Abstract
To overcome the low oral bioavailability of the highly potent and selective antiretroviral agent (R)-9-(2-phosphonylmethoxypropyl)adenine (PMPA), a new lipophilic ester derivative, i.e., the bis(isopropyloxycarbonyloxymethyl)-ester [bis(POC)-PMPA], was prepared. The usefulness of bis(POC)-PMPA as an oral prodrug for PMPA was investigated in the intestinal mucosa Caco-2 cell monolayer model. The total transport of bis(POC)-PMPA was 2.7%, whereas it was less than 0.1% for PMPA. Bis(POC)-PMPA was considerably metabolized inside the epithelial cells, since the majority of the compound was recovered after transport in the form of the monoester metabolite [mono(POC)-PMPA]. In contrast, bis(POC)-PMPA was relatively resistant to degradation at the luminal side of the Caco-2 cells. Pharmacokinetic studies with mice showed that the oral bioavailability of bis(POC)-PMPA (calculated from the curves of the concentration of free PMPA in plasma) was 20%. Neither bis(POC)-PMPA nor mono(POC)-PMPA could be recovered in plasma, suggesting the efficient release of the active drug PMPA after oral administration of bis(POC)-PMPA. Severe combined immunodeficient (SCID) mice infected with Moloney murine sarcoma virus (MSV) and treated orally with bis(POC)-PMPA for 5 or 10 days (dosages, 50, 100, or 200 mg of PMPA equivalent per kg of body weight per day) showed a significant delay in MSV-induced tumor appearance and tumor-associated death. The antiviral efficacy of oral bis(POC)-PMPA was related to the dosage and treatment period and was not significantly different from that of subcutaneous PMPA given at an equivalent dose. The favorable pharmacokinetic profile, marked antiviral efficacy, and low toxicity make bis(POC)-PMPA an attractive oral prodrug of PMPA that should be further pursued in clinical studies with patients infected with human immunodeficiency virus or hepatitis B virus.
The acyclic nucleoside phosphonate (R)-9-(2-phosphonylmethoxypropyl)adenine (PMPA) is a highly potent and selective antiretroviral agent that is currently undergoing phase I/II trials with humans infected with human immunodeficiency virus (HIV). In an initial clinical trial with individuals with CD4 cell counts of ≥200 cells/mm3, intravenous PMPA, given at daily doses of 3 mg per kg of body weight, reduced plasma HIV RNA levels by more than 1 log unit after the administration of a total of eight doses (4). Also, intravenous PMPA was found to be safe and well tolerated during this short-term study. Oral administration, as required for long-term therapy, is hindered by the low oral bioavailability of PMPA. The bis(pivaloyloxymethyl)-ester prodrug [bis(POM)-PMEA; adefovir dipivoxyl] for 9-(2-phosphonylmethoxyethyl)adenine (PMEA; adefovir), which is structurally related to PMPA, has shown potent antiretroviral activity when administered orally to retrovirus-infected mice, thus illustrating the usefulness of bis(POM)-PMEA as an oral prodrug for PMEA (12). Oral bis(POM)-PMEA was found to be readily converted to PMEA, resulting in oral bioavailabilities (as PMEA) of 53, 38, ≈25, and ≈35% in mice, rats, cynomolgus monkeys, and humans, respectively (5, 8, 12, 16). Oral bis(POM)-PMEA is now in phase III clinical trials with humans infected with HIV and in phase II clinical trials with individuals infected with hepatitis B virus (HBV) (5, 9). However, bis(pivaloyloxymethyl) esters are known to decrease the levels of carnitine, due to the formation of pivaloylcarnitin, which is excreted through the kidneys (10). In patients receiving a 2-week treatment with bis(POM)-PMEA, serum carnitine levels were reduced by ≈60%; this decrease was found to be asymptomatic and reversible after treatment was stopped (5). For long-term treatment, bis(POM)-PMEA is given with carnitine as a dietary supplement.
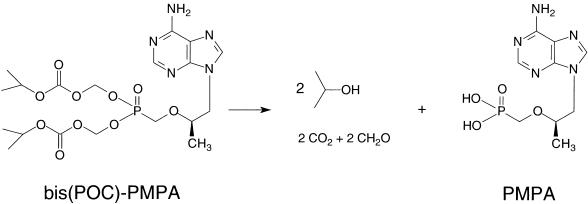
Here we report on a novel and original approach with the ester prodrug for PMPA, i.e., bis(isopropyloxycarbonyloxymethyl)-PMPA [bis(POC)-PMPA] (Fig. 1). This ester has been selected from a large number of PMPA derivatives on the basis of its chemical and enzymatic stability and its favorable octanol/water partition coefficient (log P, 1.3) (2). In vitro, the high lipophilicity of bis(POC)-PMPA results in better cellular uptake compared to that of PMPA and, hence, an increased antiviral potency in HIV-infected human lymphocyte cells (15). We have now investigated the usefulness of bis(POC)-PMPA as an oral prodrug for PMPA, i.e., its permeability in Caco-2 cell monolayers, oral bioavailability and metabolism in mice, and antiviral efficacy and safety in retrovirus-infected mice.
FIG. 1.
Metabolism of bis(POC)-PMPA to PMPA. Stochiometrically, one molecule of bis(POC)-PMPA releases two molecules of isopropanol, carbon dioxide, and formaldehyde and one molecule of PMPA. Cleavage of the first ester group yields the intermediate metabolite [mono(POC)-PMPA]; cleavage of the second ester group gives the active drug PMPA.
