Abstract
Aim: A cluster randomized trial was conducted within 41 Japanese municipalities (21 intervention and 22 usual care) to examine whether the standardized health counseling for individuals at high cardiovascular risk screened at community sites accelerates clinic visits to strengthen the primary health care system.
Methods: Among high-risk individuals aged 40–74 years screened by health checkups, 8,977 and 6,733 were allocated to the intervention and usual care groups, respectively, who were not under medical treatment but had high levels of blood pressure (systolic/diastolic ≥ 160/100 mmHg), hemoglobin A1c or glucose (≥ 7.0% or corresponding glucose levels), LDL-cholesterol (≥ 180 mg/dL for men), and/or proteinuria of ≥ 2+. The intervention was performed from May 2014 to March 2016 under a standardized health counseling program based on the health belief model primarily by public health nurses. The usual care group was provided with local counseling protocols.
Results: The cumulative proportions of clinic visits for 12 months after health checkups were 58.1% (95% confidence interval, 57.0%, 59.3%) versus 44.5% (43.2%, 45.8%), with the probability ratio of clinic visits between the groups being 1.46 (1.24, 1.72). The between-group differences between the baseline and 1-year surveys were −1.50 (−2.59, −0.41) mmHg for diastolic blood pressure in the hypertension category, −0.30% (−0.53%, −0.07%) for HbA1c in the diabetes category, −0.37 (−0.48, −0.27) mmol/L for LDL-cholesterol in the dyslipidemia category, and none for proteinuria.
Conclusion: Standardized health counseling for high-risk individuals accelerated clinic visits, with larger reductions in blood pressure, HbA1c, and LDL-cholesterol levels. The nationwide use of counseling after health checkups for high-risk individuals could help in controlling risk factors and in preventing lifestyle-related diseases.
Keywords: Health counseling, Risk factors, Lifestyle-related diseases, Referral, Outcome assessment
See editorial vol. 30: 1315-1316
Clinical Trial Registration−URL: https://www.umin.ac.jp/ctr Unique Identifier: UMIN000014012.
Introduction
Primary care systems can contribute to various health outcomes, such as lifestyle-related diseases. According to the Organization for Economic Cooperation and Development (OECD) Health Data, the scores of primary care systems were derived from the following 10 components: regulation, financing, primary care provider, access, longitudinality, first contact, comprehensiveness, coordination, family orientation, and community orientation 1) . The UK had the highest score, Japan had a score below the mean, and the USA had the lowest score among the OECD countries. In the UK, everyone has a family physician who screens people aged 40–74 years every 5 years who did not have prior diagnoses of hypertension, diabetes mellitus, hypercholesterolemia, coronary heart disease, heart failure, stroke, and so on 2) . However, not everyone in Japan and the USA has family physicians. Instead, Japan offers the universal health checkup system, run by insurers (municipalities or companies), and health counseling is primarily run by public health nurses to refer very high-risk individuals to local physicians to seek treatment 3) . This unique system may trade-off the weakness of the primary care systems and may save the costs of medical resources. A community-based stroke prevention program using this framework was demonstrated to be effective in reducing stroke incidence and prevalence at a lower cost than in a control community 4 , 5) . Since the 1960s, Japan has achieved a substantial decline in stroke mortality and a moderate decline in ischemic heart disease mortality 6) , contrasting the middle-economic countries, which have encountered increased mortality from these diseases 7) . Such trends in the middle-economic countries have made the proportion of mortality due to cardiovascular diseases approximately 60% worldwide 8) .
In 2008, a nationwide system of health checkups and counseling for people aged 40–74 years was enacted in Japan to screen for individuals with high blood pressure, high hemoglobin A1c (HbA1c) or glucose, high low-density lipoprotein (LDL) cholesterol levels, and proteinuria to reduce their risk through lifestyle modification and to refer those at a high-risk to local physicians 3) . However, whether such referrals effectively increase clinic visits is not known. Approximately 40% of patients with untreated extremely severe hypertension (systolic and/or diastolic blood pressures ≥ 180/110 mmHg) screened via community-based health checkups did not visit physicians thereafter 9) , and more than half of the patients with cardiovascular disease had not visited a physician to seek treatment for their high-risk factors 10) . We assume that this issue may have occurred because of insufficient health counseling following health checkups.
To provide evidence and prevent the advancement of vascular damage addressed by Health Japan 21 (second term: 2013 to 2022) 11) , we developed a health counseling method based on the health belief model, originally a socio-psychological model 12 , 13) , to explain and predict health-related behaviors regarding the uptake of healthcare services adopted by a municipal public health department in Amagasaki city 14) and to accelerate referral of very high-risk individuals to local physicians to seek treatment. This trial assessed the effectiveness of this counseling method for high-risk individuals screened through community-based health checkups for accelerated referral. We hypothesized that the intervention group would have a higher likelihood of visiting physicians for medical care and more improvement in risk factor levels compared to those in the usual care group.
Materials and Methods
Study Population
This study was a parallel-arm, nonblinded, cluster randomized trial conducted in a community setting. We recruited the participating municipalities among 891 municipalities having ≥ 2,000 men and women aged 40–74 years who received health checkups in the past year under the National Health Insurance (one of the major insurances for residents who do not work for governments and companies, covering a quarter of the national census population). Mayors of 43 municipalities agreed to participate in the trial.
Randomization and Masking
The 43 participating municipalities were randomized to 21 intervention and 22 usual care groups via pair-wise matching randomization 15) . Matching was performed on Mahalanobis distance computed based on the characteristics of the municipalities, including the number of high-risk individuals in the previous-year health checkups, number of people registered in the National Health Insurance, participation rate of health checkups, proportion of high educational attainment, and the number of physicians per 100,000 people using the “nbpMatching” package in R software (www.r-project.org) 16 , 17) . Randomization was performed at an independent organization (Vanderbilt University, Nashville TN, USA) while the municipality name was masked 15) . The average participation rates for health checkups at recruitment were 34.3% for the intervention group and 34.4% for the usual care group. As this trial was conducted under a legislated municipal health project, it did not require informed consent from individuals 18) . Participation involved an opt-out method via the websites, leaflets, and posters of all participating municipalities in which the study description and refusal to participate were provided by Osaka University.
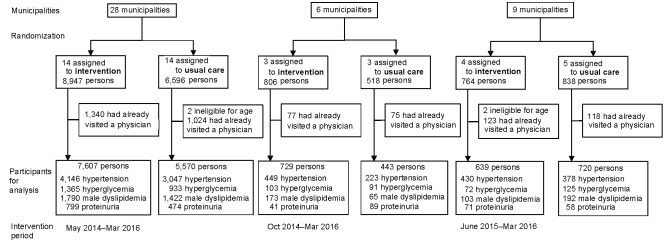
Fig.1 illustrates the selection, randomization of municipalities, and participant selection. The counselors were not blinded to the allocation of the participants to the intervention or usual care groups. There were 15,708 high-risk individuals from 43 municipalities who had very high blood pressure (systolic/diastolic blood pressures ≥ 160/100 mmHg), hemoglobin A1c (HbA1c) or glucose levels (≥ 7.0% or corresponding glucose levels and specifically for men), LDL-cholesterol (≥ 180 mg/dL) among men, and/or proteinuria of ≥ 2+ but was not treated for the corresponding risk factors (the treatment based on the information from the health checkups and the insurance claims). This program included people with self-reported histories of stroke and heart disease according to the existing national program, and the corresponding percentages were small and similar between the two groups: 1.8% and 3.2% in the intervention group and 1.7% and 2.9% in the usual care group, respectively.
Fig.1.
Flowchart of selection and randomization of municipalities to identify trial participants
The trial was conducted according to the personal information protection laws and ethical guidelines for epidemiological research and was approved by the Osaka University Ethics Committee on November 14, 2013 (No. 13 237-8). For ethical consideration, the program in the intervention group will be provided to the usual care group after the trial.
Measures
The high-risk individuals were enrolled for health counseling between May 2014 and March 2016 in 28 municipalities, between October 2014 and March 2016 in 6 municipalities, and between June 2015 and March 2016 in 9 municipalities. A total of 8,975 were in the intervention group and 6,733 in the usual care group. Follow-up for clinic visits was conducted until the end of March 2016. During follow-up, no refusal to participate and no harm-related events were reported. In addition, 300 participants (3.3%) in the intervention group and 210 (3.1%) in the usual care group were treated as censored cases as they had relocated from the original municipality or had passed away.
Health checkups included a questionnaire, interviews, physical examinations, measurements of height and weight, waist circumference at the umbilical level, blood pressure, HbA1c or blood glucose, LDL-cholesterol, high-density lipoprotein cholesterol, and triglycerides via standard methods 3) . Systolic and diastolic blood pressures were measured in a sitting position twice after a rest of at least 5 min. Fasting was defined as ≥ 8 h after the most recent meal. Interviewees reported their smoking status (nonsmoker, ex-smoker, and current smoker); alcohol consumption (never, sometimes, and daily); medication use for hypertension, diabetes, and dyslipidemia; and history of stroke, ischemic heart disease, and chronic kidney disease (and/or artificial dialysis).
