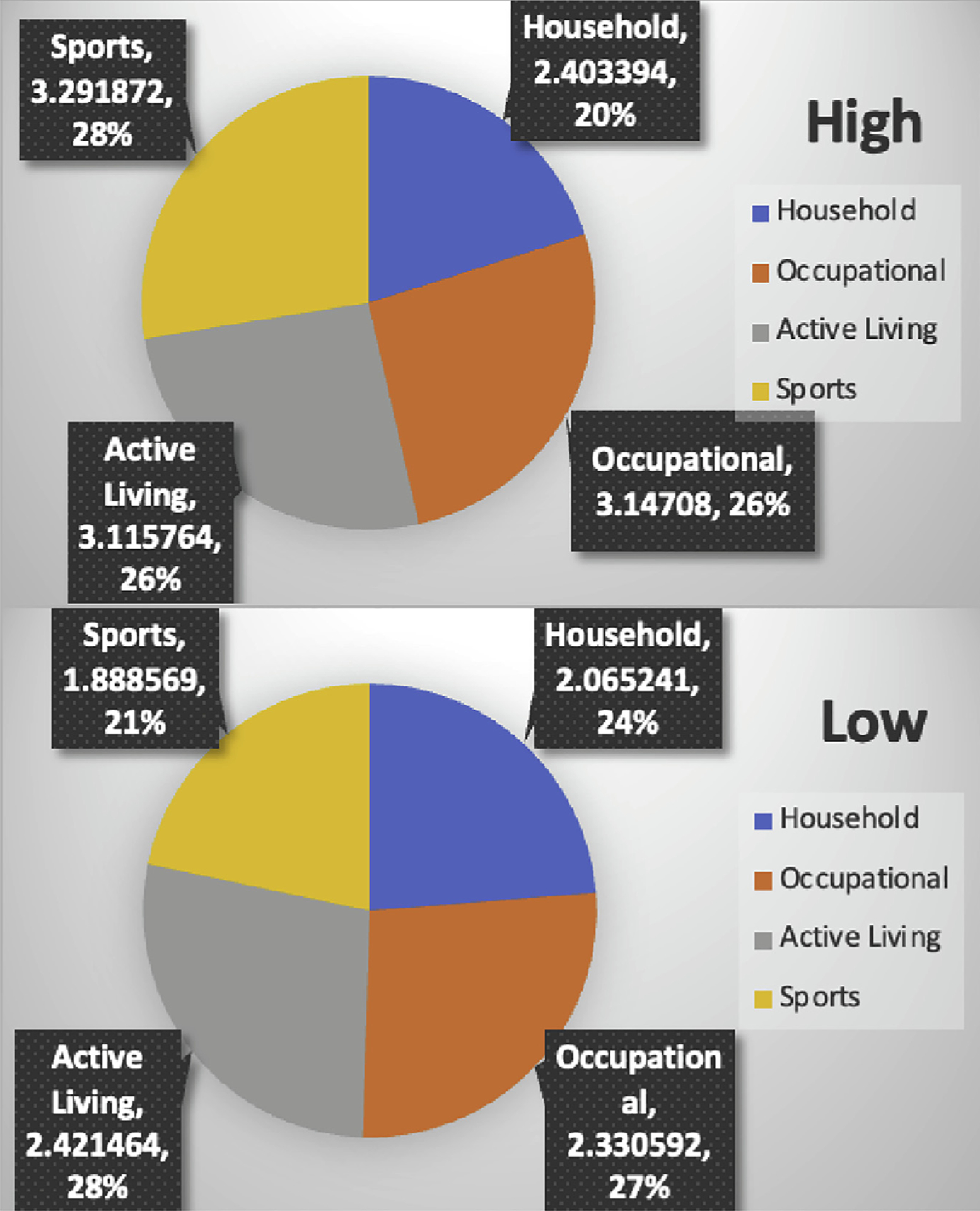
KPAS, Kaiser Physical Activity Survey.