MATERIALS AND METHODS
Compounds.
The R enantiomers of PMPA and bis(POC)-PMPA (fumarate salt) (Fig. 1) were synthesized at Gilead Sciences, Inc., Foster City, Calif. (2). Solutions of PMPA for parenteral (intravenous or subcutaneous) injection or oral administration were prepared in isotonic saline or drinking water, respectively. Oral formulations of bis(POC)-PMPA consisted of solutions in water containing 10% dimethyl sulfoxide (DMSO). To prevent any possible degradation upon storage, all solutions of bis(POC)-PMPA were freshly prepared before use.
In vitro assays.
The antiviral assays for HIV type 1 (HIV-1) were performed with human CEM T4-lymphocyte cells by previously described procedures (3). The anti-Moloney murine sarcoma virus (MSV) activities of the compounds were measured by a transformation assay with MSV-infected murine C3H/3T3 fibroblast cells (3).
Caco-2 cell studies.
The transport of bis(POC)-PMPA through human intestinal mucosa Caco-2 cells was studied by previously reported methods (1). Briefly, Caco-2 cells were plated at a density of 40,000 cells/cm2 on Costar (Cambridge, Mass.) polycarbonate membranes (pore diameter, 3 μm; diameter, 12 mm) in 12-well trays. At 18 to 24 days postseeding, the cells were washed and preincubated with transport medium, after which the integrity of the cell monolayers was verified by measurement of the transepithelial electrical resistance of the monolayers. Then, incubation with bis(POC)-PMPA was initiated by the addition to the donor side of a 100 μM solution of the test compound in transport medium containing 0.2% DMSO. Previous experiments had shown that DMSO concentrations of up to 2% had no effect on cell monolayer integrity or on transport of lipophilic compounds. Test samples were taken from the apical (donor) and basolateral (acceptor) sides at 1, 2, and 3 h after incubation. The concentrations of bis(POC)-PMPA and its metabolites mono(POC)-PMPA and PMPA were determined by high-pressure liquid chromatographic (HPLC) analysis by an ion-pairing reverse-phase method with UV detection at 260 nm similar to a previously described method (1). The column was a Symmetry Shield RP8 column (3.9 by 150 mm) from Waters (Milford, Mass.) run at a flow rate of 1 ml per min. Mobile phase A consisted of a buffer (10 mM potassium dihydrogen phosphate, 2 mM tetrabutylammonium hydrogen sulfate, 3% acetonitrile) and was adjusted to pH 5.5, while mobile phase B consisted of 100% acetonitrile. Samples were separated with a gradient system of from 4% mobile phase B to 15% mobile phase B over 9 min and then to 35% mobile phase B over 4 min, followed by an isocratic step at 35% mobile phase B (4 min) and a return to 4% mobile phase B and reequilibration. The concentrations of bis(POC)-PMPA and PMPA in the apical and basolateral samples were calculated from peak areas by using calibration curves made up from chemical standards. The concentrations of the monoester were calculated by using the bis(POC)-PMPA calibration curve.
Enzyme incubation studies.
Porcine liver carboxylesterase and phosphodiesterase were purchased from Sigma Chemical Co., St. Louis, Mo. A solution of 10 μM bis(POC)-PMPA was incubated with 0.1 IU of carboxylesterase per ml at 37°C. At various time points, 100-μl samples were collected in test tubes (containing 100 μl of ice-cold methanol to arrest enzymatic activity), mixed, and centrifuged. The supernatant was analyzed by HPLC by the method described for the Caco-2 cell studies. To examine the conversion of mono(POC)-PMPA to PMPA by phosphodiesterase, the enzyme (0.1 IU/ml) was incubated with 10 μM mono(POC)-PMPA [obtained by chemical degradation of bis(POC)-PMPA for 14 h of incubation at 60°C]. Samples were processed as described above for the carboxylesterase studies.
Pharmacokinetic studies in mice.
Female NMRI mice (weight, 25 ± 1 g) received PMPA by intravenous bolus injection (via the tail vein) or oral gavage or bis(POC)-PMPA by oral gavage. Both compounds were given at equimolar doses, i.e., 50 mg per kg for PMPA and 104 mg per kg for bis(POC)-PMPA. Blood was drawn by cardiac puncture at different time points, ranging from 2 min to 8 h (one mouse per time point). Blood samples were collected in heparin-containing tubes (0.4 IU per ml of blood) and were immediately cooled on ice. After centrifugation at 4°C, plasma samples were frozen at −20°C. For the studies of recovery in urine, PMPA or bis(POC)-PMPA was administered to the mice as described above, and the animals were placed in metabolic cages (one mouse per cage). Urine fractions (0 to 6 and 6 to 24 h) were collected, clarified by centrifugation, and frozen. The PMPA concentration in the plasma and urine samples was determined by the method described earlier for bis(POM)-PMEA (12). This method consisted of extraction, derivatization with chloroacetaldehyde, and HPLC analysis with fluorescence detection (13).
Pharmacokinetic calculations.