High-risk individuals requiring medical treatment were defined as those with at least one of the following conditions who had not visited a physician for the specified condition 15) :
1) Grade II or higher hypertension: systolic blood pressure ≥ 160 mmHg and/or diastolic blood pressure ≥ 100 mmHg
2) HbA1c ≥ 7.0%: when HbA1c results were not available, fasting glucose ≥ 130 mg/dL (7.22 mmol/L); when fasting glucose was not available, nonfasting glucose ≥ 180 mg/dL (10.00 mmol/L)
3) For men, LDL-cholesterol ≥ 180 mg/dL (4.55 mmol/L)
4) Proteinuria ≥ 2+ in urinalysis.
These criteria were obtained from clinical practice guidelines published by Japanese academic societies 19 - 23) . These measurements were standardized according to the manual published by the Ministry of Health, Welfare and Labor. The government recommends all municipalities to stick to the standardized methods because of the national health program. The criteria for high LDL-cholesterol were not applied for women because their absolute risk of ischemic heart disease was much lower than that of men 24 , 25) .
For the intervention group, health counseling was provided primarily by a certified public health nurse (PHN) and secondarily by a certified clinical nurse or nutritionist based on the health belief model 13 , 14) . The health belief model is to make a counselee perceive susceptibility, severity, benefits, and barriers and to increase self-efficacy.
In health counseling, a PHN explained how high blood pressure, hyperglycemia, and hyperlipidemia damage the brain, heart, and kidney vasculature and, if untreated, could lead to the occurrence of serious health problems, including stroke, heart attack, and renal failure (perceived susceptibility), and emphasized that these diseases would harm the patient and their family’s life physically and economically (severity). The PHN then provided information regarding the benefits of visiting a physician to seek treatment (benefits) and asked regarding barriers that prevent clinic visits (barriers). Accordingly, the patient was expected to make decisions to receive treatment and medication, improve lifestyle habits, and continue participation in the following year health checkups (self-efficacy).
Before counseling, the PHN collected information on age, sex, place of residence, occupation, and family composition. The PHN prepared a health counseling plan and provided supplemental tools for health counseling, including an overview of the following: 1) a sheet for health checkup results during the past 5 years, 2) a progression flowchart of lifestyle-related diseases, the so called “Where I am” chart (i.e., risky behaviors such as high salt intake and smoking leading to the development of metabolic risk factors, preclinical vascular disorders, overt clinical diseases, end-stage conditions, bedridden status, heart failure, dialysis, dementia, blindness, and necrosis of the extremities), and 3) 34 newly developed health education flyers for explaining risk factors, mechanisms for their initiation, progression, and complications, which were chosen and used to provide additional explanation regarding particular risks (http://www.pbhel.med.osaka-u.ac.jp/common/images/pdf/themes/jharp/hokenshidou.pdf in Japanese) 15) .
During the first year following the initial health checkups, counseling was provided three times: at 1–3, 4–6, and 7–9 months after the health checkups. The number of counseling sessions provided in the second year varied according to the health checkup results. If the results regarding the high-risk status improved regardless of medication initiation, counseling was performed once or twice: at 1–3 and/or 7–9 months after the health checkups. Otherwise, the schedule used in the first year was followed. The completeness of counseling was defined as the duration of health counseling of ≥ 10 min. Initial health counseling was performed primarily by home visits and secondarily through a face-to-face session at a municipal office or public health center. If these approaches were not suitable for the participants, telephonic counseling was performed. We targeted the provision of complete home visits and face-to-face initial counseling at 70% and the provision of complete initial counseling, including telephonic counseling, at 80%. For the usual care group, the municipality generally sent a letter or called the high-risk individuals regarding the health checkup results and the importance of visiting a physician to seek medical treatment. However, health counseling regarding disease susceptibility and severity was recommended but was not required based on local counseling protocols.
Data Collection and Management
Each municipality held the insurance data and collected data regarding health checkups from the local health examination bodies and national insurance claims from the National Health Insurance Organization. The three datasets were linked via personal identifiers to create tables for municipal and research identifiers. Municipal office identifiers, names, and date of birth were deleted from the datasets, and data were restricted to participants aged 40–74 years with high-risk factors (hypertension, diabetes, high male LDL-cholesterol, and proteinuria) at baseline health checkups.
Outcomes
There were two primary outcomes. First, the cumulative proportion of participants’ clinic visits in the intervention group was compared with that of the usual care group. Second, the cumulative incidence of composite outcomes (i.e., hospitalization for stroke, myocardial infarction, unstable angina, heart failure, chronic kidney disease, renal failure and dialysis, sudden cardiac death, and deaths from cardiovascular disease, chronic kidney disease, and renal failure) was expected to be a 10-year risk of 15% 15) . We did not make any direction of referral to either higher- or lower-level medical facilities because the choice of medical facilities is left to each participant under the universal health insurance.
The secondary outcome was the proportions of people with continued participation in health checkups for the following year, the proportions of those receiving medical treatment, and the changes in the mean levels of blood pressure, HbA1c, and LDL-cholesterol, and the proportion of proteinuria as a result of the health checkups.
We present the results of the primary and secondary outcomes. Although we planned to assess another primary outcome at the completion of the 4-year follow-up, we found insufficient statistical power. Thus, the study is in the process of data collection, and the results will be published in the future.
Statistical Analysis
The sample size of this study was computed based on the second primary outcome variable of the cumulative incidence of composite outcomes. We assumed the average number of subjects within a cluster to be 400, the 4-year cumulative incidence proportion of the composite outcomes to be 6.6% (the incidence based on our previous cohort study 16) ), and the intraclass correlation to be 0.001. We assumed that the intervention reduces the cumulative incidence proportion by 20%. The study required at least 7400 subjects with 19 clusters in each group at a two-sided statistical significance level with 80% statistical power using CRTSize package in R software (https://www.r-project.org/).
The cumulative proportion of clinic visits by all high-risk individuals and those in each high-risk category (hypertension, diabetes, dyslipidemia, and proteinuria) was calculated using the Kaplan–Meier method. Differences between the intervention and usual care groups were evaluated using the log-rank test. As all participants in both groups were followed up for 12 months, the probability (hazard) ratio of the cumulative proportions and their 95% confidence intervals (CIs) in the intervention versus usual care groups were calculated using the Cox proportional hazard model, adjusting for age, sex, smoking status (nonsmoker and current smoker), and alcohol consumption (never, sometimes, and daily) at 3, 6, and 12 months after the health checkups. The missing values of alcohol consumption were imputed using age, sex, body mass index, smoking status, and each high-risk category using a fully conditional specification model under the missing-at-random assumption 26) .
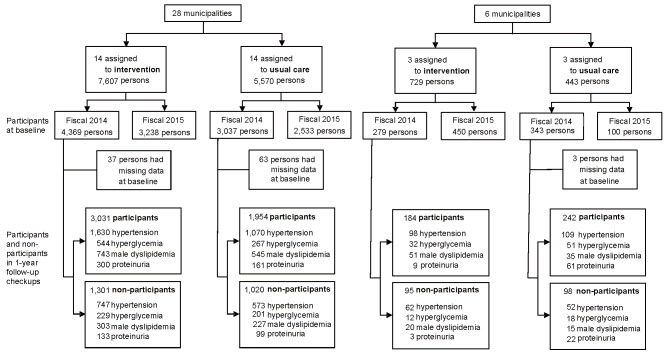
For the secondary outcome, we compared the changes in the mean levels of risk factors within each high-risk group and the proportion of proteinuria between the intervention and usual care groups during the 1-year follow-up of health checkups. We used a generalized estimating equation to estimate between-group mean differences and 95% CIs in the change from baseline for each continuous variable (blood pressure, HbA1c, and LDL-cholesterol) and odds ratio for a dichotomous variable (proteinuria) after adjustment for the baseline level of the outcome, as well as age, sex, smoking status, and alcohol consumption. The flowchart of the selection of the participants for the secondary analysis is shown in Supplementary Fig.1 . One-third of the subjects did not participate in 1-year follow-up health checkups. To conduct the secondary evaluation following the intention-to-treat principle, we handled these dropouts by constructing a pseudo-intension-to-treat population using the inverse probability propensity score weighting for participation of 1-year health checkups. To be specific, the propensity score was estimated by the logistic regression applied to the binary outcome of the participation and nonparticipation with covariates of age, sex, body mass index, smoking status, alcohol consumption, and each high-risk category.
Supplementary Fig.1.
Flow chart of selection of the participants for the secondary analysis
For the primary and secondary evaluations, intracluster (municipality) correlations were considered using robust sandwich estimates with independent working correlations 27) coupled with Rubin’s variance adjustment rule for imputation 28) with 100 multiply imputed samples for the missing alcohol consumption. All analyses used SAS 9.4 (SAS Institute Inc., Cary, NC, USA). P<0.05 (two-tailed) was considered statistically significant.
