Abstract
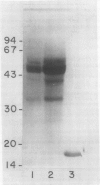
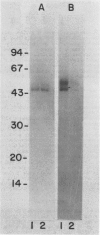
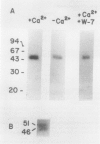
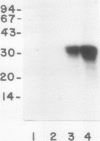
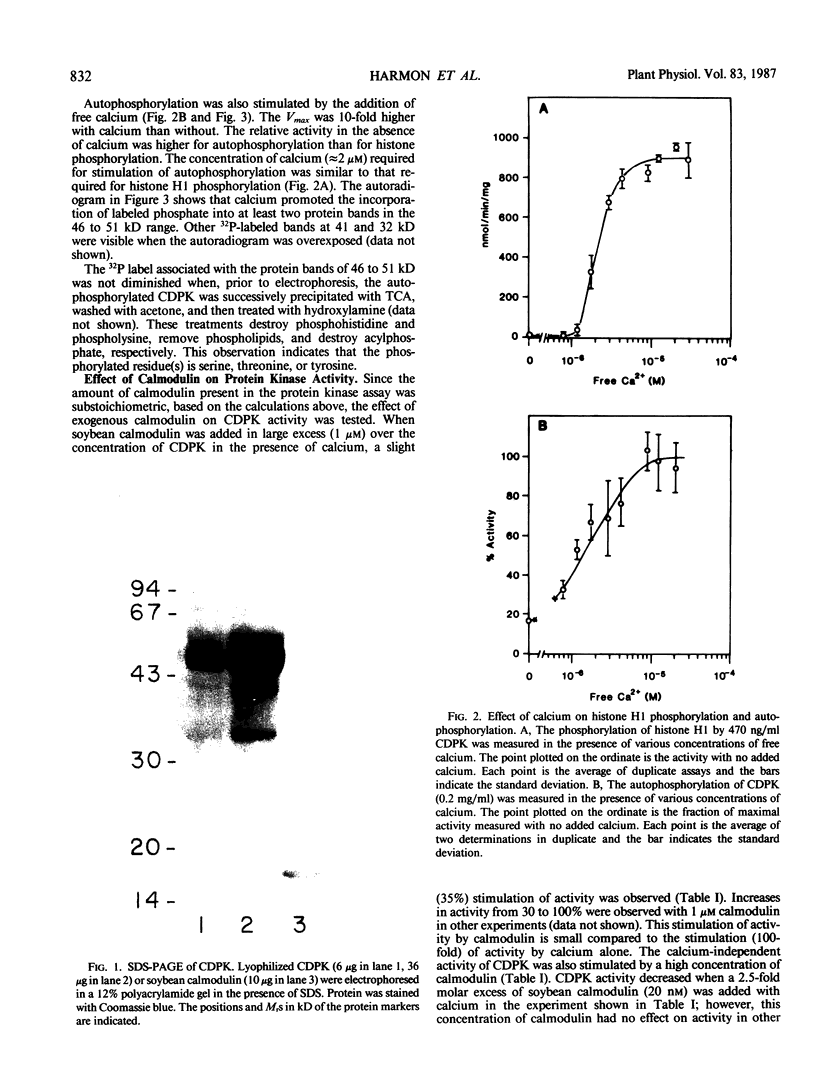
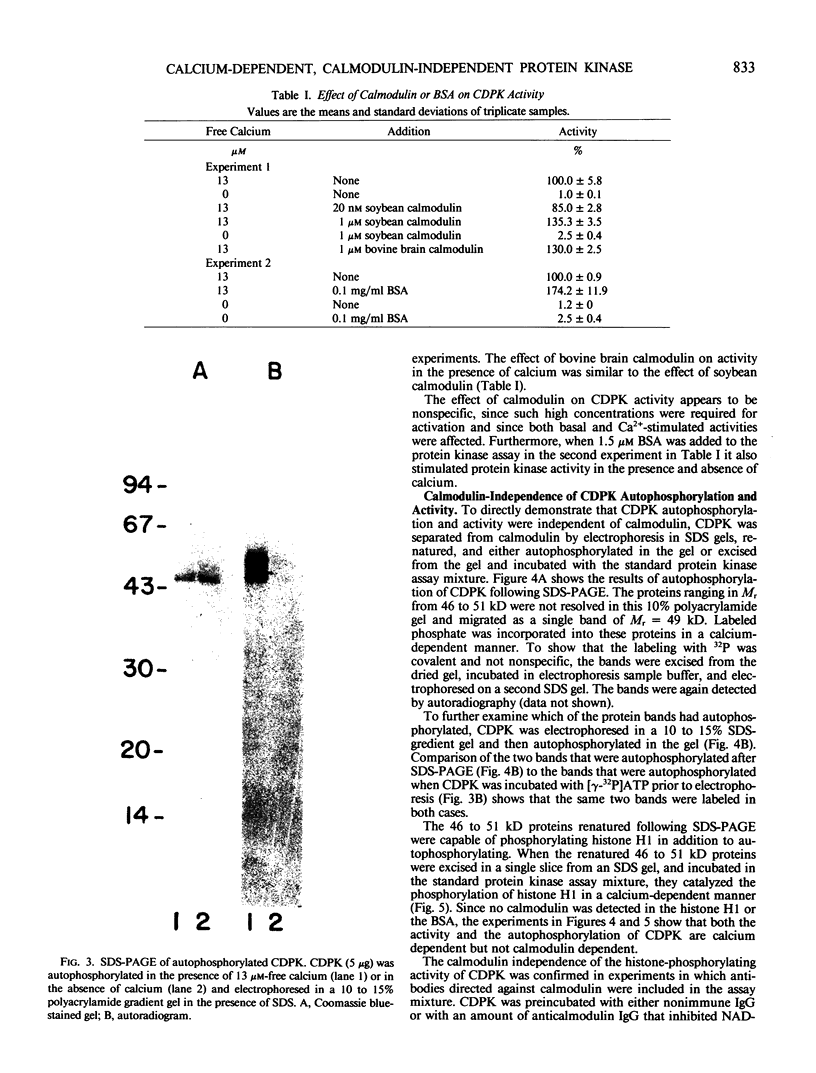
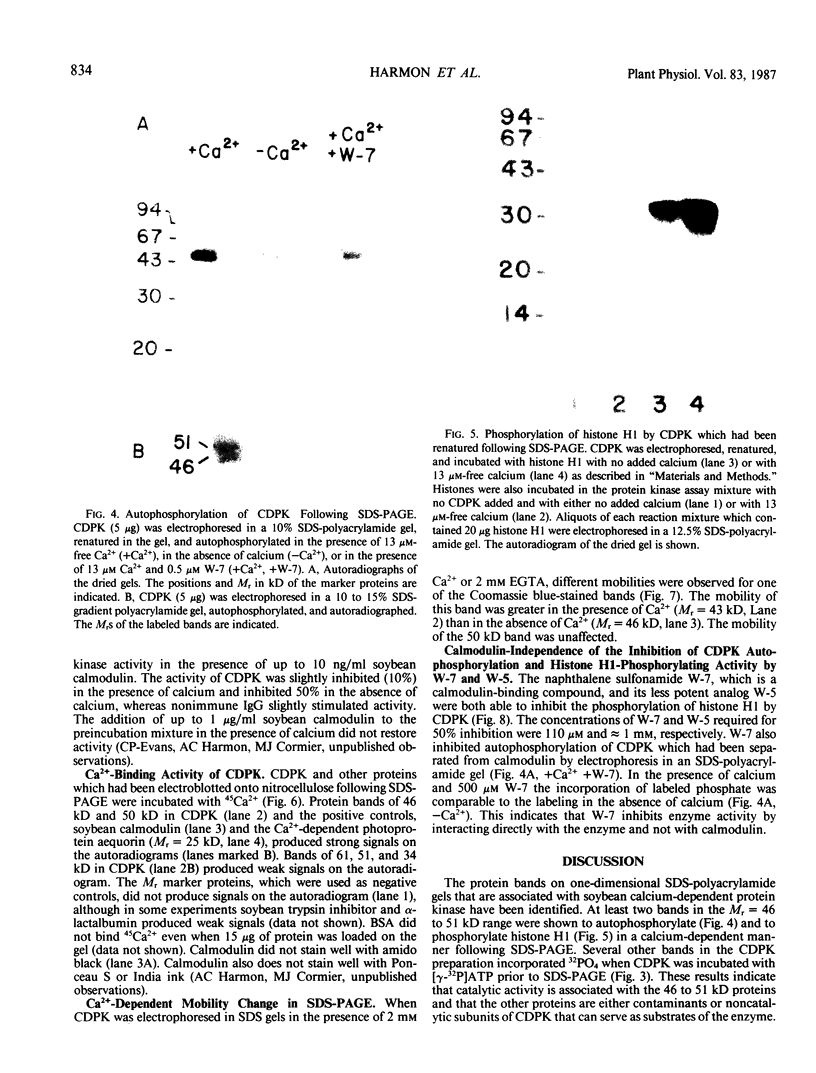
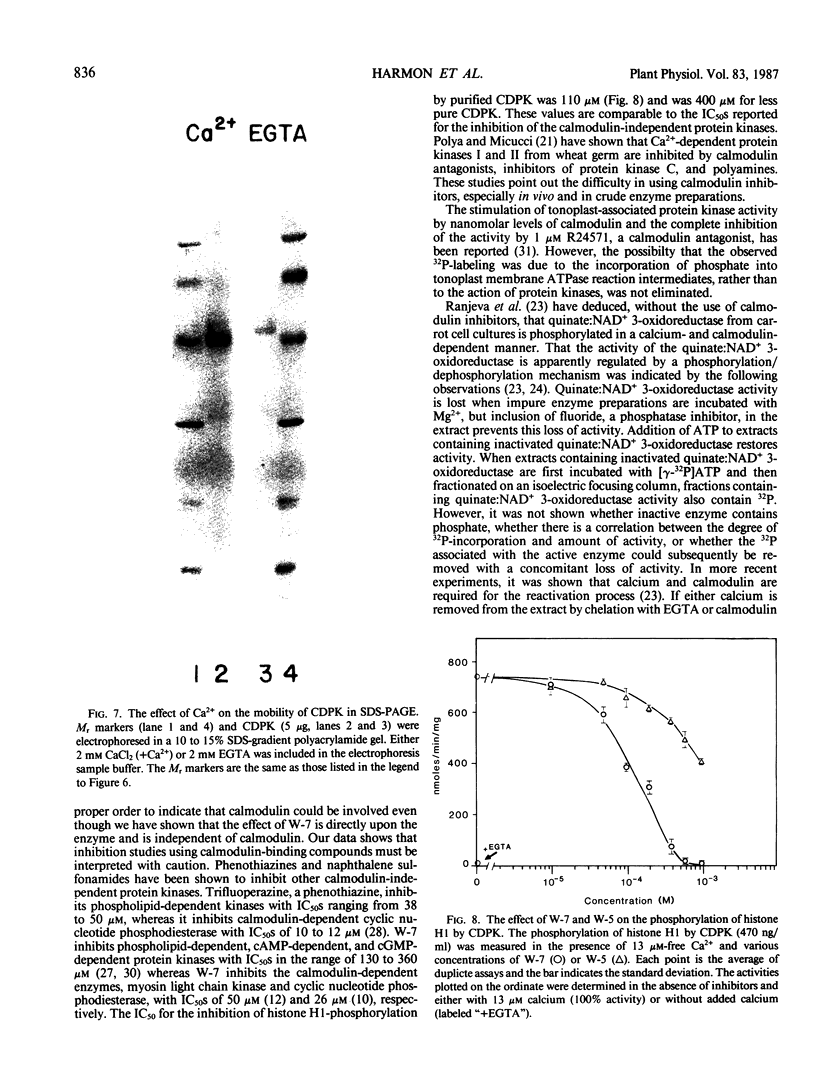
A calcium-dependent protein kinase activity from suspension-cultured soybean cells (Glycine max L. Wayne) was shown to be dependent on calcium but not calmodulin. The concentrations of free calcium required for half-maximal histone H1 phosphorylation and autophosphorylation were similar (≈2 micromolar). The protein kinase activity was stimulated 100-fold by ≥10 micromolar-free calcium. When exogenous soybean or bovine brain calmodulin was added in high concentration (1 micromolar) to the purified kinase, calcium-dependent and -independent activities were weakly stimulated (≤2-fold). Bovine serum albumin had a similar effect on both activities. The kinase was separated from a small amount of contaminating calmodulin by sodium dodecyl sulfate polyacrylamide gel electrophoresis. After renaturation the protein kinase autophosphorylated and phosphorylated histone H1 in a calcium-dependent manner. Following electroblotting onto nitrocellulose, the kinase bound 45Ca2+ in the presence of KCl and MgCl2, which indicates that the kinase itself is a high-affinity calcium-binding protein. Also, the mobility of one of two kinase bands in SDS gels was dependent on the presence of calcium. Autophosphorylation of the calmodulin-free kinase