The concentrations of PMPA in plasma after intravenous injection or oral dosing were analyzed by using the curve-fitting software package Siphar/Win (Simed, Créteil, France). The values for the area under the curve from time zero to the time of the last measurable concentration (AUC0–tlast) and the area under the first moment curve from time zero to the time of the last measurable concentration (AUMC0–tlast) were calculated by the linear trapezoidal rule. The bioavailability of PMPA following oral administration of bis(POC)-PMPA was defined as 100 × (AUCp.o., 0–tlast/AUCi.v., 0–tlast), where AUCp.o., 0–tlast equals the AUC for PMPA following oral administration of bis(POC)-PMPA, and AUCi.v., 0–tlast equals the AUC for PMPA following intravenous injection of PMPA. The mean residence time (MRT) was calculated as AUMC0–tlast/AUC0–tlast. Maximum concentrations in plasma (Cmax) and times to Cmax (Tmax) were the observed values. The plasma concentration-versus-time curves obtained after intravenous injection of PMPA were further analyzed by biexponential equations to determine the terminal elimination rate constant (kel), terminal half-life (0.693/kel), and total body clearance. The total recovery of PMPA in urine after the intravenous injection of PMPA or oral gavage of bis(POC)-PMPA was defined as the ratio of the cumulative amount of PMPA recovered in the urine collections to the amount of PMPA equivalent administered.
Antiviral studies in MSV-infected SCID mice.
Severe combined immunodeficient (SCID) mice were bred at the Rega Institute under germ-free conditions and were housed under specific-pathogen-free conditions during the antiviral experiments. Male and female mice were used at random. On day 0, MSV was inoculated intramuscularly into the left hind leg of 3-week-old SCID mice. The compounds were administered by subcutaneous injection or oral gavage once daily for 5 or 10 days postinfection. Mice were examined daily for the development of MSV-induced tumors at the injection site. Tumors were measured with calipers on days 8, 10, and 13. MSV-induced death was monitored for 30 days postinoculation. The statistical significance of the data obtained for the drug-treated versus the untreated groups was determined by the two-tailed Student’s t test.
RESULTS
Antiretroviral activity in vitro.
For HIV-1-infected human CEM lymphocyte cells, bis(POC)-PMPA was 60-fold superior to PMPA in inhibiting HIV-1 replication, with the concentrations required to inhibit viral replication by 50% (EC50s) being 0.10 and 5.9 μM for bis(POC)-PMPA and PMPA, respectively (data not shown). The concentrations causing 50% cytotoxicity for the CEM cell cultures (CC50s) were >45 and >300 μM for bis(POC)-PMPA and PMPA, respectively. Consequently, for CEM cells, the selectivity index (ratio of CC50 to EC50) was approximately ninefold higher for bis(POC)-PMPA than for PMPA. For MSV-infected murine fibroblast cells, the difference in antiviral potency was 11-fold [EC50s, 0.31 and 3.4 μM for bis(POC)-PMPA and PMPA, respectively]. For these cells, the selectivity index of PMPA was approximately threefold higher than that of bis(POC)-PMPA [MICs or concentrations causing minimal toxicity, >20 and >600 μM for bis(POC)-PMPA and PMPA, respectively].
Transport and metabolism of bis(POC)-PMPA in Caco-2 cell monolayers.
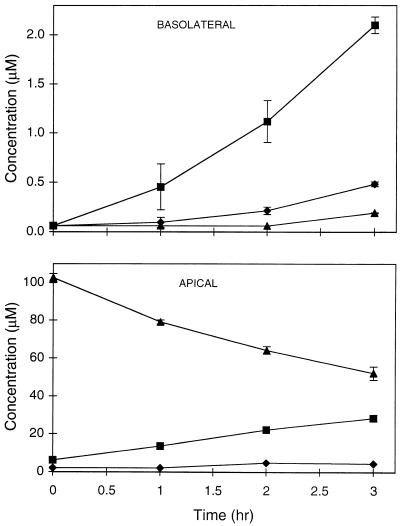
Whereas the transmembrane transport of underivatized PMPA through Caco-2 cell monolayers was found to be very low (<0.1%), permeation was considerably higher for the lipophilic ester derivative bis(POC)-PMPA. When bis(POC)-PMPA was added to the apical side at a concentration of 100 μM, the concentrations recovered at the basolateral side after 3 h of incubation were 0.2, 2, and 0.5 μM for bis(POC)-PMPA, mono(POC)-PMPA, and PMPA, respectively (Fig. 2). The total transport for bis(POC)-PMPA [i.e., the ratio of the combined basolateral concentration of bis(POC)-PMPA, mono(POC)-PMPA, and PMPA to the concentration of bis(POC)-PMPA administered apically] thus amounted to 2.7% after 3 h. The observation that mono(POC)-PMPA represented 76% of the total PMPA transported indicates that bis(POC)-PMPA undergoes considerable metabolism during its passage through the Caco-2 cell monolayers (Fig. 1). Analysis of the apical solutions showed that intact bis(POC)-PMPA, mono(POC)-PMPA, and PMPA represented 52, 28, and 2% of untransported compound, respectively, after 3 h of incubation (Fig. 2). These findings suggest that bis(POC)-PMPA is relatively sensitive to intracellular esterases (i.e., inside the epithelial cells). To identify which intracellular enzymes could be involved, studies were performed with purified enzymes from commercial sources. bis(POC)-PMPA was found to be easily converted to mono(POC)-PMPA upon incubation with carboxylesterase (100% conversion after 30 min of incubation), while phosphodiesterase was able to rapidly convert mono(POC)-PMPA to PMPA (60% conversion after 1 h of incubation) (data not shown). Although phosphodiesterase is expected to act only on mono(POC)-PMPA, small amounts of PMPA were also formed upon incubation of the enzyme with bis(POC)-PMPA. This is most likely due to the presence of low concentrations of mono(POC)-PMPA in the bis(POC)-PMPA solution (generated by chemical degradation). This chemical instability is markedly influenced by the pH, with the decomposition half-life upon incubation at 40°C being 9 and 161 h at pH 7 and pH 3, respectively.