Results
In the intervention group, among 8975 participants, 5,025, 1,540, 2,066, and 911 were at a high-risk of hypertension, diabetes, dyslipidemia, and positive proteinuria, respectively. In the usual care group, among 6733 participants, 3,648, 1,149, 1,679, and 621 were at a high-risk of hypertension, diabetes, dyslipidemia, and positive proteinuria, respectively. Baseline characteristics did not differ substantially between the two groups ( Table 1 ; Supplementary Tables 1 , 2 , 3 , 4 ) . Of the participants in the intervention group, the initial health counseling was completed for 6,851 individuals (76.3%).
Table 1. Baseline characteristics in the intervention and usual care groups.
| Intervention (n = 8,975) | Usual care (n = 6,733) | |
|---|---|---|
| Age, years | 63.3 (8.5) | 63.8 (8.1) |
| Men, n, % | 5,961 (66%) | 4,549 (68%) |
| Grade II or higher hypertension†, n, % | 5,153 (57%) | 3,730 (55%) |
| Systolic blood pressure ≥ 160 mmHg, n, % | 4,166 (46%) | 3,095 (46%) |
| Systolic blood pressure, mmHg | 150・3 (22・7) | 149・4 (23・1) |
| Diastolic blood pressure ≥ 100 mmHg, n, % | 2,161 (24%) | 1,501 (22%) |
| Diastolic blood pressure, mmHg, | 87・7 (13・8) | 87・5 (13・2) |
| Diabetes mellitus‡, n, % | 1,716 (19%) | 1,269 (19%) |
| HbA1c, % | 6.17 (1.34) | 6.13 (1.33) |
| Fasting blood glucose, mmol/L | 6.31 (2.07) | 6.64 (2.21) |
| Dyslipidemia among men§, n, % | 2,086 (23%) | 1,687 (25%) |
| LDL-cholesterol among men, mmol/L | 3.86 (1.09) | 3.91 (1.12) |
| Proteinuria of ≥ 2+, n, % | 925 (10%) | 634 (9%) |
| Current smoker, n, % | 1,734 (19%) | 1,406 (21%) |
| Current drinker, n, % | 4,893 (55%) | 3,527 (52%) |
| Overweight (Body mass index ≥ 25 kg/m2), n, % | 3,000 (33%) | 2,261 (34%) |
| Body mass index, kg/m2 | 23.9 (3.5) | 23.9 (3.5) |
Data are n (%) or mean (SD).
The numbers of measurements for intervention and usual care were 8,691 and 6,489 for HbA1c, 283 and 160 for fasting blood glucose, 0 and 84 for non-fasting glucose, 5,925 and 4,511 for serum LDL-cholesterol.
†Systolic blood pressure ≥ 160 mmHg and/or diastolic blood pressure ≥ 100 mmHg.
‡HbA1c ≥ 7.0 %; if HbA1c was missing, fasting blood glucose ≥ 7.2 mmol/L (≥ 130 mg/dL); if fasting blood glucose was also missing, casual
glucose ≥ 10.0 mmol/L (≥ 180 mg/dL).
§Serum LDL-cholesterol ≥ 4.7 mmol/L (≥ 180 mg/dL).
The number of missing for drinking status was 16 in the intervention group and 298 in the usual care group.
Supplementary Table 1. Baseline characteristics of people with grade II or higher hypertension in the intervention and usual care groups.
| Intervention (n = 5,025) | Usual care (n = 3,648) | |
|---|---|---|
| Age, years | 64.0 (7.9) | 64.9 (7.2) |
| Men, n (%) | 2,736 (55%) | 1,972 (54%) |
| Systolic blood pressure ≥ 160 mmHg, n (%) | 4,062 (81%) | 3,023 (83%) |
| Systolic blood pressure, mmHg | 165.8 (12.8) | 166.2 (12.6) |
| Diastolic blood pressure ≥ 100 mmHg, n (%) | 2,118 (42%) | 1,463 (40%) |
| Diastolic blood pressure, mmHg, | 95.3 (11.2) | 95.2 (10.5) |
| Diabetes mellitus a, n (%) | 237 (5%) | 155 (4%) |
| HbA1c, % | 5.76 (0.77) | 5.72 (0.77) |
| Fasting blood glucose, mmol/L | 5.70 (1.39) | 6.06 (1.49) |
| Dyslipidemia among men b, n (%) | 169 (3%) | 108 (3%) |
| LDL-cholesterol among men, mmol/L | 3.33 (0.84) | 3.32 (0.84) |
| Proteinuria of ≥ 2+, n (%) | 99 (2%) | 59 (2%) |
| Current smoker, n (%) | 798 (16%) | 603 (17%) |
| Current drinker, n (%) | 2,721 (54%) | 1,889 (52%) |
| Overweight (Body mass index ≥ 25 kg/m2), n (%) | 1,538 (31%) | 1,086 (30%) |
| Body mass index, kg/m2 | 23.7 (3.6) | 23.6 (3.5) |
Data are presented as n (%) or mean (SD).
The number of measurements for intervention and usual care were 4,880 and 3,554, respectively, for HbA1c, 144 and 61, respectively for fasting blood glucose, 0 and 33, respectively, for non-fasting glucose, and 2,709 and 1,951, respectively, for serum LDL-cholesterol.
a HbA1c ≥ 7.0 %, if HbA1c was not available; fasting blood glucose ≥ 7.2 mmol/L (≥ 130 mg/dL), if fasting blood glucose was not available; a non-fasting blood glucose level of ≥ 100 mmol/L (≥ 180 mg/dL) was used.
b Serum LDL cholesterol ≥ 4.7 mmol/L (≥ 180 mg/dL).
The number of missing entries for drinking status was 8 in the intervention group and 209 in the usual care group.
Supplementary Table 2. Baseline characteristics of people with diabetes mellitus in the intervention and usual care groups.
| Intervention (n = 1,540) | Usual care (n = 1,149) | |
|---|---|---|
| Age, years | 63.4 (7.9) | 63.7 (7.3) |
| Men, n (%) | 1,031 (67%) | 770 (67%) |
| Grade II or higher hypertension a, n (%) | 199 (13%) | 123 (11%) |
| Systolic blood pressure ≥ 160 mmHg, n (%) | 176 (11%) | 102 (9%) |
| Systolic blood pressure, mmHg | 136.5 (19.6) | 134.0 (18.5) |
| Diastolic blood pressure ≥ 100 mmHg, n (%) | 82 (5%) | 52 (5%) |
| Diastolic blood pressure, mmHg, | 80.0 (12.0) | 79.6 (11.3) |
| HbA1c, % | 8.37 (1.71) | 8.37 (1.68) |
| Fasting blood glucose, mmol/L | 8.95 (2.70) | 9.21 (2.63) |
| Dyslipidemia among men b, n (%) | 102 (7%) | 65 (6%) |
| LDL-cholesterol among men, mmol/L | 3.49 (0.89) | 3.43 (0.90) |
| Proteinuria of ≥ 2+, n (%) | 52 (3%) | 32 (3%) |
| Current smoker, n (%) | 336 (22%) | 288 (25%) |
| Current drinker, n (%) | 821 (53%) | 572 (50%) |
| Overweight (Body mass index ≥ 25 kg/m2), n (%) | 668 (43%) | 501 (44%) |
| Body mass index, kg/m2 | 24.7 (3.7) | 24.7 (3.9) |
Data are presented as n (%) or mean (SD).
The numbers of measurements for intervention and usual care were 1,472 and 1,082, respectively, for HbA1c, 68 and 43, respectively, for fasting blood glucose, 0 and 24, respectively, for non-fasting glucose, and 1,025 and 762, respectively, for serum LDL-cholesterol.
a Systolic blood pressure ≥ 160 mmHg and/or diastolic blood pressure ≥ 100 mmHg.
b Serum LDL cholesterol ≥ 4.7 mmol/L (≥ 180 mg/dL).
The number of missing for drinking status was 5 in the intervention group and 37 for the usual care groups.