Baseline characteristics were compared between patients with lower and higher levels of physical activity (Table 1). Maternal age was significantly greater in the higher physical activity group than the lower physical activity group (29.93±4.76 vs 28.16±5.41 years; P<.01). However, the proportion of patients with advanced maternal age was similar between the 2 groups (10.8% vs 9.2%; P=.59). Patients with higher levels of physical activity were more likely to be White (68.5% vs 41.4%; P<.01) and less likely to be African American (27.6% vs 53.8%; P<.01) than patients with lower physical activity. In addition, patients with higher levels of physical activity had a lower BMI at the start of pregnancy (26.81±6.38 vs 29.64±8.74 kg/m2;P<.01) and were less likely to be obese than patients with lower physical activity levels (23.6% vs 40.0%; P<.01). Women with higher levels of physical activity were less likely to receive oxytocin (53.2% vs 66.0%; P<.01) or an epidural in labor (75.4% vs 82.4%; P=.04). There was a significant difference in birthweight between groups, with the higher physical activity group having higher birthweights than the lower physical activity group (3321 [526] g vs 3197 [589.4] g; P<.01), but the rate of macrosomia was similar between both groups. There were no statistically significant differences in parity, pregestational and gestational diabetes, chronic hypertension, and induction of labor between the 2 groups.
TABLE 1.
Maternal demographics in patients with and without high physical activity in pregnancy
| Maternal demographics | Lower physical activity (n=608) Reference | Higher physical activity (n=203) | Pvalue |
|---|---|---|---|
| Maternal age, y | 28.16 (5.41) | 29.93 (4.76) | <.01a |
| Advanced maternal age | 56 (9.2) | 22 (10.8) | .59 |
| Race | <.01a | ||
| African American | 327 (53.8) | 56 (27.6) | |
| White | 252 (41.4) | 139 (68.5) | |
| Other | 29 (4.8) | 8 (3.9) | |
| BMI (kg/m2) | 29.64 (8.74) | 26.81 (6.38) | <.01a |
| Obesity | 243 (40.0) | 48 (23.6) | <.01a |
| GA weeks at delivery (wk) | 38.49 (1.65) | 38.82 (1.51) | .01a |
| Preterm birth | 61 (10.0) | 16 (7.9) | .44 |
| Nulliparity | 257 (42.3) | 87 (42.9) | .95 |
| Chronic hypertension | 64 (10.5) | 11 (5.4) | .07 |
| Gestational hypertension | 86 (14.1) | 16 (7.9) | .05a |
| Preeclampsia | 77 (12.7) | 10 (4.9) | <.01a |
| Pregestational diabetes | 17 (2.8) | 3 (1.5) | .49 |
| Gestational diabetes | 32 (5.3) | 11 (5.4) | .71 |
| Tobacco use | 66 (10.9) | 8 (3.9) | .01a |
| Illicit drug use | 97 (16.0) | 14 (6.9) | .01a |
| Alcohol | 29 (4.8) | 11 (5.4) | .93 |
| Induction of labor | 281 (46.2) | 81 (39.9) | .14 |
| Oxytocin | 401 (66.0) | 108 (53.2) | <.01a |
| Duration of active labor, h | 7.43 (6.29) | 5.77 (4.97) | <.01a |
| Duration of second stage, h | 0.97 (2.08) | 1.29 (2.94) | .15 |
| Cesarean delivery | 180 (29.6) | 61 (30.0) | .93 |
| Weight gain, lb | 23.1 (31.85) | 25.90 (23.83) | .25 |
| Birthweight, g | 3,197.1 (589.4) | 3,321.7 (526.5) | .01a |
| Birthweight >4000 g, % | 37 (6.1) | 14 (6.9) | .81 |
| Parity | 1.01 (1.24) | 1.02 (1.35) | .924 |
| Epidural | 501 (82.4) | 153 (75.4) | .04a |
Data represent number (percentage) or mean (standard deviation).
GA, gestational age.
Clinically significant.