FIG. 2.
Time course of transport of bis(POC)-PMPA across Caco-2 monolayers represented as cumulative basolateral (top) and apical (bottom) concentrations of PMPA (⧫), mono(POC)-PMPA (▪), and bis(POC)-PMPA (▴) after the addition of 100 μM bis(POC)-PMPA to the apical side of the monolayers. The values are the means ± standard deviations for three independent experiments.
Pharmacokinetics in mice.
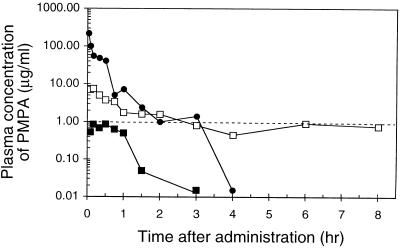
The drug concentration-versus-time curve after the intravenous bolus injection of PMPA showed a rapid and biphasic decline (Fig. 3), with a terminal half-life of 29 min (0.5 h) (Table 1). Oral administration of PMPA resulted in concentrations in plasma below 1 μg/ml, with a bioavailability (using the AUC values from 0 to 8 h) of only 1.9%. In contrast, about 10-fold higher concentrations of PMPA in plasma were obtained upon oral administration of bis(POC)-PMPA (Fig. 3). In mice receiving the prodrug at an oral dose of 50 mg of PMPA equivalent per kg, the Cmax of PMPA was 8.7 μg/ml, with a Tmax of 12 min (0.2 h). The oral bioavailability [defined as the ratio of the AUC for PMPA following oral administration of bis(POC)-PMPA to the AUC for intravenous PMPA] was 20% (Table 1). On the basis of the first-moment analysis of the drug concentration-versus-time curves, MRTs for intravenous PMPA and oral bis(POC)-PMPA were estimated to be 0.3 and 2.1 h, respectively (Table 1), thus yielding a mean absorption time of 1.8 h for the appearance of PMPA in plasma after the oral administration of bis(POC)-PMPA. The data were further confirmed in the urinary recovery studies. The cumulative urinary excretions of PMPA within 24 h after intravenous bolus injection of PMPA and oral administration of bis(POC)-PMPA at a dose of 50 mg of PMPA equivalent per kg were 79% ± 8.4% and 23% ± 5%, respectively (data not shown).
FIG. 3.
Profiles of the concentration of PMPA in the plasma of mice after intravenous bolus injection of PMPA (•) or oral gavage of PMPA (▪) or bis(POC)-PMPA (□). All compounds were given at a dose equivalent to 50 mg of PMPA per kg. Data are the average values for three independent experiments (one mouse per time point in each experiment). The dashed line represents the in vitro EC50 of PMPA for MSV-infected murine fibroblast cells.
TABLE 1.
Pharmacokinetic parameters in mice for intravenous PMPA or oral PMPA or bis(POC)-PMPAa
| Compound and route of administration | Dose (mg of PMPA equivalent/kg) | Terminal half-life (h) | Total body clearance (liter/kg/h) | Cmax in plasma (μg/ml) | Tmax in plasma (h) | AUC0–tlast (μg · h/ml) | AUMC0–tlast (μg · h2/ml) | MRT (h) | Oral bioavailability (%) |
|---|---|---|---|---|---|---|---|---|---|
| PMPA, i.v. | 50 | 0.5 ± 0.2 | 1.1 ± 0.1 | 51.6 ± 7.4 | 1,021 ± 222 | 0.3 ± 0.0 | — | ||
| PMPA, p.o. | 50 | —b | —b | 0.9 ± 0.3 | 0.6 ± 0.1 | 1.0 ± 0.7 | 52 ± 46 | 0.8 ± 0.2 | 1.9 ± 1.0 |
| Bis(POC)-PMPA, p.o. | 50 | — | — | 8.7 ± 2.4 | 0.2 ± 0.1 | 10.3 ± 2.8 | 1,416 ± 986 | 2.1 ± 1.0 | 20.3 ± 6.6 |
Data represent the means ± standard deviations for three individual experiments. Pharmacokinetic parameters were calculated by the method described in the Materials and Methods section. i.v., intravenous; p.o., per os.
—, in the absence of a clear elimination phase after oral administration, the values for terminal half-life and total body clearance could not be accurately calculated.
Antiviral efficacy in MSV-infected SCID mice.
PMPA and bis(POC)-PMPA were examined for their abilities to retard tumor development in MSV-infected SCID mice. In the first experiment, mice were treated orally with bis(POC)-PMPA or orally or subcutaneously with PMPA for 5 subsequent days at a dose of 50 or 100 mg of PMPA equivalent per kg per day. A significant delay in MSV-induced tumor appearance was observed in mice receiving subcutaneous PMPA or oral bis(POC)-PMPA (Table 2). The mean times of tumor appearance for mice receiving subcutaneous PMPA at 100 mg per kg per day or oral bis(POC)-PMPA at the equivalent dosage were 8.8 and 8.2 days, respectively; both data were statistically significant when compared to those for untreated control mice (mean day of tumor appearance, 4.9 days; P < 0.0005). The delay in tumor appearance in the mice treated subcutaneously with a daily dose of 100 mg of PMPA per kg did not differ significantly (P > 0.50) from the delay in tumor appearance in mice treated orally with bis(POC)-PMPA at the equivalent dose. The anti-MSV effect of oral PMPA was found to be marginal (P > 0.10 compared to the control). Statistical analysis of the mouse survival data did not yield significant differences for any of the drug regimens applied, probably due to the large standard deviations in the mean days of death (Table 2).