Supplementary Table 3. Baseline characteristics of men with dyslipidemia in the intervention and usual care groups.
| Intervention (n = 2,066) | Usual care (n = 1,679) | |
|---|---|---|
| Age, years | 60.5 (9.9) | 61.0 (9.7) |
| Grade II or higher hypertension a, n (%) | 177 (9%) | 117 (7%) |
| Systolic blood pressure ≥ 160 mmHg, n (%) | 134 (6%) | 90 (5%) |
| Systolic blood pressure, mmHg | 130.3 (18.1) | 129.9 (17.5) |
| Diastolic blood pressure ≥ 100 mmHg, n (%) | 101 (5%) | 61 (4%) |
| Diastolic blood pressure, mmHg, | 79.7 (11.4) | 79.7 (10.7) |
| Diabetes mellitus b, n (%) | 117 (6%) | 80 (5%) |
| HbA1c, % | 5.87 (1.00) | 5.80 (0.83) |
| Fasting blood glucose, mmol/L | 5.43 (0.66) | 5.82 (1.27) |
| LDL-cholesterol among men, mmol/L | 5.07 (0.44) | 5.09 (0.48) |
| Proteinuria of ≥ 2+, n (%) | 44 (2%) | 31 (2%) |
| Current smoker, n (%) | 564 (27%) | 455 (27%) |
| Current drinker, n (%) | 1,197 (58%) | 935 (56%) |
| Overweight (Body mass index ≥ 25 kg/m2), n (%) | 674 (33%) | 599 (36%) |
| Body mass index, kg/m2 | 24.0 (3.0) | 24.2 (2.9) |
Data are presented as n (%) or mean (SD).
The number of measurements for intervention and usual care was 1,998 and 1,610, respectively, for HbA1c, 68 and 50, respectively, for fasting blood glucose, 0 and 19, respectively, for non-fasting glucose, and 2,066 and 1,679, respectively, for serum LDL cholesterol.
a Systolic blood pressure ≥ 160 mmHg and/or diastolic blood pressure ≥ 100 mmHg.
b HbA1c ≥ 7.0 %; if HbA1c was not available, fasting blood glucose ≥ 7.2 mmol/L (≥ 130 mg/dL); if fasting blood glucose level was not available,
non-fasting blood glucose ≥ 10.0 mmol/L (≥ 180 mg/dL).
The number of missing entries for drinking status was 3 in the intervention group and 61 in the usual care group.
Supplementary Table 4. Baseline characteristics of people with proteinuria of ≥ 2+ in the intervention and usual care groups.
| Intervention (n = 911) | Usual care (n = 621) | |
|---|---|---|
| Age, years | 64.2 (8.3) | 64.5 (8.2) |
| Men, n (%) | 612 (67%) | 440 (71%) |
| Grade II or higher hypertension a, n (%) | 170 (19%) | 103 (17%) |
| Systolic blood pressure ≥ 160 mmHg, n (%) | 145 (16%) | 88 (14%) |
| Systolic blood pressure, mmHg | 138.9 (22.7) | 137.7 (21.4) |
| Diastolic blood pressure ≥ 100 mmHg, n (%) | 78 (9%) | 62 (10%) |
| Diastolic blood pressure, mmHg, | 80.6 (13.6) | 81.6 (13.4) |
| Diabetes mellitus b, n (%) | 136 (15%) | 91 (15%) |
| HbA1c, % | 6.22 (1.41) | 6.17 (1.40) |
| Fasting blood glucose, mmol/L | 6.27 (2.23) | 6.02 (1.71) |
| Dyslipidemia among men c, n (%) | 50 (5%) | 32 (5%) |
| LDL-cholesterol among men, mmol/L | 3.28 (0.93) | 3.20 (0.91) |
| Current smoker, n (%) | 169 (19%) | 155 (25%) |
| Current drinker, n (%) | 513 (56%) | 334 (54%) |
| Overweight (Body mass index ≥ 25 kg/m2), n (%) | 392 (43%) | 269 (43%) |
| Body mass index, kg/m2 | 24.6 (4.2) | 24.5 (4.4) |
Data are presented as n (%) or mean (SD).
The numbers of measurements for intervention and usual care were 889 and 590, respectively, for HbA1c, respectively, 22 and 19, respectively, for fasting blood glucose, 0 and 12, respectively, for non-fasting glucose, 605 and 428, respectively, for serum LDL-cholesterol.
a Systolic blood pressure ≥ 160 mmHg and/or diastolic blood pressure ≥ 100 mmHg.
b HbA1c ≥ 7.0 %; if HbA1c was not available, fasting blood glucose ≥ 7.2 mmol/L (≥ 130 mg/dL); if fasting blood glucose level was not available,
non-fasting blood glucose ≥ 10.0 mmol/L (≥ 180 mg/dL).
c Serum LDL cholesterol ≥ 4.7 mmol/L (≥180 mg/dL).
The number of missing entries for drinking status was 10 in the usual care group.
Supplementary Table 5 shows the distribution of the initial counseling participants according to the time from health checkups, mode of counseling, number of counseling sessions, and number of people reached. The initial counseling was completed within 90 days of the health checkups for three-quarters of the participants, was conducted either as a home visit or face-to-face interview in a public place for over 90% of the participants and two or three times for over 50% of the participants, and had reached more than 90% of the participants.
Supplementary Table 5. Distributions of completed initial health counseling according to time from health checkups, mode of counseling, number of counseling sessions, and number of people reached in the intervention group.
| Time from health checkups | |
|---|---|
| ≤ 45 days | 2,881 (42.1%) |
| 46–90 days | 2,088 (30.5%) |
| ≥ 91 days | 1,882 (27.5%) |
| Mode of health counseling | |
| Home visit | 4,269 (62.3%) |
| Face-to-face interview in a public place | 2,042 (29.8%) |
| Telephone | 379 (5.5%) |
| Missing record | 161 (2.4%) |
| Number of counseling sessions in a year | |
| Initial counseling only (once) | 3,096 (45.2%) |
| Two times | 2,216 (32.4%) |
| Three times | 1,539 (22.5%) |
| People reached | |
| Participants | 6,409 (93.6%) |
| Family members | 402 (5.9%) |
| Missing records | 40 (0.6%) |
Data are presented as n (%).
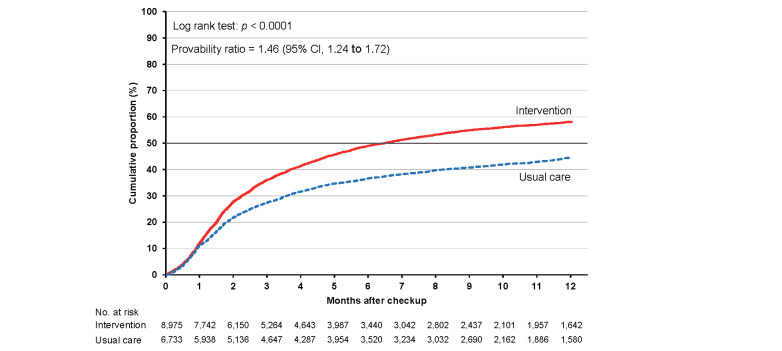
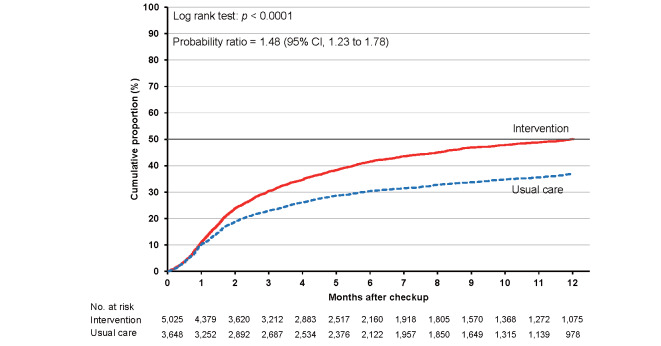
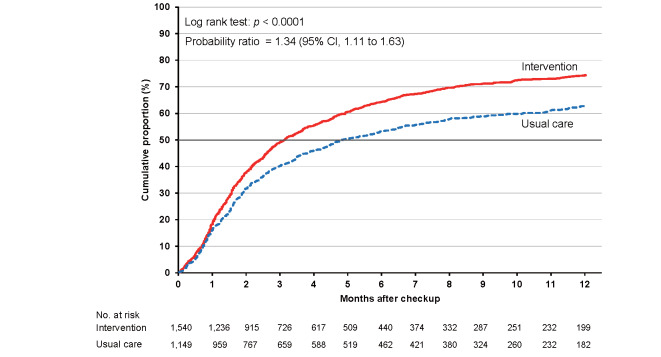
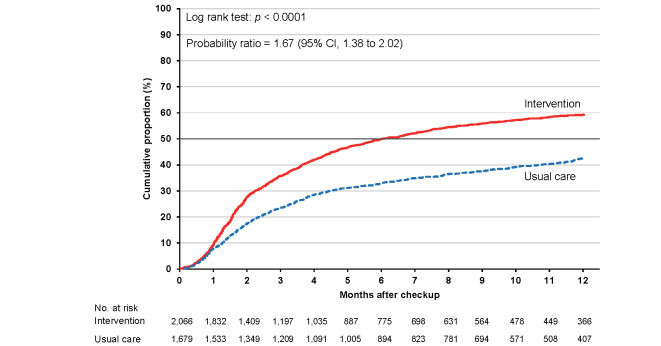
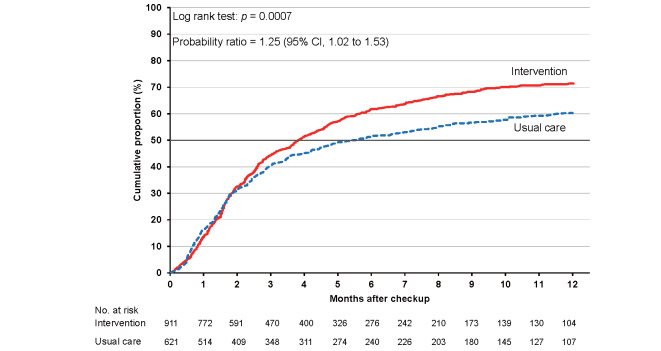
As shown in Supplementary Table 6 , the cumulative proportions of clinic visits in the intervention and usual care groups were 36.0% (95% CI, 35.0% to 37.1%) and 27.4% (26.4% to 28.5%), respectively, at 3 months. The corresponding proportions were 49.0% (47.9% to 50.1%) and 36.6% (35.5% to 37.8%) at 6 months and 58.1% (57.0% to 59.3%) and 44.5% (43.2% to 45.8%) at 12 months. The cumulative proportion was consistently higher in the intervention group than in the usual care group for the total category ( Fig.2 ) and each high-risk category ( Supplementary Figs.2 , 3 , 4 , 5 ) , with larger differences between the intervention and usual care groups for hypertension and dyslipidemia.