was inhibited by the calmodulin-binding compound N-(6-aminohexyl)-5-chloro-1-naphthalene sulfonamide (W-7), showing that the inhibition of activity by W-7 is independent of calmodulin. These results show that soybean calcium-dependent protein kinase represents a new class of protein kinase which requires calcium but not calmodulin for activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burgess W. H., Jemiolo D. K., Kretsinger R. H. Interaction of calcium and calmodulin in the presence of sodium dodecyl sulfate. Biochim Biophys Acta. 1980 Jun 26;623(2):257–270. doi: 10.1016/0005-2795(80)90254-8. [DOI] [PubMed] [Google Scholar]
- Charbonneau H., Hice R., Hart R. C., Cormier M. J. Purification of calmodulin by Ca2+-dependent affinity chromatography. Methods Enzymol. 1983;102:17–39. doi: 10.1016/s0076-6879(83)02005-4. [DOI] [PubMed] [Google Scholar]
- Geahlen R. L., Anostario M., Jr, Low P. S., Harrison M. L. Detection of protein kinase activity in sodium dodecyl sulfate-polyacrylamide gels. Anal Biochem. 1986 Feb 15;153(1):151–158. doi: 10.1016/0003-2697(86)90074-6. [DOI] [PubMed] [Google Scholar]
- Harmon A. C., Jarrett H. W., Cormier M. J. An enzymatic assay for calmodulins based on plant NAD kinase activity. Anal Biochem. 1984 Aug 15;141(1):168–178. doi: 10.1016/0003-2697(84)90441-x. [DOI] [PubMed] [Google Scholar]
- Hart R. C., Bates M. D., Cormier M. J., Rosen G. M., Conn P. M. Synthesis and characterization of calmodulin antagonistic drugs. Methods Enzymol. 1983;102:195–204. doi: 10.1016/s0076-6879(83)02020-0. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Asano M., Iwadare S., Matsumoto I., Totsuka T., Aoki N. A novel vascular relaxing agent, N-(6--aminohexyl)-5-chloro-1-naphthalensulfonamide which affects vascular smooth muscle actomyosin. J Pharmacol Exp Ther. 1978 Oct;207(1):8–15. [PubMed] [Google Scholar]
- Hidaka H., Asano M., Tanaka T. Activity-structure relationship of calmodulin antagonists, Naphthalenesulfonamide derivatives. Mol Pharmacol. 1981 Nov;20(3):571–578. [PubMed] [Google Scholar]
- Hidaka H., Sasaki Y., Tanaka T., Endo T., Ohno S., Fujii Y., Nagata T. N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide, a calmodulin antagonist, inhibits cell proliferation. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4354–4357. doi: 10.1073/pnas.78.7.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka H., Yamaki T., Naka M., Tanaka T., Hayashi H., Kobayashi R. Calcium-regulated modulator protein interacting agents inhibit smooth muscle calcium-stimulated protein kinase and ATPase. Mol Pharmacol. 1980 Jan;17(1):66–72. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lukas T. J., Iverson D. B., Schleicher M., Watterson D. M. Structural characterization of a higher plant calmodulin : spinacia oleracea. Plant Physiol. 1984 Jul;75(3):788–795. doi: 10.1104/pp.75.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K., Mikawa T., Ebashi S. Detection of calcium binding proteins by 45Ca autoradiography on nitrocellulose membrane after sodium dodecyl sulfate gel electrophoresis. J Biochem. 1984 Feb;95(2):511–519. doi: 10.1093/oxfordjournals.jbchem.a134633. [DOI] [PubMed] [Google Scholar]