TABLE 2.
Anti-MSV effects of subcutaneous PMPA, oral PMPA, and oral bis(POC)-PMPA in a 5-day treatment schedulea
| Compound and route of administration | Dosage (mg of PMPA equivalent/ kg/day) | No. of mice | Mean day of tumor appearance | Mean day of death |
|---|---|---|---|---|
| bis(POC)-PMPA, p.o. | 100 | 5 | 8.2 ± 1.6b | 16.8 ± 3.0c |
| 50 | 5 | 7.2 ± 0.4b | 22.0 ± 5.1c | |
| PMPA, s.c. | 100 | 5 | 8.8 ± 1.3b | 23.4 ± 9.2c |
| 50 | 5 | 7.2 ± 0.4b | 15.4 ± 0.6c | |
| PMPA, p.o. | 100 | 5 | 5.6 ± 0.6c | 19.6 ± 6.1c |
| 50 | 5 | 4.6 ± 0.9c | 14.8 ± 3.5c | |
| Control | 0 | 10 | 4.9 ± 1.1 | 17.3 ± 6.1 |
Data represent the means ± standard deviations. p.o., per os; s.c., subcutaneous.
Statistical significance (two-tailed Student’s t test), P < 0.001.
Not significant.
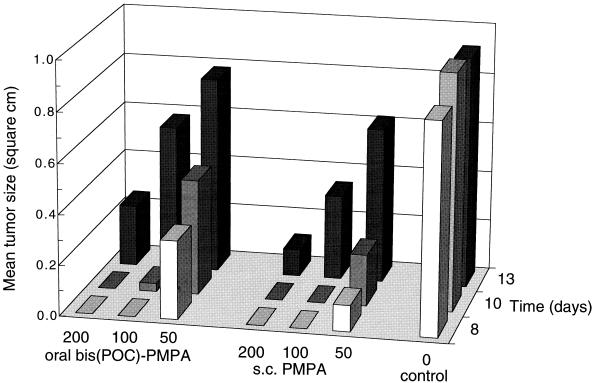
In the 5-day treatment study, both PMPA and bis(POC)-PMPA were devoid of any visible toxicity (manifested by body weight loss or early deaths) with a regimen of 100 mg of PMPA equivalent per kg per day. In the subsequent studies, both compounds were investigated at dosages of up to 200 mg of PMPA equivalent per kg per day given for 10 successive days. Both oral bis(POC)-PMPA and subcutaneous PMPA demonstrated a significant delay in MSV-induced tumor appearance and associated death (Table 3) that was clearly more pronounced than that in the 5-day treatment study (Table 2). Thus, the antiviral efficacies of oral bis(POC)-PMPA and subcutaneous PMPA were found to be dependent on dose and treatment duration. For instance, at a dosage of 100 mg of PMPA equivalent per kg per day, oral bis(POC)-PMPA delayed the mean time of tumor appearance from 4.9 to 6.0 days (control mice) to 8.2 and 12.2 days when it was administered for 5 and 10 days, respectively (P < 0.0005 for comparison of both drug regimens) (Tables 2 and 3). Similarly, the efficacy of subcutaneous PMPA (dosage, 100 mg per kg per day) was significantly lower after 5 days than after 10 days of treatment (mean times of tumor appearance, 8.8 and 13.7 days, respectively; P < 0.0005). Overall, the efficacy of oral bis(POC)-PMPA was about equal to that of subcutaneous PMPA at an equivalent dose. At a daily dose equivalent to 200 mg of PMPA per kg given for 10 days, both regimens delayed tumor appearance by ≈150%, i.e., from 6.0 days (control mice) to 14.4 and 16.1 days for oral bis(POC)-PMPA and subcutaneous PMPA, respectively (Table 3). Their equivalence with respect to antiviral efficacy is also apparent from Fig. 4, which shows the tumor sizes of drug-treated and untreated control mice at different time points after infection.
TABLE 3.
Anti-MSV effects of subcutaneous PMPA and oral bis(POC)-PMPA in a 10-day treatment schedulea
| Compound and route of administration | Dosage (mg of PMPA equivalent/ kg/day) | No. of mice | Mean day of tumor appearance | Mean day of death |
|---|---|---|---|---|
| bis(POC)-PMPA, p.o. | 200 | 10 | 14.4 ± 1.4b | 23.4 ± 2.1b |
| 100 | 10 | 12.2 ± 1.5b | 21.5 ± 2.9b | |
| 50 | 10 | 8.2 ± 2.0b | 20.2 ± 1.9b | |
| PMPA, s.c. | 200 | 10 | 16.1 ± 1.7b | 24.9 ± 2.8b |
| 100 | 10 | 13.7 ± 1.0b | 21.8 ± 1.8b | |
| 50 | 10 | 11.1 ± 2.9b | 19.9 ± 1.4b | |
| Control | 0 | 20 | 6.0 ± 0.7 | 14.2 ± 1.1 |
Data represent the means ± standard deviations. p.o., per os; s.c., subcutaneous.