Supplementary Table 6. Cumulative proportions (95% confidence intervals, CIs) of clinical visits at 3, 6, and 12 months.
| Intervention | Usual care | p value* | |
|---|---|---|---|
| Total (n) | 8,975 | 6,733 | |
| 3 months, % | 36.0 (35.0 to 37.1) | 27.4 (26.4 to 28.5) | <0.001 |
| 6 months, % | 49.0 (47.9 to 50.1) | 36.6 (35.5 to 37.8) | <0.001 |
| 12 months, % | 58.1 (57.0 to 59.3) | 44.5 (43.2 to 45.8) | <0.001 |
| Hypertension, n | 5,025 | 3,648 | |
| 3 months, % | 30.4 (29.1 to 31.7) | 22.9 (21.6 to 24.3) | <0.001 |
| 6 months, % | 41.6 (40.1 to 43.0) | 30.4 (28.9 to 31.9) | <0.001 |
| 12 months, % | 50.1 (48.5 to 51.6) | 36.8 (35.1 to 38.5) | <0.001 |
| Diabetes mellitus, n | 1,540 | 1,149 | |
| 3 months, % | 49.1 (46.6 to 51.7) | 40.2 (37.4 to 43.1) | <0.001 |
| 6 months, % | 64.3 (61.8 to 66.8) | 53.2 (50.2 to 56.1) | <0.001 |
| 12 months, % | 74.3 (71.9 to 76.8) | 62.7 (59.6 to 65.8) | <0.001 |
| Dyslipidemia (men), n | 2,066 | 1,679 | |
| 3 months, % | 35.7 (33.6 to 37.9) | 23.5 (21.5 to 25.6) | <0.001 |
| 6 months, % | 49.9 (47.6 to 52.2) | 32.9 (30.7 to 35.3) | <0.001 |
| 12 months, % | 59.3 (56.9 to 61.7) | 42.6 (40.0 to 45.3) | <0.001 |
| Proteinuria (≥ 2), n | 911 | 621 | |
| 3 months, % | 44.3 (41.1 to 47.7) | 40.5 (36.7 to 44.5) | 0.18 |
| 6 months, % | 61.7 (58.4 to 65.0) | 51.5 (47.5 to 55.6) | 0.001 |
| 12 months, % | 71.4 (68.0 to 74.7) | 60.2 (56.0 to 64.5) | <0.001 |
Data are % (95% confidence interval).
*Adjusted for age, sex, smoking status, and alcohol consumption with multiple imputation.
Fig.2.
Cumulative proportions of clinic visits for participants in the intervention and usual care groups
Supplementary Fig.2.
Cumulative proportions of clinic visits for participants with grade II or higher hypertension in the intervention and usual care groups
Supplementary Fig.3.
Cumulative proportions of clinic visits for participants with diabetes mellitus in the intervention and usual care groups
Supplementary Fig.4.
Cumulative proportions of clinic visits for men with dyslipidemia in the intervention and usual care groups
Supplementary Fig.5.
Cumulative proportions of clinic visits for participants with proteinuria of ≥ 2+ in the intervention and usual care groups
The probability ratios (95% CI) of clinic visits during 24 months in the intervention versus usual care groups after adjusting for age, sex, smoking status, and alcohol consumption were 1.46 (1.24–1.72) for the total study population, 1.48 (1.23–1.78) for hypertension, 1.34 (1.11–1.63) for diabetes, 1.67 (1.38–2.02) for dyslipidemia, and 1.25 (1.02–1.53) for proteinuria ( Table 2 ) .
Table 2. Probability ratios (95% confidence intervals, CIs) for clinic visits in the intervention and usual care groups.
| Intervention | Usual care | Probability ratio (95%CI) | Multivariable Probability ratio (95%CI)* | |||||
|---|---|---|---|---|---|---|---|---|
| n | Person- years | No. of clinic visits | n | Person- years | No. of clinic visits | |||
| Total | 8,975 | 4,082 | 4,626 | 6,733 | 3,731 | 2,727 | 1.45 (1.24 to 1.70) | 1.46 (1.24 to 1.72) |
| Hypertension | 5,025 | 2,487 | 2,208 | 3,648 | 2,178 | 1,228 | 1.47 (1.22 to 1.76) | 1.48 (1.23 to 1.78) |
| Diabetes mellitus | 1,540 | 566 | 1,044 | 1,149 | 522 | 670 | 1.35 (1.12 to 1.62) | 1.34 (1.11 to 1.63) |
| Dyslipidemia (men) | 2,066 | 933 | 1,075 | 1,679 | 962 | 631 | 1.65 (1.38 to 1.97) | 1.67 (1.38 to 2.02) |
| Proteinuria (≥ 2+) | 911 | 354 | 585 | 621 | 280 | 343 | 1.26 (1.02 to 1.55) | 1.25 (1.02 to 1.53) |
*Adjusted for age, sex, smoking status, and alcohol consumption with multiple imputation.
For the participants at baseline, the number of participants in the 1-year follow-up health checkups was 3,215 (69.7%) in the intervention group and 2,196 (66.3%) in the usual care group ( Supplementary Table 7 ) . Among them, the number of people who had visited a physician was 1,860 (57.9%) in the intervention group and 959 (43.7%) in the usual care group. The baseline characteristics of participants and nonparticipants did not differ substantially in both the intervention and usual care groups.
Supplementary Table 7. Baseline characteristics of participants and non-participants in 1-year follow-up health checkups in the intervention and usual care groups.
| Participants in 1-year follow-up checkups | Non-participants in 1-year follow-up checkups | |
|---|---|---|
| Intervention | ||
| No. of participants | 3,215 | 1,396 |
| Age, years | 63.6 (7.9) | 62.6 (9.7) |
| Men, n (%) | 2,230 (69%) | 896 (64%) |
| Grade Ⅱ or higher hypertension a, n (%) | 1,779 (55%) | 828 (59%) |
| Systolic blood pressure ≥ 160 mmHg, n (%) | 1,446 (45%) | 684 (49%) |
| Systolic blood pressure, mmHg | 149.6 (22.8) | 151.7 (23.3) |
| Diastolic blood pressure ≥ 100 mmHg, n (%) | 712 (22%) | 361 (26%) |
| Diastolic blood pressure, mmHg | 86.9 (13.7) | 88.5 (14.2) |
| Diabetes mellitus b, n (%) | 625 (19%) | 263 (19%) |
| HbA1c, % | 6.14 (1.31) | 6.21 (1.48) |
| Fasting blood glucose, mmol/L | 5.40 (0.56) | 5.63 (0.82) |
| Dyslipidemia among men c, n (%) | 799 (25%) | 327 (23%) |
| LDL-cholesterol among men, mmol/L | 3.89 (1.10) | 3.91 (1.14) |
| Proteinuria of ≥ 2+, n (%) | 312 (10%) | 140 (10%) |
| Current smoker, n (%) | 575 (18%) | 316 (23%) |
| Current drinker, n (%) | 1,789 (56%) | 743 (53%) |
| Overweight (Body mass index ≥25 kg/m2), n (%) | 1,013 (32%) | 506 (36%) |
| Body mass index, kg/m2 | 23.8 (3.50) | 24.2 (3.68) |
| Usual care | ||
| No. of participants | 2,196 | 1,118 |
| Age, years | 64.1 (7.6) | 63.7 (9.0) |
| Men, n (%) | 1,511 (69%) | 734 (66%) |
| Grade Ⅱ or higher hypertension a, n (%) | 1,201 (55%) | 642 (57%) |
| Systolic blood pressure ≥ 160 mmHg, n (%) | 984 (45%) | 531 (48%) |
| Systolic blood pressure, mmHg | 148.7 (22.9) | 150.6 (24.0) |
| Diastolic blood pressure ≥ 100 mmHg, n (%) | 487 (22%) | 290 (26%) |
| Diastolic blood pressure, mmHg | 87.8 (13.2) | 88.0 (13.9) |
| Diabetes mellitus b, n (%) | 355 (16%) | 240 (21%) |
| HbA1c, % | 6.09 (1.28) | 6.27 (1.52) |
| Fasting blood glucose, mmol/L | 5.72 (1.04) | 5.67 (0.71) |
| Dyslipidemia among men c, n (%) | 582 (27%) | 244 (22%) |
| LDL-cholesterol among men, mmol/L | 3.92 (1.11) | 3.87 (1.14) |
| Proteinuria of ≥ 2+, n (%) | 224 (10%) | 125 (11%) |
| Current smoker, n (%) | 405 (18%) | 254 (23%) |
| Current drinker, n (%) | 1,112 (51%) | 564 (50%) |
| Overweight (Body mass index ≥ 25 kg/m2), (%) | 699 (32%) | 386 (35%) |
| Body mass index, kg/m2 | 23.8 (3.38) | 24.0 (3.69) |
Data are presented as n (%) or mean (SD).