- Polya G. M., Micucci V. Interaction of wheat germ ca-dependent protein kinases with calmodulin antagonists and polyamines. Plant Physiol. 1985 Dec;79(4):968–972. doi: 10.1104/pp.79.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjeva R., Refeno G., Boudet A. M., Marmé D. Activation of plant quinate:NAD 3-oxidoreductase by Ca and calmodulin. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5222–5224. doi: 10.1073/pnas.80.17.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzman R. C., Raynor R. L., Kuo J. F. N-(6-Aminohexyl)-5-chloro-1-naphthalenesulfonamide(W-7), a calmodulin antagonist, also inhibits phospholipid-sensitive calcium-dependent protein kinase. Biochim Biophys Acta. 1983 Jan 4;755(1):144–147. doi: 10.1016/0304-4165(83)90284-2. [DOI] [PubMed] [Google Scholar]
- Schatzman R. C., Wise B. C., Kuo J. F. Phospholipid-sensitive calcium-dependent protein kinase: inhibition by antipsychotic drugs. Biochem Biophys Res Commun. 1981 Feb 12;98(3):669–676. doi: 10.1016/0006-291x(81)91166-9. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Ohmura T., Yamakado T., Hidaka H. Two types of calcium-dependent protein phosphorylations modulated by calmodulin antagonists. Naphthalenesulfonamide derivatives. Mol Pharmacol. 1982 Sep;22(2):408–412. [PubMed] [Google Scholar]
- Veluthambi K., Poovaiah B. W. In vitro and in vivo protein phosphorylation in Avena sativa L. coleoptiles: effects of Ca2+, calmodulin antagonists, and auxin. Plant Physiol. 1986 Jul;81(3):836–841. doi: 10.1104/pp.81.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]