Statistical significance (two-tailed Student’s t test), P < 0.0005.
FIG. 4.
Inhibition of MSV-induced tumor development in SCID mice receiving a 10-day treatment with subcutaneous (s.c.) PMPA or oral bis(POC)-PMPA. The indicated doses are in milligrams of PMPA equivalent per kilogram per day. Data are the mean tumor sizes, measured on days 8, 10, and 13 postinfection. Drug-treated groups consisted of 10 mice; the untreated control group contained 20 mice.
DISCUSSION
PMPA is one of the most potent and selective antiretroviral agents that have been described to date (3). In contrast to the closely related compound PMEA, which is active against retroviruses (i.e., HIV), hepadnaviruses (i.e., HBV), and herpesviruses, PMPA is active only against HIV and HBV (14). In a phase I/II study, intravenous PMPA has been found to have impressive anti-HIV activity (4). Prolonged therapy (>6 months) with bis(POM)-PMEA has so far not been associated with the considerable selection of PMEA-resistant HIV mutants (11). Whether PMPA also leads to a slow (or virtually no) resistance development must be ascertained by further studies.
Given the anionic nature of the phosphonate moiety of PMPA, resulting in low oral bioavailability (<2% in mice), the synthesis of lipophilic ester derivatives was undertaken. Clinical experience with the bis(pivaloyloxymethyl)-ester of PMEA [bis(POM)-PMEA] indicated that the pivaloyl moiety of the prodrug conjugates to carnitine, resulting in decreased serum carnitine levels after the administration of higher doses of bis(POM)-PMEA. This adverse effect can, however, easily be corrected by the addition of carnitine to the diet (5). In addition, the pivaloyl moiety may possibly be associated with mild to moderate gastrointestinal side effects that are seen with the higher doses of bis(POM)-PMEA (5). Hence, a slightly different prodrug approach was pursued for PMPA, namely, the bis(isopropyloxycarbonyloxymethyl)-ester. The latter moiety differs from the pivaloyl unit in two aspects: it contains an additional oxygen atom, and it contains an isopropyl group instead of the tert-butyl group (Fig. 1). Consequently, bis(POC)-PMPA would not have the drawback of carnitine interactions. The exact metabolic pathway for bis(POC)-PMPA, as proposed in Fig. 1, is still under investigation. In particular, the breakdown of the isopropyloxycarbonyloxymethyl moiety and the extent of formaldehyde release are as yet unknown.
Despite these structural differences, bis(POC)-PMPA and bis(POM)-PMEA have similar pharmacokinetic properties. (i) After oral administration, both prodrugs rapidly release the active compound (PMPA or PMEA), and neither the intact prodrug nor its monoester metabolite can be recovered from the plasma. (ii) For bis(POC)-PMPA, the oral bioavailability (calculated from the concentrations of free PMPA in plasma) was estimated to be 20% in mice and 30% in dogs, as reported recently by Shaw et al. (17). This is of the same order reported for bis(POM)-PMEA (53, 38, 25, and 35% in mice, rats, monkeys, and humans, respectively) (5, 8, 12, 16). (iii) The transmembrane transport through intestinal mucosa Caco-2 cell monolayers is much higher for bis(POC)-PMPA and bis(POM)-PMEA (2.7 and 8.8% total transport, respectively) than for the free nucleotide analogue (<0.1%) (1). Interestingly, bis(POC)-PMPA undergoes considerable metabolism inside the epithelial cells, since the monoester [mono(POC)-PMPA] represents 78% of the total compound recovered after passage through the Caco-2 monolayers. In contrast, degradation of bis(POC)-PMPA at the luminal side of the Caco-2 cells was found to be less than 37%. These studies illustrate that use of the ester prodrug has a clear advantage in terms of oral bioavailability, since the monoesters as well as the free nucleotides (PMEA and PMPA) show very limited epithelial transport. Similarly, the slightly higher oral bioavailability of bis(POC)-PMPA in dogs than in mice (30% compared to 20%) could be related to the fact that chemical hydrolysis of bis(POC)-PMPA is much slower at lower pH (an acidic formulation was used in the studies with dogs but not in the studies with mice) (17). Similarly, the effect of 10% DMSO in the formulation used for the studies with mice is not known (although this effect may be assumed to be minimal due to strong dilution of the oral solution in the gut). In other words, the oral bioavailability of these prodrugs may be influenced by their formulation and administration with food. Indeed, for bis(POM)-PMEA, the oral bioavailabilities were found to be 40 and 30% when administered with or without food, respectively (5).
The data from the Caco-2 model suggest that after oral administration of bis(POC)-PMPA or bis(POM)-PMEA and their passage through the intestinal mucosa, the prodrugs reach the liver mainly as the monoester, which is then further metabolized (by liver esterases) to the active drug (PMPA or PMEA). However, further studies are needed to confirm this hypothesis. Also, the involvement of carboxylesterase and phosphodiesterase in the formation of the monoester and the free drug, respectively, needs further investigation.