The duration of the second stage of labor was similar between patients with higher and lower levels of physical activity (1.29±2.94 vs 0.97±2.08 hours; P=.15) (Figure 3). The active stage of labor was significantly shorter in patients with higher physical activity levels (5.77±4.97 vs 7.43±6.29 hours; P<.01). Patients with higher physical activity levels were less likely to have a prolonged first stage of labor than those with lower physical activity (9.8% vs 19.4%; P<.01; aRR, 0.55; 95% confidence interval,0.34–0.83). Rates of prolonged second stage of labor, second-stage cesarean delivery, operative vaginal delivery, perineal lacerations, and postpartum hemorrhage were similar between the groups (Table 2).
TABLE 2.
Secondary outcomes in patients with and without high physical activity in pregnancy
| Outcome | Lower physical activity (n=608) Reference | Higher physical activity (n=203) | Pvalue | RR (95% CI) | aRR (95% CIa) |
|---|---|---|---|---|---|
| Duration of second stage, h | 0.97 (2.08) | 1.29 (2.94) | .15 | ||
| Duration of first stage, h | 14.4 (9.46) | 10.7 (7.30) | <.01b | ||
| Duration of active labor, h | 7.43 (6.29) | 5.77 (4.97) | <.01b | ||
| Prolonged first stage | 118 (19.4) | 20 (9.8) | <.01b | 0.51 (0.32–0.79)b | 0.55 (0.34–0.83)b |
| Prolonged second stage | 35 (5.8) | 13 (6.4) | .73 | 1.11 (0.60–2.06) | 1.19 (0.62–2.14) |
| Second-stage cesarean delivery | 13 (2.1) | 6 (3.0) | .59 | 1.38 (0.53–3.59) | 1.40 (0.49–3.51)c |
| Operative vaginal delivery | 27 (4.4) | 9 (4.4) | 1 | 1.00 (0.48–2.09) | 1.00 (0.45–2.03) |
| All perineal lacerations | 264 (43.4) | 100 (49.3) | .17 | 1.13 (0.96–1.34) | 1.11 (0.94–1.29) |
| Severe perineal laceration | 20 (3.3) | 5 (2.5) | .65 | 0.75 (0.28–1.97) | 0.68 (0.23–1.68)c |
| PPH | 24 (3.9) | 7 (3.4) | .84 | 0.87 (0.38–2.0) | 0.86 (0.34–1.88) |
Data represent number (percentage) or mean (standard deviation).
aRR, adjusted relative risk; CI, confidence interval; PPH, postpartum hemorrhage; RR, relative risk.
Adjusted for obesity and epidural;
Clinically significant;
Adjusted only for obesity.
Similar results were seen in a subgroup analysis of only nulliparous patients, where higher physical activity levels were associated with a shorter active stage of labor and lower likelihood of a prolonged first stage. There was no difference in the duration of the second stage of labor or other adverse labor outcomes between the groups (Table 3).
TABLE 3.
Primary and secondary outcomes in nulliparous patients
| Outcome | Lower physical activity (n=257) Reference | Higher physical activity (n=87) | Pvalue | RR (95% CI) | aRR (95% CIa) |
|---|---|---|---|---|---|
| Duration of second stage, h | 1.32 (1.20) | 1.98 (3.10) | .10 | ||
| Duration of active labor, h | 9.17 (6.66) | 6.98 (6.15) | .02b | ||
| Prolonged first stage | 74 (28.8) | 12 (13.8) | .01b | 0.48 (0.27–0.84)b | 0.51 (0.27–0.84)b |
| Prolonged second stage | 25 (9.7) | 10 (11.5) | .68 | 1.18 (0.59–2.36) | 1.27 (0.61–2.44) |
| Second-stage cesarean delivery | 10 (3.9) | 6 (6.9) | .25 | 1.77 (0.66–4.73) | 1.85 (0.65–4.84) |
| Operative vaginal delivery | 18 (7.0) | 4 (4.6) | .61 | 0.66 (0.29–1.89) | 0.66 (0.19–1.72) |
| All perineal lacerations | 154 (59.9) | 54 (62.1) | .80 | 1.04 (0.85–1.26) | 1.04 (0.85–1.23) |
| Severe perineal laceration | 13 (5.1) | 3 (3.4) | .77 | 0.68 (0.20–2.34) | 0.62 (0.14–1.86)c |
| PPH | 14 (5.4) | 3 (3.4) | .58 | 0.75 (0.19–2.15) | 0.62 (0.14–1.77) |
Data represent number (percentage) or mean (standard deviation).
aRR, adjusted relative risk; CI, confidence interval; PPH, postpartum hemorrhage; RR, relative risk.