a Systolic blood pressure ≥ 160 mmHg and/or diastolic blood pressure ≥ 100 mmHg.
b HbA1c ≥ 7.0 %; if HbA1c was not available, fasting blood glucose ≥ 7.2 mmol/L (≥ 130 mg/dL); if fasting blood glucose was not available, a non-fasting blood glucose level of ≥ 100 mmol/L (≥ 180 mg/dL) was used.
c Serum LDL cholesterol ≥ 4.7 mmol/L (≥ 180 mg/dL).
The number of missing entries for drinking status was 4 for participants in the 1-year follow-up health checkups and 5 for non-participants in the intervention group. The respective numbers in the usual care group were 202 and 84.
Table 3 summarizes the between-group differences in risk factors between the intervention and usual care groups. In the intention-to-treat analysis, a larger reduction in the intervention group was observed for mean diastolic blood pressure (−1.50 [−2.58 to −0.41] mmHg) in the hypertension category, mean HbA1c (−0.30% [−0.53% to −0.08%]) in the diabetes category, and mean LDL-cholesterol (−0.37 [−0.48 to −0.27] mmol/L) in the dyslipidemia category, but it was not observed in the prevalence of proteinuria.
Table 3. Between-group differences in mean values of blood pressures, HbA1c and LDL-cholesterol and proportion of proteinuria between the intervention and usual care group.
| Intervention | Usual care | |||||
| Baseline Mean±SD, % | 1-year Mean±SD, % | p for difference a | Baseline Mean±SD, % | 1-year Mean±SD, % | p for difference a | |
| Hypertension | ||||||
| Number of participants | 1,728 | 1,179 | ||||
| Systolic blood pressure, mmHg | 165.9±12.5 | 150.6±18.5 | <0.0001 | 165.6±12.3 | 152.1±18.9 | <0.0001 |
| Diastolic blood pressure, mmHg | 94.7±11.3 | 87.1±11.9 | <0.0001 | 95.6±10.3 | 88.8±12.1 | <0.0001 |
| Diabetes mellitus | ||||||
| Number of participants | 576 | 318 | ||||
| HbA1c, % | 8.23±1.58 | 7.40±1.35 | <0.0001 | 8.37±1.71 | 7.74±1.65 | <0.0001 |
| Dyslipidemia (men) | ||||||
| Number of participants | 794 | 580 | ||||
| LDL-cholesterol among men, mmol/L | 5.08±0.48 | 4.19±0.98 | <0.0001 | 5.06±0.44 | 4.55±0.85 | <0.0001 |
| Proteinuria | ||||||
| Number of participants | 309 | 222 | ||||
| Proteinuria of ≥ 2+, % | 100.0 | 42.4 | - | 100.0 | 44.1 | - |
| Between-group difference over time | ||||||
| Subpopulation b | Pseudo-intension-to-treat population c | |||||
|
Mean (95% CI) / OR d (95% CI) |
p for difference |
Mean (95% CI) / OR d (95% CI) |
p for difference | |||
| Hypertension | ||||||
| Number of participants | ||||||
| Systolic blood pressure, mmHg | -1.64 (-3.79 to 0.52) | 0.14 | -1.67 (-3.82 to 0.49) | 0.13 | ||
| Diastolic blood pressure, mmHg | -1.45 (-2.56 to -0.34) | 0.01 | -1.50 (-2.58 to -0.41) | 0.007 | ||
| Diabetes mellitus | ||||||
| Number of participants | ||||||
| HbA1c, % | -0.29 (-0.52 to -0.06) | 0.01 | -0.30 (-0.53 to -0.08) | 0.009 | ||
| Dyslipidemia (men) | ||||||
| Number of participants | ||||||
| LDL-cholesterol among men, mmol/L | -0.37 (-0.47 to -0.27) | <0.0001 | -0.37 (-0.48 to -0.27) | <0.0001 | ||
| Proteinuria | ||||||
| Number of participants | ||||||
| Proteinuria of ≥ 2+, % | 0.88 (0.58 to 1.33) | 0.55 | 0.88 (0.59 to 1.32) | 0.54 | ||
SD, standard deviation; OR, odds ratio; CI, confidence interval.
a Generalized estimating equations without covariates
b People with continued participation in the 1-year follow-up health checkups. We used a generalized estimating equation to estimate between-group mean differences and 95%CIs in the change from baseline for each continuous variable (blood pressure, HbA1c, and LDL-cholesterol) and odds ratio for a dichotomous variable (proteinuria) after adjustment for the baseline level of the outcome, as well as age, sex, smoking status, and alcohol consumption.
c Following the intention-to-treat principle, we handled the dropouts for 1-year follow-up health checkups using the inverse probability propensity score weighting for participation of 1-year health checkups. The propensity score was estimated by the logistic regression applied to the binary outcome of the participation and non-participation with covariates of age, sex, body mass index, smoking status, alcohol consumption, and each high-risk category.
Discussion
This is the first cluster randomized trial investigating the impact of health counseling using a model of accelerated referral of high-risk individuals to local physicians for the prevention of lifestyle-related diseases, primarily cardiovascular disease. The results demonstrated that the program facilitated more clinic visits. The program led to larger reductions in risk factors such as diastolic blood pressure, HbA1c, and LDL-cholesterol between the baseline and 1-year follow-up checkups. The clinic visits at 12 months increased to 58% as a total, with 50% to 71% for each risk factor group. The probable barrier for reaching 100% was no or few signs and symptoms for these risk factors because the presence of robust symptoms is a strong driver of clinic visits.
Studies have shown that lifestyle interventions generally yield small changes in cardiovascular risk factors 29 - 31) and few changes in the incidence and mortality resulting from a cardiovascular disease or total mortality in general populations 31 - 33) . The combination of lifestyle and medical interventions yielded larger effects on cardiovascular disease or total mortality. The Multiple Risk Factor Intervention Trial showed a long-term legacy effect on the primary prevention of coronary heart disease. After 10.5 years (an average of 3.8 years after intervention termination), mortality from acute myocardial infarction and all-cause mortality was lower by 10.6% and 7.7%, respectively, in the intervention group than in the usual care group 34) . A benefit of interventions was observed in a community with a high prevalence of hypertension 4) .
The current trial demonstrated that health counseling within a theoretical framework was feasible and effective in accelerating visits to physicians for treatment, which is an important health behavior for high-risk individuals. The accelerated clinic visits led to greater reductions in mean values of diastolic blood pressure (by −1.5 mmHg), HbA1c (by −0.30% point), and blood LDL-cholesterol (by −0.37 mmol/L) in our trial, primarily due to the initiation of drug treatment, which is expected to lead to a larger decline in the cardiovascular disease in the future. According to meta-analyses of clinical trials 35 - 38) , the above mean reductions in diastolic blood pressure, HbA1c, and LDL-cholesterol corresponded to risk reduction for cardiovascular disease by 9%, 8%–10%, and 5%, respectively.
This study had several strengths, including its large population-based sample of over 15,000 individuals from 43 Japanese municipalities. The intervention involved a combination of established health belief models and could be applied to other populations. Blinding for cluster randomization was performed thoroughly to avoid bias. Monitoring participants’ clinic visits using national digital records ensured the accuracy and completeness of the data.