In the pharmacokinetic studies with mice, elimination of PMPA from plasma was found to be much slower after oral administration of bis(POC)-PMPA than after intravenous administration of PMPA, since the AUC from 4 to 8 h represented ≈36 and 0% of the total AUC from 0 to 8 h, respectively. This is also reflected in the different MRTs for both administration routes. The long-lasting presence of PMPA in the plasma may be the basis for the marked antiviral efficacy of oral bis(POC)-PMPA in MSV-infected SCID mice. Indeed, oral bis(POC)-PMPA was equipotent to subcutaneous PMPA, even though the oral bioavailability of the prodrug was only 20% in mice. These observations suggest that the continued presence of PMPA at lower concentrations may have a greater effect on antiviral efficacy than the effect achievable with higher but short-lived peak values. Theoretically, the long-lasting levels of PMPA in plasma following oral administration of bis(POC)-PMPA could be explained in at least three ways: (i) slow absorption of the prodrug in the gut, (ii) slow release of PMPA after first-pass metabolism in the liver, and (iii) efflux of free PMPA from the blood cells after its initial cellular uptake and metabolism (7). Further studies are required to address these issues.
As an animal model used to test the antiviral efficacy of prodrugs of nucleoside or nucleotide analogs, the MSV-infected SCID mouse model has proven to be most useful. This model allows far more rapid assessment than larger animal models, such as the model of simian immunodeficiency virus infection in monkeys. In the latter model, subcutaneous PMPA already found to be remarkably active against acute as well as chronic simian immunodeficiency virus infection (18, 19, 20). With the MSV-infected SCID mouse model, it was previously shown that oral bis(POM)-PMEA has antiviral activity equivalent to that of systemic PMEA (12). Similarly, it has now been demonstrated that the antiviral efficacy of oral bis(POC)-PMPA is comparable to that of systemic PMPA given at an equivalent dose and for the same treatment period. In addition, oral bis(POC)-PMPA was found to be relatively nontoxic to rats and dogs that received daily doses of the prodrug for 28 days (6). The favorable oral bioavailability of bis(POC)-PMPA, its efficiency in releasing the free nucleotide PMPA, and its equivalence to PMPA in terms of antiviral efficacy and safety support its further clinical development as an oral prodrug for PMPA. Phase I/II clinical trials designed to test the oral bioavailability, pharmacokinetics, safety, and antiviral activity of bis(POC)-PMPA in HIV-infected patients have recently been initiated.
ACKNOWLEDGMENTS
We thank Hubert Herbots, Reza Oliyai, J.-P. Shaw, Ann Absillis, Lizette Van Berckelaer, Ria Van Berwaer, and Willy Zeegers for excellent technical assistance.
This study was supported in part by the Biomedical Research Programme of the European Commission, the Belgian Nationaal Fonds voor Wetenschappelijk Onderzoek, and the Belgian Geconcerteerde Onderzoeksacties. Pieter Annaert acknowledges a fellowship of the Flemish Institute for the promotion of scientific-technological research in the Industry (IWT).
REFERENCES
- 1.Annaert P, Kinget R, Naesens L, De Clercq E, Augustijns P. Transport, uptake and metabolism of the bis(pivaloyloxymethyl)-ester prodrug of 9-(2-phosphonylmethoxyethyl)adenine in an in vitro cell culture system of the intestinal mucosa (Caco-2) Pharm Res. 1997;14:492–496. doi: 10.1023/a:1012155717819. [DOI] [PubMed] [Google Scholar]
- 2.Arimilli M N, Kim C U, Dougherty J, Mulato A, Oliyai R, Shaw J P, Cundy K C, Bischofberger N. Synthesis, in vitro biological evaluation, and oral bioavailability of 9-[2-phosphonylmethoxy)propyl]adenine (PMPA) prodrugs. Antivir Chem Chemother. 1997;8:557–564. [Google Scholar]
- 3.Balzarini J, Holy A, Jindrich J, Naesens L, Snoeck R, Schols D, De Clercq E. Differential antiherpesvirus and antiretrovirus effects of the (S) and (R) enantiomers of acyclic nucleoside phosphonates: potent and selective in vitro and in vivo antiretrovirus activities of (R)-9-(2-phosphonomethoxypropyl)-2,6-diaminopurine. Antimicrob Agents Chemother. 1993;37:332–338. doi: 10.1128/aac.37.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barditch-Crovo P, Deeks S, Kahn J, Redpath I, Smith A, Hwang F, Hellmann N, Cundy K, Rooney J, Lietman P. PMPA: safety, pharmacokinetics, and antiretroviral activity when administered as a single dose and for seven consecutive days to patients with HIV infection. Presented at the 10th International Conference on Antiviral Research. 1997. [Google Scholar]