Adjusted for obesity and epidural;
Clinically significant;
Adjusted only for obesity.
The results were unchanged when using first- and second-trimester KPAS scores. Patients with higher physical activity scores in the first and second trimester had a significantly shorter duration of active labor and a lower likelihood of a prolonged first stage of labor with no difference in the duration of the second stage of labor.
Discussion
This secondary analysis of a prospective cohort study reported that higher physical activity levels during pregnancy are associated with a shorter duration of the active stage of labor and reduced likelihood of prolonged first stage with no difference in the duration of the second stage. This suggests that exercise during pregnancy may play a larger role in uterine contractility than pelvic floor strength, which is responsible for a successful second stage.
Previous studies looking at the effects of physical activity on labor outcomes have shown mixed results. In a randomized controlled trial in which patients were randomly assigned to either an aerobic exercise group or a control group, Barakat et al17 found that patients in the exercise group had a shorter first stage and total duration of labor. Another trial of nulliparous patients comparing an exercise group with a nonexercising control group found that women who exercised had a shorter duration of the first and second stages of labor, were less likely to require oxytocin augmentation, and were more likely to have spontaneous vaginal deliveries.18 Conversely, a randomized controlled trial in which patients were randomized to a 12-week exercise program or control group found no difference in the duration of the active stage of labor or the proportion of women with a prolonged second stage.10 However, none of these studies accounted for active living habits outside of a standardized exercise program that may affect labor outcomes.
Contrary to our initial hypothesis, there was no difference in the second stage of labor between patients with and without high physical activity levels. In the second stage, maximum contraction of the diaphragm increases the intrauterine pressure allowing descent of the fetal head through the pelvic floor muscles, more specifically, the levator ani muscles—puborectalis, pubococcygeus, and iliococcygeus.19 Previous studies have argued that a lack of elasticity of these muscles and increased muscle stiffening increases the force required for delivery and may in fact impede the second stage of labor.20 In fact, more recent studies have used shear wave elastography to measure the elastic properties of the pelvic floor muscles during pregnancy as a risk assessment tool for severe perineal trauma.21 Using this information, experts propose that athletes are more likely to have rigid pelvic floor muscles that may prolong the second stage of labor and argue that pelvic floor muscle training may in fact decrease the elasticity of these key muscles.22,23 For this reason, studies have concluded that the role of pelvic floor muscle exercises during pregnancy is not supported by scientific evidence, with a recent study showing no change in adverse labor outcomes with this intervention.24,25 Therefore, it is plausible that high levels of physical activity during pregnancy may not affect or potentially worsen the elasticity of the pelvic floor muscles that facilitate a shorter second stage of labor.
The association between higher levels of physical activity and a shorter active phase of labor is biologically plausible and may be attributed to increased uterine contractility that has been demonstrated with exercise during pregnancy. Spinnewijin et al26 measured intrauterine pressure before, during, and after maternal exercise in women admitted for an induction of labor and found that maternal exercise was associated with an increase in uterine activity. Although the physiology behind this finding is understudied, there are several theories. It is possible that noradrenaline, which is released during exercise, acts as a uterine stimulant that increases uterine contractility during pregnancy.27 Other studies have found that hyperlipidemia may play a role independently of obesity, demonstrating that the addition of cholesterol can inhibit both spontaneous and oxytocin-induced contractions and that its clearance, perhaps with increasing physical activity levels, may improve myometrial contractility.28,29 Furthermore, exercise is a key component in the formation of cross-bridges between actin and myosin in the skeletal muscles, which leads to the release of calcium ions and results in excitation–contraction coupling.30 It is believed that this calcium channel activation may also be of relevance in smooth muscle contraction and may play an important role in the physiological process of uterine contractility.31
The findings of this study are further supported by previous animal and human studies demonstrating that increased physical activity was associated with higher serum oxytocin levels anda decreased need for oxytocin augmentation in labor.18,32,33 Ferreira et al34 demonstrated that pregnant patients who exercise were less likely to require an induction of labor compared with sedentary patients, possibly secondary to a naturally elevated oxytocin level. Although this study showed no difference in the rates of induction of labor between the 2 groups, it showed that patients with high physical activity levels were significantly less likely to receive oxytocin during labor.