Limitations
First, there could be a “Hawthrone effect” from the close follow-up and attention so that the improvement of clinic visits and risk factor control were not attributable solely to our health counseling. Because the close follow-up and attention were an important part of our intervention, it is difficult to separate the Hawthrone effect from the overall effect of our intervention. Second, we demonstrated the effect of accelerated clinic visits, although the initial health counseling was not completed for 25% of the intervention group’s members. The proportion of clinic visits could have been underestimated, particularly for people with hypertension, wherein the insurance claim of hypertension was less likely to occur unless physicians started drug treatment or further examinations for hypertensive end-organ defects. Third, the quality and intensity of health counseling could have varied among intervention communities, although we ensured the standardization of health counseling through training, supervision, seminars, and site visits. Fourth, the effects of health counseling could have varied according to the health counselors’ backgrounds, the number of participants per counselor, mode of initial counseling, or unknown participant characteristics. However, the characteristics of high-risk individuals were likely similarly distributed between the intervention and usual care groups because we performed cluster randomization controlling for the residence location, number of high-risk individuals and insured persons, participation rates in health checkups, and number of high educational attainment and physicians per 100,000 population. Lastly, the participants were under one of the major health insurances, covering a quarter of the total population in Japan. However, generalizability to people covered under other insurances is not guaranteed.
In conclusion, this trial presents a feasible and effective method of health counseling to accelerate the referral to physicians to seek treatment. Our findings suggest that the nationwide use of such counseling after health checkups could control risk factors and could possibly prevent lifestyle-related diseases. This trial also provided evidence to boost the effectiveness of the Japanese primary care system. Policymakers who wish to enhance primary care can use our findings to develop strategies to facilitate the prevention and control of life-threatening lifestyle-related diseases, which account for the large global health burden of mortality.
Acknowledgement
The authors would like to thank all J-HARP staff members, health professionals, and workers involved in the J-HARP study for their valuable help in conducting data collection and follow-up, Professor Satoshi Hattori for his statistical advice, Professor Ichiro Kawachi for his conceptual advice, Mari Tanaka for her assistance in preparing the manuscript, and Editage (www.editage.com) for English language editing.
Conflict of Interest
None for all authors.
Financial Disclosure
None for all authors.
Funding
Health, Labour, and Welfare Sciences Research Grant (20132016) from the Ministry of Health, Labour, and Welfare as a Japan Strategic Clinical Trial.
Data Sharing Statement
The data collected for the study will not be available to the others, except for academic scientists with investigator support after the approval of the study committee whose members are the present authors. The related documents (study protocol and statistical analysis plan) will be available with publication on the website of http://www.pbhel.med.osaka-u.ac.jp/themes/j-harp.html.
Transparency Statement
The lead author (the manuscript’s guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported and that no important aspects of the study have been omitted.
Substantial Contributors
The members of the J-HARP study group are Hiroyasu Iso (chairperson of the study), Osaka University Graduate School of Medicine; Iichiro Shimomura (chairperson of the research support team), Osaka University Graduate School of Medicine; Midori Noguchi (team leader for intervention support), Osaka University Graduate School of Medicine; Tetsuji Yokoyama (team leader for training), National Institute of Public Health; Toshiko Yoshida (team leader for monitoring), School of Nursing, Miyagi University; Isao Saito (team leader for determining endpoints), Ehime University Graduate School of Medicine; Gen Kobashi (team leader for data collection and management), Dokkyo Medical University School of Medicine; Ayumi Shintani (team leader for data analysis), Osaka City University Graduate School of Medicine.
Members of each team are as follows:
Research Coordination: Hitoshi Nishizawa, Akihiko Kitamura, Hironori Imano, Minako Kinuta, Mitsuyoshi Takahara, Takekazu Kimura, and Mari Tanaka, Osaka University Graduate School of Medicine
Intervention Support: Sumi Kojima, Osaka University Graduate School of Medicine; Kazue Matsuo, Fukuoka Jogakuin Nursing University; Shizuko Omote, Kanazawa University, Faculty of Health Sciences, Institute of Medical, Pharmaceutical and Health Sciences; Miyae Yamakawa, Osaka University Graduate School of Medicine; Shoko Katsura, Miyagi University School of Nursing; Keiko Koide, Shitennoji University Faculty of Education; Michie Nomura, Ehime Prefectural University of Health Sciences; and Kyoko Izumi, Kyoko Izumi School of Nursing, Mukogawa Women’s University
Training: Yukari Sugita, Chiba University Graduate School of Nursing; Yumiko Morinaga, National Institute of Public Health; Kiyoko Makimoto, Konan Woman’s University; and Siko Makimoto, Osaka University Graduate School of Medicine
Monitoring: Yukiko Anzai, Miyagi University School of Nursing; Miyuki Makaya, School of Nursing, Kitasato University; Akiko Kadoguchi, Sakakibara Heart Institute; Sayaka Kotera, Kobe University Graduate School of Health Sciences; Chikako Miura, Japan Association for Development of Community Medicine; Shino Bando, Miyagi University School of Nursing; Tomomi Yamada, Department of Medical Innovation, Osaka University Hospital; Daisuke Furushima, University of Shizuoka; and Kanae Takahashi, Department of Medical Statistics, Osaka City University Graduate School of Medicine
Endpoint Determination: Kazumasa Yamagishi, Faculty of Medicine, University of Tsukuba; Yoshihiro Kokubo, National Cerebral and Cardiovascular Center; Hiroshi Yatsuya, Fujita Health University; and Masako Kakizaki, Fujita Health University
Data Collection and Management: Toshimi Sairenchi, Dokkyo Medical University School of Medicine
Data Analysis: Tomomi Yamada, Daisuke Furushima, and Kanae Takahashi, Data Coordinating Center, Department of Medical Innovation, Osaka University Hospital and Ai Noda, Department of Public Health, Juntendo University Graduate School of Medicine
Participating municipalities (alphabetical order): Annaka, Aomori, Azumino, Fuefuki, Fujieda, Fukuyama, Hatsukaichi, Higashiomi, Hikone, Ibaraki, Iida, Imabari, Imari, Ishioka, Itoshima, Iwaki, Izumi, Kagoshima, Karatsu, Kashima, Kinokawa, Kitakami, Kitsuki, Kure, Matsumoto, Makinohara, Moriya, Nakagawa, Nakatsu, Ninohe, Ohtawara, Onomichi, Sado, Sakai, Shizuoka, Takarazuka, Takatsuki, Tamba, Tanabe, Takasaki, Toride, Tottori, and Yokote
References
- 1).Macinko J, Starfield B, Shi L: The contribution of primary care systems to health outcomes within Organization for Economic Cooperation and Development (OECD) countries, 1970-1998. Health Serv Res, 2003; 38: 831-865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Public Health England: NHS Health Check practice guideline for commissioners and providers. October 2019. https: //www.healthcheck.nhs.uk/commissioners-and-providers/national-guidance/ Date accessed: December 1, 2022 [Google Scholar]
- 3).Kohro T, Furui Y, Mitsutake N, Fujii R, Morita H, Oku S, Ohe K, Nagai R: The Japanese national health screening and intervention program aimed at preventing worsening of the metabolic syndrome. Int Heart J, 2008; 49: 193-203 [DOI] [PubMed] [Google Scholar]
- 4).Iso H, Shimamoto T, Naito Y, Sato S, Kitamura A, Iida M, Konishi M, Jacobs DR Jr., Komachi Y: Effects of a long-term hypertension control program on stroke incidence and prevalence in a rural community in northeastern Japan. Stroke, 1998; 29: 1510-1518 [DOI] [PubMed] [Google Scholar]
- 5).Yamagishi K, Sato S, Kitamura A, Kiyama M, Okada T, Tanigawa T, Ohira T, Imano H, Kondo M, Okubo I, Ishikawa Y, Shimamoto T, Iso H: Cost-effectiveness and budget impact analyses of a long-term hypertension detection and control program for stroke prevention. J Hypertens, 2012; 30: 1874-1879 [DOI] [PubMed] [Google Scholar]
- 6).Ikeda N, Saito E, Kondo N, Inoue M, Ikeda S, Satoh T, Wada K, Stickley A, Katanoda K, Mizoue T, Noda M, Iso H, Fujino Y, Sobue T, Tsugane T, Naghavi M, Ezzati M, Shibuya K: What has made the population of Japan healthy? Lancet, 2011; 378: 1094-1105 [DOI] [PubMed] [Google Scholar]