- 5.Barditch-Crovo P, Toole J, Hendrix C W, Cundy K C, Ebeling D, Jaffe H S, Lietman P S. Anti-human immunodeficiency virus (HIV) activity, safety, and pharmacokinetics of adefovir dipivoxyl (9-[2-(bis(pivaloyloxymethyl)phosphonylmethoxyethyl]adenine) in HIV-infected patients. J Infect Dis. 1997;176:406–413. doi: 10.1086/514057. [DOI] [PubMed] [Google Scholar]
- 6.Bischofberger N, Naesens L, De Clercq E, Fridland A, Srinivas R V, Robbins B L, Arimilli M, Cundy K C, Kim C, Lacy S, Lee W, Shaw J-P, Oliyai R. Bis(POC)PMPA, an orally bioavailable prodrug of the antiretroviral agent PMPA, poster 214. Presented at the Fourth Conference on Retroviruses and Opportunistic Infections. 1997. [Google Scholar]
- 7.Cundy K C, Barditch-Crovo P, Walker R E, Collier A C, Ebeling D, Toole J, Jaffe H S. Clinical pharmacokinetics of adefovir in human immunodeficiency virus type 1-infected patients. Antimicrob Agents Chemother. 1995;39:2401–2405. doi: 10.1128/aac.39.11.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cundy K C, Fishback J A, Shaw J-P, Lee M L, Soike K F, Visor G C, Lee W A. Oral bioavailability of the antiretroviral agent 9-(2-phosphonylmethoxyethyl)adenine (PMEA) from three formulations of the prodrug bis(pivaloyloxymethyl)-PMEA in fasted male cynomolgus monkeys. Pharm Res. 1994;11:839–843. doi: 10.1023/a:1018925723889. [DOI] [PubMed] [Google Scholar]
- 9.Gilson R J, Chopra K, Murray-Lyon I, Newell A, Nelson M, Tedder R S, Toole J, Jaffe H S, Hellmann N, Weller I V D. 36th ICAAC Program Addendum. Washington, D.C: American Society for Microbiology; 1996. Adefovir dipivoxyl (bis-POM PMEA) treatment for chronic hepatitis B infection: a placebo-controlled Phase I/II study, abstr. LB1. [Google Scholar]
- 10.Holme E, Greter J, Jacobsen C-E, Lindstedt S, Nordin I, Kristiansson B, Jodal U. Carnitine deficiency induced by pivampicillin and pivmecillinam therapy. Lancet. 1989;ii:469–473. doi: 10.1016/s0140-6736(89)92086-2. [DOI] [PubMed] [Google Scholar]
- 11.Mulato A S, Lamy P L, Li W, Miller M D, Cherrington J M. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Genotypic characterization of HIV-1 variants isolated from AIDS patients after prolonged therapy with adefovir dipivoxyl (bis-POM PMEA), abstr. I-114. [Google Scholar]
- 12.Naesens L, Balzarini J, Bischofberger N, De Clercq E. Antiretroviral activity and pharmacokinetics in mice of oral bis(pivaloyloxy- methyl)-9-(2-phosphonylmethoxyethyl)adenine, the bis(pivaloyloxymethyl) ester prodrug of 9-(2-phosphonylmethoxyethyl)adenine. Antimicrob Agents Chemother. 1996;40:22–28. doi: 10.1128/aac.40.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naesens L, Balzarini J, De Clercq E. Acyclic nucleoside phosphonates in plasma determined by high-performance liquid chromatography with fluorescence detection. Clin Chem. 1992;38:480–485. [PubMed] [Google Scholar]
- 14.Naesens L, Snoeck R, Andrei G, Balzarini J, Neyts J, De Clercq E. HPMPC (cidofovir), PMEA (adefovir) and related acyclic nucleoside phosphonate analogues: a review of their pharmacology and clinical potential in the treatment of viral infections. Antivir Chem Chemother. 1997;8:1–23. [Google Scholar]
- 15.Robbins B, Srinivas R, Kim C, Bischofberger N, Fridland A. Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phosphonomethoxypropyl)adenine (PMPA), bis(isopropyloxymethylcarbonyl)PMPA. Antimicrob Agents Chemother. 1998;42:612–617. doi: 10.1128/aac.42.3.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw J-P, Louie M S, Krishnamurthy V V, Arimilli M N, Jones R J, Bidgood A M, Lee W A, Cundy K C. Pharmacokinetics and metabolism of selected prodrugs of PMEA in rats. Drug Metab Dispos. 1997;25:362–366. [PubMed] [Google Scholar]
- 17.Shaw J-P, Sueoka C M, Oliyai R, Lee W A, Arimilli M N, Kim C U, Cundy K C. Metabolism and pharmacokinetics of novel oral prodrugs of 9-[(R)-2-phosphonomethoxy)propyl]adenine (PMPA) in dogs. Pharm Res. 1997;14:1824–1829. doi: 10.1023/a:1012108719462. [DOI] [PubMed] [Google Scholar]
- 18.Tsai C-C, Follis K E, Beck T W, Sabo A, Bischofberger N, Dailey P J. Effects of (R)-9-(2-phosphonylmethoxypropyl)adenine monotherapy on chronic SIV infection in macaques. AIDS Res Hum Retroviruses. 1997;13:707–712. doi: 10.1089/aid.1997.13.707. [DOI] [PubMed] [Google Scholar]
- 19.Tsai C-C, Follis K E, Sabo A, Beck T W, Grant R F, Bischofberger N, Benveniste R E, Black R. Prevention of SIV infection in macaques by (R)-9-(2-phosphonylmethoxypropyl)adenine. Science. 1995;270:1197–1199. doi: 10.1126/science.270.5239.1197. [DOI] [PubMed] [Google Scholar]
- 20.Van Rompay K K A, Cherrington J M, Marthas M L, Berardi C J, Mulato A S, Spinner A, Tarara R P, Canfield D R, Telm S, Bischofberger N, Pedersen N C. 9-[2-Phosphonylmethoxy)propyl]adenine therapy of established simian immunodeficiency virus infection in infant rhesus macaques. Antimicrob Agents Chemother. 1997;40:2586–2591. doi: 10.1128/aac.40.11.2586. [DOI] [PMC free article] [PubMed] [Google Scholar]