Our study has several strengths. First, the large sample size included in this prospective cohort study allowed us to detect differences in the duration of different stages of labor and adjust for an important confounder in this analysis, obesity, as well as epidural use. In addition, this study used a valid and reliable survey whose questions reflect physical activity over the past 12 months as a quantitative measure to assess physical activity levels specifically during pregnancy. This study had broad inclusion criteria and included patients with medical comorbidities, allowing for generalizability. Furthermore, this study is novel in that it uses a quantitative analysis tool that accounts for activity that is not necessarily intended exercise and measures physical activity in many forms.
There are some limitations of this study that should be considered. First, we were unable to confirm the accuracy of patient-reported data. For example, the addition of pedometer data to validate survey responses would have further strengthened the study. Second, our post hoc analysis demonstrated that our sample size of 811 patients had 80% power to detect a 65% difference in the duration of second stage between patients with and without high physical activity. Therefore, it is possible that smaller differences in the duration of the second stage between groups were not detected. Smaller differences in the duration of the second stage, however, are less likely to be clinically meaningful. In addition, there may be an element of selection bias as there was a subset of patients excluded from the study who did not complete the KPAS in all trimesters. When the patients who never completed any KPAS were compared with the patients who completed in once or more, it was found that African American women were significantly less likely to take the survey (68.4% vs 54.1%; P<.01). This highlights an important population of patients with missing physical activity data who should be the focus of future work in this area. This study placed equal weights for each of the KPAS domains; however, the single domains may not carry the same risk of adverse outcomes during labor and delivery. The utility and effect of a weighted score based on domain-specific differences should be considered for future studies. Finally, because there are no published cutoffs for what is considered high or low activity in pregnancy, an arbitrary percentile-based cutoff was selected. Given the rarity of the exposure, this cutoff allowed for adequate power to detect differences in clinically relevant outcomes.
This study concluded that higher physical activity levels during pregnancy are associated with a shorter active stage of labor and decreased likelihood of a prolonged first stage with no difference in the second stage of labor. Future studies should investigate the potential benefits of exercise on uterine contractility and explore what forms of physical activity improve labor outcomes to identify domain-specific interventions during pregnancy.
AJOG at a Glance.
Why was this study conducted?
There is mixed evidence regarding the effects of physical activity during pregnancy on labor outcomes. Previous studies have not included an assessment of various forms of physical activities, in addition to exercise, on labor outcomes.
Key findings
This secondary analysis of a prospective cohort study found that higher levels of global physical activity during pregnancy were associated with a shorter duration of the active stage of labor and decreased likelihood of a prolonged first stage, with no difference in the duration of the second stage.
What does this add to what is known?
The results of this study suggested that physical activity during pregnancy may play a role in improved labor outcomes. This calls for a closer look at the potential physiological benefits of higher global physical activity that is not necessarily centered on exercise alone.
Acknowledgments
N.R. is supported by the Foundation for SMFM/AAOGF and the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number K23HD098315. This work was also supported by The March of Dimes Foundation.
Footnotes
The authors report no conflict of interest.
Presented at the 41st Annual Pregnancy Meeting, Society for Maternal Fetal Medicine, January 25–30, 2021.