- 7).Wagner KH, Brath H: A global view on the development of non communicable diseases. Prev Med, 2012; 54 Suppl: S38-41 [DOI] [PubMed] [Google Scholar]
- 8).World Health Organization: The top 10 causes of death. https: //www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death. Date accessed: December 1, 2022 [Google Scholar]
- 9).Kitamura A: Changes in the prevalence of hypertension and related factors in a cohort study of the Osaka Prefectural Health Science Center. Proceedings of the 33rd Annual Meeting of the Japanese Society of Hypertension 2010: 185 (in Japanese) [Google Scholar]
- 10).Kojo T, Innami I: Analysis of the patients’ prior consultation behavior with vascular dementia, ischemic heart diseases and ischemic stroke. Jap J Cardiovasc Dis Prev, 2010; 45: 22-31 (English abstract) [Google Scholar]
- 11).Health Japan 21 (the second term) Official website. 2018/ http: //www.nibiohn.go.jp/eiken/kenkounippon21/en/. Date accessed: December 1, 2022 [Google Scholar]
- 12).Janz NK, Becker MH: The Health Belief Model: a decade later. Health Educ Q, 1984; 11: 1-47 [DOI] [PubMed] [Google Scholar]
- 13).Rosenstock IM, Strecher VJ, Becker MH: Social learning theory and the Health Belief Model. Health Educ Q, 1988; 15: 175-183 [DOI] [PubMed] [Google Scholar]
- 14).Ryo M, Nakamura T, Funahashi T, Noguchi M, Kishida K, Okauchi Y, Nishizawa H, Ogawa T, Kojima S, Ohira T, et al.: Health education “Hokenshido” program reduced metabolic syndrome in the Amagasaki visceral fat study. Three-year follow-up study of 3,174 Japanese employees. Intern Med, 2011; 50: 1643-1648 [DOI] [PubMed] [Google Scholar]
- 15).Noguchi M, Kojima S, Sairenchi T, Kinuta M, Yamakawa M, Nishizawa H, Takahara M, Imano H, Kitamura A, Yoshida T, Shintani A, Saito I, Yokoyama T, Shimomura I, Iso H: Japan Trial in High-Risk Individuals to Enhance Their Referral to Physicians (J-HARP)-A nurse-led, community-based prevention program of lifestyle-related disease. J Epidemiol, 2020; 30: 194-199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Iso H, Cui R, Takamoto I, Kiyama M, Saito I, Okamura T, Miyamoto Y, Higashiyama A, Kiyohara Y, Ninomiya T, Yamada M, Nakagawa H, Sakurai M, Shimabukuro M, Higa M, Shimamoto K, Saito S, Daimon M, Kayama T, Noda M, Sadayoshi Ito S, Yokote K, Ito C, Nakao K, Yamauchi T, Kadowaki T: Risk Classification for Metabolic Syndrome and the Incidence of Cardiovascular Disease in Japan With Low Prevalence of Obesity: A Pooled Analysis of 10 Prospective Cohort Studies. J Am Heart Assoc, 2021; 10: e020760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Greevy RA, Jr., Grijalva CG, Roumie CL, Beck C, Hung AM, Murff HJ, Liu X, Griffin MR: Reweighted Mahalanobis distance matching for cluster-randomized trials with missing data. Pharmacoepidemiol Drug Saf, 2012; 21 Suppl 2: 148-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Imai K, King G, Nall C: The Essential Role of Pair Matching in Cluster-Randomized Experiments, with Application to the Mexican Universal Health Insurance Evaluation. Statist Sci, 2009; 24: 29-53 [Google Scholar]
- 19).The Ministry of Health, Labour and Welfare: Ethical Guidelines for Medical and Health Research. 2015. Involving Human Subjects. https: //www.mhlw.go.jp/site_kensaku_english.html?q=ethical%20guidline. Date accessed: December 1, 2022 [Google Scholar]
- 20).Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ishimitsu T, Ito M, Ito S, Itoh H, Iwao H, Kai H, Kario K, Kashihara N, Kawano Y, Kim-Mitsuyama S, Kimura G, Kohara K, Komuro I, Kumagai H, Matsuura H, Miura K, Morishita R, Naruse M, Node K, Ohya Y, Rakugi H, Saito I, Saitoh S, Shimada K, Shimosawa T, Suzuki H, Tamura K, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S, Umemura S: The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res, 2014; 37: 253-390 [Google Scholar]
- 21).Tajima N, Noda M, Origasa H, Noto H, Yabe D, Fujita Y, Goto A, Fujimoto K, Sakamoto M, Haneda M: Evidence-based practice guideline for the treatment for diabetes in Japan 2013. Diabetology International, 2015; 6: 151-187 [Google Scholar]
- 22).Teramoto T, Sasaki J, Ishibashi S, Birou S, Daida H, Dohi S, Egusa G, Hiro T, Hirobe K, Iida M, Kihara S, Kinoshita M, Maruyama C, Ohta T, Okamura T, Yamashita S, Yokode M, Yokote K: Executive summary of the Japan Atherosclerosis Society (JAS) guidelines for the diagnosis and prevention of atherosclerotic cardiovascular diseases in Japan -2012 version. J Atheroscler Thromb, 2013; 20: 517-523 [DOI] [PubMed] [Google Scholar]
- 23).Fukagawa M, Yokoyama K, Koiwa F, Taniguchi M, Shoji T, Kazama JJ, Komaba H, Ando R, Kakuta T, Fujii H, Nakayama M, Shibagaki Y, Fukumoto S, Fujii N, Hattori M, Ashida A, Iseki K, Shigematsu T, Tsukamoto Y, Tsubakihara Y, Tomo T, Hirakata H, Akizawa T: Clinical practice guideline for the management of chronic kidney disease-mineral and bone disorder. Ther Apher Dial, 2013; 17: 247-288 [DOI] [PubMed] [Google Scholar]
- 24).Iso H: Changes in coronary heart disease risk among Japanese. Circulation, 2008; 118: 2725-2729 [DOI] [PubMed] [Google Scholar]
- 25).Noda H, Iso H, Irie F, Sairenchi T, Ohtaka E, Ohta H: Gender difference of association between LDL cholesterol concentrations and mortality from coronary heart disease amongst Japanese: the Ibaraki Prefectural Health Study. J Intern Med, 2010; 267: 576-587 [DOI] [PubMed] [Google Scholar]
- 26).Lee KJ, Carlin JB: Multiple imputation for missing data: Fully conditional specification versus multivariate normal imputation. Am J Epidemiol, 2010; 171: 624632 [DOI] [PubMed] [Google Scholar]
- 27).Roberts C, Roberts SA: Design and analysis of clinical trials with clustering effects due to treatment. Clin Trials, 2005; 2: 152-162 [DOI] [PubMed] [Google Scholar]
- 28).Rubin DB: Inference and missing data. Biometrika, 1976; 63: 581-592 [Google Scholar]
- 29).Umezawa A, Maruyama C, Endo Y, Suenaga Y, Shijo Y, Kameyama N, Sato A, Nishitani A, Ayaori M, Waki M, Teramoto T, Ikewaki K: Effects of Dietary Education Program for the Japan Diet on Cholesterol Efflux Capacity: A Randomized Controlled Trial. J Atheroscler Thromb, 2022; 29: 881-893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Umemoto S, Onaka U, Kawano R, Kawamura A, Motoi S, Honda N, Kanazashi H, Mitarai M: Effects of a Japanese Cuisine-Based Antihypertensive Diet and Fish Oil on Blood Pressure and Its Variability in Participants with Untreated Normal High Blood Pressure or Stage I Hypertension: A Feasibility Randomized Controlled Study. J Atheroscler Thromb, 2022; 29: 152-173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Ebrahim S, Smith GD: Systematic review of randomised controlled trials of multiple risk factor interventions for preventing coronary heart disease. BMJ (Clinical research ed), 1997; 314: 1666-1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Krogsbøll LT, Jørgensen KJ, Grønhøj Larsen C, Gøtzsche PC: General health checks in adults for reducing morbidity and mortality from disease: Cochrane systematic review and meta-analysis. BMJ, 2012; 345: e7191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Jørgensen T, Jacobsen RK, Toft U, Aadahl M, Glümer C, Pisinger C: Effect of screening and lifestyle counselling on incidence of ischaemic heart disease in general population: Inter99 randomised trial. BMJ, 2014; 348: g3617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).The Multiple Risk Factor Intervention Trial Research Group: Mortality rates after 10.5 years for participants in the Multiple Risk Factor Intervention Trial. Findings related to a priori hypotheses of the trial. JAMA, 1990; 263: 1795-1801 [DOI] [PubMed] [Google Scholar]
- 35).Verdecchiaa P, Gentileb G, Angelia F, Mazzottaa G, Manciac G, Reboldib G: Influence of blood pressure reduction on composite cardiovascular endpoints in clinical trials. J Hypertens, 2010; 28: 1356-1365 [DOI] [PubMed] [Google Scholar]
- 36).Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, Braunwald E, Sabatine MS: Association Between Lowering LDL-C and Cardiovascular Risk Reduction Among Different Therapeutic Interventions: A Systematic Review and Meta-analysis. JAMA, 2016; 316: 1289-1297 [DOI] [PubMed] [Google Scholar]
- 37).Cholesterol Treatment Trialists' (CTT) Collaboration, Fulcher J, O'Connell R, Voysey M, Emberson J, Blackwell L, Mihaylova B, Simes J, Collins R, Kirby A, Colhoun H, Braunwald E, La Rosa J, Pedersen TR, Tonkin A, Davis B, Sleight P, Franzosi MG, Baigent C, Keech A: Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet, 2015; 385: 1397-1405 [DOI] [PubMed] [Google Scholar]
- 38).Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, Golden SH: Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med, 2004; 141: 421-431 [DOI] [PubMed] [Google Scholar]