References
- 1.Aune D, Sen A, Henriksen TT, Saugstad OD, Tonstad S. Physical activity and the risk of gestational diabetes mellitus: a systematic review and dose-response meta-analysis of epidemiological studies. Eur J Epidemiol 2016;31:967–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dempsey JC, Butler CL, Sorensen TK, et al. A case-control study of maternal recreational physical activity and risk of gestational diabetes mellitus. Diabetes Res Clin Pract 2004;66:203–15. [DOI] [PubMed] [Google Scholar]
- 3.Sorensen TK, Williams MA, Lee IM, Dashow EE, Thompson ML, Luthy DA. Recreational physical activity during pregnancy and risk of preeclampsia. Hypertension 2003;41:1273–80. [DOI] [PubMed] [Google Scholar]
- 4.Marcoux S, Brisson J, Fabia J. The effect of leisure time physical activity on the risk of preeclampsia and gestational hypertension. J Epidemiol Community Health 1989;43:147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Currie LM, Woolcott CG, Fell DB, Armson BA, Dodds L. The association between physical activity and maternal and neonatal outcomes: a prospective cohort. Matern Child Health J 2014;18:1823–30. [DOI] [PubMed] [Google Scholar]
- 6.The American College of Obstetricians and Gynecologists. Physical activity and exercise during pregnancy and the postpartum period. 2020. Available at: https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2020/04/physical-activity-and-exercise-during-pregnancy-and-the-postpartum-period. Accessed November 16, 2020.
- 7.World Health Organization. Physical activity. 2020. Available at: https://www.who.int/news-room/fact-sheets/detail/physical-activity. Accessed November 16, 2020.
- 8.Perales M, Calabria I, Lopez C, Franco E, Coteron J, Barakat R. Regular exercise throughout pregnancy is associated with a shorter first stage of labor. Am J Health Promot 2016;30:149–54. [DOI] [PubMed] [Google Scholar]
- 9.Ghodsi Z, Asltoghiri M, Hajiloomohajerani M. Exercise and pregnancy: duration of labor stages and perinea tear rates. Procedia Soc Behav Sci 2012;31:441–5. [Google Scholar]
- 10.Salvesen KÅ, Stafne SN, Eggebø TM, Mørkved S. Does regular exercise in pregnancy influence duration of labor? A secondary analysis of a randomized controlled trial. Acta Obstet Gynecol Scand 2014;93:73–9. [DOI] [PubMed] [Google Scholar]
- 11.Masoud AT, AbdelGawad MM, Elshamy NH, et al. The effect of antenatal exercise on delivery outcomes: a systematic review and meta-analysis of randomized controlled trials. J Gynecol Obstet Hum Reprod 2020;49:101736. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt MD, Freedson PS, Pekow P, Roberts D, Sternfeld B, Chasan-Taber L. Validation of the Kaiser Physical Activity Survey in pregnant women. Med Sci Sports Exerc 2006;38:42–50. [DOI] [PubMed] [Google Scholar]
- 13.Schuster S, Šklempe Kokić I, Sindik J. Measuring physical activity in pregnancy using questionnaires: a meta-analysis. Acta Clin Croat 2016;55:440–52. [DOI] [PubMed] [Google Scholar]
- 14.Harrison MS, Ali S, Pasha O, et al. A prospective population-based study of maternal, fetal, and neonatal outcomes in the setting of prolonged labor, obstructed labor and failure to progress in low- and middle-income countries. Reprod Health 2015;12:S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The American College of Obstetricians and Gynecologists. Safe prevention of the primary cesarean delivery. 2014. Available at: https://www.acog.org/clinical/clinical-guidance/obstetric-care-consensus/articles/2014/03/safe-prevention-of-the-primary-cesarean-delivery.
- 16.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998;280:1690–1. [DOI] [PubMed] [Google Scholar]
- 17.Barakat R, Franco E, Perales M, López C, Mottola MF. Exercise during pregnancy is associated with a shorter duration of labor. A randomized clinical trial. Eur J Obstet Gynecol Reprod Biol 2018;224:33–40. [DOI] [PubMed] [Google Scholar]
- 18.Beckmann CR, Beckmann CA. Effect of a structured antepartum exercise program on pregnancy and labor outcome in primiparas. J Reprod Med 1990;35:704–9. [PubMed] [Google Scholar]
- 19.Ashton-Miller JA, DeLancey JOL. On the biomechanics of vaginal birth and common sequelae. Annu Rev Biomed Eng 2009;11:163–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Kruger JA, Nash MP, Nielsen PMF. Effects of nonlinear muscle elasticity on pelvic floor mechanics during vaginal childbirth. J Biomech Eng 2010;132:111010. [DOI] [PubMed] [Google Scholar]
- 21.Gachon B, Fritel X, Pierre F, Nordez A. In vivo assessment of the elastic properties of women’s pelvic floor during pregnancy using shear wave elastography: design and protocol of the ELASTOPELV study. BMC Musculoskelet Disord 2020;21:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horsley K Fitness in childbearing years. In: Sapsford R, Bullock-Saxton J, Markwell S, eds. Women’s Press’ Health. A textbook for physiotherapists London (United Kingdom of Great Britain and Northern Ireland): WB Saunders Company Ltd; 1998. p. 168–91. [Google Scholar]
- 23.Raadgers M, Ramkers MJ, van Lunsen RHW. Treatment of sexual and pelvic floor dysfunction. In: Carriere B, Feldt CM, eds. The pelvic floor. New York (NY): Thieme Verlag;2006. [Google Scholar]
- 24.Artal R, O’Toole M. Guidelines for the American College of Obstetricians and Gynecologists for exercise during pregnancy and the postpartum period. Br J Sports Med 2003;37:6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bø K, Fleten C, Nystad W. Effect of antenatal pelvic floor muscle training on labor and birth. Obstet Gynecol 2009;113:1279–84. [DOI] [PubMed] [Google Scholar]
- 26.Spinnewijn WE, Lotgering FK, Struijk PC, Wallenburg HCS. Fetal heart rate and uterine contractility during maternal exercise at term. Am J Obstet Gynecol 1996;174:43–8. [DOI] [PubMed] [Google Scholar]
- 27.Artal R Hormonal responses to exercise in pregnancy. In: Artal R, Wiswell RA, eds. Exercise in pregnancy. Baltimore (MD): Williams & Wilkins; 1986. p. 727–30. [Google Scholar]
- 28.Zhang J, Bricker L, Wray S, Quenby S. Poor uterine contractility in obese women. BJOG 2007;114:343–8. [DOI] [PubMed] [Google Scholar]
- 29.Smith RD, Babiychuk EB, Noble K, Draeger A, Wray S. Increased cholesterol decreases uterine activity: functional effects of cholesterol alteration in pregnant rat myometrium. Am J Physiol Cell Physiol 2005;288:C982–8. [DOI] [PubMed] [Google Scholar]
- 30.Suhr F, Gehlert S, Grau M, Bloch W. Skeletal muscle function during exercise-Fine-tuning of diverse subsystems by nitric oxide. Int J Mol Sci 2013;14:7109–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wray S, Kupittayanant S, Shmygol A, Smith RD, Burdyga T. The physiological basis of uterine contractility: a short review. Exp Physiol 2001;86:239–46. [DOI] [PubMed] [Google Scholar]
- 32.Yüksel O, Ateş M, Kızıldağ S, et al. Regular aerobic voluntary exercise increased oxytocin in female mice: the cause of decreased anxiety and increased empathy-like behaviors. Balkan Med J 2019;36:257–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irianti S, Ginandjar AB, Krisnadi SR, Effendi JS, Nataprawira D, Gandamihardja S. Aerobic exercise and its effect on oxytocin level and labor progression. IOP Conf Ser.: Mater Sci Eng 2017;180. [Google Scholar]
- 34.Ferreira CLM, Guerra CML, Silva AITJ, do Rosário HRV, Pereira MBFLO. Exercise in pregnancy: the impact of an intervention program in the duration of labor and mode of delivery. Rev Bras Ginecol Obstet 2019;41:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]


